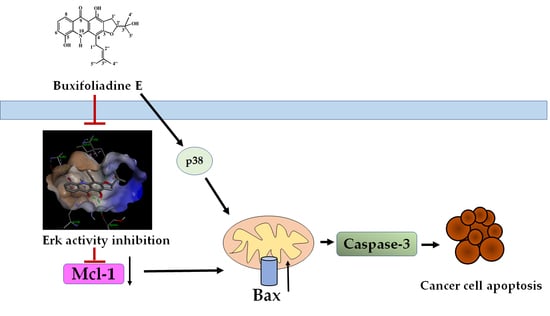
Acridone Derivatives from Atalantia monophyla Inhibited Cancer Cell Proliferation through ERK Pathway
Abstract
:1. Introduction
2. Results
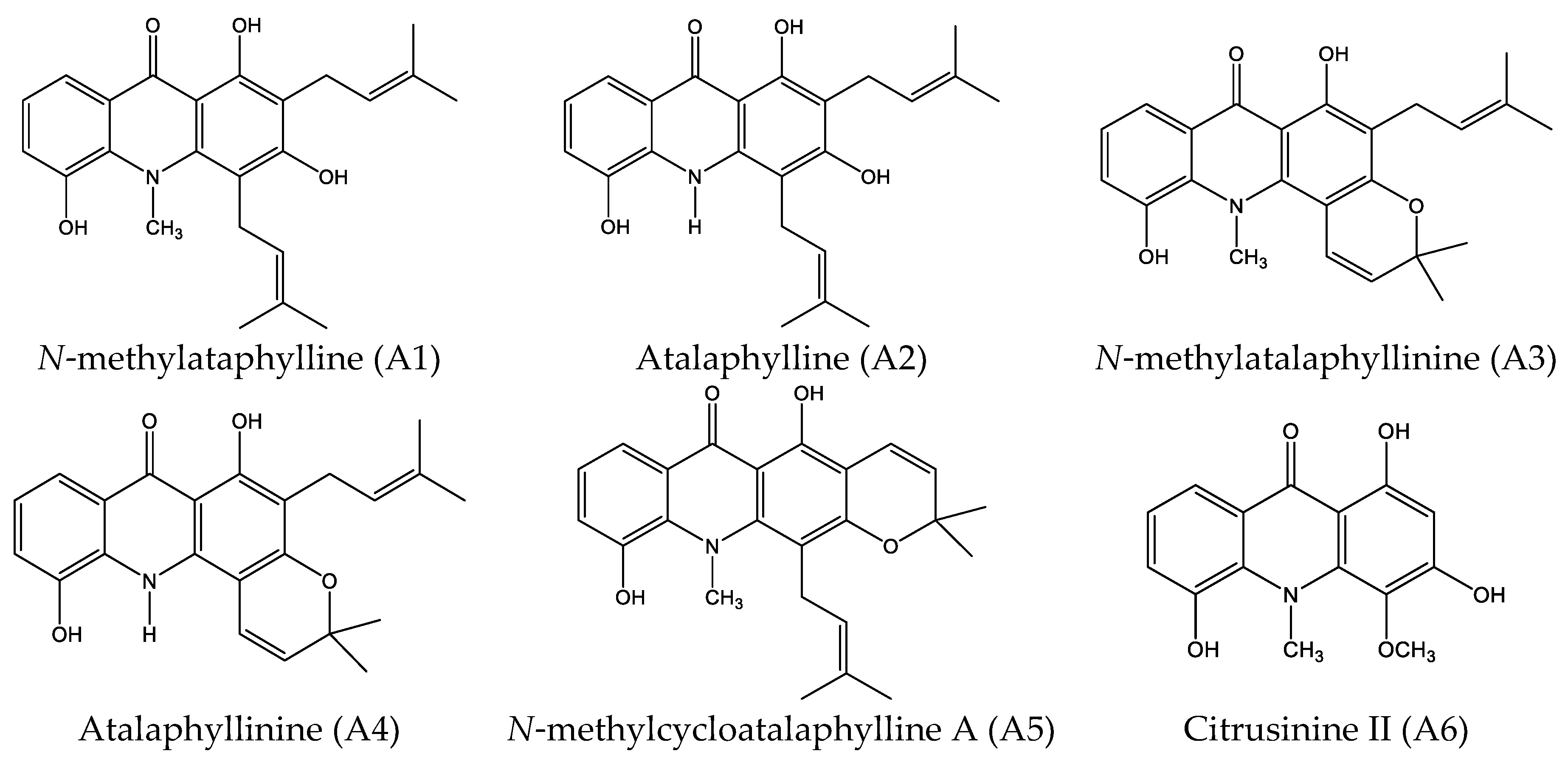
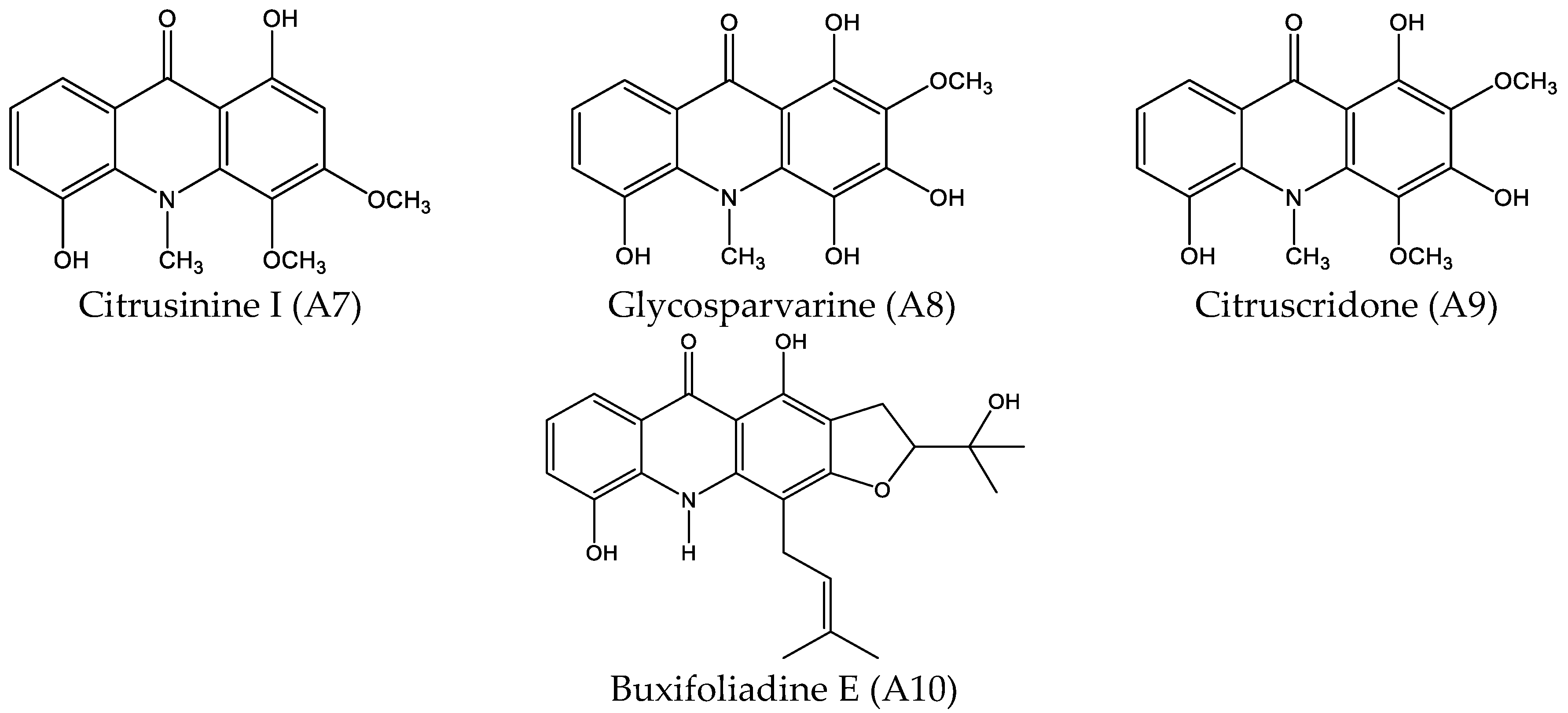
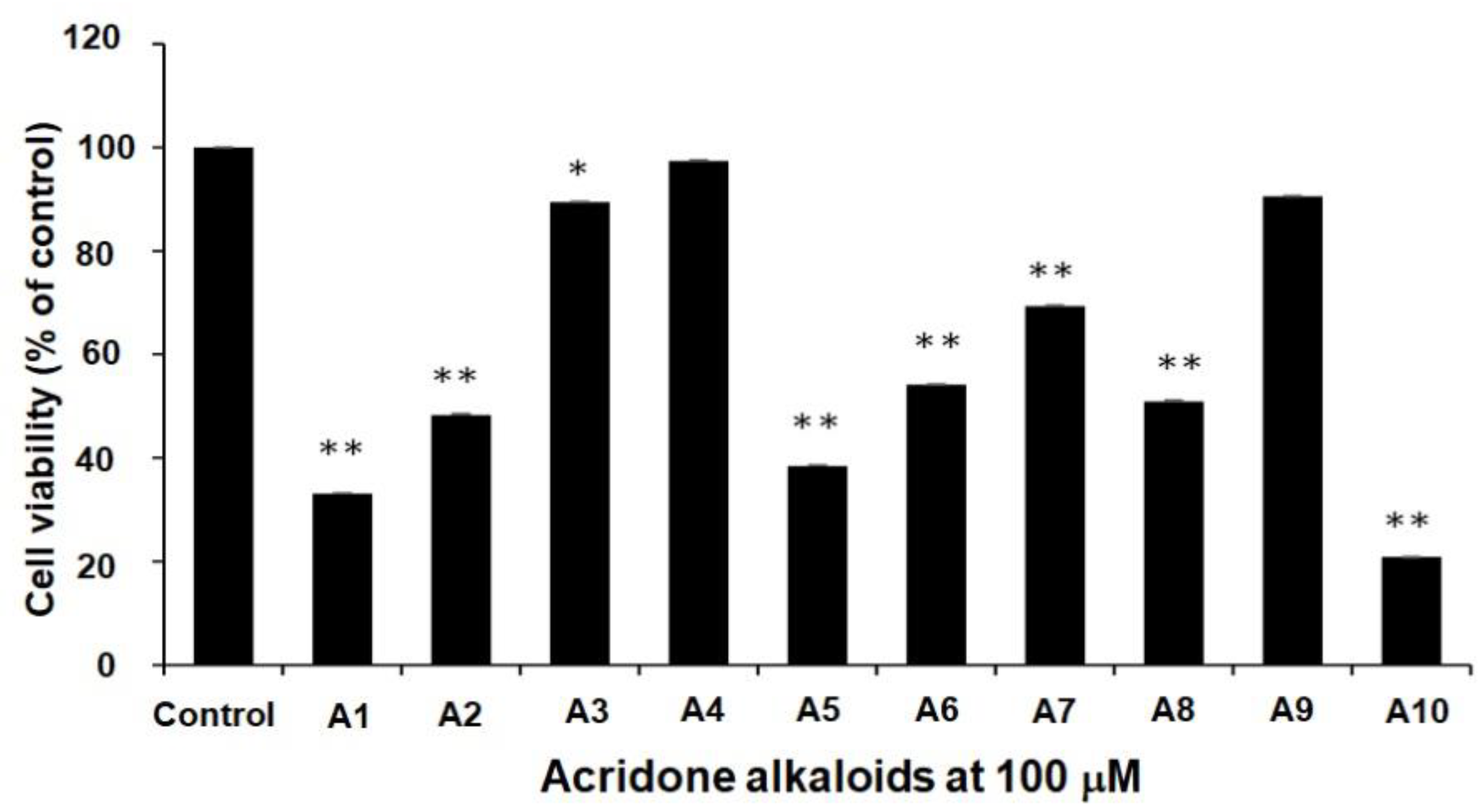
2.1. Cytotoxicity Effect of 10 Acridone Alkaloids
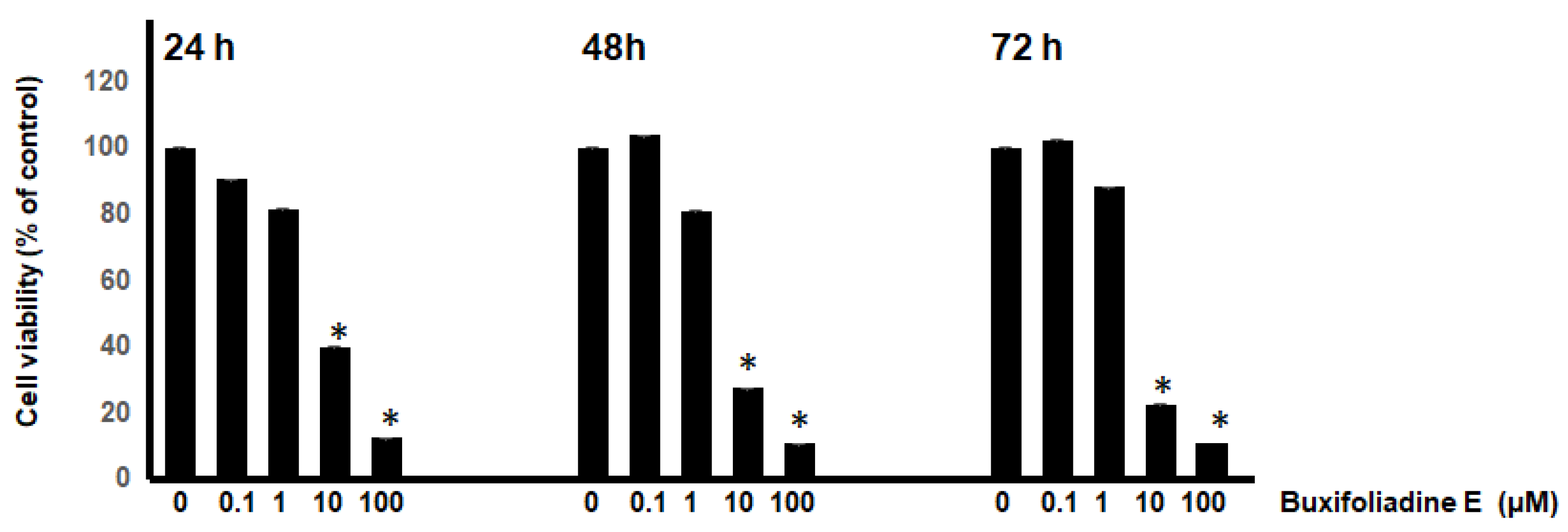
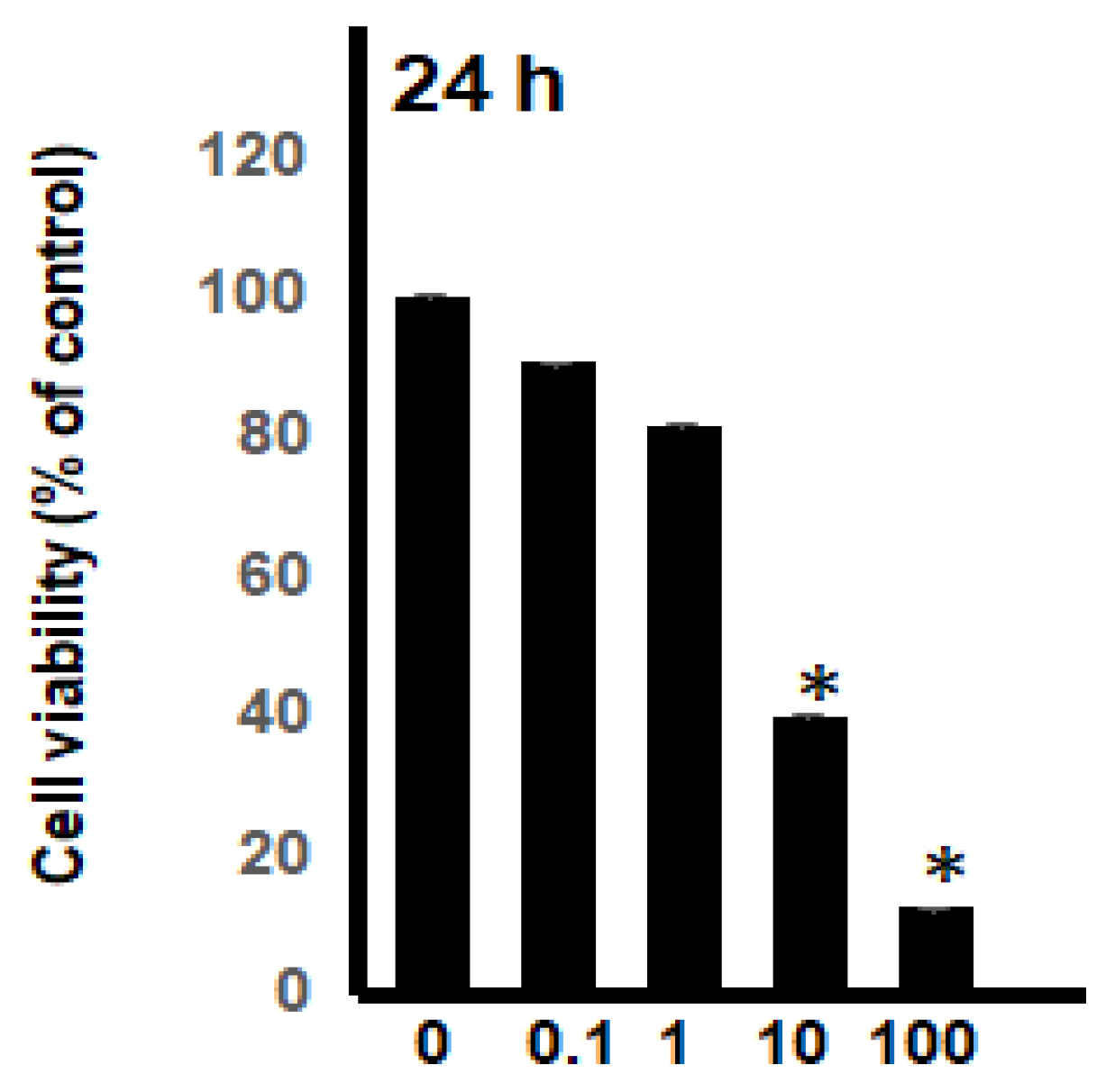
2.2. Dose-Response and Time-Course Studies
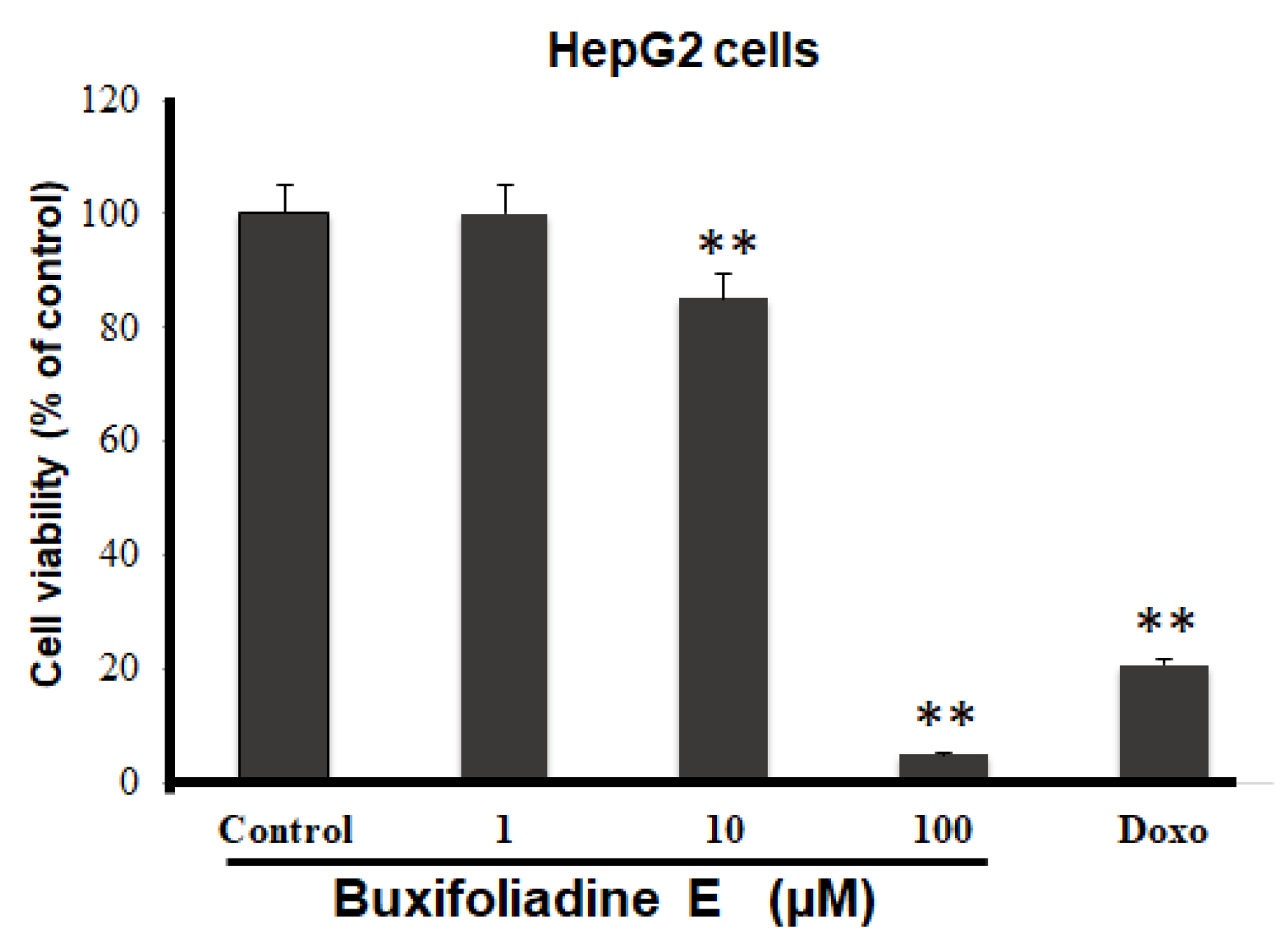
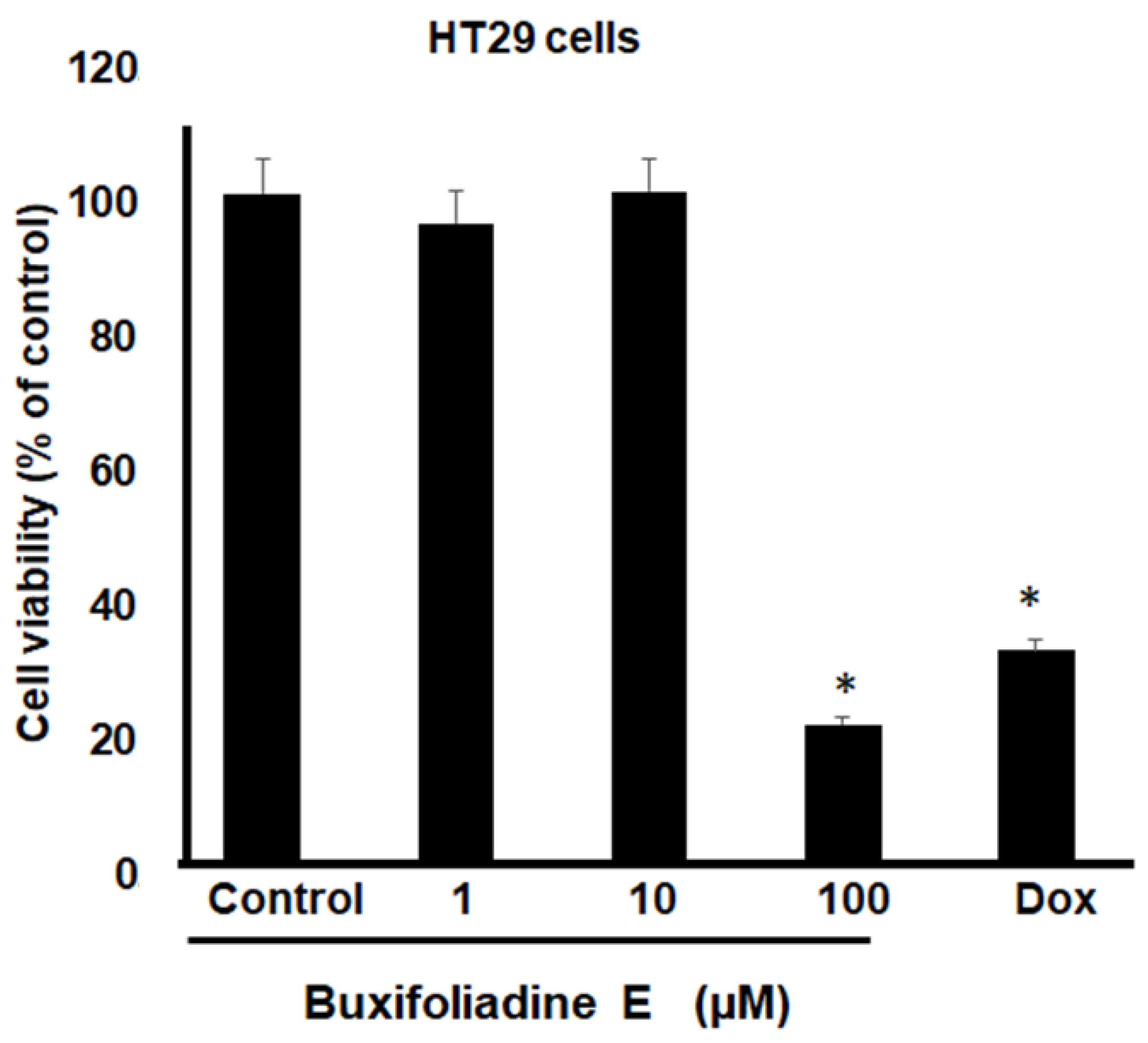
2.3. Cytotoxic Effects of Buxifoliadine E on Prostate Cancer (LNCaP), Neuroblastoma (SH SY5Y), Hepatoblastoma (HepG2), and Colorectal Cancer (HT29)
2.4. Effects of Buxifoliadine E on Apoptotic Signaling Proteins in HepG2 Cells
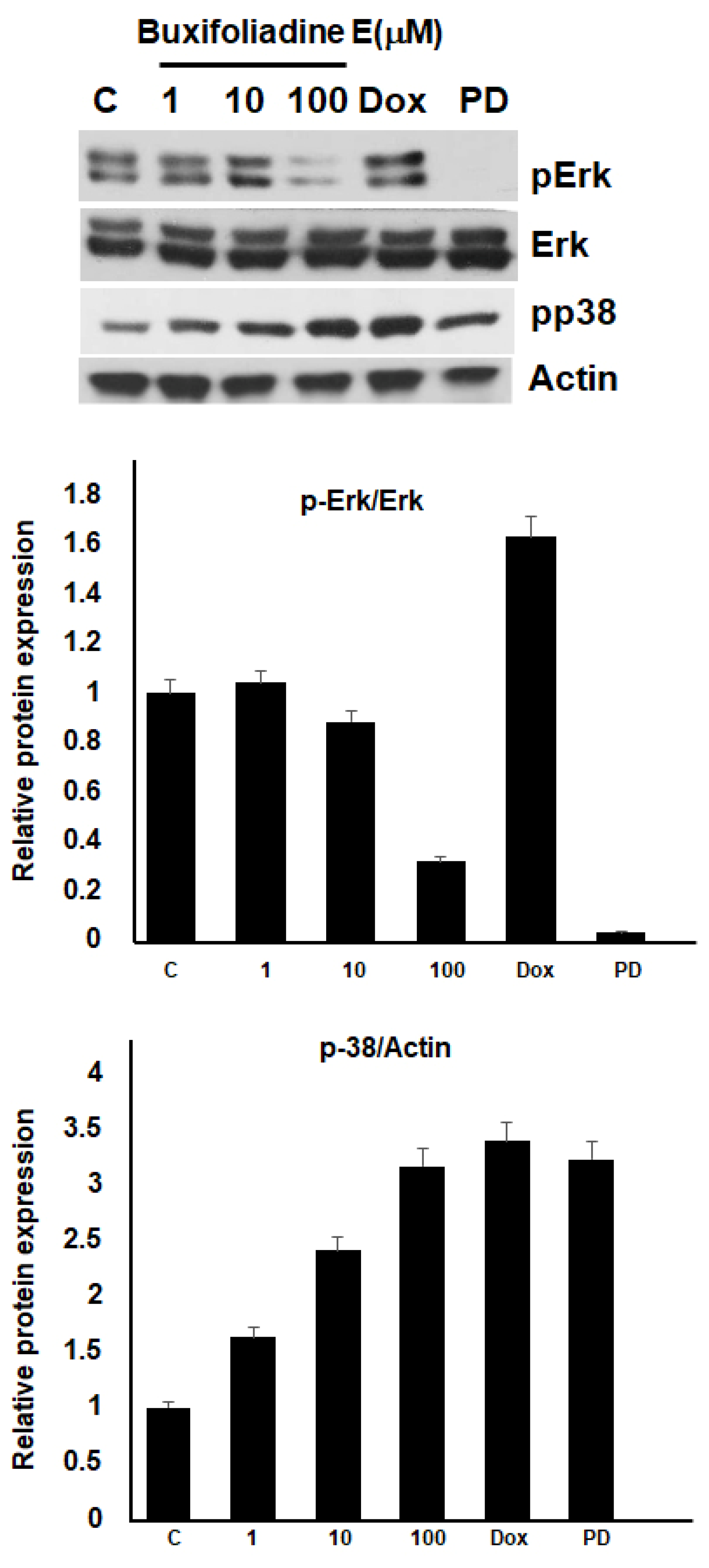
2.5. Effects of Buxifoliadine E on the Expression Levels of Proteins Related to ERK/MAPK Signaling Pathway in the HepG2 Cells
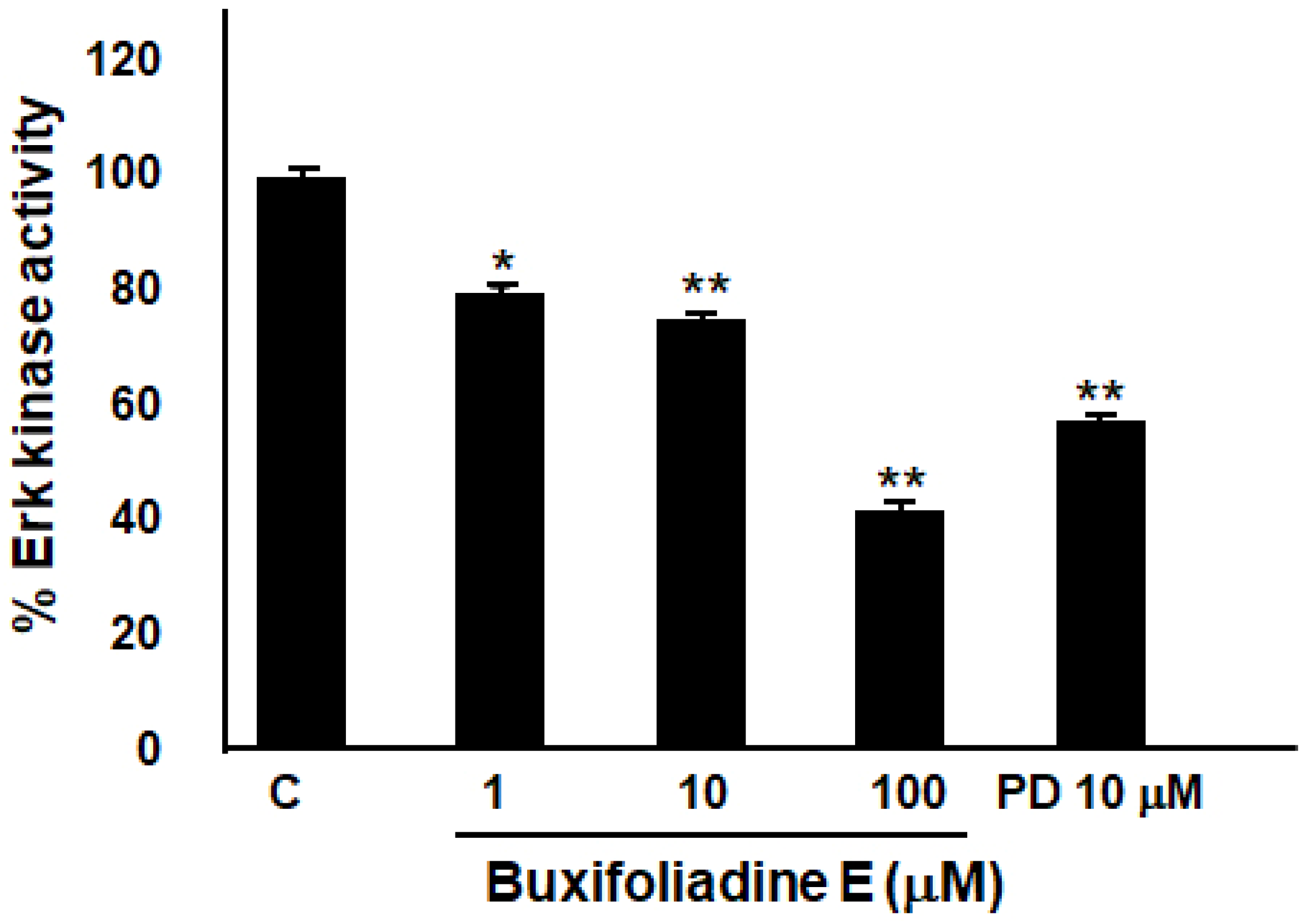
2.6. Buxifoliadine E Inhibited Erk Kinase Activity
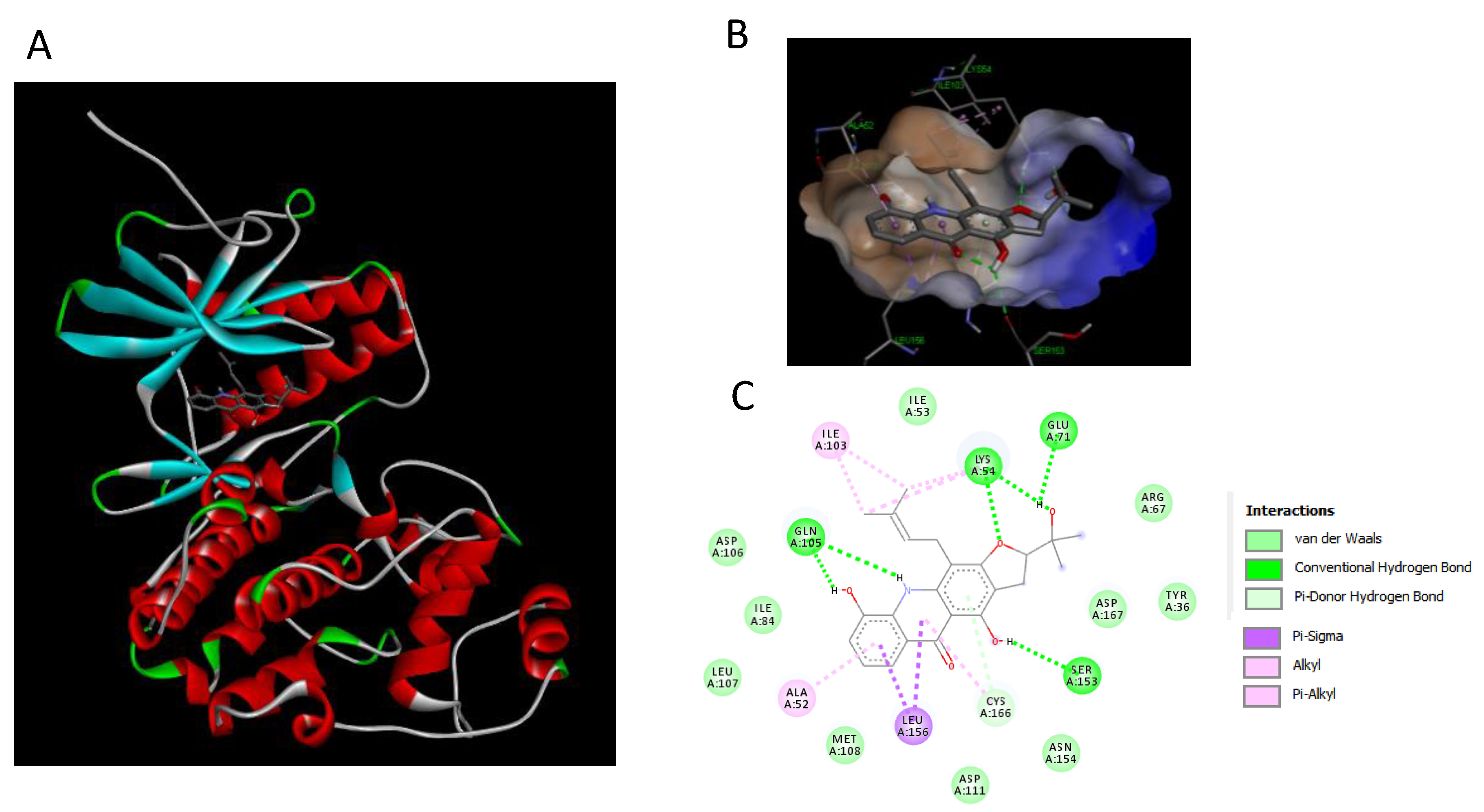
2.7. Binding Interaction Studies between Buxifoliadine E and Target Erk
2.8. Pharmacokinetic and Toxicity Profiles of Buxifoliadine E
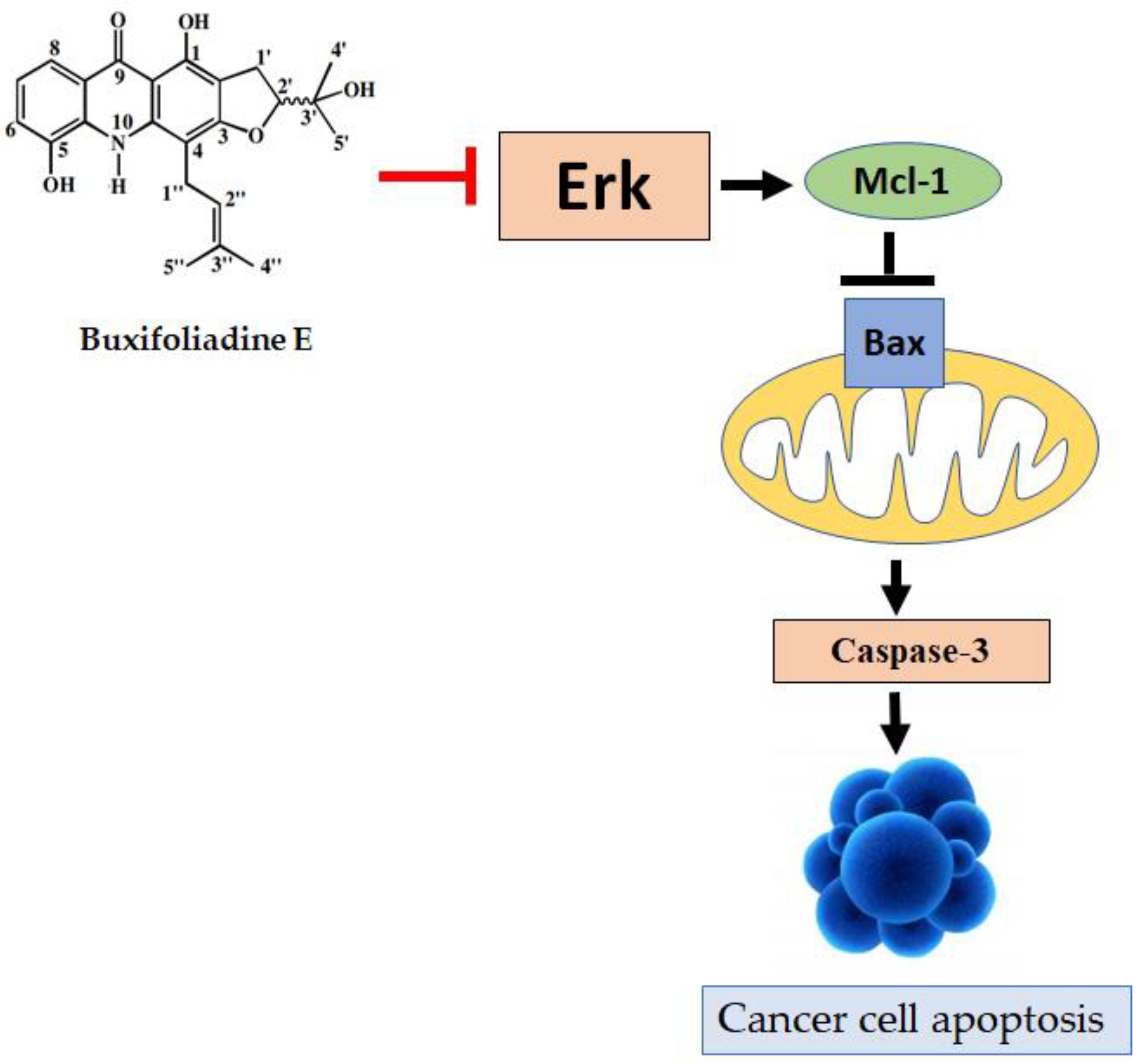
3. Discussion
4. Materials and Methods
4.1. Materials
4.1.1. Acridone Alkaloids
4.1.2. Agents
4.2. Cell Culture and Treatment
4.3. Cell Cytotoxicity
4.4. Western Blot Analysis
4.5. Erk Kinase Activity Assay
4.6. In Silico Binding Interaction Studies between Buxifoliadine E and Target Erk
4.7. In Silico Pharmacokinetic Properties of Buxifoliadine E
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Bax | Bcl-2-associated X protein |
| Bid | BH3 interacting domain death agonist |
| DTT | Dithiothreitol |
| ECL | Enhanced chemiluminescence |
| EDTA | Ethylenediamine tetraacetic acid |
| ERK | Extracellular regulated kinase |
| HEPES | N-2-Hydroxyethylpiperazine-N’-2-Ethanesulfonic Acid |
| JNK | Jun-N-terminal kinase |
| MAPKs | Mitogen-activated protein kinases |
| Mcl-1 | B-cell lymphoma 1 |
| PMSF | Phenylmethylsulfonyl fluoride |
| SAPK | Stress-activated protein kinase |
| WHO | World Health Organization |
| WST-8 | 2-(2-methoxy-4-nitrophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H-tetrazolium, monosodium salt |
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN Estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Byung, H.C.; Shigeo, H.; Chiong, E. The incidence, mortality, and risk factors of prostate cancer in Asian men. Prostate Int. 2019, 7, 1–8. [Google Scholar]
- Gann, P.H. Risk factors for prostate cancer. Rev. Urol. 2002, 4, S3–S10. [Google Scholar] [PubMed]
- Patel, A.R.; Klein, E.A. Risk factors for prostate cancer. Nat. Clin. Pract. Urol. 2009, 6, 87–95. [Google Scholar] [CrossRef]
- GBD 2015 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1659–1724. [Google Scholar] [CrossRef] [Green Version]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef] [Green Version]
- Stewart, B.W.; Wild, C.P. (Eds.) World Cancer Report 2014. Lyon: International Agency for Research on Cancer; WHO Press: Geneve, Switzerland, 2015. [Google Scholar]
- Parker, C.; Gillessen, S.; Heidenreich, A.; Horwich, A. Cancer of the prostate: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2015, 26, v69–v77. [Google Scholar] [CrossRef] [PubMed]
- Perlmutter, M.A.; Lepor, H. Androgen deprivation therapy in the treatment of advanced prostate cancer. Rev. Urol. 2007, 9, 3–8. [Google Scholar]
- Wen, S.; Niu, Y.; Lee, S.O.; Chang, C. Androgen receptor (AR) positive vs negative roles in prostate cancer cell deaths including apoptosis, anoikis, entosis, necrosis and autophagic cell death. Cancer Treat. Rev. 2013, 40, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, N.; Furukawa, H.; Ito, Y.; Yoshida, S.; Maeno, K.; Nishiyama, Y. Anti-herpesvirus activity of citrusinine-I, a new acridone alkaloid, and related compounds. Antivir. Res. 1989, 12, 21–36. [Google Scholar] [CrossRef]
- Wouatsa, V.N.; Misra, L.; Kumar, S.; Prakash, O.; Khan, F.; Tchoumbougnang, F.; Venkatesh, R.K. Aromatase and glycosyltransferase inhibiting acridone alkaloids from fruits of Cameroonian zanthoxylum species. Chem. Cent. J. 2013, 7, 125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Luo, A.; Kamau, P.; Takomthong, P.; Hu, J.; Boonyarat, C.; Luo, L.; Lai, R. A plant-derived TRPV3 inhibitor suppresses pain and itch. Br. J. Pharmacol. 2021, 178, 1669–1683. [Google Scholar] [CrossRef] [PubMed]
- Parveen, M.; Aslam, A.; Nami, S.A.A.; Malla, A.M.; Alam, M.; Lee, D.U.; Rehman, S.; Silva, P.S.P.; Silva, M.R. Potent acetylcholinesterase inhibitors: Synthesis, biological assay and docking study of nitro acridone derivatives. J. Photochem. Photobiol. Biol. 2016, 161, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Takomthong, P.; Waiwut, P.; Yenjai, C.; Sombatsri, A.; Reubroycharoen, P.; Lei, L.; Lai, R.; Chaiwiwatrakul, S.; Boonyarat, C. Multi-target actions of acridones from Atalantia monophylla towards Alzheimer’s pathogenesis and their pharmacokinetic properties. Pharmaceuticals 2021, 14, 888. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, D.; Chen, Z.; Li, S.; Gao, C.; Cao, D.; Liu, F.; Liu, H.; Jiang, Y. Acridone derivative 8a induces oxidative stress-mediated apoptosis in CCRF-CEM leukemia cells: Application of metabolomics in mechanistic studies of antitumor agents. PLoS ONE 2013, 8, e63572. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Li, S.; Liang, Z.; Lin, H.; Fu, R. Acridone suppresses the proliferation of human breast cancer cells in vitro via ATP-binding cassette subfamily G member 2. Oncol. Lett. 2018, 15, 2651–2654. [Google Scholar] [CrossRef] [Green Version]
- Sombatsri, A.; Thummanant, Y.; Sribuhom, T.; Boonmak, J.; Youngme, S.; Phusrisom, S.; Kukongviriyapan, V.; Yenjai, C. New limonophyllines A–C from the stem of Atalantia monophylla and cytotoxicity against cholangiocarcinoma and HepG2 cell lines. Arch. Pharmacal Res. 2018, 41, 431–437. [Google Scholar] [CrossRef]
- Munshi, A.; Ramesh, R. Mitogen-Activated Protein Kinases and Their Role in Radiation Response. Genes Cancer 2013, 4, 401–408. [Google Scholar] [CrossRef]
- Rodríguez-Berriguete, G.; Fraile, B.; Martínez-Onsurbe, P.; Olmedilla, G.; Paniagua, R.; Royuela, M. MAP kinases and prostate cancer. J. Signal Transduct. 2011, 2012, 169170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nitulescu, G.M.; Van De Venter, M.; Nitulescu, G.; Ungurianu, A.; Juzenas, P.; Peng, Q.; Olaru, O.T.; Grădinaru, D.; Tsatsakis, A.; Tsoukalas, D.; et al. The Akt pathway in oncology therapy and beyond (Review). Int. J. Oncol. 2018, 53, 2319–2331. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Liu, H.T. MAPK signal pathways in the regulation of cell proliferation in mammalian cells. Cell Res. 2002, 12, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Chansriniyom, C.; Ruangrungsi, N.; Lipipun, V.; Kumamoto, T.; Ishikawa, T. Isolation of acridone alkaloids and N-[(4-Monoterpenyloxy)phenylethyl]-substituted sulfur-containing propanamide derivatives from Glycosmis parva and their anti-herpes simplex virus activity. Chem. Pharm. Bull. 2009, 57, 1246–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukaew, A.; Ponglimanont, C.; Karalai, C.; Tewtrakul, S. Potential anti-allergic acridone alkaloids from the roots of Atalantia monophylla. Phytochemistry 2008, 69, 2616–2620. [Google Scholar] [CrossRef]
- Rawla, P. Epidemiology of prostate cancer. World J. Oncol. 2019, 10, 63–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, C.; Wan, J.; Mei, Z. Acridone alkaloids with cytotoxic and antimalarial activities from Zanthoxylum simullans Hance. Pharmacogn. Mag. 2014, 10, 73–76. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Yang, X.; Geng, M.; Huang, M. Targeting ERK, an Achilles’ Heel of the MAPK pathway, in cancer therapy. Acta Pharm. Sin. B. 2018, 8, 552–562. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Hou, Y.; Gu, C.; Zhao, D.; Duan, Y.; Ran, Z.; Li, Q.; Li, X. Inhibitory effect of the mitogen activated protein kinase specific inhibitor PD98059 on Mtb-Ag-activated γδΤ cells. Int. J. Clin. Exp. Pathol. 2017, 10, 9644–9648. [Google Scholar]
- Pires, D.E.V.; Blundell, T.L.; Ascher, D.B. pkCSM: Predicting small-molecule pharmacokinetic and toxicity properties using graph-based signatures. J. Med. Chem. 2015, 58, 4066–4072. [Google Scholar] [CrossRef]
- Ohori, M.; Kinoshita, T.; Okubo, M.; Sato, K.; Yamazaki, A.; Arakawa, H.; Nishimura, S.; Inamura, N.; Nakajima, H.; Neya, M.; et al. Identification of a selective ERK inhibitor and structural determination of the inhibitor-ERK2 complex. Biochem. Biophys. Res. Commun. 2005, 336, 357–363. [Google Scholar] [CrossRef]



| Pharmacokinetic Properties | Model Name | Predicted Value |
|---|---|---|
| Absorption | Human intestinal bsorption (% absorbed) | 68.876 |
| Distribution | VDss (human) (log L/kg) (L/kg) | 0.255 (1.8 L/kg) |
| Metabolism | CYP2D6 substrate | No |
| CYP3A4 substrate | No | |
| CYP1A2 inhibitor | No | |
| CYP2C19 inhibitor | Yes | |
| CYP2C9 inhibitor | No | |
| CYP2D6 inhibitor | No | |
| CYP3A4 inhibitor | No | |
| Excretion | Total clearance (log mL/min/kg) | 0.198 |
| Renal OCT2 substrate | No | |
| Toxicity | Hepatotoxicity | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.-Y.; Boonyarat, C.; Takomthong, P.; Plekratoke, K.; Hayakawa, Y.; Yenjai, C.; Kaewamatawong, R.; Chaiwiwatrakul, S.; Waiwut, P. Acridone Derivatives from Atalantia monophyla Inhibited Cancer Cell Proliferation through ERK Pathway. Molecules 2022, 27, 3865. https://doi.org/10.3390/molecules27123865
Gao W-Y, Boonyarat C, Takomthong P, Plekratoke K, Hayakawa Y, Yenjai C, Kaewamatawong R, Chaiwiwatrakul S, Waiwut P. Acridone Derivatives from Atalantia monophyla Inhibited Cancer Cell Proliferation through ERK Pathway. Molecules. 2022; 27(12):3865. https://doi.org/10.3390/molecules27123865
Chicago/Turabian StyleGao, Wen-Yong, Chantana Boonyarat, Pitchayakarn Takomthong, Kusawadee Plekratoke, Yoshihiro Hayakawa, Chavi Yenjai, Rawiwun Kaewamatawong, Suchada Chaiwiwatrakul, and Pornthip Waiwut. 2022. "Acridone Derivatives from Atalantia monophyla Inhibited Cancer Cell Proliferation through ERK Pathway" Molecules 27, no. 12: 3865. https://doi.org/10.3390/molecules27123865
APA StyleGao, W.-Y., Boonyarat, C., Takomthong, P., Plekratoke, K., Hayakawa, Y., Yenjai, C., Kaewamatawong, R., Chaiwiwatrakul, S., & Waiwut, P. (2022). Acridone Derivatives from Atalantia monophyla Inhibited Cancer Cell Proliferation through ERK Pathway. Molecules, 27(12), 3865. https://doi.org/10.3390/molecules27123865