Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants
Abstract
:1. Introduction
2. Plant Selection and Literature Review Methods
3. Ethnomedical Uses of Selected Medicinal Plants
| Species and Family | Ethnomedical Uses | Countries from Where the Plant Has Been Collected for Chemical Studies | Parts Used for Chemical Isolation | Isolated Compounds |
|---|---|---|---|---|
| Acalypha wilkesiana Müll.Arg. (Euphorbiaceae) | Pulped shoots (i.e., collected when leaves are still red) are applied to cuts and open sores [42]. | Nigeria | Leaves; stem and root barks | Gallic acid, Corilagin, Geraniin, Rutin, Kaempferol 3-O-rutinoside [48]. |
| Ageratum conyzoides (L.) L. (Asteraceae) | Meshed whole plant applied to wounds to enhance healing [32,42]. | Brazil, India | Whole plant | 5,6,7,8,3′,4′,5′-Heptamethoxyflavone, Coumarin [49]; Ageconyflavones A-C, Linderoflavone B, Eupalestin, Nobiletin, 5,6,7,5′-Tetramethoxy-3′,4′-methylenedioxyflavone, Sinensetin, 5,6,7,3′,4′,5′-Hexamethoxyflavone, 5,6,7,8,3′-Pentamethoxy-4′-hydroxyflavone, 5,6,7,8,3′,5′-Hexamethoxy-4′-hydroxyflavone [49,50]. |
| Alphitonia excelsa (Fenzl) Reissek ex Benth. (Rhamnaceae) | Leaves are applied to sore eyes; warm aqueous leaves infusion is used as a bath to ease headaches; decoction from bark, wood, and roots is applied externally to relieve body pains; bark and wood decoction are used as a mouth wash to relieve toothache [34,42]. | Philippines | Twigs | Betulinic acid [51]. |
| Alphitonia petriei Braid & C.T.White (Rhamnaceae) | A decoction made from the bark is applied externally to relieve body pain [34]. | Australia | Leaves; stems | Embolic acid, Alphitolic acid, trans- and cis-Coumaroyl esters of alphitolic acid, Betulinic acid [52]. |
| Angophora costata (Gaertn.) Hochr. ex Britten (Myrtaceae) | An aqueous solution of reddish exudate from the trunk is taken orally against diarrhoea [8,53]. | Australia | Leaves | Costatamins A-C [54]. |
| Antidesma bunius (L.) Spreng. (Phyllanthaceae) | Indicated for headaches, colds, and fevers [32]. | Vietnam | Leaves; fruits | Antidesoside, Podocarpusflavone A, Amentoflavone, Byzantionoside B, Roseoside [55]. |
| Barringtonia racemosa (L.) Spreng. (Lecythidaceae) | Pulverized roots are applied to skin sores [42]. | Bangladesh, China, India, Taiwan, and Vietnam | Stem bark; seeds; roots; leaves | Olean-18-en-3β-O-E-coumaroyl ester, Olean-18-en-3β-O-Z-coumaroyl ester, Germanicol, Germanicone, Betulinic acid, Lupeol, Taraxerol [56]; 3,3’-Dimethoxy ellagic acid, Dihydromyticetin, Gallic acid, Bartogenic acid, Stigmasterol [57,58]; Rutin [59,60]; Nasimalun A and B [61]; Barringtin D1-D3, and M1, Casuarictin, Tellimagrandin I, Valoneic acid dilactone, Schimawalin A [62]; Isoracemosol A, Racemosaceramide A, Racemosol A and E [58,63]; Barringtogenol C [58]; 3β-p-E-Coumaroymaslinic acid, cis-Careaborin, Careaborin, Maslinic acid, 2α,3β,19α-Trihydroxyolean-12-ene-24,28-dioic acid, 3β-p-Z-coumaroylcorosolic acid, Corosolic acid, 1α,2α,3β,19α-Tetrahydroxyurs-12-en-28-oic acid, 19α-Hydroxyl ursolic acid, 3α,19α-Dihydroxyurs-12-en-24,28-dioic acid, Tormentic acid, 3-Hydroxy-7,22-dien-ergosterol [64]; Barringosides G-I [65]. |
| Brasenia schreberi J.F.Gmel. (Combretaceae) | Astringent leaves are used for dysentery [31,42]. | Canada | Quercetin-7-O-glucoside, Gallic acid [66]. | |
| Brucea javanica (L.) Merr. (Simaroubaceae) | Roots and leaves are used as analgesics [32]. | China and Thailand | Aerial; seeds; roots | Brusatol [67]; Demethyl-dehydrobrusatol, Deacetyl-yadanzioside I, Javanicoside G, Yadanziolide C and E, Bruceine A-D and H, Bruceoside A-E, Yadanzioside C and I, Yadanzioside K and L, Dehydrobruceine B, Dehydro-bruceantinol, Deacetylated isobrucein B [68]; brujavanol A and B, bruceine, 11-dehydroklaineanone, 15β-hydroxyklaineanone, 14,15β-dihydroxyklaineanone, 15β-O-acetyl-14hydroxyklaieanone [69] |
| Calophyllum inophyllum L. (Calophyllaceae) | Nut kernel ground with red pigment is mixed with water and rubbed to ease body pain [42]. | China, France, Fiji, French Polynesia, India, Indonesia, Malaysia, Thailand, Taiwan, and Vietnam | Leaves; seeds; twigs; stems; roots | Inophinnin, Inophinone [70,71]; Inophyllin A, Friedelin, Stigmasterol [71,72,73]; Macluraxanthone, Pyranojacareubin, 4-Hydroxyxanthone, Betulinic acid, Inophyxanthone A, Pancixanthone A, Gerontoxanthone B, Jacareubin [71,74,75,76]; Inocalophyllin A and B [77]; Caloxanthone O and P [78]; Tamanolide, Tamanolide D, E1, E2, and P [79,80]; Calophyllolide [81,82]; 3β,23-epoxy-Friedelan-28-oic acid, Epifriedelanol, Canophyllal, Canophyllol, Canophyllic acid, 3-oxo-Friedelan-28-oic acid, Oleanolic acid, 3,4-Secofriedelan-3,28-dioic acid, 27-Hydroxyacetate canophyllic acid, 3-oxo-27-Hydroxyacetate friedelan-28-oic acid [73,83,84]; Caloxanthone Q, 2-Deprenylrheediaxanthone B, 6-Deoxyjacareubin [75,85]; 1,3,6,7-Tetrahydroxy-5-methoxy-4-(1′,1′-dimethyl-2′-propenyl)-8-(3″,3″-dimethyl-2″-propenyl)-xanthone, (2′S)-7-Hydroxycaloxanthone B, Caloxanthone A-C, 7-Prenyljacareubin, Daphnifolin, Tovopyrifolin C, 1,3,5-Trihydroxyxanthone, 2-Hydroxyxanthone [76]; Inophyllums G-1, G-2, and P [86]; Isocalophyllic acid, Amentoflavone [84,87]; 27-[(E)-p-Coumaroyloxy]canophyllic acid, 27-[(Z)-p-coumaroyloxy]canophyllic acid, Methyl shikimate, (3S,5R,6R,7E,9R)-3,5,6-Trihydroxy-β-ionyl-3-O-β-d-glucopyranoside, Benzyl-O-α-l-rhanmopyranosyl (1→6)-β-d-glucopyranoside, Hexylrutinoside, Kaempferol-3-O-α-l-rhamnoside, 27-[(Z)-p-Coumaroyloxy]friedelin-28-carboxylic acid, (22E,24R)-24-Methyl-5α-cholesta-7,22-diene-3β,5,6β-triol, 3-oxo-Friedelan-28-oicacid [87]; trans-2-[2-(Trifluoromethyl)phenyl]-10b,10c-dimethyl-10b,10c-dihydropyrene, anti-4-aza-B-Homo-5α-cholestane-3-one [88]. |
| Centella asiatica (L.) Urb. (Apiaceae) | Juice derived from the plant is taken orally or applied locally for non-specific ulcerations. Powered leaves mixed with lime are applied to sores on babies, and the plant is also indicated for skin diseases [31,32,42,89]. | China, Japan, India, Madagascar, USA, and Vietnam | Whole plant | Asiaticoside, Asiaticoside C, F, G-I, 23-O-Acetyl madecassoside, Asiatic acid, Madecassic acid, Madecassoside, 23-O-Acetylasiaticoside B, Stigmasterol 3-O-β-glucoside, Quercetin 3-O-glucuronide [90,91,92,93,94,95]; Inositol, Centellose [92]; 4′-Hydroxyl-7-methoxyl-6-prenyl-3-O-trans-p-Coumaroyl-flavonol, (2R,3R,2′′S)-3-Furanoyl-brosimacutin E, Epigallocatechin 3-O-p-coumaroate, Pinobanksin-3-propanoate, Kaempferol, Pachypodol, Coryaurone A [94,96]; Asiaticoside B [93,97]; Isomadecassoside [98]; Quadranoside IV, Quercetin, Astragalin, Isoquercetrin [94]; Centelloside E-G, 11-oxo-Asiaticoside B, 11-oxo-Madecassoside, 11(β)-Methoxy asiaticoside B, 11(β)-Methoxy madecassoside, Centellasaponin A, Isoasiaticoside, Scheffoleoside A [93]; 2α,3β,20,23-Tetrahydroxyurs-28-oic acid [99]; Ursolic acid lactone, Ursolic acid, Pomolic acid, Epi-maslinic acid, Corosolic acid, Rosmarinic acid [95]. |
| Centipeda minima (L.) A.Braun & Asch. (Asteraceae) | Infusion and decoction from the whole plant, along with other two species (C. cunninghamii and C. thespidioides) is used to wash eye inflammation due to conjunctiva and purulent ophthalmia [42,43]. | China, Japan, Nepal, South Korea, and Thailand | Whole plant | Brevilin A [100,101]; Apigenin, Quercetin-3-Me-ether, Quercetin-3,3′-diMe-ether, Quercetin-3,7,3′-trimethyl-ether, Quercetin-3,7,3′,4′-tetramethyl-ether, Isobutyroylplenolin, Senecioylplenolin, Aurantiamide acetate, Tetrahydrohelenalin, α-Cyperone [102]; 6-O-Methylacrylylplenolin, 6-O-Isobutyroylplenolin, 6-O-Angeloylplenolin [103]; 2β-(Isobutyryloxy)florilenalin [104]; 2R,3R)-(+)-7,4′-di-O-Methyldihydrokaempferol, Iristectorin A, 4′,5,8-Trihydroxy-7-methoxyisoflavone, 3-Trimethoxyquercetin, 3-O-Caffeoyl-α-glueopyranose, 3-O-Caffeoyl-β-glucopyranose, Quercetin, Epipinoresinol, Hispidulin [105]; Minimaoside A and B [106]; Minimolides G and H [107]; Minimolide A-F, J-L, Cenminolide A, B, Centiplide A, (1S,2S,4R,5S,7R,8S,10R)-2α-Tigloyloxy-4α-angeloyloxyguaia- 11(13)-en-8α,12-olide, Centiplide C-I [101,108,109]; 8,10-Dihydroxy-9(2)-methylbutyryloxythymol, 10-Hydroxy-8,9-dioxyisopropylidene-thymol, 8,9,10-Trihydroxythymol, Thymol-β-glucopyranoside, 9-Hydroxythymol, 8,10-Dihydroxy-9-isobutyryloxythymol, 8-Hydroxy-9,10-diisobutyryloxythymol [110]; 4,5β-Dihydroxy-2β-(isobutyryloxy)-10βH-guai-11(13)-en-12,8β-olide, 4-Hydroxyguaia-9,11(13)-dien-12,8β-olide, 2β-(Isobutyryloxy) florilenalin, Pulchellin-2α-O-tiglate, Florilenalin-2α-O-tiglate [111]; Microhelenalin B and C, Arnicolides B-D, Helenalin-angelate, Helenalin-isovalerate, Helenalin-isobutyrate, Helenalin-3-methyl-2-butanoate, Minimolide E, Minimolide B, 2α-Methoxy-6α-angeloyl-2,3-helenalin [101]; Caloinophyllin A, Nobiletin, Quercetin pentamethyl ether, 3′,4′5,7-Tetramethoxyflavone, 4′,5,7-Trimethoxyflavone, 1,5-Dihydroxyxanthone, 1,8-Dimethoxy-2-hydroxyxanthone, 1,6-Dihydroxy-7-methoxyxanthone, 4-Methoxycaffeic acid [112]. |
| Cleome viscosa L. (Cleomaceae) | The whole meshed plant is applied externally to relieve rheumatism, swellings, headaches, colds, ulcers, and open-sores; seeds are eaten to relieve fever and diarrhoea [8,42]. | India, USA, Nigeria, and Vietnam | Seeds; aerial; leaves | Quercetin 3-O-(2″-acetyl)-glucoside [113]; Malabaric acid, Stigmast-4-en-3-one, Stigmast-4-ene-3,6-dione [114]; Cleomaldeic acid [115]; Lupeol [116]; Astragalin, Visconoside A-C, Vincetoxicoside A and B, Kaempferitrin, Kaempferide 3-O-β-d-glucopyranoside 7-O-α-l-rhamnopyranoside, Kaempferol 3-O-β-d-glucopyranoside 7-O-α-l-rhamnopyranoside, Isorhamnetin 3-O-β-d-glucopyranoside [117,118]; Lactam nonanoic acid [119]. |
| Clerodendrum inerme (L.) Gaertn. (Heliotropiaceae) | Crushed leaves and bark are applied on sores [32,42]. | China, Egypt, India, Taiwan, Thailand, and Vietnam | Aerial; flowers; roots; leaves | 3-Hydroxy-3′,4′-dimethoxychalcone, 3,2′-Dihydroxy-3′,4′-dimethoxychalcone, 5-Hydroxy-7,8-dimethoxyflavone, Eucalyptin [120]; 2-(3-Methoxy-4-hydroxylphenyl) ethyl-O-2”,3”-diacetyl-α-l-rhamnopyranosyl-(1→3)-4-O-(E)-feruloyl-β-d-glucopyranoside, monomelittoside, Melittoside, Inerminoside A1, Acteoside, Isoacteoside, Campneoside I [121,122,123]; 4α-Methyl-24β-ethyl-5α-cholesta-14,25-dien-3β-ol; 24β-Ethylcholesta-5,9(11),22E-trien-3β-ol; 11-Pentacosanone; 6-Nonacosanone, Clerodermic acid [124]; Inerminoside A-D [125,126]; Sammangaosides A-C, Leucosceptoside A, Decaffeoyl-acteoside, Darendoside B, Monomelittoside, Melittoside, (7S,8R)-Dehydrodiconiferyl alcohol 9-O-β-glucopyranoside, (7S,8R)-Dehydrodiconiferyl alcohol 4-O-β-glucopyranoside, β-Glucopyranoside, β-(2′-O-β-Xylopyranosyl) glucopyranoside, Salidroside, (Z)-3-Hexenyl-β-glucopyranoside, 2,6-Dimethoxy-p-hydroquinone 1-O-β-glucopyranoside, Seguinoside K [123]; Lup-1,5,20(29)-trien-3-O-β-d-glucopyranoside [122]; Octacosane, Friedelin, β-Amyrin [127]; Crolerodendrum A and B, Uncinatone, Harwickiic acid, Acacetin, Kaempferol 3,7,4′-trimethyl ether, 5α,8α-Epidioxyergosta-6,22-diene-3β-ol [128,129]; Inermes A and B, 14,15-Dihydro-15β-methoxy-3-epicaryoptin [130]; Hispidulin, Diosmetin [129]. |
| Corymbia terminalis (F.Muell.) K.D.Hill & L.A.S.Johnson (Myrtaceae) | The plant is used for dysentery [131]. | Australia | Gum | Cianidanol, Taxifolin, Aromadendrin, Farrerol [132]. |
| Crinum pedunculatum R.Br. (Amaryllidaceae) | Crushed whole plant-rubbed on body parts stung by marine organism [32,42]. | NA | NA | NA |
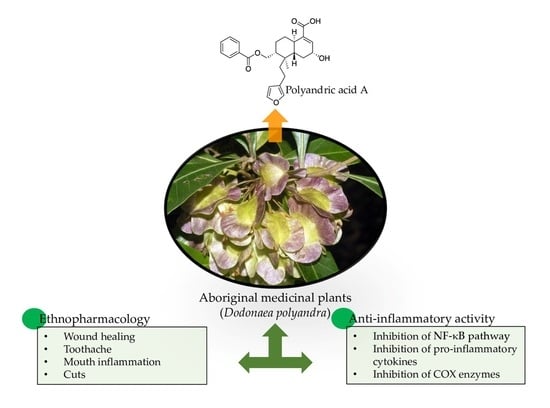
| Dodonaea polyandra Merr. & L.M.Perry (Sapindaceae) | The plant is used for toothache, mouth inflammation, cuts, and open wounds [32]. | Australia | Leaves; stems; leaf resins | Polyandric acid A [133]; 13,17-Epoxy-13-methyl-15-oxo-labda-7-ene, 17-Hydroxy-13-methyl-labda-7,13Z-diene-15-oic acid, 13-Methyl-17-oxo-labda-7,13Z-diene-15-oic acid, Labdane [134]; 15,16-Epoxy-8α-(benzoyloxy)methylcleroda-3,13(16),14-trien-18-oic acid, 15,16-Epoxy-8α-(benzoyloxy)methyl-2α-hydroxycleroda-3,13(16),14-trien-18-oic acid, 15,16-Epoxy-8α-(benzoyloxy)methyl-2-oxocleroda-3,13(16),14-trien-18-oic acid, 15,16-Epoxy-2α-benzoyloxycleroda-3,13(16),14-trien-18-oic acid [135]; 5,7,4′-Trihydroxy-3′(3-methylbut-2-enyl)-3-methoxy flavone, 5,7-Dihydroxy-3′(3-methylbut-2-enyl)-3,4′-dimethoxy flavone, 5,7,4′-Trihydroxy-3′,5′(3-methylbut-2-enyl)-3-methoxy flavone, 5,7,4′-Trihydroxy-3′,5′(3-methylbut-2-enyl)-3,6-dimethoxy flavone, Viscosol, 5,4′-Dihydroxy-3,7-dimethoxyflavone [136]. |
| Dodonaea viscosa (L.) Jacq. (Sapindaceae) | Leaves are chewed to relieve toothache; root juice is used as a mouthwash; leaf juice is used to heal stonefish and stingray wounds; root decoction is applied to wounds [34,42]. | Cameroon, China, and Mexico | Stems; bark | Dodovisins A-F, Dodovisnoid E, (+)-hardwickiic acid, ent-15,16-Epoxy-1,3,13(16),14-clerodatetraen-18-oic acid, Hautriwaic lactone, Dodovisnoid G, Methyl-dodovisate B, 5α-Hydroxy-1,2-dehydro-5,10-dihydroprintziasaure-methylester, Strictic acid, Dodonolide [137]; Hautriwaic acid [138]; 2,18-Dihydroxylabda-7,13(E)-dien-15-oic acid, 5,7-Dihydroxy-3,6,4′-trimethoxy-3′-(4-hydroxy-3-methyl-but-2-enyl)flavone, 2,17-Dihydroxylabda-7,13(E)-dien-15-oic acid, 2-Hydroxylabda-7,13(E)-dien-15-oic acid, 3,6-Dimethoxy-5,7,4′-trihydroxyflavone, Penduletin, Santin [139]. |
| Eleocharis dulcis (Burm.f.) Trin. ex Hensch. (Cyperaceae) | Whole plant infusion in saltwater (preferred for those growing in or near saltwater) is applied to wounds and sealed with a hollow stem of the same plant [25]. | China | Whole plant; peel | 6′-(4″-Hydroxy-3″-methoxy-phenylpropenyl)-1-(10-methoxy-phenylacetone)-1′-O-β-d-glucopyranoside, Susaroyside A, Clausenaglycoside A-D, Emarginone A and B, Thoreliin B, 4-O-(1′,3′-Dihydroxypropan-2′-yl)-dihydroconiferyl alcohol 9-O-β-d-glucopyranoside, 2-[4-(3-Methoxy-1-propenyl)-2-methoxy-phenoxy]-propane-1,3-diol, 6′-O-(E-Cinnamoyl)-coniferin, Methyl 3-(2-O-β-d-glucopyranosyl-3,4,5,6-tetramethoxyphenyl) propanoate, 9-O-(E-Cinnamoyl)-coniferin, 6′-O-(E-Cinnamoyl)-syringin, 2′-O-(E-Cinnamoyl)-syringin [140]. |
| Eucalyptus camaldulensis Dehnh. (Myrtaceae) | Gum (or kino) mixed with water is taken orally (recommended not more than 1.3 g of kino) against diarrhoea; infusion made from aerial parts is used for washing head to heal colds and fevers [42,141,142]. | NA | NA | |
| Euphorbia hirta L. (Euphorbiaceae) | A decoction from dried herb (whole plant) is used for deworming, dysentery, bowel problems, and colic warts [31,42]. | India | Whole plant | Kaempferol, Rutin, Quercetin [143]. |
| Euphorbia tirucalli L. (Euphorbiaceae) | The plant is known for healing skin cancer [32]. | China | Aerial; latex | 12-O-(2E,4E,6E,8E-Tetradecatetraenoyl)-13-O-isobutyroyl-4β-deoxyphorbol, 13-O-acetyl-12-O-(2Z,4E-Octadienoyl)-4β-deoxyphorbol, Pedilstatin, 4β-Deoxy-phorbol-13-acetate, 4α-deoxy-phorbol-13-acetate, 3-O-(2,4,68-Tetradecatetraenoyl) ingenol [144]. |
| Excoecaria agallocha L. (Euphorbiaceae) | Toxic juice from this plant is applied externally to relieve painful punctures caused by marine organisms, such as the sharp spines of some fish. Infusion from the bark is rubbed against body pain [32,42]. | Australia, China, India, Japan, and Vietnam | Leaves; stems; resinous wood; roots; twigs; bark | 12-Deoxyphorbol 13-(3E,5E-decadienoate) [145]; Excoecarins R1 and R2 [146]; 3α,11β-Dihydroxy-ent-isopimara-8(14),15-dien-2-one, 16β-Hydroxy-ent-atisan-3-one, Ribenone, ent-labda-8(17),13E-diene-3β,15-diol, ent-3β-Hydroxybeyer-15-ene-2,12-dione [147]; Excoecarins S, T1-T2, ent-12-oxo-2,3-Secobeyer-15-ene-2,3-dioic acid, ent-15-epoxy-Beyerane-3α-ol, Agallochin H [148]; Excoecarins V1—V3, 3,5,7,3′,5′-Pentahydroxy-2R,3R-flavanonol 3-O-α-l-rhamnopyranoside, ent-Atisane-16α-ol, ent-2,3-Secobeyer-15-ene-2,3-dioic acid, ent-15,18-Dihydroxybezoate, 3,4,5-Trimethoxyphenol 1-O-β-d-(6-galloyl)-glucopyranoside [149]; 3β-[(2E,4E)-5-oxo-Decadienoyloxy]-olean-12-ene, β-Amyrin acetate, Taraxerone, 3-Epitaraxerol, Epilupeol, Taraxerol, Taraxerone, 3β-[(2E,4E)-6-oxo-Decadienoyloxy]-olean-12-ene, Acetyl aleuritolic acid, Cycloart-22-ene-3β,25-diol, β-Sitostenone, (24R)-24-Ethylcholesta-4,22-dien-3-one, β-Sitosterol [150,151]; Excoagallochaols A–E [152]; Agallochins A-E [153,154]; Excoecarins D, E, and K [155]; Agallochins J-L [154,156]; Agallochins F-I, 2-Acetoxy-1,15-beyeradiene-3,12-dione, 2-Hydroxy-1,15-beyeradiene-3,12-dione, ent-kauran-16β-ol-3-one [148,154,157]; Excoecariphenols A-D [158]; Agallochaols K–P, Agallochaol Q, ent-17-Hydroxykaur-15-en-3-one, ent-Kaur-15-en-3β,17-diol, 7-Deoxogeayine, ent-15-Hydroxylabd-8(17),13E-dien-3-one, ent-15,18-Dihydroxylabd-8(17),13E-diene, ent-3β,11α-Dihydroxyisopimara-8(14),15-dien-2-one, ent-3β-Hydroxybeyer-15-en-2,12-dione [159]; ent-16α-Hydroxy-atisane-3,4-lactone, ent-16α-Hydroxy-atisane-3-one, ent-Atisane-3β,16α-diol, ent-3,4-seco-16α-Hydroxyatis-4(19)-en-3-oic acid [160]; Triacontane [161]; Agallochins M-P [159,162,163]; Excagallonoid A, ent-(3α,5β,8α,9β,10α,12α)-3-Hydroxyatis-16-en-14-one, Atis-16-ene-3,14-dione, 2-Hydroxy-atis-1,16-diene-3,14-dione, 12-Hydroxy-13-methylpodocarpa-8,11,13-trien-3-one [164]; Excolides A-B [165]; Afzelin, Quercitrin, Rutin, Kaempferol-3-O-(2-O-acetyl)-α-l-rhamnopyranoside, Kaempferide 3-O-α-l-rhamnopyranoside, Kaempferol 3-O-α-l-arabinofuranoside [166]; Agallolides A-M [167] |
| Flueggea virosa (Roxb. ex Willd.) Royle (Phyllanthaceae) | An aqueous leaf infusion is taken orally to heal internal pains, such as toothache; the liquid is applied to skin sores [42,168]. | China and Taiwan | Aerial; roots | Flueggether A, Virosinine A [169]; Flueggenines A, B, and D, Norsecurinine [170,171,172]; Flueggines A and B [173]; Fluevirosines A-C [174]; Virosaines A and B [171,175]; 3β,12-Dihydroxy-13-methylpodocarpa-6,8,11,13-tetraene, 3β,12-Dihydroxy-13-methylpodocarpa-8,11,13-triene, Spruceanol, ent-3β,12α-Dihydroxypimara-8(14),15-diene, 3α-Hydroxy-12-methoxy-13-methyl-entpodocarp-6,8,11,13-tetraene, 3α-Hydroxy-13-hydroxymethyl-12-methoxy-ent-podocarp-6,8,11,13-tetraene, 3β-Hydroxy-13-hydroxymethyl-12-methoxy-ent-podocarp-6,8,11,13-tetraene, 12-Hydroxy-13-methylent-podocarp-6,8,11,13-tetraen-3-one, 12-Methoxy-13-methyl-ent-podocarp-6,8,11,13-tetraen-3-one, 6β,12-Dihydroxy-13-methyl-ent-podocarp-8,11,13-trien-3-one, 7α,20-Epoxy-3α-hydroxy-12-methoxy-13-methyl-ent-podocarp-8,11,13-triene, 3α,20-Epoxy-3β-hydroxy-12-methoxy-13-methyl-ent-podocarp-8,11,13-triene [176,177]; Fluvirosaones A and B, Virosecurinine [172,178]; 9(10→20)-Abeo-ent-podocarpane; 3,10-Dihydroxy-12-methoxy-13-methyl-9(10→20)-abeo-ent-podocarpa-6,8,11,13-tetraene; 4E-Dehydrochebulic acid trimethyl ester; 12-Hydroxy-20(10→5)-abeo-4,5-seco-podocarpa-5(10),6,8,11,13-pentaen-3-one; Betulinic acid 3β-calfeate, (+)-Ampelosin E [177]; Flueggrenes A and B [179]; Flueggenoids A–E, 6,12-Dihydroxy-13-methyl-7-oxo-ent-podocarpa−5,8,11,13-tetraeno-20,3α-lactone; 10α,12-Dihydroxy-13-methyl-9(10→20)-abeo-ent-podocarpa−6,8,11,13-tetraen-3-one; 12-Hydroxy-20(10→5)-abeo-4,5-seco-podocarpa-5(10),6,8,11,13-pentaen-3-one; Securinine, Bergenin, Norbergenin [171]; Fluevirines E and F, Viroallosecurinine [172]; Flueindolines A–C, Donaxanine, Methyltryptamine, N,N-Dimethyltryptamine, 1-Acetyl-β-carboline, 1-Hydroxymethyl-β-carboline, N-Methyl-1,2,3,4-tetrahydro-β-carboline, Strychnocarpine, Racemate, Hydromethyl-2-methyl-tetrahydro-β-carboline [180]. |
| Heliotropium ovalifolium Forss (Heliotropiaceae) | Herb extract is used to relieve fevers [181]. | India, Egypt, and Zimbabwe | Aerial | Heliophenanthrone [182]; Retronecine, Helifoline [183]; Supinine, 7-Angelyl-heliotridine [184]; 4,7,8-Trimethoxy-naphthalene-2-carboxylic acid, 6-Hydroxy-5,7-dimethoxy-naphthalene-2-carbaldehyde [185]; Heliotropamide [186]. |
| Hibiscus tiliaceus L. (Malvaceae) | Infusions from bark and sapwood (with salt or freshwater) are applied to wounds and covered with the bark of the same plant [25,42]. | China, Japan, and Taiwan | Stem; wood; bark | Hibiscusin, Hibiscusamide, Vanillic acid, 4-Hydroxybenzoic acid, Syringic acid, 4-Hydroxybenzaldehyde, Scopoletin, N-trans-Feruloyltyramine, N-cis-Feruloyltyramine [187]; 27-oic-3-oxo-28-Friedelanoic acid, 3α-Hydroxyfriedelane-2-one, 4α-Hydroxyfriedelane-3-one, Friedelin, Epifriedelanol, Pachysandiol A, 3β-O-(p-Hydroxy-Z-cinnamoyl)oleanolic acid, 3β-O-(p-hydroxy-E-cinnamoyl)oleanolic acid, oleanolic acid [188]; Hibiscusterpene I-V, Hibiscone B and C, Isohemigossypol-1-methyl ether, Virginicin, Parvifloral A, Syriacusin A [189]. |
| Ipomoea brasiliensis (L.) Sweet (I. pes-caprae (L.) R. Br.) (Combretaceae) | Leaves decoction is applied externally for sores; the heated leaves are used to discharge boils [32,42]. | China, India, Mexico, and Thailand | Whole plant | Pescapreins X-XVII [190]; β-Damascenone, Phytol [191]; Pescaproside A and B, Pescapreins I-IX, Stoloniferin III [192]; Ipomeolides A and B, Presqualene alcohol, Icosyl (E)-3-(4-hydroxyphenyl)acrylate, β-Sitosterol-3-O-β-d-glucopyranoside, Stigmasterol, Lupeol [193]. |
| Litsea glutinosa (Lour.) C.B.Rob. (Heliotropiaceae) | Leaves and bark decoctions are applied to sores and to relieve body pain; sometimes, chewed leaves are applied to cuts and sores [32,34,42]. | China and India | Leaves; twigs; heartwood | Glutin, β-sitosterol, Stigmasterol, (−)-Epicatechin, Sitosterol-β-d-glucopyranoside [194]; (3R,4S,5S)-2-Hexadecyl-3-hydroxy-4-methylbutanolide, Litsealactone C, D, and G, Eusmoside C [195]. |
| Macaranga tanarius (L.) Müll.Arg. (Euphorbiaceae) | The plant is known for wound healing [196]. | Japan, Taiwan, Thailand, and Vietnam | Bark; leaves; fruits; glandular trichomes | (2β,5β,10α,13α)-2-Hydroxypimara-9(11),15-dien-12-one, Methyl 2α-hydroxy-3β-[(4-hydroxybenzoyl)oxy]taraxer-14-en-28-oate, 2α-Acetoxy-3β-[(4-hydroxybenzoyl)oxy]-taraxer-14-en-28-oic acid, β-Sitosterol, Friedelin, Friedelin-3β-ol, β-Amyline, Macarangonol, 3β-Acetoxytaraxer-14-en-28-oic acid, 2α-Hydroxy-3β-[(4-hydroxybenzoyl)oxy]taraxer-14-en-28-oic acid [197]; (+)-Pinoresinol 4-O-[6″-O-galloyl]-β-d-glucopyranoside, Roseoside, Icariside B5, (6R,9R)-3-oxo-α-ionol β-d-glucoside, (6R,9S)-3-oxo-α-Ionol β-d-glucoside, (2S,3R)-Dihydrodehydrodiconiferyl alcohol β-d-glucoside, (+)-Pinoresinol 4-O-β-d-glucopyranoside, Scopoline, Rutin, Quercetin 3-O-galactopyranoside, Quercetin 3-O-arabinopyranoside, Isovitexin, Methyl gallate, Hexenyl β-d-glucoside, (E)-2-Hexenyl β-d-glucoside, Malloapeltine [198]; Macarangiosides A-F, Mallophenol B, Lauroside E [199]; Tanariflavanones A-D [198,200,201]; Macaflavanones A-G, Kolavenol [202]; 3′-Geranyl-naringenin [203]; Nymphaeol A-C, Isonymphaeol B, 3′-Geranyl naringenin [200,201,202,203,204]; Macatanarin D, Schweinfurthins E-H, and K-Q,5-((E)-3,5-Dihydroxystyryl)-3-((E)-3,7-dimethylocta-2,6-dien-1-yl)benzene-1,2-diol [205]; Tanarifuranonol, Vomifoliol, Blumenol B, vedelianin, mappain, methyl-mappain [201,206]. |
| Manihot esculenta Crantz (Euphorbiaceae) | The plant is known to be effective against belly aches and diarrhoea [196]. | NA | NA | NA |
| Melaleuca leucadendra (L.) L. (Myrtaceae) | The plant is known to be effective against headache, sinusitis, cough and colds, and skin sores [32,42]. | Egypt | Essential oil | Stachyurin (or casuarinin), Ellagitannin [207]. |
| Merremia tridentata (L.) Hallier f. (Combretaceae) | The whole plant is chewed or soaked in the water before applying it to the sores [131]. | Vietnam | Stem bark | Apigenin, Cynaroside, Luteolin, Cosmosiin, Quercitrin [208]. |
| Morinda citrifolia L. (Rubiaceae) | Leaves extract used to ease headaches [26,31]. | French Polynesia and Japan | Fruits | (+)-3,4,3′,4′-Tetrahydroxy-9,7′α-epoxylignano-7α,9′-lactone, (+)-3,3′-Bisdemethyltanegool, (−)-Pinoresinol, (−)-3,3″-Bisdemethylpinoresinol, Quercetin, Kaempferol, Scopoletin, Isoscopoletin, Vanillin [209]; 1,5,15-Tri-O-methylmorindol, 2-O-(β-d-glucopyranosyl)-1-O-hexanoyl-β-d-gluropyranose, 2-O-(β-d-glucopyranosyl)-1-O-octanoyl-β-d-gluropyranose, 5,15-Di-O-methylmorindol, 1,3-Dihydroxy-2-methoxyanthracene-9,10-dione, 6-O-(β-d-Glucopyranosyl)-1-O-hexanoyl-β-d-glucopyranose, 6-O-(β-d-glucopyranosyl)-1-O-octanoyl-β-d-glucopyranose, 2,6-Di-O-(β-d-Glucopyranosyl)-1-O-hexanoyl-β-d-glucopyranose, 3-Methylbut-3-enyl-β-d-glucopyranose, 3-Methylbut-3-enyl-6-O-β-d-glucopyranosyl-β-d-glucopyranose, Asperulosidic acid, Rutin [210,211]; Nonioside A, (2E,4E,7Z)-deca-2,4,7-trienoate-2-O-β-d-glucopyranosyl-β-d-glucopyranoside, Tricetin [211]. |
| Nauclea orientalis (L.) L. (Rubiaceae) | Aqueous bark infusion is used for sore belly; it is also applied externally to relieve rheumatic pains; the wood infusion is used for relieving fevers [32,43]. | China, Japan, Laos, Papua New Guinea, Thailand, and Vietnam | Heartwood; bark; leaves; stems; roots; | Noreugenin, Naucleoside [212]; Angustine, 18,19-Dihydroangustine, 10-Hydroxyangustine, 3,14,18,19-Tetrahydroangustine, Parvine, Angustoline [213]; Nauclealines A and B, Naucleosides A and B, Strictosamide, Vincosamide, Pumiloside, Kelampayoside A, β-Sitosterol, Sitosteryl β-d-glucoside [214,215]; Naucleaorals A and B [216]; 10-Hydroxystrictosamide, 6′-O-Acetylstrictosamide [215]; α-Pinene, Loganetin, Loganin, Sweroside, Grandifloroside, Methyl 3,4-dihydroxybenzoate, 4-Hydroxycinnamic acid, 3-(2,4-Dihydroxylphenyl)propanoic acid, Methyl 3-(2,4-dihydroxylphenyl)propanoate, Skimmin, Adicardin, Aloe emodin, Pinoresinol [217]; Naucleaorine, Epimethoxynaucleaorine, Strictosidine lactam, 3,4,5-Trimethoxyphenol, 3α-Hydroxyurs-12-en-28-oic acid methyl ester, 3α,23-Dihydroxyurs-12-en-28-oic acid, 3α,19α,23-Trihydroxyurs-12-en-28-oic acid methyl ester, Oleanolic acid [218]; Nauclorienine, Antirhine, Iso-antirhine, Alangine, Naucline, Neonaucline, Angustidine, Subditine [219]. |
| Nelumbo nucifera Gaertn. (Nelumbonaceae) | Milky juice from leaves is used against diarrhoea [31]. | China, India, and Japan | Flowers; rhizome; leaves; seed embryo | 2α,24-Diacetoxy-3β-hydroxyolean-12-en-28-oic acid, Hyptatic acid A, Maslinic acid, Botulin, Lupeol [220]; (R)-Coclaurine, (S)-norcoclaurine, Quercetin 3-O-β-d-glucuronide [221]; Neferine [222,223]; Liensinine, Isoliensinine [224]; Betulinic acid [225]. |
| Ochrosia elliptica Labill. (Apocynaceae) | Bark is known to be good for dysentery [26]. | China and Egypt | Stems and leaves | 10-Methoxyconolidine, Apparicine, Vallesamine, Yunnanensine A, Angustilodine, Isositsirikine, (−)-Echitainine, Pseudo akuammigine [226]; Ursolic acid [227,228]; Ellipticine, elliptinine, methoxyellipticine, reserpiline (elliptine) [229]. |
| Ocimum tenuiflorum L. (Heliotropiaceae) | The plant is used to relieve fevers [230]. | NA | NA | NA |
| Phyllanthus urinaria L. (Phyllanthaceae) | The plant is used against colds [26,131]. | China and Taiwan | Whole plant | Phyllanthin, Phyltetralin, Trimethyl-3,4-dehydrochebulate, Methylgallate, Rhamnocitrin, Methyl brevifolincarboxylate, β-Sitosterol-3-O-β-d-glucopyranoside, Quercitrin, Rutin [231]; Geraniin [232]; Corilagin, Ellagic acid [233]. |
| Phragmites australis (Cav.) Trin. ex Steud. (Plantaginaceae) | The plant is used to treat sore throat [234,235]. | China | Roots | N-p-Coumaroyl serotonin, N-p-Coumaroyl-trypamine, phranisines A-B [236]. |
| Sarcostemma viminale (L.) R. Br (Apocynaceae) | The plant is indicated for skin sores and eye complaints [237]. | NA | NA | NA |
| Scaevola taccada (Gaertn.) Roxb. (Euphorbiaceae) | Leaves decoction is applied externally to skin sores [8,32]. | Thailand | Fruits | Scataccanol, ent-ammirin, Nodachenetin, Marmesin, Xanthyletin, Umbelliferone, 4-Formylsyringol, 6-Hydroxy-7-methyl-1-oxo-4-carbomethoxy octahydrocyclopenta[c]pyran, Loganetin, Matairesinol, 2-(4-Hydroxyphenyl) 3-(3,4-dihydroxyphenyl)-2-propenoate [238]. |
| Scoparia dulcis L. (Plantaginaceae) | Leaves infusion is taken orally to heal stomach pain; the pulped whole plant is used for covering sores and cuts to enhance healing [32]. | Bangladesh and Brazil | Whole plant | Glutinol [239]; Scoparinol [240]; iso-dulcinol, 4-epi-scopadulcic acid B, dulcidiol, scopanolal, dulcinol, and scopadiol [241]. |
| Terminalia catappa L. (Combretaceae) | The plant is indicated for sore throat [196]. | China and New Caledonia | Leaves; bark | Ursolic acid, 2,3,23-Trihydroxyurs-12-en-28-oic acid [242]; 3,4,5-Trimethoxyphenyl-1-O-(4-sulfo)-β-d-glucopyranoside, Chebuloside II, Arjunoglucoside II, Arjunolic acid, Betulinic acid, β-Sitosterol-3-O-β-d-glucopyranoside [243]. |
| Terminalia muelleri Benth. (Combretaceae) | The plant is indicated for skin sores [196]. | Egypt | Leaves | Apigenin-8-C-(2″-O-galloyl) glucoside 1, Luteolin-8-C-(2″-O-galloyl) glucoside 2, 1-O-Galloyl-2,3,4,6-dihexahydroxydiphenoyl-β-d-glucopyranoside, 1,4,6-Tri-O-galloyl-2,3-hexahydroxydiphenoyl-β-d-glucopyranoside, 1,2-Di-O-galloyl-4,6-hexahydroxydiphenoyl-β-d-glucopyranoside, Isostrictinin, 1-O-Galloyl-β-d-glucopyranoside, Combretum caffrum, Ellagic acid, Gallic acid [244,245]; Isoorientin, Vitexin, Chebulinic acid [245]. |
| Verbena officinalis L. (Verbenaceae) | A decoction made from the whole plant is applied externally to overcome fever and rheumatic pain [31,42,246]. | China and India | Aerial | 3,4-Dihydroverbenalin, Daucosterol [247]; Ursolic acid [248]; Verbenalin, Hastatoside, Acteoside, β-sitosterol-d-glucoside [249]. |
4. Overview of the Anti-Inflammatory Mechanism of Action/Pathways

5. Phytochemistry and Pharmacology of Medicinal Plants
5.1. Anti-Inflammatory Crude Extracts
5.2. Anti-Inflammatory Compounds
5.2.1. Terpenes and Terpenoids
5.2.2. Flavonoids
5.2.3. Alkaloids
| Plant Species | Crude Extracts Tested | Isolated Compounds Tested | Anti-Inflammatory Activities |
|---|---|---|---|
| Acalypha wilkesiana | Leaves extract | NT | Suppressed lipopolysaccharide (LPS)-induced nitric oxide (NO), prostaglandins E2 (PGE2), and inducible nitric oxide synthase (iNOS) productions, and cyclooxygenase-2 (COX-2) expression in RAW 264.7 cells; also reduced secretion of tumour necrosis factor-alpha (TNF-α), interleukins 1beta (IL-1β), and IL-6 in LPS-stimulated RAW 264.7 cells [290]; also attenuated carrageenin-induced inflammation/oedema [291,292]. |
| Ageratum conyzoides | Leaves extract; aerial extract | Coumarin, 5′-Methoxy nobiletin, and Eupalestin | Leaves extract is anti-inflammatory in subacute (cotton pellet-induced granuloma) and chronic (formaldehyde-induced arthritis) models of inflammation in rats [293]; also reduced paw oedema [275]; pure compounds reduced p65 nuclear factor kappa B (NF-κB) and p-p38 mitogen-activated protein kinase (MAPK) [276] activities. |
| Alphitonia excelsa | NT | Betulinic acid | Reduced levels of COX-2, NO, TNF-α, and IL1-β in tissues obtained from λ-carrageenan-induced paw oedema mice [294]. |
| Alphitonia petriei | NT | Alphitolic acid, trans- and cis-Coumaroyl esters of alphitolic acid, and Betulinic acid | Reduced or inhibited NO production and TNF-α level in LPS + interferon-gamma (IFN-γ) activated RAW 264.7 cells [52]. |
| Angophora costata | NT | Costatamins A-C | Reduced NO production and TNF-α secretion in RAW 264.7 cells [54]. |
| Antidesma bunius | NT | Antidesoside, Podocarpusflavone A, and Amentoflavone | Reduced NO production in LPS-stimulated BV2 cells and RAW 264.7 cells [55]. |
| Barringtonia racemosa | Inflorescence axes extract | Barringoside I | Inflorescence axes extract inhibited xanthine oxidase (XO) activity [295]; fruit extract—attenuated acute inflammation induced by inflammogens in rat paw oedema and also in carrageenin-induced rat paw oedema [296]. Barringoside I moderately inhibited LPS-induced NO production in RAW 264.7 cells [65]. |
| Brasenia schreberi | NT | Quercimeritrin | Reduced the expression of iNOS and NO in LPS-stimulated RAW 264.7 cells; also prevented the overexpression of COX-2 and granulocyte macrophage-colony-stimulating factor [66]. |
| Brucea javanica | Oil emulsion; seeds extract | Brusatol | Oil emulsion attenuated pathology in dextran sodium sulphate (DSS)-induced colitis in mice and reduced levels of TNF-α, IL-1β, IL-6, IL-8, IL-17, and IFN-γ [265]; seed extract inhibited the production of NO, PGE2, TNF-α, IL-1β, and IL-6 but increased anti-inflammatory IL-10 cytokine [297]. Brusatol reduced TNF-α, pro-IL-1β, PGE2, and NO levels; also suppressed NF-κB signalling pathway in LPS-stimulated macrophages; significantly attenuated pathology in 2,4,6-trinitrobenze sulfonic acid (TNBS)-induced mice colitis; suppressed IL-1β and IL-18 levels, and elevates levels of catalase (CAT), glutathione (GSH), and superoxide dismutase (SOD) enzymes in the colon tissue [269]. |
| Calophyllum inophyllum | Leaves extract | Calophyllolide, and 27-[(E)-p-coumaroyl] canophyllic acid | Leaves extract suppressed LPS-induced NO production, and the expression of iNOS, COX-2, and NF-κB in RAW 264.7 cells [298]. Pure compounds downregulated IL-1β, IL-6, TNF-α, and NO production but upregulated IL-10 in RAW 264.7 cells [82,87]. |
| Centella asiatica | Whole plant extract | Asiatic acid, Isomadecassoside, Asiaticoside G, 11-oxo-Asiaticoside B, and Rosmarinic acid | Crude extract reduced IL-13 and inhibited activation of NF-κB pathway [299,300]. Pure compounds reduced NO production in LPS-stimulated RAW 264.7 cells [94,98,299]. |
| Centipeda minima | Whole plant extract | Brevilin A, Centiplide A, Centiplides H, and Helenalin-isovalerate | Whole plant extract reduced NO production in LPS-induced RAW 264.7 cells and λ-carrageenan-induced paw oedema [262]; also inhibited monocyte chemotaxis and macrophage infiltration in DSS-induced acute colitis mouse model [263]; also inhibited the LPS-induced production of TNF-α and IL-1β [264]. Pure compounds attenuated LPS-induced NF-κB pathway activation and oxidative stress, and thus, suppressed neuroinflammation [301]; also reduced NO production in LPS-activated RAW 264.7 cells [101]; reduced IL-1β secretion to suppress NOD-, LRR- and pyrin domain-containing protein 3 (NLRP3) inflammasome in LPS-induced macrophage cells and monosodium urate (MSU)-challenged peritonitis model [270]. |
| Cleome viscosa | NT | Quercetin 3-O-(2″-acetyl)-glucoside, Cleomiscosins A-C, and Malabaric acid | Reduced carrageenan-induced rat paw oedema [113]; reduced IL-4, TNF-α, and NO production in LPS-stimulated mouse solenocytes [302]; also reduced COX-1 and 2 activities [114]. |
| Clerodendrum inerme | Leaves extract | Hispidulin | Leaves extract inhibited NO production in LPS-stimulated RAW 264.7 cells [274]. Hispidulin inhibited PGE2 production, and iNOS and COX-2 expressions via the blockade of NF-κB DNA-binding activity and c-Jun N-terminal Kinase (JNK) pathway [274]. |
| Corymbia terminalis | NT | Taxifolin, Aromadendrin, Cianidanol, and Farrerol | Suppressed IL-6 level in LPS-stimulated cells; also suppress IL-8 and COX-1 and 2 enzyme activities in keratinocytes [132]. |
| Crinum pedunculatum | Bulb extract | NT | Bulb extract inhibited carrageenin-induced rat paw oedema [303]. |
| Dodonaea polyandra | Leaves extract | Polyandric acid A, 15,16-Epoxy-8α-(benzoyloxy) methyl-2α-hydroxycleroda-3,13(16), and 15,16-Epoxy-2α-benzoyloxycleroda-3,13(16),14-trien-18-oic acid. | Leaves extract reduced 12-O-Tetradecanoylphorbol acetate (TPA)-induced mouse ear oedema [304]. Pure compounds’ topical application significantly reduced IL-1β production in mouse ear tissue in an acute model [133]; attenuated TPA-induced mouse ear oedema [135]. |
| Dodonaea viscosa | Leaves extract | Hautriwaic acid. | Leaves extract reduced carrageenin-induced rat paw oedema [305]. Hautriwaic acid reduced inflammation in TPA-induced mice ear oedema [138]. |
| Eleocharis dulcis | NT | Susaroyside A. | Reduced TNF-α level in LPS-activated macrophage cells [306]. |
| Eucalyptus camaldulensis | Crude EO | NS | Reduced carrageenan-induced paw oedema, xylene-induced ear oedema, and cotton pellet-induced granuloma [307]. |
| Euphorbia hirta | Leaves extract, whole plant extract | β-Amyrin. | Leaves extract inhibited TNF-α production in LPS-treated rats [308]; inhibited LPS-induced NO production in peritoneal macrophages [308]; inhibited NO production and iNO protein expressions in LPS-activated RAW 264.7 cells [309]; reduced carrageenin-induced oedema in rats [310]; inhibited PGE2 production in rabbit synovial fibroblast cells (HIG-82) [311]; whole plant extract inhibited NO production in LPS-induced RAW 264.7 cells [312]; reduced pro-inflammatory cytokines in adjuvant-induced arthritis mice [313]. β-amyrin inhibited the cellular molecules (E-selectin, sICAM-1, and sVCAM-1) involved in the development of atherosclerotic initiation induced by pro-inflammatory cytokines in SVEC4-10 endothelial cells via activation of the endothelial nitric oxide synthase (eNOS) and attenuation of adhesion molecules expressions [314]. |
| Euphorbia tirucalli | Roots extract | NT | Roots extract reduced acetic acid-induced pain/inflammation by inhibiting TNF-α and IFN-γ productions [315]. |
| Excoecaria agallocha | NT | Agallochaols K, O, and Q, ent-17-Hydroxykaur-15-en-3-one, ent-Kaur-15-en-3β,17-diol, ent-15,18-Dihydroxylabd-8(17),13E-diene, Agallolides I and J, and Agallochanin K. | Suppressed the expression of NF-κB and activator protein-1 (AP-1) targeted genes and TNF-α and IL-6 LPS-activated Raw 264.7 cells [154,159,167]. |
| Flueggea virosa | NT | Flueggrenes A. | Inhibited superoxide anion generation and elastase release in N-Formylmethionyl-leucyl-phenylalanine (FMLP)/cytochalasin B (CB) activated-human neutrophils [179]. |
| Heliotropium ovalifolium | NT | 4,7,8-Trimethoxy-naphthalene-2-carboxylic acid and 6-Hydroxy-5,7-dimethoxy-naphthalene-2-carbaldehyde. | Reduced IL-6 and TNF-α in LPS activated human leukemia monocytic cell line (THP-1) cells [185]. |
| Hibiscus tiliaceus | Whole plant extract | NT | Whole plant extract reduced acute auricle swelling induced by dimethylbenzene in mice [316]. |
| Ipomoea pes-caprae | Stem and leaves extract | Eugenol and 2-Methoxy-4-vinylphenol. | Stems and leaves extract reduced trypsin-, histamine-, and bradykinin-induced paw oedema in mice [317]; inhibited prostaglandins synthesis [191]. |
| Litsea glutinosa | Leaves extract | NT | Leaves extract reduced carrageenin-induced paw oedema in mice [318]. |
| Macaranga tanarius | NT | Nymphaeol B. | Nymphaeol B inhibited COX-2 activity and reduced PGE2 production [201] |
| Manihot esculenta | Leaves extract | NS | Leaves extract reduced carrageenan-induced rat paw oedema and xylene-induced ear swelling in mice [319]. |
| Melaleuca leucadendra | Whole plant extract | Stachyurin. | Whole plant extract suppressed LPS-induced NO and PGE2 production, and COX-2 expression in RAW 264.7 cells [320]. Stachyurin (or Casuarinin) reduced ethanol-induced gastric ulceration in rats by increasing mucin production and reducing acidity; it also increased glutathione and catalase levels; it suppressed the immunoexpressing of NF-κB, COX-2, and iNOS to their normal values [207]. |
| Merremia tridentata | NT | Apigenin and Quercetrin. | Apigenin suppressed TNF-α, IL-1β, and IL-6 production in LPS-induced murine BV2 microglia cells, and also suppressed LPS-induced NF-κB pathway activation [321]; Quercetrin inhibited NO production and suppressed pro-inflammatory cytokines (TNF-α, IL-1β and IL-6) in LPS-induced RAW 264.7 cells [322]. |
| Morinda citrifolia | Fruits extract; fruits juice; seeds extract | (+)-3,4,3′,4′-Tetrahydroxy-9,7′α-epoxylignano-7α,9′-lactone, (+)-3,3′-Bisdemethyltanegool, (−)-Pinoresinol, (−)-3,3′-Bisdemethylpinoresinol, Kaempferol, Scopoletin, Isoscopoletin, Vanillin, Asperulosidic acid, Rutin, Nonioside A, (2E,4E,7Z)-deca-2,4,7-Trienoate-2-O-β-d-glucopyranosyl-β-d-glucopyranoside, and Tricetin. | Fruits extract inhibited matrix metalloproteinase-9 (MMP-9) release from LPS-stimulated human monocytes [209]; fruit juice reduced both MPO activity and pro-inflammatory cytokines (TNF-α and IFN-γ) in the intestine of C57BL/6 mice exposed to DSS chemical [323]; fruits extract also reduced intracellular reactive oxygen species (ROS) and suppressed COX-2, IL-8, and PGE2 in Caco-2 cells, and neutrophil chemotaxis by suppressing the translocation of the p65 subunit [324]; seed extract inhibited NO production in LPS-stimulated RAW 264 cells [325]. Pure compounds decreased NO production, the expression of IKKα/β, I-κBα, and NF-κB p65 in LPS-stimulated macrophages [209,211]. |
| Nauclea orientalis | Bark extract | NT | Bark extract protected the myocardium inflammation as a result of doxorubicin (Dox)-induced oxidative stress in Wistar rats [326]. |
| Nelumbo nucifera | Flower extract; fruits extract; rhizome extract; leaves extract | Neferine, Quercetin, Cianidanol, and Betulinic acid. | Flowers extract suppressed TNF-α secretion in LPS-stimulated macrophages [327]; fruits extract—reduced carrageenin-induced paw oedema in rats [328]; also upregulated anti-inflammatory cytokines (IL-10 and 12), and downregulated pro-inflammatory cytokines (IL-6, IL-1β, TNF-α, and IFN-γ) [329]; rhizome extract reduced carrageenin- and serotonin-induced paw oedema in male albino Wistar rats [225]; leaves extract reduces the expression and productions of IL-1β, IL-6, TNF-α, PGE2, and NO; also reduced NF-κB activity by inhibiting NF-κB phosphorylation [330]. Pure compounds reduced LPS and LPS + a pan-caspase inhibitor (Z-VAD)-induced secretion of nitrite, inflammatory cytokines, and expression of iNOS and COX-2; oral administration of Neferine reduced inflammation in DSS-induced colitis model [281,282]; it also reduced IL-6 and TNF-α production in LPS-activated RAW 264.7 cells, and activated peroxisome proliferator-activated receptor (PPARα and PPARγ) [331]; quercetin 3-O-β-d-glucuronid reduced NO release in LPS-treated RAW 264.7 cells [332]; quercetin and Cianidanol inhibited JNK- and NF-κB-regulated pathways [333]; Betulinic acid reduced carrageenin and serotonin-induced rat paw oedema [225]. |
| Ochrosia elliptica | NT | 10-Methoxyconolidine, Ellipticine, apparicine, yunnanensine, isositsirikine | All compounds reduced the production of NO and pro-inflammatory cytokines (TNF-α and IL-6) in LPS-stimulated RAW 264.7 cells and human peripheral blood monocytes [226,280]. |
| Ocimum tenuiflorum | Leaves extract | NS | Leaves extract reduced the LPS-induced inflammation in RAW 264.7 cells [334]. |
| Phragmites australis | Aerial extract | NT | Aerial crude extract inhibited the production of NO and ROS, and pro-inflammatory cytokines (TNF-α and IL-1β) in LPS-induced RAW 264.7 cells [335]. |
| Phyllanthus urinaria | NT | β-Sitosterol-3-O-β-d-glucopyranoside and Corilagin. | Both compounds inhibited the NO production in LPS- and IFN-γ-activated peritoneal macrophages [231]; decreased NF-κB/DNA interactions affecting the IL-8 gene expression in TNF-α treated IB3-1 cells. Inhibited TNF-α induced secretion of monocyte chemoattractant protein-1 (MCP-1) and RANTSE (Regulated on Activation, Normal T cell Expressed and Secreted) as well, suggesting its potential as an anti-inflammatory therapy for cystic fibrosis [336]. |
| Sarcostemma viminale | Whole plant extract | NS | Whole plant extract inhibited the production of pro-inflammatory cytokines in RAW 264.7 cells [337]. |
| Scaevola taccada | Leaves extract | NT | Leaves extract reduced IL-1β levels in the tissues of Sprague-Dawley rats suffering from mastitis [338]. |
| Scoparia dulcis | Whole plant extract | Betulinic acid. | Whole plant extract reduced levels of COX-2, NO, TNF-α, and IL-1β in mice tissues from λ-carrageenan-induced paw oedema [271]; also inhibited both LOX and XO activities [294]. Betulinic acid reduced levels of COX-2, NO, TNF-α, and IL-1β in tissues obtained from mice with λ-carrageenan-induced paw oedema [294]. |
| Terminalia catappa | Leaves extract, bark extract, stem extract | Ursolic acid and Asiatic acid (2α,3β,23-trihydroxyurs-12-en-28-oic acid). | Leaves extract reduced TPA-induced ear oedema in both acute and chronic male ICR (Institute of Cancer Research) mice models by inhibiting myeloperoxidase (MPO) activity [242]; bark extract reduced carrageenan-induced paw oedema by inhibiting cellular infiltration and MPO activity [266]; stem bark extract inhibited IL-1β and nitrite production in RAW 264.7 cells; also reduced colonic damage and weight/length ratio in TNBS-induced colitis model [339]. Ursolic acid and 2α,3β,23-trihydroxyurs-12-en-28-oic acid reduced TPA-induced ear oedema and inhibited MPO activity [242]. |
| Terminalia muelleri | Leaves extract | NT | Leaves extract reduced carrageenan-induced paw oedema and lowers PGE2 and inflammatory cytokines (TNF-α, IL-1β, and IL-6) [245]. |
| Verbena officinalis | Leaves extract | NT | Leaves extract reduced carrageenin-induced paw oedema in mice [249]; also reduced TPA-induced ear oedema [340]. |
5.2.4. Coumarins
5.2.5. Glycosides
6. Biodiscovery Potential and Challenges
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Schippmann, U.; Leaman, D.J.; Cunningham, A.B. Impact of culitvation and gathering of medicinal plants on biodiversity: Global trends and issues (Case Study No 7). In Proceedings of the Biodiversity and the Ecosystem Approach in Agriculture, Forestry and Fisheries, Rome, Italy, 12–13 October 2002; pp. 140–167. [Google Scholar]
- Verpoorte, R. Pharmacognosy in the New Millennium: Leadfinding and Biotechnology. J. Pharm. Pharmacol. 2000, 52, 253–262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calixto, J.B. Twenty-five years of research on medicinal plants in Latin America: A personal view. J Ethnopharmacol 2005, 100, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Wangchuk, P.; Yeshi, K.; Vennos, C.; Mandal, S.C.; Kloos, S.; Nugraha, A.S.; Tashi; Samten. Three medicinal Corydalis species of the Himalayas: Their ethnobotany, pharmacognosy, phytochemistry and pharmacology. J. Herbal Med. 2020, 23, 100384. [Google Scholar] [CrossRef]
- Australian Bureau of Statistics. Cultural Identification. National Aboriginal and Torres Strait Islander Health Survey Table Builder. 2019. Available online: https://www.abs.gov.au/statistics/people/aboriginal-and-torres-strait-islander-peoples/national-aboriginal-and-torres-strait-islander-health-survey/latest-release (accessed on 21 January 2022).
- Australian Bureau of Statistics. Estimates and projections, Aboriginal and Torres Strait Islander Australians, 2006 to 2031; ABS cat. no. 3238.0; ABS: Canberra, Australia, 2019.
- McConvell, P.; Thieberger, N. Keeping track of Indigenous language endangerment in Australia. In Language Diversity in the Pacific: Endangerment and Survival; Cunningham, D., Ingram, D.E., Sumbuk, K., Eds.; Multilingual Matters: Clevedon, Austrailia, 2006; pp. 54–84. [Google Scholar]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; New Holland Publishers: Wahroonga, Australia, 2006. [Google Scholar]
- Barr, A. Aboriginal communities of the Northern Territory of Australia. In Traditional Bush Medicines. An Aboriginal Pharmacopoeia; Greenhouse Publications: Darwin, Australia, 1988. [Google Scholar]
- Locher, C.; Semple, S.J.; Simpson, B.S. Traditional Australian Aboriginal medicinal plants: An untapped resource for novel therapeutic compounds? Future. Med. Chem. 2013, 5, 733–736. [Google Scholar] [CrossRef] [PubMed]
- Packer, J.; Turpin, G.; Ens, E.; Venkataya, B.; Mbabaram, C.; Yirralka, R.; Hunter, J. Building partnerships for linking biomedical science with traditional knowledge of customary medicines: A case study with two Australian Indigenous communities. J. Ethnobiol. Ethnomed. 2019, 15, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barr, A. Traditional Bush Medicines: An Aboriginal Pharmacopoeia; Greenhouse Publications: Richmond, VIC, Australia, 1988. [Google Scholar]
- Guo, Y.; Sakulnarmrat, K.; Konczak, I. Anti-inflammatory potential of native Australian herbs polyphenols. Toxicol. Rep. 2014, 1, 385–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeshi, K.; Crayn, D.; Ritmejeryte, E.; Wangchuk, P. Plant Secondary Metabolites Produced in Response to Abiotic Stresses Has Potential Application in Pharmaceutical Product Development. Molecules 2022, 27, 313. [Google Scholar] [CrossRef]
- Zubair, M.; Rizwan, K.; Rashid, U.; Saeed, R.; Saeed, A.A.; Rasool, N.; Riaz, M. GC/MS profiling, in vitro antioxidant, antimicrobial and haemolytic activities of Smilax macrophylla leaves. Arab. J. Chem. 2017, 10, S1460–S1468. [Google Scholar] [CrossRef] [Green Version]
- Ashraf, I.; Zubair, M.; Rizwan, K.; Rasool, N.; Jamil, M.; Khan, S.A.; Tareen, R.B.; Ahmad, V.U.; Mahmood, A.; Riaz, M.; et al. Chemical composition, antioxidant and antimicrobial potential of essential oils from different parts of Daphne mucronata Royle. Chem. Cent. J. 2018, 12, 135. [Google Scholar] [CrossRef] [Green Version]
- Khalid, A.; Shahid, S.; Khan, S.A.; Kanwal, S.; Yaqoob, A.; Rasool, Z.G.; Rizwan, K. Antioxidant activity and hepatoprotective effect of Cichorium intybus (Kasni) seed extract against carbon tetrachloride-induced liver toxicity in rats. Trop. J. Pharm. Res. 2018, 17. [Google Scholar] [CrossRef] [Green Version]
- Adegboye, O.; Field, M.A.; Kupz, A.; Pai, S.; Sharma, D.; Smout, M.J.; Wangchuk, P.; Wong, Y.; Loiseau, C. Natural-product-based solutions for tropical infectious diseases. Clin. Microbiol. Rev. 2021, 34, e0034820. [Google Scholar] [CrossRef] [PubMed]
- Cock, I.E. Medicinal and aromatic plants—Australia. In Ethnopharmacology Section, Biological, Physiological and Health Sciences, Encyclopedia of Life Support Systems (EOLSS), Developed under the Auspices of the UNESCO; EOLSS Publishers: Oxford, UK, 2011; Available online: http://www.eolss.net (accessed on 30 April 2022).
- Bureau of Meteorology. Map of climate zones of Australia: Government of Australia. 2001. Available online: http://www.bom.gov.au/climate/how/newproducts/images/zones.shtml (accessed on 5 May 2022).
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2017, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, W.J.; Currier, B.L. Analgesic pharmacology: II. Specific analgesics. J. Am. Acad. Orthop. Surg. 2004, 12, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Risser, A.; Donovan, D.; Heintzman, J.; Page, T. NSAID prescribing precautions. Am. Fam. Physician 2009, 15, 1371–1378. [Google Scholar]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; Mandarin, Octopus Publishing Group: Melbourne, Australia, 1992. [Google Scholar]
- Levitt, D. Unwritten pharmacopoeia. Hemisphere 1979, 23, 244–249. [Google Scholar]
- Webb, L.J. Guide to the Medicinal and Poisonous Plants of Queensland; CSIRO Bulletin Number 232; Government Printer: Melbourne, Australia, 1948. [Google Scholar]
- Williams, C. Medicinal Plants in Australia. Bush Medicine; Rosenberg Publishing Pty Ltd.: Kenthurst, Australia, 2010; Volume 1, pp. 76–81. [Google Scholar]
- Williams, C. Medicinal Plants in Australia. Gums, Resins, Tannin and Essential Oils; Rosenberg Publishing Pty Ltd.: Kenthurst, Australia, 2011; Volume 2. [Google Scholar]
- Levitt, D. Plants and People: Aboriginal Uses of Plants on Groote Eyelandt; Australian Institute of Aboriginal Studies: Canberra, Austrailia, 1981. [Google Scholar]
- Low, T. Wild Food Plants of Australia; Angus and Robertson: Melbourne, Australia, 1989. [Google Scholar]
- Maiden, J.H. The useful native plants of Australia; Turner and Henderson: Sydney, Australia, 1889. [Google Scholar]
- Webb, L.J. Some New Records of Medicinal Plants Used by the Aborigines of Tropical Queensland and New Guinea; Royal Society of Queensland: Brisbane, Australia, 1959; Volume 71. [Google Scholar]
- Cribb, A.B.; Cribb, J.W. Wild Medicine in Australia; Collins Publications: Sydney, Australia, 1981. [Google Scholar]
- Webb, L.J. The use of plant medicines and poisons by Australian aborigines. Mankind 1969, 7, 137. [Google Scholar] [CrossRef]
- The Australasian Virtual Herbarium; Council of Heads of Australasian Herbaria. Available online: https://avh.chah.org.au (accessed on 15 February 2022).
- Low, T. Bush Medicine: A Pharmacopoeia of Natural Remedies; Angus and Robertbson: Melbourne, Australia, 1990. [Google Scholar]
- Traditional Aboriginal Medicines: Aboriginal Communities of the Northern Territory; Conservation Commission of the Northern Territory: Darwin, Austrailia, 1993.
- Gott, B. Indigenous use of plants in south-eastern Australia. Telopea 2008, 12, 215–226. [Google Scholar] [CrossRef]
- National Institute of Health (NIH), National Library of Medicine: National Center for Biotechnology Information; Rockville Pike, Bethesda, MD, USA. PubChem Homepgae. Available online: https://pubchem.ncbi.nlm.nih.gov (accessed on 30 April 2022).
- Royal Society of Chemistry, ChemSpider Home Page. Available online: http://www.chemspider.com/ (accessed on 30 April 2022).
- Carlsen, M.H.; Halvorsen, B.L.; Holte, K.; Bohn, S.K.; Dragland, S.; Sampson, L.; Willey, C.; Senoo, H.; Umezono, Y.; Sanada, C.; et al. The total antioxidant content of more than 3100 foods, beverages, spices, herbs and supplements used worldwide. Nutr. J. 2010, 9, 3. [Google Scholar] [CrossRef]
- Lassak, E.V.; McCarthy, T. Australian Medicinal Plants; Methuen: Sydney, Australia, 1983; ISBN 9780454004380. [Google Scholar]
- Reid, E.; Betts, T.J. The records of Western Australian plants used by Aboriginals as medicinal agents. Planta Med. 1979, 36, 164–173. [Google Scholar] [CrossRef]
- Phua, D.H.; Zosel, A.; Heard, K. Dietary supplements and herbal medicine toxicities-when to anticipate them and how to manage them. Int. J. Emerg. Med. 2009, 2, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Wojcikowski, K.; Johnson, D.W.; Gobé, G. Medicinal herbal extracts-renal friend or foe. Part one: The toxicities of medicinal herbs? Nephrology 2004, 9, 313–318. [Google Scholar] [CrossRef] [PubMed]
- Tuechler, A.; Ferrier, A.; Cosgrove, R. Transforming the inedible to the edible: An analysis of the nutritional returns from Aboriginal nut processing in Queensland’s Wet Tropics. Aust. Archaeol. 2014, 79, 1–8. [Google Scholar] [CrossRef]
- Hegarty, M.P.; Hegarty, E.E. Food Safety of Australian Plant Bushfoods; RIRDC Publication: Barton, Australia, 2001; pp. 1–75. [Google Scholar]
- Adesina, S.K.; Idowu, O.; Ogundaini, A.O.; Oladimeji, H.; Olugbade, T.A.; Onawunmi, G.O.; Pais, M. Antimicrobial constituents of the leaves of Acalypha wilkesiana and Aacalypha hispida. Phytother Res. 2000, 14, 371–374. [Google Scholar] [CrossRef]
- Moreira, M.D.; Picanco, M.C.; Barbosa, L.C.; Guedes, R.N.; Barros, E.C.; Campos, M.R. Compounds from Ageratum conyzoides: Isolation, structural elucidation and insecticidal activity. Pest Manag. Sci. 2007, 63, 615–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vyas, A.V.; Mulchandani, N.B. Polyoxygenated flavones from Ageratum conyzoides. Phytochemistry 1986, 25, 2625–2627. [Google Scholar] [CrossRef]
- Fuentes, R.G.; Valenciano, A.L.; Cassera, M.B.; Kingston, D.G.I. Antiproliferative and antiplasmodial investigation of Alphitonia excelsa and Arcangelesia flava. Philipp. J. Sci. 2020, 149, 115–120. [Google Scholar]
- Raju, R.; Gunawardena, D.; Ahktar, M.A.; Low, M.; Reddell, P.; Munch, G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules 2016, 21, 1521. [Google Scholar] [CrossRef] [Green Version]
- Lauterer, J. Chemical and physiological notes on native and acclimatised mydriatic plants of Queensland. Australas. Med. Gaz. 1895, 14, 457–460. [Google Scholar]
- Raju, R.; Singh, A.; Bodkin, F.; Munch, G. Costatamins A-C, new 4-phenylcoumarins with anti-inflammatory activity from the Australian woodland tree Angophora costata (Myrtaceae). Fitoterapia 2019, 133, 171–174. [Google Scholar] [CrossRef]
- Trang, D.T.; Huyen, L.T.; Nhiem, N.X.; Quang, T.H.; Hang, D.T.T.; Yen, P.H.; Tai, B.H.; Anha, H.L.T.; Binh, N.Q.; Van Minha, C.; et al. Tirucallane glycoside from the leaves of Antidesma bunius and inhibitory NO production in BV2 cells and RAW264.7 macrophages. Nat. Prod. Commun. 2016, 11, 935–937. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Deng, Z.; Proksch, P.; Lin, W. Two new 18-en-oleane derivatives from marine mangrove plant, Barringtonia racemosa. Pharmazie 2006, 61, 365–366. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.Y.; Long, L.J.; Wu, J. Chemical constituents of mangrove plant Barringtonia racemosa. Zhong Yao Cai. 2006, 29, 671–672. (In Chinese) [Google Scholar] [PubMed]
- Gowri, P.M.; Radhakrishnan, S.V.; Basha, S.J.; Sarma, A.V.; Rao, J.M. Oleanane-type isomeric triterpenoids from Barringtonia racemosa. J. Nat. Prod. 2009, 72, 791–795. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.K.; Bhattacharya, K.; Mandal, C.; Pal, B.C. Identification and quantification of the active component quercetin 3-O-rutinoside from Barringtonia racemosa, targets mitochondrial apoptotic pathway in acute lymphoblastic leukemia. J. Asian Nat. Prod. Res. 2010, 12, 639–648. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.R.; Patil, C.R.; Jadhav, R.B.; Mahajan, V.K.; Patil, P.R.; Gaikwad, P.S. Anti-arthritic activity of bartogenic acid isolated from fruits of Barringtonia racemosa Roxb. (Lecythidaceae). Evid. Based Complement. Alternat. Med. 2011, 2011, 785245. [Google Scholar] [CrossRef] [Green Version]
- Hasan, C.M.; Khan, S.; Jabbar, A.; Rashid, M.A. Nasimaluns A and B: Neo-clerodane diterpenoids from Barringtonia racemosa. J. Nat. Prod. 2000, 63, 410–411. [Google Scholar] [CrossRef]
- Yoshikawa, S.; Chen, L.G.; Yoshimura, M.; Amakura, Y.; Hatano, T.; Taniguchi, S. Barricyclin D1-a dimeric ellagitannin with a macrocyclic structure-and accompanying tannins from Barringtonia racemosa. Biosci. Biotechnol. Biochem. 2021, 85, 1609–1620. [Google Scholar] [CrossRef]
- Ponnapalli, M.G.; Dangeti, N.; Sura, M.B.; Kothapalli, H.; Akella, V.S.; Shaik, J.B. Self gelating isoracemosol A, new racemosaceramide A, and racemosol E from Barringtonia racemosa. Nat. Prod. Res. 2017, 31, 63–69. [Google Scholar] [CrossRef]
- Xia, H.; Zhang, X.L.; Wang, G.H.; Tong, Y.C.; He, L.; Wang, H.F.; Pei, Y.H.; Chen, Y.J.; Sun, Y. Chemical constituents from Barringtonia racemosa. Zhongguo Zhong Yao Za Zhi. 2016, 41, 2460–2465. (In Chinese) [Google Scholar] [CrossRef]
- Van, Q.T.T.; Vien, L.T.; Hanh, T.T.H.; Huong, P.T.T.; Cuong, N.T.; Thao, N.P.; Thuan, N.H.; Dang, N.H.; Thanh, N.V.; Cuong, N.X.; et al. Acylated flavonoid glycosides from Barringtonia racemosa. Nat. Prod. Res. 2020, 34, 1276–1281. [Google Scholar] [CrossRef]
- Legault, J.; Perron, T.; Mshvildadze, V.; Girard-Lalancette, K.; Perron, S.; Laprise, C.; Sirois, P.; Pichette, A. Antioxidant and anti-inflammatory activities of quercetin 7-O-beta-D-glucopyranoside from the leaves of Brasenia schreberi. J. Med. Food 2011, 14, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Tan, L.; Xie, J.; Lai, Z.; Huang, Y.; Qu, C.; Luo, D.; Lin, Z.; Huang, P.; Su, Z.; et al. Characterization of brusatol self-microemulsifying drug delivery system and its therapeutic effect against dextran sodium sulfate-induced ulcerative colitis in mice. Drug Deliv. 2017, 24, 1667–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Wu, J.; Tan, T.; Guo, W.; Xiong, Z.; Yang, S.; Feng, Y.; Wen, Q. Quassinoids from Brucea javanica and attenuates lipopolysaccharide-induced acute lung injury by inhibiting PI3K/Akt/NF-kappaB pathways. Fitoterapia 2021, 153, 104980. [Google Scholar] [CrossRef] [PubMed]
- Chumkaew, P.; Srisawat, T. Antimalarial and cytotoxic quassinoids from the roots of Brucea javanica. J. Asian Nat. Prod. Res. 2017, 19, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Mah, S.H.; Lian Ee, G.C.; Teh, S.S.; Sukari, M.A. Antiproliferative xanthone derivatives from Calophyllum inophyllum and Calophyllum soulattri. Pak. J. Pharm. Sci. 2015, 28, 425–429. [Google Scholar] [PubMed]
- Lian Ee, G.C.; Mah, S.H.; Rahmani, M.; Taufiq-Yap, Y.H.; Teh, S.S.; Lim, Y.M. A new furanoxanthone from the stem bark of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 956–960. [Google Scholar] [CrossRef]
- Lian Ee, G.C.; Kua, A.S.; Lim, C.K.; Jong, V.; Lee, H.L. Inophyllin A, a new pyranoxanthone from Calophyllum inophyllum (Guttiferae). Nat. Prod. Res. 2006, 20, 485–491. [Google Scholar] [CrossRef]
- Li, Y.Z.; Li, Z.L.; Yin, S.L.; Shi, G.; Liu, M.S.; Jing, Y.K.; Hua, H.M. Triterpenoids from Calophyllum inophyllum and their growth inhibitory effects on human leukemia HL-60 cells. Fitoterapia 2010, 81, 586–589. [Google Scholar] [CrossRef]
- Li, Y.; Li, Z.L.; Liu, M.S.; Li, D.Y.; Zhang, H.; Hua, H.M. Xanthones from leaves of Calophyllum inophyllum Linn. Yao Xue Xue Bao 2009, 44, 154–157. (In Chinese) [Google Scholar]
- Wei, D.J.; Mei, W.L.; Zhong, H.M.; Zeng, Y.B.; Wu, X.D.; Dai, H.F. A new prenylated xanthone from the branches of Calophyllum inophyllum. J. Asian Nat. Prod. Res. 2011, 13, 265–269. [Google Scholar] [CrossRef]
- Haerani, S.N.; Raksat, A.; Pudhom, K. Two new xanthones from the root of Thai Calophyllum inophyllum and their toxicity against colon and liver cancer cells. J. Nat. Med. 2021, 75, 670–674. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.C.; Hung, M.C.; Wang, L.T.; Chen, C.Y. Inocalophyllins A, B and their methyl esters from the seeds of Calophyllum inophyllum. Chem. Pharm. Bull. 2003, 51, 802–806. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, H.F.; Zeng, Y.B.; Xiao, Q.; Han, Z.; Zhao, Y.X.; Mei, W.L. Caloxanthones O and P: Two new prenylated xanthones from Calophyllum inophyllum. Molecules 2010, 15, 606–612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leu, T.; Raharivelomanana, P.; Soulet, S.; Bianchini, J.P.; Herbette, G.; Faure, R. New tricyclic and tetracyclic pyranocoumarins with an unprecedented C-4 substituent. Structure elucidation of tamanolide, tamanolide D and tamanolide P from Calophyllum inophyllum of French Polynesia. Magn. Reson. Chem. 2009, 47, 989–993. [Google Scholar] [CrossRef]
- Ginigini, J.; Lecellier, G.J.; Nicolas, M.; Nour, M.; Hnawia, E.; Lebouvier, N.; Herbette, G.; Lockhart, P.; Raharivelomanana, P. Chemodiversity of Calophyllum inophyllum L. oil bioactive components related to their specific geographical distribution in the South Pacific region. PeerJ 2019, 7, e6896. [Google Scholar] [CrossRef] [Green Version]
- Kalyanaraman, L.; Mohan Kumar, R.; Vishweshwar, P.; Pichai, R.; Narasimhan, S. 5-Meth-oxy-2,2-dimethyl-6-[(2E)-2-methyl-but-2-eno-yl]-10-phenyl-2H,8H-pyrano[2,3-f]chromen-8-one (calophyllolide). Acta Crystallogr. Sect. E Struct. Rep. Online 2010, 66, o1115. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, V.L.; Truong, C.T.; Nguyen, B.C.Q.; Vo, T.V.; Dao, T.T.; Nguyen, V.D.; Trinh, D.T.; Huynh, H.K.; Bui, C.B. Anti-inflammatory and wound healing activities of calophyllolide isolated from Calophyllum inophyllum Linn. PLoS ONE 2017, 12, e0185674. [Google Scholar] [CrossRef] [Green Version]
- Laure, F.; Herbette, G.; Faure, R.; Bianchini, J.P.; Raharivelomanana, P.; Fogliani, B. Structures of new secofriedelane and friedelane acids from Calophyllum inophyllum of French Polynesia. Magn. Reson. Chem. 2005, 43, 65–68. [Google Scholar] [CrossRef]
- Prasad, J.; Shrivastava, A.; Khanna, A.K.; Bhatia, G.; Awasthi, S.K.; Narender, T. Antidyslipidemic and antioxidant activity of the constituents isolated from the leaves of Calophyllum inophyllum. Phytomedicine 2012, 19, 1245–1249. [Google Scholar] [CrossRef]
- Li, Z.L.; Liu, D.; Li, D.Y.; Hua, H.M. A novel prenylated xanthone from the stems and leaves of Calophyllum inophyllum. Nat. Prod. Res. 2011, 25, 905–908. [Google Scholar] [CrossRef]
- Patil, A.D.; Freyer, A.J.; Eggleston, D.S.; Haltiwanger, R.C.; Bean, M.F.; Taylor, P.B.; Caranfa, M.J.; Breen, A.L.; Bartus, H.R.; Johnson, R.K.; et al. The inophyllums, novel inhibitors of HIV-1 reverse transcriptase isolated from the Malaysian tree, Calophyllum inophyllum Linn. J. Med. Chem. 1993, 36, 4131–4138. [Google Scholar] [CrossRef] [PubMed]
- Van Thanh, N.; Jang, H.J.; Vinh, L.B.; Linh, K.T.P.; Huong, P.T.T.; Cuong, N.X.; Nam, N.H.; Van Minh, C.; Kim, Y.H.; Yang, S.Y. Chemical constituents from Vietnamese mangrove Calophyllum inophyllum and their anti-inflammatory effects. Bioorg. Chem. 2019, 88, 102921. [Google Scholar] [CrossRef] [PubMed]
- Susanto, D.F.; Aparamarta, H.W.; Widjaja, A.; Jadid, N.; Gunawan, S. Isolation and identification of cholestane and dihydropyrene from Calophyllum inophyllum. Heliyon 2019, 5, e02893. [Google Scholar] [CrossRef] [PubMed]
- Hurst, E. The Poison Plants of NSW; Snelling Printing Works Pty Ltd.: Sydney, Australia, 1942. [Google Scholar]
- Ren, B.; Luo, W.; Xie, M.-J.; Zhang, M. Two new triterpenoid saponins from Centella asiatica. Phytochem. Lett. 2021, 44, 102–105. [Google Scholar] [CrossRef]
- Rumalla, C.S.; Ali, Z.; Weerasooriya, A.D.; Smillie, T.J.; Khan, I.A. Two new triterpene glycosides from Centella asiatica. Planta Med. 2010, 76, 1018–1021. [Google Scholar] [CrossRef]
- Singh, B.; Rastogi, R.P. A reinvestigation of the triterpenes of Centenella asiatica. Phytochem 1968, 8, 917–921. [Google Scholar] [CrossRef]
- Wu, Z.-W.; Li, W.-B.; Zhou, J.; Liu, X.; Wang, L.; Chen, B.; Wang, M.-K.; Ji, L.; Hu, W.-C.; Li, F. Oleanane- and ursane-type triterpene saponins from Centella asiatica exhibit neuroprotective effects. J. Agric. Food Chem. 2020, 68, 6977–6986. [Google Scholar] [CrossRef]
- Nhiem, N.X.; Tai, B.H.; Quang, T.H.; Kiem, P.V.; Minh, C.V.; Nam, N.H.; Kim, J.H.; Im, L.R.; Lee, Y.M.; Kim, Y.H. A new ursane-type triterpenoid glycoside from Centella asiatica leaves modulates the production of nitric oxide and secretion of TNF-alpha in activated RAW 264.7 cells. Bioorg. Med. Chem. Lett. 2011, 21, 1777–1781. [Google Scholar] [CrossRef]
- Yoshida, M.; Fuchigami, M.; Nagao, T.; Okabe, H.; Matsunaga, K.; Takata, J.; Karube, Y.; Tsuchihashi, R.; Kinjo, J.; Mihashi, K.; et al. Antiproliferative Constituents from Umbelliferae Plants VII.1) Active Triterpenes and Rosmarinic Acid from Centella asiatica. Biol. Pharm. Bull. 2005, 28, 173–175. [Google Scholar] [CrossRef] [Green Version]
- Zheng, J.; Zhou, Q.; Cao, X.; Meng, Y.; Jiang, G.; Xu, P. Two new flavonol derivatives from the whole plants of Centella asiatica and their cytotoxic activities. Phytochem. Lett. 2022, 47, 34–37. [Google Scholar] [CrossRef]
- Sahu, N.P.; Roy, S.K.; Mahato, S.B. Spectroscopic determination of structures of triterpenoid trisaccharides from Centella asiatica. Phytochemistry 1989, 28, 2852–2854. [Google Scholar] [CrossRef]
- Chianese, G.; Masi, F.; Cicia, D.; Ciceri, D.; Arpini, S.; Falzoni, M.; Pagano, E.; Taglialatela-Scafati, O. Isomadecassoside, a New Ursane-Type Triterpene Glycoside from Centella asiatica Leaves, Reduces Nitrite Levels in LPS-Stimulated Macrophages. Biomolecules 2021, 11, 494. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.-L.; Duan, H.-Q.; Takaishi, Y.; Gao, W.-Y. A novel triterpene from Centella asiatica. Molecules 2006, 11, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Oh, H.M.; Kwon, B.-M.; Baek, N.I.; Kim, S.H.; Lee, J.H.; Eun, J.S.; Yang, J.H.; Kim, D.K. Inhibitory activity of 6-O-angeloylprenolin from Centipeda minima on farnesyl protein transferase. Arch Pharm Res. 2006, 29, 64–66. [Google Scholar] [PubMed]
- Xue, P.H.; Zhang, N.; Liu, D.; Zhang, Q.R.; Duan, J.S.; Yu, Y.Q.; Li, J.Y.; Cao, S.J.; Zhao, F.; Kang, N.; et al. Cytotoxic and anti-inflammatory sesquiterpenes from the whole plants of Centipeda minima. J. Nat. Prod. 2021, 84, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-B.; Chun, Y.-T.; Ebizuka, Y.; Sankawa, U. Biologically active constituents of Centipeda minima: Sesquiterpenes of potential anti-allergy activity. Chem. Pharm. Bull. 1991, 39, 3272–3275. [Google Scholar] [CrossRef] [Green Version]
- Taylor, R.S.; Towers, G.H. Antibacterial constituents of the Nepalese medicinal herb, Centipeda minima. Phytochemistry 1998, 47, 631–634. [Google Scholar] [CrossRef]
- Su, M.; Li, Y.; Chung, H.Y.; Ye, W. 2beta-(Isobutyryloxy)florilenalin, a sesquiterpene lactone isolated from the medicinal plant Centipeda minima, induces apoptosis in human nasopharyngeal carcinoma CNE cells. Molecules 2009, 14, 2135–2146. [Google Scholar] [CrossRef] [Green Version]
- Cao, J.; Li, G. Chemical constituents of Centipeda minima. China J. Chin. Mater. Med. 2012, 37, 2301–2303. [Google Scholar]
- Ding, L.F.; Liu, Y.; Liang, H.X.; Liu, D.P.; Zhou, G.B.; Cheng, Y.X. Two new terpene glucosides and antitumor agents from Centipeda minima. J. Asian Nat. Prod. Res. 2009, 11, 732–736. [Google Scholar] [CrossRef]
- Wu, P.; Li, X.G.; Liang, N.; Wang, G.C.; Ye, W.C.; Zhou, G.X.; Li, Y.L. Two new sesquiterpene lactones from the supercritical fluid extract of Centipeda minima. J. Asian Nat. Prod. Res. 2012, 14, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Su, M.X.; Wang, Y.; Wang, G.C.; Ye, W.C.; Chung, H.Y.; Li, J.; Jiang, R.W.; Li, Y.L. Supercritical fluid extraction assisted isolation of sesquiterpene lactones with antiproliferative effects from Centipeda minima. Phytochemistry 2012, 76, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zeng, Y.; Huang, Q.; Wen, S.; Wei, Y.; Chen, Y.; Zhang, X.; Bai, F.; Lu, Z.; Wei, J.; et al. Helenalin from Centipeda minima ameliorates acute hepatic injury by protecting mitochondria function, activating Nrf2 pathway and inhibiting NF-kappaB activation. Biomed Pharmacother. 2019, 119, 109435. [Google Scholar] [CrossRef]
- Liang, H.; Bao, F.; Dong, X.; Tan, R.; Zhang, C.; Lu, Q.; Cheng, Y. Antibacterial thymol derivatives isolated from Centipeda minima. Molecules 2007, 12, 1606–1613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, H.-X.; Bao, F.-K.; Dong, X.-P.; Zhu, H.-J.; Lu, X.-J.; Shi, M.; Lu, Q.; Cheng, Y.-X. Two New Antibacterial Sesquiterpenoids from Centipeda minima. Chem 2007, 4, 2810–2816. [Google Scholar] [CrossRef]
- Ponguschariyagul, S.; Sichaem, J.; Khumkratok, S.; Siripong, P.; Lugsanangarm, K.; Tip-Pyang, S. Caloinophyllin A, a new chromanone derivative from Calophyllum inophyllum roots. Nat. Prod. Res. 2018, 32, 2535–2541. [Google Scholar] [CrossRef] [PubMed]
- Senthamilselvi, M.M.; Kesavan, D.; Sulochana, N. An anti-inflammatory and anti-microbial flavone glycoside from flowers of Cleome viscosa. Org. Med. Chem. Lett. 2012, 2, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, A.A.; Georges, K.; Nair, M.G. Cyclooxygenase enzyme and lipid peroxidation inhibitory terpenoids and steroidal compounds as major constituents in Cleome viscosa leaves. Planta Med. 2021, 88. [Google Scholar] [CrossRef]
- Jente, R.; Jakupovic, J.; Olatunji, G.A. A cembranoid diterpene from Cleome viscosa. Phytochemistry 1990, 29, 666–667. [Google Scholar] [CrossRef]
- Singh, H.; Ali, S.S.; Khan, N.A.; Mishra, A.; Mishra, A.K. Wound healing potential of Cleome viscosa Linn. seeds extract and isolation of active constituent. S. Afr. J. Bot. 2017, 112, 460–465. [Google Scholar] [CrossRef]
- Phan, N.M.; Nguyen, T.P.; Le, T.D.; Mai, T.C.; Phong, M.T.; Mai, D.T. Two new flavonol glycosides from the leaves of Cleome viscosa L. Phytochem. Lett. 2016, 18, 10–13. [Google Scholar] [CrossRef]
- Nguyen, T.P.; Tran, C.L.; Vuong, C.H.; Do, T.H.T.; Le, T.D.; Mai, D.T.; Phan, N.M. Flavonoids with hepatoprotective activity from the leaves of Cleome viscosa L. Nat. Prod. Res. 2017, 31, 2587–2592. [Google Scholar] [CrossRef] [PubMed]
- Jana, A.; Biswas, S.M. Lactam nonanic acid, a new substance from Cleome viscosa with allelopathic and antimicrobial properties. J. Biosci. 2011, 36, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Shahabuddin, S.K.; Munikishore, R.; Trimurtulu, G.; Gunasekar, D.; Devillee, A.; Bodo, B. Two new chalcones from the flowers of Clerodendrum inerme. Nat. Prod. Commun. 2013, 8, 459–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nan, H.; Wu, J.; Zhang, S. A new phenylethanoid glycoside from Clerodendrum inerme. Pharmazie 2005, 60, 798–799. [Google Scholar] [PubMed]
- Fauvel, M.T.; Gleye, J.; Andary, C. Verbascoside: A constituent of Clerodendrum inerme. Planta Med. 1989, 55, 577. [Google Scholar] [CrossRef]
- Kanchanapoom, T.; Kasai, R.; Chumsri, P.; Hiraga, Y.; Yamasaki, K. Megastigmane and iridoid glucosides from Clerodendrum inerme. Phytochemistry 2001, 58, 333–336. [Google Scholar] [CrossRef]
- Pandey, R.; Verma, R.K.; Singh, S.C.; Gupta, M.M. 4α-Methyl-24β-ethyl-5α-cholesta-14,25-dien-3β-ol and 24β-ethylcholesta-5, 9(11), 22E-trien-3β-ol, sterols from Clerodendrum inerme. Phytochemistry 2003, 63, 415–420. [Google Scholar] [CrossRef]
- Caliş, I.; Hosny, M.; Yürüker, A.; Wright, A.D.; Sticher, O. Inerminosides A and B, two novel complex iridoid glycosides from Clerodendrum inerme. J. Nat. Prod. 1994, 57, 494–500. [Google Scholar] [CrossRef]
- Caliş, I.; Hosny, M.; Yürüker, A. Inerminosides A1, C and D, three iridoid glycosides from Clerodendrum inerme. Phytochemistry 1994, 37, 1083–1085. [Google Scholar] [CrossRef]
- Parveen, M.; Khanam, Z.; Ali, M.; Rahman, S.Z. A novel lupene-type triterpenic glucoside from the leaves of Clerodendrum inerme. Nat. Prod. Res. 2010, 24, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Ba Vinh, L.; Thi Minh Nguyet, N.; Young Yang, S.; Hoon Kim, J.; Thi Vien, L.; Thi Thanh Huong, P.; Van Thanh, N.; Xuan Cuong, N.; Hoai Nam, N.; Van Minh, C.; et al. A new rearranged abietane diterpene from Clerodendrum inerme with antioxidant and cytotoxic activities. Nat. Prod. Res. 2018, 32, 2001–2007. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.J.; Lee, H.J.; Chen, H.L.; Fan, P.C.; Ku, Y.L.; Chiou, L.C. Hispidulin, a constituent of Clerodendrum inerme that remitted motor tics, alleviated methamphetamine-induced hyperlocomotion without motor impairment in mice. J. Ethnopharmacol. 2015, 166, 18–22. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Verma, R.K.; Gupta, M.M. Neo-clerodane diterpenoids from Clerodendrum inerme. Phytochem. 2005, 66, 643–648. [Google Scholar] [CrossRef] [PubMed]
- Roth, W.E. Superstition, Magic and Medicine; North Queensland Ethnography Bulletin Number 5; Government Printer: Brisbane, Australia, 1903. [Google Scholar]
- Marzieh, N. The Medicinal Effects of Two Australian Native Plants. Ph.D. Thesis, Queensland University of Technology, Brisbane, Austrailia, 2020. [Google Scholar]
- Simpson, B.S.; Luo, X.; Costabile, M.; Caughey, G.E.; Wang, J.; Claudie, D.J.; McKinnon, R.A.; Semple, S.J. Polyandric acid A, a clerodane diterpenoid from the Australian medicinal plant Dodonaea polyandra, attenuates pro-inflammatory cytokine secretion in vitro and in vivo. J. Nat. Prod. 2014, 77, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Simpson, B.S.; Claudie, D.J.; Smith, N.M.; McKinnon, R.A.; Semple, S.J. Rare, seven-membered cyclic ether labdane diterpenoid from Dodonaea polyandra. Phytochemistry 2012, 84, 141–146. [Google Scholar] [CrossRef]
- Simpson, B.S.; Claudie, D.J.; Gerber, J.P.; Pyke, S.M.; Wang, J.; McKinnon, R.A.; Semple, S.J. In vivo activity of benzoyl ester clerodane diterpenoid derivatives from Dodonaea polyandra. J. Nat. Prod. 2011, 74, 650–657. [Google Scholar] [CrossRef]
- Simpson, B.S.; Claudie, D.J.; Smith, N.M.; Gerber, J.P.; McKinnon, R.A.; Semple, S.J. Flavonoids from the leaves and stems of Dodonaea polyandra: A Northern Kaanju medicinal plant. Phytochemistry 2011, 72, 1883–1888. [Google Scholar] [CrossRef]
- Lei, C.; Wang, X.H.; Liu, Y.N.; Zhao, T.; Hu, Z.; Li, J.Y.; Hou, A.J. Clerodane diterpenoids from Dodonaea viscosa and their inhibitory effects on ATP citrate lyase. Phytochemistry 2021, 183, 112614. [Google Scholar] [CrossRef]
- Salinas-Sanchez, D.O.; Herrera-Ruiz, M.; Perez, S.; Jimenez-Ferrer, E.; Zamilpa, A. Anti-inflammatory activity of hautriwaic acid isolated from Dodonaea viscosa leaves. Molecules 2012, 17, 4292–4299. [Google Scholar] [CrossRef]
- Wabo, H.K.; Chabert, P.; Tane, P.; Note, O.; Tala, M.F.; Peluso, J.; Muller, C.; Kikuchi, H.; Oshima, Y.; Lobstein, A. Labdane-type diterpenes and flavones from Dodonaea viscosa. Fitoterapia 2012, 83, 859–863. [Google Scholar] [CrossRef]
- Wei, R.R.; Ma, Q.G.; Sang, Z.P.; Dong, J.H. Studies on phenylpropanoids from Eleocharis dulcis and their hepatoprotective activities. Zhongguo Zhong Yao Za Zhi. 2021, 46, 1430–1437. [Google Scholar] [CrossRef] [PubMed]
- Campbell, A. Pharmacy of Victorian Aborigines. Aust. J. Pharm. 1973, 54, 894–900. [Google Scholar]
- Maiden, J.H. The Forest Flora of New South Wales; Government Printer: Sydney, Australia, 1922; Volume 7. [Google Scholar]
- Gupta, S.S.; Azmi, L.; Mohapatra, P.K.; Rao, C.V. Flavonoids from whole plant of Euphorbia hirta and their evaluation against experimentally induced gastroesophageal reflux disease in rats. Pharmacogn. Mag. 2017, 13, S127–S134. [Google Scholar] [CrossRef] [PubMed]
- Weng, H.Z.; Tian, Y.; Zhang, J.S.; Huang, J.L.; Tang, G.H.; Yin, S. A new tigliane-type diterpenoid from Euphorbia tirucalli. Nat. Prod. Res. 2021, 35, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Erickson, K.L.; Beutler, J.A.; Cardellina, J.H.; McMahon, J.B.; Newman, D.J.; Boyd, M.R. A novel phorbol ester from Excoecaria agallocha. J. Nat. Prod. 1995, 58, 769–772. [Google Scholar] [CrossRef]
- Konishi, T.; Yamazoe, K.; Konoshima, T.; Maoka, T.; Fujiwara, Y.; Miyahara, K. New bis-secolabdane diterpenoids from Excoecaria agallocha. J. Nat. Prod. 2003, 66, 108–111. [Google Scholar] [CrossRef]
- Kang, J.; Chen, R.Y.; Yu, D.Q. A new isopimarane-type diterpene and a new natural atisane-type diterpene from Excoecaria agallocha. J. Asian Nat. Prod. Res. 2005, 7, 729–734. [Google Scholar] [CrossRef]
- Konishi, T.; Yamazoe, K.; Konoshima, T.; Fujiwara, Y. Seco-labdane type diterpenes from Excoecaria agallocha. Phytochemistry 2003, 64, 835–840. [Google Scholar] [CrossRef]
- Konishi, T.; Yamazoe, K.; Kanzato, M.; Konoshima, T.; Fujiwara, Y. Three diterpenoids (excoecarins V1-V3) and a flavanone glycoside from the fresh stem of Excoecaria agallocha. Chem. Pharm. Bull. 2003, 51, 1142–1146. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.H.; Dai, J.; Chen, X.; Yuan, J.Q. Pentacyclic triterpenoids from leaves of Excoecaria agallocha. Chem. Pharm. Bull. 2006, 54, 920–921. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, M.Q.; Bao, G.M.; Ji, N.Y.; Li, X.M.; Wang, B.G. Triterpenoids and steroids from Excoecaria agallocha. Zhongguo Zhong Yao Za Zhi 2008, 33, 405–408. [Google Scholar] [PubMed]
- Wang, J.D.; Zhang, W.; Li, Z.Y.; Xiang, W.S.; Guo, Y.W.; Krohn, K. Elucidation of excogallochaols A-D, four unusual diterpenoids from the Chinese mangrove Excoecaria agallocha. Phytochemistry 2007, 68, 2426–2431. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, A.S.; Rao, V.L. Five diterpenoids (agallochins A-E) from the mangrove plant Excoecaria agallocha Linn. Phytochemistry 2000, 55, 891–901. [Google Scholar] [CrossRef]
- Jiang, Z.P.; Zou, B.H.; Li, X.J.; Liu, J.J.; Shen, L.; Wu, J. Ent-kauranes from the Chinese Excoecaria agallocha L. and NF-kappaB inhibitory activity. Fitoterapia 2019, 133, 159–170. [Google Scholar] [CrossRef]
- Konishi, T.; Konoshima, T.; Fujiwara, Y.; Kiyosawa, S. Excoecarins D, E, and K, from Excoecaria agallocha. J. Nat. Prod. 2000, 63, 344–346. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.; Rao, V.L.; Sreedhar, K. Agallochins J-L, new isopimarane diterpenoids from Excoecaria agallocha L. Nat. Prod. Res. 2003, 17, 27–32. [Google Scholar] [CrossRef]
- Anjaneyulu, A.S.; Rao, V.L.; Sreedhar, K. ent-Kaurane and beyerane diterpenoids from Excoecaria agallocha. J. Nat. Prod. 2002, 65, 382–385. [Google Scholar] [CrossRef]
- Li, Y.; Yu, S.; Liu, D.; Proksch, P.; Lin, W. Inhibitory effects of polyphenols toward HCV from the mangrove plant Excoecaria agallocha L. Bioorg. Med. Chem. Lett. 2012, 22, 1099–1102. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Yu, S.; Proksch, P.; Gu, J.; Lin, W. TNF-alpha inhibitory diterpenoids from the Chinese mangrove plant Excoecaria agallocha L. Phytochemistry 2010, 71, 2124–2131. [Google Scholar] [CrossRef]
- Wang, Z.C.; Lin, Y.M.; Feng, D.Q.; Ke, C.H.; Lin, P.; Yan, C.L.; Chen, J.D. A new atisane-type diterpene from the bark of the mangrove plant Excoecaria agallocha. Molecules 2009, 14, 414–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Satyan, R.S.; Sakthivadivel, M.; Shankar, S.; Dinesh, M.G. Mosquito larvicidal activity of linear alkane hydrocarbons from Excoecaria agallocha L. against Culex quinquefasciatus Say. Nat. Prod. Res. 2012, 26, 2232–2234. [Google Scholar] [CrossRef] [PubMed]
- Anjaneyulu, A.R.S.; Rao, V.L. Seco diterpenoids from Excoecaria agallocha L. Phytochemistry 2003, 62, 585–589. [Google Scholar] [CrossRef]
- Huyen, T.L.; Nguyen, V.T.; Nguyen, H.M.; Nguyen, T.A.; Tran, T.Q.; Tran, T.H.; Tran, T.H. Agallochin P, a new diterpene from Vietnamese mangrove Excoecaria agallocha L. Nat. Prod. Res. 2021, 35, 1–6. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Z.; Wang, Y.; Li, X. Highly oxygenated ent-atisane and podocarpane diterpenoids from Excoecaria agallocha. Nat. Prod. Res. 2021, 34, 1–7. [Google Scholar] [CrossRef]
- Rao Annam, S.C.V.; Ankireddy, M.; Sura, M.B.; Ponnapalli, M.G.; Sarma, A.V.S.; Basha, S.J. Epimeric excolides from the stems of Excoecaria agallocha and structural revision of rhizophorin A. Org. Lett. 2015, 17, 2840–2843. [Google Scholar] [CrossRef]
- Rifai, Y.; Arai, M.A.; Sadhu, S.K.; Ahmed, F.; Ishibashi, M. New Hedgehog/GLI signaling inhibitors from Excoecaria agallocha. Bioorg. Med. Chem. Lett. 2011, 21, 718–722. [Google Scholar] [CrossRef]
- Jiang, Z.-P.; Yu, Y.; Shen, L. Agallolides A-M, including two rearranged ent-atisanes featuring a bicyclo[3.2.1]octane motif, from the Chinese Excoecaria agallocha. Bioorg. Chem. 2020, 104, 104206. [Google Scholar] [CrossRef]
- Fletcher, T.G. Thanakupi’s Guide to Language & Culture: A Thaynakwith Dictionary; Jennifer Isaacs Arts & Publishing: North Sydney, NSW, USA, 2007. [Google Scholar]
- Zhang, H.; Zhu, K.K.; Han, Y.S.; Luo, C.; Wainberg, M.A.; Yue, J.M. Flueggether A and Virosinine A, Anti-HIV Alkaloids from Flueggea virosa. Org. Lett. 2015, 17, 6274–6277. [Google Scholar] [CrossRef]
- Gan, L.S.; Fan, C.Q.; Yang, S.P.; Wu, Y.; Lin, L.P.; Ding, J.; Yue, J.M. Flueggenines A and B, two novel C,C-linked dimeric indolizidine alkaloids from Flueggea virosa. Org. Lett. 2006, 8, 2285–2288. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, F.F.; Zhu, Z.; Fang, Q.Q.; Qu, S.J.; Zhu, W.; Yang, L.; Zuo, J.P.; Tan, C.H. Flueggenoids A-E, new dinorditerpenoids from Flueggea virosa. Fitoterapia 2019, 133, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, J.; Huo, Z.; Yuwen, H.; Li, Y.; Zhang, Y. Fluevirines E and F, two new alkaloids from Flueggea virosa. Nat. Prod. Res. 2020, 34, 2001–2006. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.X.; Wang, Y.; Zhang, D.M.; Jiang, R.W.; Wang, G.C.; Shi, J.M.; Huang, X.J.; Chen, W.M.; Che, C.T.; Ye, W.C. Flueggines A and B, two new dimeric indolizidine alkaloids from Flueggea virosa. Org. Lett. 2011, 13, 3888–3891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, C.R.; Zhu, K.K.; Gao, A.H.; Luo, C.; Li, J.; Yue, J.M. Fluevirosines A-C: A biogenesis inspired example in the discovery of new bioactive scaffolds from Flueggea virosa. Org. Lett. 2013, 15, 120–123. [Google Scholar] [CrossRef]
- Zhao, B.X.; Wang, Y.; Zhang, D.M.; Huang, X.J.; Bai, L.L.; Yan, Y.; Chen, J.M.; Lu, T.B.; Wang, Y.T.; Zhang, Q.W.; et al. Virosaines A and B, two new birdcage-shaped Securinega alkaloids with an unprecedented skeleton from Flueggea virosa. Org. Lett. 2012, 14, 3096–3099. [Google Scholar] [CrossRef]
- Chao, C.H.; Cheng, J.C.; Shen, D.Y.; Wu, T.S. Anti-hepatitis C virus dinorditerpenes from the roots of Flueggea virosa. J. Nat. Prod. 2014, 77, 22–28. [Google Scholar] [CrossRef]
- Chao, C.H.; Lin, Y.J.; Cheng, J.C.; Huang, H.C.; Yeh, Y.J.; Wu, T.S.; Hwang, S.Y.; Wu, Y.C. Chemical constituents from Flueggea virosa and the structural revision of dehydrochebulic acid trimethyl ester. Molecules 2016, 21, 1239. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.K.; Cai, J.; Yin, Z.Y.; Luo, P.; Li, C.J.; Ma, H.; Seeram, N.P.; Gu, Q.; Xu, J. Fluvirosaones A and B, Two indolizidine alkaloids with a pentacyclic skeleton from Flueggea virosa. Org. Lett. 2018, 20, 991–994. [Google Scholar] [CrossRef]
- Chao, C.H.; Cheng, J.C.; Hwang, T.L.; Shen, D.Y.; Wu, T.S. Trinorditerpenes from the roots of Flueggea virosa. Bioorg. Med. Chem. Lett 2014, 24, 447–449. [Google Scholar] [CrossRef]
- Xie, Q.-J.; Zhang, W.-Y.; Wu, Z.-L.; Xu, M.-T.; He, Q.-F.; Huang, X.-J.; Che, C.-T.; Wang, Y.; Ye, W.-C. Alkaloid constituents from the fruits of Flueggea virosa. Chin. J. Nat. Med. 2020, 18, 385–392. [Google Scholar] [CrossRef]
- Palmer, E. On plants used by the natives of North Queensland, Flinders and Mitchell Rivers for food, medicine and clothing. In Journal and Proceedings of the Royal Society of New South Wales; Acting Government Printer: Sydney, Australia, 1883; Volume 17, pp. 93–113. [Google Scholar]
- Guilet, D.; Guntern, A.; Loset, J.R.; Queiroz, E.F.; Ndjoko, K.; Foggin, C.M.; Hostettmann, K. Absolute configuration of a tetrahydrophenanthrene from Heliotropium ovalifolium by LC-NMR of its Mosher esters. J. Nat. Prod. 2003, 66, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Mohanraj, S.; Kulanthaivel, P.; Subramanian, P.S.; Herz, W. Helifoline, a pyrrolizidine alkaloid from Heliotropium ovalifolium. Phytochemistry 1981, 20, 1991–1995. [Google Scholar] [CrossRef]
- Rizk, A.M.; Hammouda, F.M.; Hassan, N.M. Pyrrolizidine alkaloids from Heliotropium arbainense and Heliotropium ovalifolium. Qatar Univ. Sci. Bull. 1991, 11, 113–119. [Google Scholar]
- Kulkarni-Almeida, A.; Suthar, A.; Goswami, H.; Vishwakarma, R.; Chauhan, V.S.; Balakrishnan, A.; Sharma, S. Novel leads from Heliotropium ovalifolium, 4,7,8-trimethoxy-naphthalene-2-carboxylic acid and 6-hydroxy-5,7-dimethoxy-naphthalene-2-carbaldehyde show specific IL-6 inhibitory activity in THP-1 cells and primary human monocytes. Phytomedicine 2008, 15, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Guntern, A.; Ioset, J.R.; Queiroz, E.F.; Sándor, P.; Foggin, C.M.; Hostettmann, K. Heliotropamide, a novel oxopyrrolidine-3-carboxamide from Heliotropium ovalifolium. J. Nat. Prod. 2003, 66, 1550–1553. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.J.; Huang, S.Y.; Duh, C.Y.; Chen, I.S.; Wang, T.C.; Fang, H.Y. A new cytotoxic amide from the stem wood of Hibiscus tiliaceus. Planta Med. 2006, 72, 935–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Huang, X.; Sattler, I.; Fu, H.; Grabley, S.; Lin, W. Structure elucidation of a new friedelane triterpene from the mangrove plant Hibiscus tiliaceus. Magn. Reson. Chem. 2006, 44, 624–628. [Google Scholar] [CrossRef]
- Muatsumoto, T.; Imahori, D.; Achiwa, K.; Saito, Y.; Ohta, T.; Yoshida, T.; Kojima, N.; Yamashita, M.; Nakayama, Y.; Watanabe, T. Chemical structures and cytotoxic activities of the constituents isolated from Hibiscus tiliaceus. Fitoterapia 2020, 142, 104524. [Google Scholar] [CrossRef]
- Tao, H.; Hao, X.; Liu, J.; Ding, J.; Fang, Y.; Gu, Q.; Zhu, W. Resin glycoside constituents of Ipomoea pes-caprae (beach morning glory). J. Nat. Prod. 2008, 71, 1998–2003. [Google Scholar] [CrossRef]
- Pongprayoon, U.; Baeckström, P.; Jacobsson, U.; Lindström, M.; Bohlin, L. Antispasmodic activity of beta-damascenone and E-phytol isolated from Ipomoea pes-caprae. Planta Med. 1992, 58, 19–21. [Google Scholar] [CrossRef]
- Escobedo-Martínez, C.; Pereda-Miranda, R. Resin glycosides from Ipomoea pes-caprae. J. Nat. Prod. 2007, 70, 974–978. [Google Scholar] [CrossRef] [PubMed]
- Sura, M.B.; Ponnapalli, M.G.; Annam, S.C.V.A.; Bobbili, V.V.P. Ipomeolides A and B, resin glycosides from Ipomoea pes-caprae and combination therapy of ipomeolide A with doxorubicin. J. Nat. Prod. 2019, 82, 1292–1300. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.S.; Huang, R.; Lu, H.; Li, F.Y.; Yang, J.H. A new 2′-oxygenated flavone glycoside from Litsea glutinosa (Lour.) C. B. Rob. Biosci Biotechnol Biochem. 2010, 74, 652–654. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.; Pareek, D.; Dobhal, S.; Sharma, M.C.; Joshi, Y.C.; Dobhal, M.P. Butanolides from methanolic extract of Litsea glutinosa. Chem Biodivers. 2013, 10, 394–400. [Google Scholar] [CrossRef]
- Nai Agama Aboriginal Corporation. Bush Medicine of the Northern Peninsula Area of Cape York; Nai Agama Aboriginal Corporation: Bamaga, Australia, 1995. [Google Scholar]
- Wada, S.; Tanaka, R. Isolation, DNA topoisomerase-II inhibition, and cytotoxicity of three new terpenoids from the bark of Macaranga tanarius. Chem Biodivers. 2006, 3, 473–479. [Google Scholar] [CrossRef]
- Matsunami, K.; Otsuka, H.; Kondo, K.; Shinzato, T.; Kawahata, M.; Yamaguchi, K.; Takeda, Y. Absolute configuration of (+)-pinoresinol 4-O-[6″-O-galloyl]-beta-D-glucopyranoside, macarangiosides E, and F isolated from the leaves of Macaranga tanarius. Phytochemistry 2009, 70, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Matsunami, K.; Takamori, I.; Shinzato, T.; Aramoto, M.; Kondo, K.; Otsuka, H.; Takeda, Y. Radical-scavenging activities of new megastigmane glucosides from Macaranga tanarius (L.) MULL.-ARG. Chem. Pharm. Bull. 2006, 54, 1403–1407. [Google Scholar] [CrossRef] [Green Version]
- Tseng, M.H.; Chou, C.H.; Chen, Y.M.; Kuo, Y.H. Allelopathic prenylflavanones from the fallen leaves of Macaranga tanarius. J. Nat. Prod. 2001, 64, 827–828. [Google Scholar] [CrossRef]
- Phommart, S.; Sutthivaiyakit, P.; Chimnoi, N.; Ruchirawat, S.; Sutthivaiyakit, S. Constituents of the leaves of Macaranga tanarius. J. Nat. Prod. 2005, 68, 927–930. [Google Scholar] [CrossRef]
- Kawakami, S.; Harinantenaina, L.; Matsunami, K.; Otsuka, H.; Shinzato, T.; Takeda, Y. Macaflavanones A-G, prenylated flavanones from the leaves of Macaranga tanarius. J. Nat. Prod. 2008, 71, 1872–1876. [Google Scholar] [CrossRef]
- Nam, S.H.; Yamano, A.; Kim, J.A.; Lim, J.; Baek, S.H.; Kim, J.E.; Kwon, T.G.; Saito, Y.; Teruya, T.; Choi, S.Y.; et al. Prenylflavonoids isolated from Macaranga tanarius stimulate odontoblast differentiation of human dental pulp stem cells and tooth root formation via the mitogen-activated protein kinase and protein kinase B pathways. Int. Endod. J. 2021, 54, 1142–1154. [Google Scholar] [CrossRef] [PubMed]
- Natsume, N.; Yonezawa, T.; Saito, Y.; Woo, J.T.; Teruya, T. Prenylflavonoids from fruit of Macaranga tanarius promote glucose uptake via AMPK activation in L6 myotubes. J. Nat. Med. 2021, 75, 813–823. [Google Scholar] [CrossRef] [PubMed]
- Doan, T.M.H.; Nguyen, T.L.; Trinh, T.T.V.; Vu, V.N.; Phi, T.D.; Litaudon, M.; Roussi, F.; Chau, V.M.; Pham, V.C. Cytotoxic Phenolic Compounds from Fruit Glandular Trichomes of Macaranga tanarius. J. Anal. Methods Chem. 2019, 2019, 2917032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peresse, T.; Jezequel, G.; Allard, P.M.; Pham, V.C.; Huong, D.T.M.; Blanchard, F.; Bignon, J.; Levaique, H.; Wolfender, J.L.; Litaudon, M.; et al. Cytotoxic prenylated stilbenes isolated from Macaranga tanarius. J. Nat. Prod. 2017, 80, 2684–2691. [Google Scholar] [CrossRef] [PubMed]
- Al-Sayed, E.; Michel, H.E.; Khattab, M.A.; El-Shazly, M.; Singab, A.N. Protective role of casuarinin from Melaleuca leucadendra against ethanol-induced gastric ulcer in rats. Planta Med. 2020, 86, 32–44. [Google Scholar] [CrossRef] [PubMed]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.). Biomed. Pharmacother. 2022, 146. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Palu, K.; West, B.J.; Su, C.X.; Zhou, B.N.; Jensen, J.C. Lipoxygenase inhibitory constituents of the fruits of noni (Morinda citrifolia) collected in Tahiti. J. Nat. Prod. 2007, 70, 859–862. [Google Scholar] [CrossRef] [PubMed]
- Akihisa, T.; Matsumoto, K.; Tokuda, H.; Yasukawa, K.; Seino, K.; Nakamoto, K.; Kuninaga, H.; Suzuki, T.; Kimura, Y. Anti-inflammatory and potential cancer chemopreventive constituents of the fruits of Morinda citrifolia (Noni). J. Nat. Prod. 2007, 70, 754–757. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Yu, J.S.; Huang, P.; Qader, M.; Manavalan, A.; Wu, X.; Kim, J.C.; Pang, C.; Cao, S.; Kang, K.S.; et al. Identification of Anti-Inflammatory Compounds from Hawaiian Noni (Morinda citrifolia L.) Fruit Juice. Molecules 2020, 25, 4968. [Google Scholar] [CrossRef] [PubMed]
- Fujita, E.; Fujita, T.; Suzuki, T. On the constituents of Nauclea orientalis L. I. Noreugenin and naucleoside, a new glycoside. (Terpenoids V). Chem. Pharm. Bull. 1967, 15, 1682–1686. [Google Scholar] [CrossRef] [Green Version]
- Erdelmeier, C.A.; Regenass, U.; Rali, T.; Sticher, O. Indole alkaloids with in vitro antiproliferative activity from the ammoniacal extract of Nauclea orientalis. Planta Med. 1992, 58, 43–48. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; ElSohly, H.N.; Jacob, M.R.; Pasco, D.S.; Walker, L.A.; Clark, A.M. New Indole Alkaloids from the Bark of Nauclea orientalis. J. Nat. Prod. 2001, 64, 1001–1005. [Google Scholar] [CrossRef] [PubMed]
- Erdelmeier, C.A.J.; Wright, A.D.; Orjala, J.; Baumgartner, B.; Ralt, T.; Sticher, O. New Indole Alkaloid Glycosides from Nauclea orientalis. Planta Med. 1991, 57, 149–152. [Google Scholar] [CrossRef]
- Sichaem, J.; Surapinit, S.; Siripong, P.; Khumkratok, S.; Jong-aramruang, J.; Tip-pyang, S. Two new cytotoxic isomeric indole alkaloids from the roots of Nauclea orientalis. Fitoterapia 2010, 81, 830–833. [Google Scholar] [CrossRef] [PubMed]
- Dao, P.T.; Quan, T.L.; Mai, N.T. Constituents of the stem of Nauclea orientalis. Nat. Prod. Commun. 2015, 10, 1901–1903. [Google Scholar] [CrossRef] [Green Version]
- He, Z.D.; Ma, C.Y.; Zhang, H.J.; Tan, G.T.; Tamez, P.; Sydara, K.; Bouamanivong, S.; Southavong, B.; Soejarto, D.D.; Pezzuto, J.M.; et al. Antimalarial constituents from Nauclea orientalis (L.) L. Chem Biodivers. 2005, 2, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.P.; Ju, P.K.; Long, J.T.; Lai, L.; Zhao, W.H.; Zhang, C.; Zhang, Z.J.; Fu, Y.H. Cytotoxic indole alkaloids from Nauclea orientalis. Nat. Prod. Res. 2018, 32, 2922–2927. [Google Scholar] [CrossRef]
- Chaudhuri, P.K.; Singh, D. A new triterpenoid from the rhizomes of Nelumbo nucifera. Nat. Prod. Res. 2013, 27, 532–536. [Google Scholar] [CrossRef]
- Kashiwada, Y.; Aoshima, A.; Ikeshiro, Y.; Chen, Y.P.; Furukawa, H.; Itoigawa, M.; Fujioka, T.; Mihashi, K.; Cosentino, L.M.; Morris-Natschke, S.L.; et al. Anti-HIV benzylisoquinoline alkaloids and flavonoids from the leaves of Nelumbo nucifera, and structure-activity correlations with related alkaloids. Bioorg. Med. Chem. 2005, 13, 443–448. [Google Scholar] [CrossRef]
- Tomita, M.; Furukawa, H.; Yang, T.H.; Liu, T.J. Hiroshi Furukawa: On the alkaloids of Nelumbo nucifera Gaertn, IX. alkaloids of Loti embryo. (2). Structure of Neferine, a new Biscoclaurine alkaloid. Chem. Pharm. Bull. 1965, 13, 39. [Google Scholar] [CrossRef] [Green Version]
- Itoh, A.; Saitoh, T.; Tani, K.; Uchigaki, M.; Sugimoto, Y.; Yamada, J.; Nakajima, H.; Oshiro, H.; Sun, S.; Tanahashi, T. Bisbenzylisoquinoline Alkaloids from Nelumbo nucifera. Chem. Pharm. Bull. 2011, 59, 947–951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, M.; Furukawa, H.; Yang, T.-H.; Lin, T.-J. On the Alkaloids of Nelumbo nucifera Gaertn. VIII. Studies on the alkaloids of Loti embryo. (1). Structure of Isoliensinine a New Biscoclaurine Type Alkaloid. Chem. Pharm. Bull. 1965, 13, 39–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mukherjee, P.K.; Saha, K.; Das, J.; Pal, M.; Saha, B.P. Studies on the anti-inflammatory activity of rhizomes of Nelumbo nucifera. Planta Med. 1997, 63, 367–369. [Google Scholar] [CrossRef]
- Chen, A.-H.; Liu, Y.-P.; Wang, Z.-X.; Ma, Y.-L.; Jiang, Z.-H.; Lai, L.; Guo, R.-R.; Long, J.-T.; Lin, S.-X.; Xu, W.; et al. Structurally diverse indole alkaloids from Ochrosia elliptica. Heterocycles 2017, 94, 743–749. [Google Scholar]
- Labib, R.M.; Ebada, S.S.; Youssef, F.S.; Ashour, M.L.; Ross, S.A. Ursolic acid, a natural pentacylcic triterpene from Ochrosia elliptica and its role in the management of certain neglected tropical diseases. Pharmacogn. Mag. 2016, 12, 319–325. [Google Scholar] [CrossRef] [PubMed]
- Elshamy, A.I.; Farrag, A.-R.H.; Mohamed, S.H.; Ali, N.A.; Mohamed, T.A.; Menshawy, M.M.; Zaglool, A.W.; Efferth, T.; Hegazy, M.-E.F. Gastroprotective effects of ursolic acid isolated from Ochrosia elliptica on ethanol-induced gastric ulcer in rats. Med. Chem. Res. 2019, 29, 113–125. [Google Scholar] [CrossRef]
- Goodwin, S.; Smith, A.F.; Horning, E.C. Alkaloids of Ochrosia elliptica Labill. J. Am. Chem. Soc. 1959, 81, 1903–1908. [Google Scholar] [CrossRef]
- Bailey, F.M. Medicinal plants of Queensland. Proc. Linn. Soc. New South Wales 1880, 5, 1–29. [Google Scholar] [CrossRef]
- Fang, S.H.; Rao, Y.K.; Tzeng, Y.M. Anti-oxidant and inflammatory mediator’s growth inhibitory effects of compounds isolated from Phyllanthus urinaria. J. Ethnopharmacol. 2008, 116, 333–340. [Google Scholar] [CrossRef]
- Lin, S.Y.; Wang, C.C.; Lu, Y.L.; Wu, W.C.; Hou, W.C. Antioxidant, anti-semicarbazide-sensitive amine oxidase, and anti-hypertensive activities of geraniin isolated from Phyllanthus urinaria. Food Chem. Toxicol. 2008, 46, 2485–2492. [Google Scholar] [CrossRef]
- Jikai, L.; Yue, H.; Henkel, T.; Weber, K. One step purification of corilagin and ellagic acid from Phyllanthus urinaria using high-speed countercurrent chromatography. Phytochem. Anal. 2002, 13, 1–3. [Google Scholar] [CrossRef]
- Gott, B. SAUSE Database, South Australian Plants Used by Aborigines; Department of Ecology and Evolutionary Biology, Monash University: Melbourne, Australia, 1992. [Google Scholar]
- Clarke, P.A. Aboriginal uses of plants as medicines, narcotics and poisons in southern South Australia. J. Anthropol. Soc. South Aust. 1987, 25, 3–23. [Google Scholar]
- Chen, Y.; Li, L.; Jiang, L.R.; Tan, J.Y.; Guo, L.N.; Wang, X.L.; Dong, W.; Wang, W.B.; Sun, J.K.; Song, B. Alkaloids constituents from the roots of Phragmites australis (Cav.) Trin. ex Steud. with their cytotoxic activities. Nat. Prod. Res. 2022, 36, 1454–1459. [Google Scholar] [CrossRef] [PubMed]
- Forster, P.I. A taxonomic revision of Sarcostemma R.Br. subgenus Sarcostemma (Asclepiadaceae: Asclepiadeae) in Australia. Austral. Syst. Bot. 1992, 5, 53–70. [Google Scholar] [CrossRef]
- Suthiwong, J.; Thongsri, Y.; Yenjai, C. A new furanocoumarin from the fruits of Scaevola taccada and antifungal activity against Pythium insidiosum. Nat. Prod. Res. 2017, 31, 453–459. [Google Scholar] [CrossRef] [PubMed]
- Freire, S.M.; Torres, L.M.; Roque, N.F.; Souccar, C.; Lapa, A.J. Analgesic activity of a triterpene isolated from Scoparia dulcis L. (Vassourinha). Mem. Inst. Oswaldo Cruz. 1991, 86, 149–151. [Google Scholar] [CrossRef]
- Ahmed, M.; Shikha, H.A.; Sadhu, S.K.; Rahman, M.T.; Datta, B.K. Analgesic, diuretic, and anti-inflammatory principle from Scoparia dulcis. Pharmazie. 2001, 56, 657–660. [Google Scholar]
- Ahsan, M.; Islam, S.K.N.G.; Gray, A.I.; Stimson, W.H. Cytotoxic Diterpenes from Scoparia dulcis. J. Nat. Prod. 2003, 66, 958–961. [Google Scholar] [CrossRef]
- Fan, Y.M.; Xu, L.Z.; Gao, J.; Wang, Y.; Tang, X.H.; Zhao, X.N.; Zhang, Z.X. Phytochemical and antiinflammatory studies on Terminalia catappa. Fitoterapia 2004, 75, 253–260. [Google Scholar] [CrossRef]
- Pertuita, D.; Mitaine-Offera, A.-C.; Miyamotob, T.; Tanakab, C.; Delemasurec, S.; Dutartrec, P.; Lacaille-Duboisa, M.-A. A new aromatic compound from the stem bark of Terminalia catappa. Nat. Prod. Commun. 2015, 10, 1005–1007. [Google Scholar] [CrossRef] [Green Version]
- El-Kashak, W.A.; Osman, S.M.; Gaara, A.H.; El-Toumy, S.A.; Mohamed, T.K.; Brouard, I. Phenolic metabolites, biological activities, and isolated compounds of Terminalia muelleri extract. Pharm Biol 2017, 55, 2277–2284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fahmy, N.M.; Al-Sayed, E.; Abdel-Daim, M.M.; Singab, A.N. Anti-inflammatory and analgesic activities of Terminalia muelleri Benth. (Combretaceae). Drug Dev. Res. 2017, 78, 146–154. [Google Scholar] [CrossRef] [PubMed]
- Woolls, W. A Contribution to the Flora of Australia; F. White: Sydney, Australia, 1867. [Google Scholar]
- Zhang, T.; Ruan, J.L.; Lu, Z.M. Studies on chemical constituents of aerial parts of Verbena officinalis L. Zhongguo Zhong Yao Za Zhi. 2000, 25, 676–678. (In Chinese) [Google Scholar] [PubMed]
- Liu, C.H.; Liu, Y. Determination of ursolic acid in herba of Verbena officinalis by HPLC. Zhongguo Zhong Yao Za Zhi. 2002, 27, 916–918. (In Chinese) [Google Scholar]
- Deepak, M.; Handa, S.S. Antiinflammatory activity and chemical composition of extracts of Verbena officinalis. Phytother. Res. 2000, 14, 463–465. [Google Scholar] [CrossRef]
- Wang, N.; Liang, H.; Zen, K. Molecular mechanisms that influence the macrophage m1-m2 polarization balance. Front Immunol. 2014, 5, 614. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-kappaB signaling in inflammation. Signal Transduct Target 2017, 2. [Google Scholar] [CrossRef] [Green Version]
- Newton, K.; Dixit, V.M. Signaling in innate immunity and inflammation. Cold Spring Harb. Perspect. Biol. 2012, 4, a006049. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Knowles, R.G.; Moncada, S. Nitric oxide synthases in mammals. Biochem J. 1994, 298, 249–258. [Google Scholar] [CrossRef]
- Solà, C.; Barrón, S.; Tusell, J.M.; Serratosa, J. The Ca2+/calmodulin system in neuronal hyperexcitability. Int. J. Biochem. Cell Biol. 2001, 33, 439–455. [Google Scholar] [CrossRef]
- Wink, D.A.; Mitchell, J.B. Chemical biology of nitric oxide: Insights into regulatory, cytotoxic, and cytoprotective mechanisms of nitric oxide. Free Radic. Biol. Med. 1998, 25, 434–456. [Google Scholar] [CrossRef]
- Simon, L.S. Role and regulation of cyclooxygenase-2 during inflammation. Am. J. Med. 1999, 106, 37S–42S. [Google Scholar] [CrossRef]
- McDaniel, M.M.; Kottyan, L.C.; Singh, H.; Pasare, C. Suppression of inflammasome activation by IRF8 and IRF4 in cDCs is critical for T cell priming. Cell Rep. 2020, 31, 107604. [Google Scholar] [CrossRef] [PubMed]
- Sugita, R.; Kuwabara, H.; Kubota, K.; Sugimoto, K.; Kiho, T.; Tengeiji, A.; Kawakami, K.; Shimada, K. Simultaneous Inhibition of PGE2 and PGI2 Signals Is Necessary to Suppress Hyperalgesia in Rat Inflammatory Pain Models. Mediators Inflamm. 2016, 2016, 9847840. [Google Scholar] [CrossRef] [Green Version]
- Tegeder, I. COX-1 and COX-2 in Pain. In Encyclopedia of Pain; Gebhart, G.F., Schmidt, R.F., Eds.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Huang, S.S.; Chiu, C.S.; Lin, T.H.; Lee, M.M.; Lee, C.Y.; Chang, S.J.; Hou, W.C.; Huang, G.J.; Deng, J.S. Antioxidant and anti-inflammatory activities of aqueous extract of Centipeda minima. J. Ethnopharmacol. 2013, 147, 395–405. [Google Scholar] [CrossRef]
- Chan, B.D.; Wong, W.Y.; Lee, M.M.; Leung, T.W.; Shum, T.Y.; Cho, W.C.; Chen, S.; Tai, W.C. Centipeda minima extract attenuates dextran sodium sulfate-induced acute colitis in mice by inhibiting macrophage activation and monocyte chemotaxis. Front Pharmacol. 2021, 12, 738139. [Google Scholar] [CrossRef]
- Li, S.Y.; Zhou, Y.L.; He, D.H.; Liu, W.; Fan, X.Z.; Wang, Q.; Pan, H.F.; Cheng, Y.X.; Liu, Y.Q. Centipeda minima extract exerts antineuroinflammatory effects via the inhibition of NF-kappaB signaling pathway. Phytomedicine 2020, 67, 153164. [Google Scholar] [CrossRef]
- Huang, Y.F.; Zhou, J.T.; Qu, C.; Dou, Y.X.; Huang, Q.H.; Lin, Z.X.; Xian, Y.F.; Xie, J.H.; Xie, Y.L.; Lai, X.P.; et al. Anti-inflammatory effects of Brucea javanica oil emulsion by suppressing NF-kappaB activation on dextran sulfate sodium-induced ulcerative colitis in mice. J. Ethnopharmacol. 2017, 198, 389–398. [Google Scholar] [CrossRef]
- Daram, P.; Jitta, S.R.; Shreedhara, C.S.; Misra, C.S.; Gourishetti, K.; Lobo, R. Investigation of anti-inflammatory and anti-arthritic potentials of Terminalia catappa bark using in vitro assays and carrageenan-induced inflammation, complete Freund’s adjuvant induced arthritis model in rats. S. Afr. J. Bot. 2021, 141, 313–321. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar]
- Feng, Z.; Cao, J.; Zhang, Q.; Lin, L. The drug likeness analysis of anti-inflammatory clerodane diterpenoids. Chin. Med. 2020, 15, 126. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wang, T.; Dou, Y.; Huang, Y.; Qu, C.; Gao, J.; Huang, Z.; Xie, Y.; Huang, P.; Lin, Z.; et al. Brusatol ameliorates 2,4,6-trinitrobenzenesulfonic acid-induced experimental colitis in rats: Involvement of NF-kappaB pathway and NLRP3 inflammasome. Int. Immunopharmacol. 2018, 64, 264–274. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; Xu, G.; Zhan, X.; Wang, Z.; Wang, Y.; Liu, H.; Hou, X.; Shi, W.; Ma, J.; Bai, Z.; et al. Brevilin A inhibits NLRP3 inflammasome activation in vivo and in vitro by acting on the upstream of NLRP3-induced ASC oligomerization. Mol. Immunol. 2021, 135, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.C.; Peng, W.H.; Chiu, T.H.; Lai, S.C.; Lee, C.Y. Anti-inflammatory effects of Scoparia dulcis L. and betulinic acid. Am. J. Chin. Med. 2011, 39, 943–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki, S.J.; Crespo, J.F.; Cabanillas, B. Anti-inflammatory effects of flavonoids. Food Chem. 2019, 299, 125124. [Google Scholar] [CrossRef]
- Choy, K.W.; Murugan, D.; Leong, X.F.; Abas, R.; Alias, A.; Mustafa, M.R. Flavonoids as natural anti-inflammatory agents targeting nuclear factor-Kappa B (NFkappaB) signaling in cardiovascular diseases: A Mini Review. Front Pharmacol. 2019, 10, 1295. [Google Scholar] [CrossRef] [Green Version]
- Srisook, K.; Srisook, E.; Nachaiyo, W.; Chan-In, M.; Thongbai, J.; Wongyoo, K.; Chawsuanthong, S.; Wannasri, K.; Intasuwan, S.; Watcharanawee, K. Bioassay-guided isolation and mechanistic action of anti-inflammatory agents from Clerodendrum inerme leaves. J. Ethnopharmacol. 2015, 165, 94–102. [Google Scholar] [CrossRef]
- Faqueti, L.G.; Brieudes, V.; Halabalaki, M.; Skaltsounis, A.L.; Nascimento, L.F.; Barros, W.M.; Santos, A.R.; Biavatti, M.W. Antinociceptive and anti-inflammatory activities of standardized extract of polymethoxyflavones from Ageratum conyzoides. J. Ethnopharmacol. 2016, 194, 369–377. [Google Scholar] [CrossRef]
- Vigil de Mello, S.V.; da Rosa, J.S.; Facchin, B.M.; Luz, A.B.; Vicente, G.; Faqueti, L.G.; Rosa, D.W.; Biavatti, M.W.; Frode, T.S. Beneficial effect of Ageratum conyzoides Linn (Asteraceae) upon inflammatory response induced by carrageenan into the mice pleural cavity. J. Ethnopharmacol. 2016, 194, 337–347. [Google Scholar] [CrossRef]
- Bairwa, K.; Singh, I.N.; Roy, S.K.; Grover, J.; Srivastava, A.; Jachak, S.M. Rotenoids from Boerhaavia diffusa as potential anti-inflammatory agents. J. Nat. Prod. 2013, 76, 1393–1398. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Zheng, T.T.; Li, X.; Liang, Y.; Wang, L.J.; Huang, Y.C.; Xiao, H.T. Plant-derived alkaloids: The promising disease-modifying agents for inflammatory bowel disease. Front. Pharmacol. 2019, 10, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa-Filho, J.M.; Piuvezam, W.R.; Moura, M.D.; Silva, M.S.; Lima, K.V.B.; da-Cunhra, E.V.L.; Fechine, I.M.; Takemura, O.S. Anti-inflammatory activity of alkaloids: A twenty-century review. Braz. J. Pharmacogn. 2006, 16, 109–139. [Google Scholar] [CrossRef] [Green Version]
- Tian, L.X.; Li, X.Y.; Tang, X.; Zhou, X.Y.; Luo, L.; Ma, X.Y.; Tang, W.Q.; Yu, J.; Ma, W.; Yang, X.; et al. Ellipticine conveys protective effects to lipopolysaccharide-activated macrophages by targeting the JNK/AP-1 signaling pathway. Inflammation 2020, 43, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Guo, Y.; Min, X.; Pei, L.; Chen, X. Neferine, a bisbenzylisoquinoline alkaloid, ameliorates dextran sulfate sodium-induced ulcerative colitis. Am. J. Chin. Med. 2018, 46, 1263–1279. [Google Scholar] [CrossRef] [PubMed]
- Min, X.; Guo, Y.; Zhou, Y.; Chen, X. Protection against Dextran Sulfate Sodium-Induced Ulcerative Colitis in Mice by Neferine, A Natural Product from Nelumbo nucifera Gaertn. Cell J. 2021, 22, 523–531. [Google Scholar] [CrossRef]
- Wiedenfeld, H. Plants containing pyrrolizidine alkaloids-toxicity and problems. Food Addit. Contam. 2011, 28, 282–292. [Google Scholar] [CrossRef] [Green Version]
- Jamtsho, T.; Yeshi, K.; Samten; Wangchuk, P. Comparative analysis of two Himalayan Aconitum species for their phytopharmaceutical properties. J. Herb. Med. 2021. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, M.; Chen, L.; Qiao, Y.; Ma, S.; Sun, D.; Si, J.; Liao, Y. Determination of Toxic Pyrrolizidine Alkaloids in Traditional Chinese Herbal Medicines by UPLC-MS/MS and Accompanying Risk Assessment for Human Health. Molecules 2021, 26, 1648. [Google Scholar] [CrossRef]
- Letsyo, E.; Jerz, G.; Winterhalter, P.; Beuerle, T. Toxic pyrrolizidine alkaloids in herbal medicines commonly used in Ghana. J. Ethnopharmacol. 2017, 202, 154–161. [Google Scholar] [CrossRef]
- World Health Organisation. Pyrrolizidine alkaloids health and safety guide. In Proceedings of the World Health Organization for the International Programme on Chemical Safety, Geneva, Switzerland. 1989. Available online: https://apps.who.int/iris/bitstream/handle/10665/39808/9241543477-eng.pdf?sequence=1&isAllowed=y (accessed on 25 April 2022).
- Bosi, C.F.; Rosa, D.W.; Grougnet, R.; Lemonakis, N.; Halabalaki, M.; Skaltsounis, A.L.; Biavatti, M.W. Pyrrolizidine alkaloids in medicinal tea of Ageratum conyzoides. Rev. Bras. De Farmacogn. 2013, 23, 425–432. [Google Scholar] [CrossRef] [Green Version]
- Diallo, A.; Eklu-Gadegbeku, K.; Amegbor, K.; Agbonon, A.; Aklikokou, K.; Creppy, E.; Gbeassor, M. In vivo and in vitro toxicological evaluation of the hydroalcoholic leaf extract of Ageratum conyzoides L. (Asteraceae). J. Ethnopharmacol. 2014, 155, 1214–1218. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Pang, H.; Chen, Y.; Huang, L.; Liu, H.; Zheng, Y.; Sun, C.; Zhang, G.; Wang, G. Anti-Inflammatory Effect of a Polyphenol-Enriched Fraction from Acalypha wilkesiana on Lipopolysaccharide-Stimulated RAW 264.7 Macrophages and Acetaminophen-Induced Liver Injury in Mice. Oxid. Med. Cell Longev. 2018, 2018, 7858094. [Google Scholar] [CrossRef] [Green Version]
- Owoyele, B.V.; Okoye, O.C.; Dolor, R.O.; Oloruntola, O.P.; Soladoye, A.O. Analgesic, anti-inflammatory and antipyretic effects of the ethanol extract of Acalypha wilkesiana leaves in rats. Niger J. Physiol. Sci. 2011, 26, 77–82. [Google Scholar] [PubMed]
- Olukunle, J.O.; Adenubi, O.T.; Biobaku, K.T.; Sogebi, E.A. Anti-inflammatory and analgesic effects of methanol extract and fractions of Acalypha wilkesiana leaves. J. Basic Clin. Physiol. Pharmacol. 2015, 26, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Moura, A.C.; Silva, E.L.; Fraga, M.C.; Wanderley, A.G.; Afiatpour, P.; Maia, M.B. Antiinflammatory and chronic toxicity study of the leaves of Ageratum conyzoides L. in rats. Phytomedicine 2005, 12, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Coulibaly, A.Y.; Kiendrebeogo, M.; Kehoe, P.G.; Sombie, P.A.; Lamien, C.E.; Millogo, J.F.; Nacoulma, O.G. Antioxidant and anti-inflammatory effects of Scoparia dulcis L. J. Med. Food. 2011, 14, 1576–1582. [Google Scholar] [CrossRef]
- Osman, N.I.; Sidik, N.J.; Awal, A.; Adam, N.A.; Rezali, N.I. In vitro xanthine oxidase and albumin denaturation inhibition assay of Barringtonia racemosa L. and total phenolic content analysis for potential anti-inflammatory use in gouty arthritis. J. Intercult Ethnopharmacol. 2016, 5, 343–349. [Google Scholar] [CrossRef]
- Patil, K.R.; Patil, C.R. Anti-inflammatory activity of bartogenic acid containing fraction of fruits of Barringtonia racemosa Roxb. in acute and chronic animal models of inflammation. J. Tradit. Complement. Med. 2017, 7, 86–93. [Google Scholar] [CrossRef]
- Yang, J.; Li, S.; Xie, C.; Ye, H.; Tang, H.; Chen, L.; Peng, A. Anti-inflammatory activity of ethyl acetate fraction of the seeds of Brucea javanica. J. Ethnopharmacol. 2013, 147, 442–446. [Google Scholar] [CrossRef]
- Tsai, S.C.; Liang, Y.H.; Chiang, J.H.; Liu, F.C.; Lin, W.H.; Chang, S.J.; Lin, W.; Wu, C.H.; Weng, J.R. Anti-inflammatory effects of Calophyllum inophyllum L. in RAW264.7 cells. Oncol. Rep. 2012, 28, 1096–1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmood, A.; Tiwari, A.K.; Şahin, K.; Küçük, Ö.; Ali, S. Triterpenoid saponin-rich fraction of Centella asiatica decreases IL-1β andNF-κB, and augments tissue regeneration and excision wound repair. Turk. J. Biol. 2016, 40, 399–409. [Google Scholar] [CrossRef]
- Viswanathan, G.; Dan, V.M.; Radhakrishnan, N.; Nair, A.S.; Rajendran Nair, A.P.; Baby, S. Protection of mouse brain from paracetamol-induced stress by Centella asiatica methanol extract. J. Ethnopharmacol. 2019, 236, 474–483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.L.; Yan, Y.M.; Li, S.Y.; He, D.H.; Xiong, S.; Wei, S.F.; Liu, W.; Hu, L.; Wang, Q.; Pan, H.F.; et al. 6-O-angeloylplenolin exerts neuroprotection against lipopolysaccharide-induced neuroinflammation in vitro and in vivo. Acta Pharmacol. Sin. 2020, 41, 10–21. [Google Scholar] [CrossRef]
- Bawankule, D.U.; Chattopadhyay, S.K.; Pal, A.; Saxena, K.; Yadav, S.; Faridi, U.; Darokar, M.P.; Gupta, A.K.; Khanuja, S.P. Modulation of inflammatory mediators by coumarinolignoids from Cleome viscosa in female swiss albino mice. Inflammopharmacology 2008, 16, 272–277. [Google Scholar] [CrossRef]
- Doe, P.; Danquah, C.A.; Ohemeng, K.A.; Opare, A.E.; Sharif, A.; Akua-Abora, D.; Kwame Akuoko, A.; Kpabitey, A.; Quarshie, E.; Asante, O.O.; et al. Analgesic, Anti-inflammatory, and Anti-pyretic Activities of Crinum pedunculatum R.Br. Bulb Extracts. Pharmacogn. Res. 2021, 14, 24–29. [Google Scholar] [CrossRef]
- Simpson, B.; Claudie, D.; Smith, N.; Wang, J.; McKinnon, R.; Semple, S. Evaluation of the anti-inflammatory properties of Dodonaea polyandra, a Kaanju traditional medicine. J. Ethnopharmacol. 2010, 132, 340–343. [Google Scholar] [CrossRef]
- Khalil, N.M.; Sperotto, J.S.; Manfron, M.P. Antiinflammatory activity and acute toxicity of Dodonaea viscosa. Fitoterapia 2006, 77, 478–480. [Google Scholar] [CrossRef]
- Shao, S.Y.; Yang, Y.N.; Feng, Z.M.; Jiang, J.S.; Zhang, P.C. Anti-inflammatory phenylpropanoid glycosides from the fruits of Forsythia suspensa. Bioorg. Med. Chem. Lett. 2019, 29, 126635. [Google Scholar] [CrossRef]
- Mondal, M.; Quispe, C.; Sarkar, C.; Bepari, T.C.; Alam, M.J.; Saha, S.; Ray, P.; Rahim, M.A.; Islam, M.T.; Setzer, W.N.; et al. Analgesic and Anti-Inflammatory Potential of Essential Oil of Eucalyptus camaldulensis Leaf: In Vivo and in Silico Studies. Nat. Prod. Commun. 2021, 16, 1–16. [Google Scholar] [CrossRef]
- Ahmad, S.F.; Bani, S.; Sultan, P.; Ali, S.A.; Bakheet, S.A.; Attia, S.M.; Abd-Allah, A.R. TNF-alpha inhibitory effect of Euphorbia hirta in rats. Pharm. Biol. 2013, 51, 411–417. [Google Scholar] [CrossRef] [PubMed]
- Shih, M.F.; Cheng, Y.D.; Shen, C.R.; Cherng, J.Y. A molecular pharmacology study into the anti-inflammatory actions of Euphorbia hirta L. on the LPS-induced RAW 264.7 cells through selective iNOS protein inhibition. J. Nat. Med. 2010, 64, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Lanhers, M.C.; Fleurentin, J.; Dorfman, P.; Mortier, F.; Pelt, J.M. Analgesic, antipyretic and anti-inflammatory properties of Euphorbia hirta. Planta Med. 1991, 57, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Er, H.M.; Mohamed, S.M.; Chen, Y.S. In vitro anti-inflammatory activity of fractionated Euphorbia hirta aqueous extract on rabbit synovial fibroblasts. Biomed J. 2015, 38, 301–306. [Google Scholar] [CrossRef]
- Sharma, N.; Samarakoon, K.W.; Gyawali, R.; Park, Y.H.; Lee, S.J.; Oh, S.J.; Lee, T.H.; Jeong, D.K. Evaluation of the antioxidant, anti-inflammatory, and anticancer activities of Euphorbia hirta ethanolic extract. Molecules 2014, 19, 14567–14581. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, S.F.; Attia, S.M.; Bakheet, S.A.; Ashour, A.E.; Zoheir, K.M.; Abd-Allah, A.R. Anti-inflammatory effect of Euphorbia hirta in an adjuvant-induced arthritic murine model. Immunol. Investig. 2014, 43, 197–211. [Google Scholar] [CrossRef]
- Shih, M.F.; Cherng, J.Y. Reduction of adhesion molecule production and alteration of eNOS and endothelin-1 mRNA expression in endothelium by Euphorbia hirta L. through its beneficial beta-amyrin molecule. Molecules 2014, 19, 10534–10545. [Google Scholar] [CrossRef] [Green Version]
- Palit, P.; Mukherjee, D.; Mahanta, P.; Shadab, M.; Ali, N.; Roychoudhury, S.; Asad, M.; Mandal, S.C. Attenuation of nociceptive pain and inflammatory disorders by total steroid and terpenoid fraction of Euphorbia tirucalli Linn root in experimental in vitro and in vivo model. Inflammopharmacology 2018, 26, 235–250. [Google Scholar] [CrossRef]
- Qiu, F.; Tian, H.; Zhang, Z.; Yuan, X.L.; Tan, Y.F.; Ning, X.Q. Pharmacological study on hemostasis, analgesic and anti inflammation effects of the alcohol extract of Hibiscus tiliaceus. Zhong Yao Cai. 2013, 36, 1648–1651. (In Chinese) [Google Scholar]
- da Silva Barth, C.; Tolentino de Souza, H.G.; Rocha, L.W.; da Silva, G.F.; Dos Anjos, M.F.; Pastor, V.D.; Belle Bresolin, T.M.; Garcia Couto, A.; Roberto Santin, J.; Meira Quintao, N.L. Ipomoea pes-caprae (L.) R. Br (Convolvulaceae) relieved nociception and inflammation in mice—A topical herbal medicine against effects due to cnidarian venom-skin contact. J. Ethnopharmacol. 2017, 200, 156–164. [Google Scholar] [CrossRef]
- Bhowmick, R.; Sarwar, M.S.; Dewan, S.M.; Das, A.; Das, B.; Uddin, M.M.; Islam, M.S.; Islam, M.S. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Biol. Res. 2014, 47, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Adeyemi, O.O.; Yemitan, O.K.; Afolabi, L. Inhibition of chemically induced inflammation and pain by orally and topically administered leaf extract of Manihot esculenta Crantz in rodents. J. Ethnopharmacol. 2008, 119, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Surh, J.; Yun, J.M. Antioxidant and anti-inflammatory activities of butanol extract of Melaleuca leucadendron L. Prev. Nutr. Food Sci. 2012, 17, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Huo, X.; Liu, W.; Li, K.; Sun, Z.; Tian, J. Apigenin exhibits anti-inflammatory effects in LPS-stimulated BV2 microglia through activating GSK3beta/Nrf2 signaling pathway. Immunopharmacol. Immunotoxicol. 2020, 42, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Diao, P.; Shu, X.; Li, L.; Xiong, L. Quercetin and Quercitrin Attenuates the Inflammatory Response and Oxidative Stress in LPS-Induced RAW264.7 Cells: In Vitro Assessment and a Theoretical Model. Biomed Res. Int. 2019, 2019, 7039802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho de Sousa, B.; Reis Machado, J.; da Silva, M.V.; da Costa, T.A.; Lazo-Chica, J.E.; Degasperi, T.D.; Rodrigues Junior, V.; Sales-Campos, H.; Uber Bucek, E.; Freire Oliveira, C.J. Morinda citrifolia (Noni) Fruit Juice Reduces Inflammatory Cytokines Expression and Contributes to the Maintenance of Intestinal Mucosal Integrity in DSS Experimental Colitis. Mediators Inflamm. 2017, 2017, 6567432. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.L.; Liu, C.T.; Chou, M.C.; Ko, C.H.; Wang, C.K. Noni (Morinda citrifolia L.) fruit extracts improve colon microflora and exert anti-inflammatory activities in Caco-2 Cells. J. Med. Food 2015, 18, 663–676. [Google Scholar] [CrossRef]
- Tanikawa, T.; Kitamura, M.; Hayashi, Y.; Tomida, N.; Uwaya, A.; Isami, F.; Inoue, Y. Anti-Inflammatory Effects of Morinda citrifolia Extract against Lipopolysaccharide-Induced Inflammation in RAW264 Cells. Medicines 2021, 8, 43. [Google Scholar] [CrossRef]
- Sandamali, J.A.N.; Hewawasam, R.P.; Jayatilaka, K.A.P.W.; Mudduwa, L.K.B. Nauclea orientalis (L.) Bark Extract Protects Rat Cardiomyocytes from Doxorubicin-Induced Oxidative Stress, Inflammation, Apoptosis, and DNA Fragmentation. Oxid. Med. Cell Longev. 2022, 2022, 1714841. [Google Scholar] [CrossRef]
- Sranujit, R.P.; Noysang, C.; Tippayawat, P.; Kooltheat, N.; Luetragoon, T.; Usuwanthim, K. Phytochemicals and immunomodulatory effect of Nelumbo nucifera flower extracts on human macrophages. Plants 2021, 10, 2007. [Google Scholar] [CrossRef]
- Rajput, M.A.; Zehra, T.; Ali, F.; Kumar, G. Evaluation of Antiinflammatory Activity of Ethanol Extract of Nelumbo nucifera Fruit. Turk. J. Pharm. Sci. 2021, 18, 56–60. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; He, Y.; Yang, Y.; Gou, Y.; Li, S.; Wang, R.; Zeng, S.; Zhao, X. Antioxidant and Inflammatory Effects of Nelumbo nucifera Gaertn. Leaves. Oxid. Med. Cell Longev. 2021, 2021, 8375961. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Kim, G.D.; Go, M.S.; Kwon, D.; Jung, I.K.; Auh, J.H.; Kim, J.H. Anti-inflammatory effects of Nelumbo leaf extracts and identification of their metabolites. Nutr. Res. Pract. 2017, 11, 265–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, C.; Deng, J.; Liu, D.; Tuo, X.; Yu, Y.; Yang, H.; Wang, N. Nuciferine Inhibits Proinflammatory Cytokines via the PPARs in LPS-Induced RAW264.7 Cells. Molecules 2018, 23, 2723. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Sun, X.Y.; Li, X.W.; Yang, T.; Qi, L.W. Enrichment and separation of quercetin-3-O-beta-d-glucuronide from lotus leaves (Nelumbo nucifera gaertn.) and evaluation of its anti-inflammatory effect. J. Chromatogr. B Analyt. Technol. Biomed Life Sci. 2017, 1040, 186–191. [Google Scholar] [CrossRef]
- Liu, S.H.; Lu, T.H.; Su, C.C.; Lay, I.S.; Lin, H.Y.; Fang, K.M.; Ho, T.J.; Chen, K.L.; Su, Y.C.; Chiang, W.C.; et al. Lotus leaf (Nelumbo nucifera) and its active constituents prevent inflammatory responses in macrophages via JNK/NF-kappaB signaling pathway. Am. J. Chin. Med. 2014, 42, 869–889. [Google Scholar] [CrossRef]
- Narasimhulu, C.A.; Vardhan, S. Therapeutic potential of Ocimum tenuiflorum as MPO inhibitor with implications for atherosclerosis prevention. J. Med. Food 2015, 18, 507–515. [Google Scholar] [CrossRef]
- Zhu, L.; Zhang, D.; Yuan, C.; Ding, X.; Shang, Y.; Jiang, Y.; Zhu, G. Anti-Inflammatory and antiviral effects of water-soluble crude extract from Phragmites australis in vitro. Pak. J. Pharm. Sci. 2017, 30, 1357–1362. [Google Scholar]
- Gambari, R.; Borgatti, M.; Lampronti, I.; Fabbri, E.; Brognara, E.; Bianchi, N.; Piccagli, L.; Yuen, M.C.; Kan, C.W.; Hau, D.K.; et al. Corilagin is a potent inhibitor of NF-kappaB activity and downregulates TNF-alpha induced expression of IL-8 gene in cystic fibrosis IB3-1 cells. Int. Immunopharmacol. 2012, 13, 308–315. [Google Scholar] [CrossRef]
- Brestovac, B.; Coghlan, O.; Jackaman, C.; Nelson, D.; Townsend, D. Sarcostemma viminale activates macrophages to a pro-inflammatory phenotype. Comp. Clin. Pathol. 2014, 24, 817–826. [Google Scholar] [CrossRef]
- Prihantono, P.; Ardi Syamsu, S.; Smaradhania, N.; Ahmad, M.; Siagian, N.A.; Lubis, K.; Umrah, A.S. Application of Scaevola taccada (Gaertn.) Roxb. Reduce Pro-inflammatory Cytokines Interleukin-1β in Sprague Dawley Mice Suffering from Mastitis. Open Access Maced. J. Med. Sci. 2020, 8, 423–427. [Google Scholar] [CrossRef]
- Abiodun, O.O.; Rodriguez-Nogales, A.; Algieri, F.; Gomez-Caravaca, A.M.; Segura-Carretero, A.; Utrilla, M.P.; Rodriguez-Cabezas, M.E.; Galvez, J. Antiinflammatory and immunomodulatory activity of an ethanolic extract from the stem bark of Terminalia catappa L. (Combretaceae): In vitro and in vivo evidences. J. Ethnopharmacol. 2016, 192, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Calvo, M.I.; Vilalta, N.; San Julián, A.; Fernández, M. Anti-inflammatory activity of leaf extract of Verbena officinalis L. Phytomedicine 1998, 5, 465–467. [Google Scholar] [CrossRef]
- Wang, J.; Fang, X.; Ge, L.; Cao, F.; Zhao, L.; Wang, Z.; Xiao, W. Antitumor, antioxidant and anti-inflammatory activities of kaempferol and its corresponding glycosides and the enzymatic preparation of kaempferol. PLoS ONE 2018, 13, e0197563. [Google Scholar] [CrossRef]
- Jansson, D.; Dieriks, V.B.; Rustenhoven, J.; Smyth, L.C.D.; Scotter, E.; Aalderink, M.; Feng, S.; Johnson, R.; Schweder, P.; Mee, E.; et al. Cardiac glycosides target barrier inflammation of the vasculature, meninges and choroid plexus. Commun. Biol. 2021, 4, 260. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhang, J.H.; He, Y.Q.; Zhang, Q.L.; Zhu, B.; Shen, Y.; Liu, M.Q.; Zhu, L.L.; Xin, H.L.; Qin, L.P.; et al. Iridoid glycosides from Morinda officinalis How. exert anti-inflammatory and anti-arthritic effects through inactivating MAPK and NF-kappaB signaling pathways. BMC Complement. Med. Ther. 2020, 20, 172. [Google Scholar] [CrossRef]
- Fabricant, D.S.; Tarnsworth, N.R. The value of plants used in traditional medicine for drug discovery. Enviromental Health 2001, 109, 69–75. [Google Scholar]
- Atanasov, A.G.; Waltenberger, B.; Pferschy-Wenzig, E.M.; Linder, T.; Wawrosch, C.; Uhrin, P.; Temml, V.; Wang, L.; Schwaiger, S.; Heiss, E.H.; et al. Discovery and resupply of pharmacologically active plant-derived natural products: A review. Biotechnol. Adv. 2015, 33, 1582–1614. [Google Scholar] [CrossRef] [Green Version]
- Canter, P.H.; Thomas, H.; Ernst, E. Bringing medicinal plants into cultivation: Opportunities and challenges for biotechnology. Trends Biotechnol. 2005, 23, 180–185. [Google Scholar] [CrossRef]
- United Nations. Convention on Biological Diversity. Available online: http://www.cbd.int/doc/legal/cbd-en.pdf (accessed on 25 April 2022).
- United Nations. Nagoya Protocol on Access to Genetic Resources and the Fair and Equitable Sharing of Benefits Arising from Their Utilization to the Convention on Biological Diversity. Available online: https://www.cbd.int/abs/doc/protocol/nagoya-protocol-en.pdf (accessed on 25 April 2022).
- United Nations. United Nations Declaration on the Rights of Indigenous Peoples. 2008, pp. 1–29. Available online: https://www.un.org/development/desa/indigenouspeoples/wp-content/uploads/sites/19/2018/11/UNDRIP_E_web.pdf (accessed on 26 April 2022).
- Golvan, C.; Janke, T. Indigenous intellectual property issues. In Proceedings of the 35th Australian Legal Convention, Sydney, Australia, 25 March 2007. [Google Scholar]
- Queensland Government. Reforms of the Biodiscovery Act 2004: Collecting Biological Native Resources-Biodiscovery (30 August 2021). Available online: https://environment.des.qld.gov.au/licences-permits/plants-animals/biodiscovery/biodiscovery-act-reform (accessed on 8 May 2022).
- Elsharkawy, E.R.; Alghanem, S.M.; Elmorsy, E. Effect of habitat variations on the chemical composition, antioxidant, and antimicrobial activities of Achillea fragrantissima (Forssk) Sch. Bip. Biotechnol. Rep. (Amst.) 2021, 29, e00581. [Google Scholar] [CrossRef]
- Al-Hmadi, H.; El Mokni, R.; Joshi, R.K.; Ashour, M.L.; Hammami, S. The Impact of Geographical Location on the Chemical Compositions of Pimpinella lutea Desf. Growing in Tunisia. Appl. Sci. 2021, 11, 7739. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, P.; Zhou, M.; Wang, T.; Fang, S.; Shang, X.; Fu, X. Geographic Variation in the Chemical Composition and Antioxidant Properties of Phenolic Compounds from Cyclocarya paliurus (Batal) Iljinskaja Leaves. Molecules 2018, 23, 2440. [Google Scholar] [CrossRef] [PubMed] [Green Version]

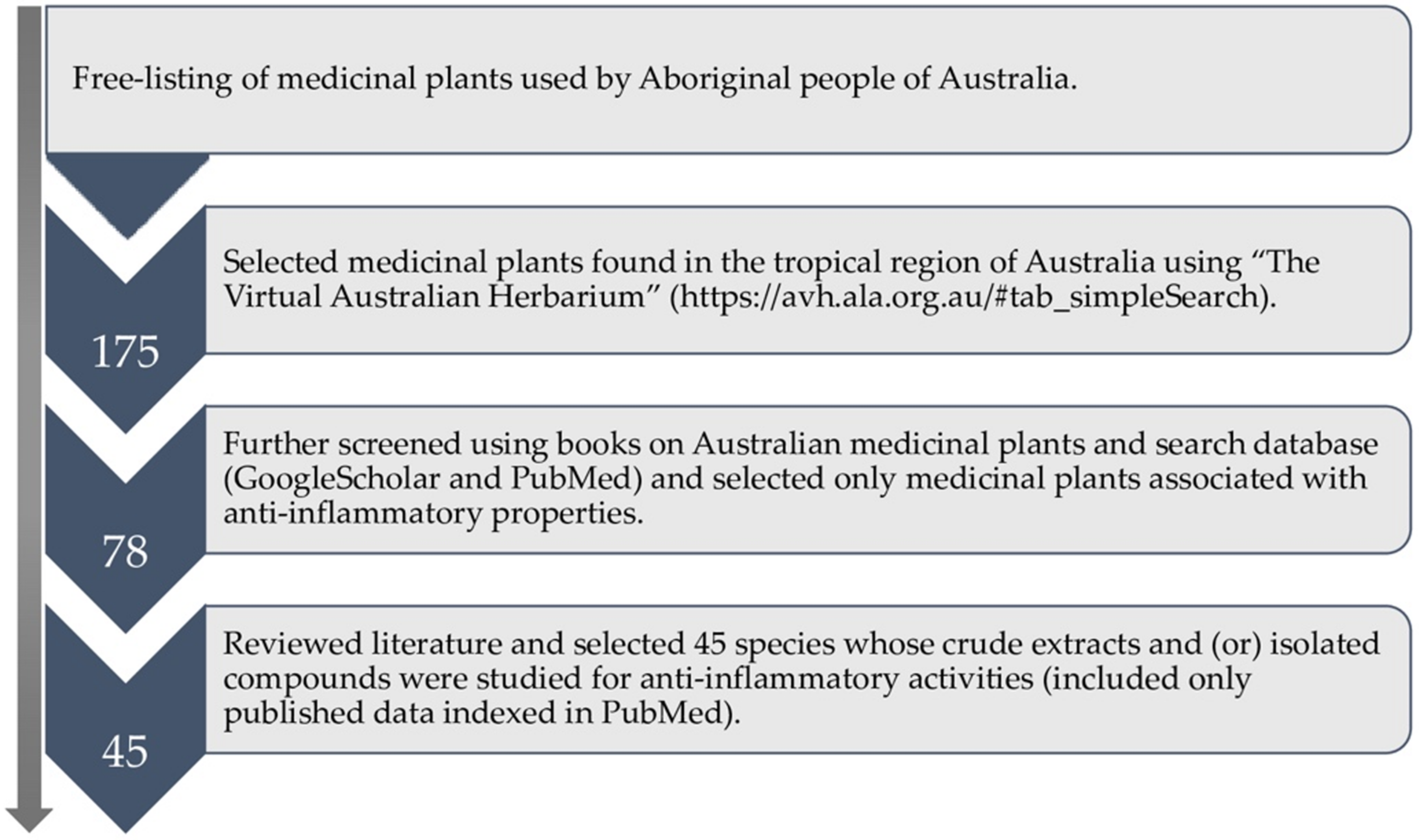






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeshi, K.; Turpin, G.; Jamtsho, T.; Wangchuk, P. Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants. Molecules 2022, 27, 3849. https://doi.org/10.3390/molecules27123849
Yeshi K, Turpin G, Jamtsho T, Wangchuk P. Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants. Molecules. 2022; 27(12):3849. https://doi.org/10.3390/molecules27123849
Chicago/Turabian StyleYeshi, Karma, Gerry Turpin, Tenzin Jamtsho, and Phurpa Wangchuk. 2022. "Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants" Molecules 27, no. 12: 3849. https://doi.org/10.3390/molecules27123849
APA StyleYeshi, K., Turpin, G., Jamtsho, T., & Wangchuk, P. (2022). Indigenous Uses, Phytochemical Analysis, and Anti-Inflammatory Properties of Australian Tropical Medicinal Plants. Molecules, 27(12), 3849. https://doi.org/10.3390/molecules27123849