Effect of TiO2 Calcination Pretreatment on the Performance of Pt/TiO2 Catalyst for CO Oxidation
Abstract
:1. Introduction
2. Experiments
2.1. TiO2 Pretreatment
2.2. Catalyst Preparation
2.3. Catalyst Characterization
2.4. Catalytic Testing
3. Results and Discussion
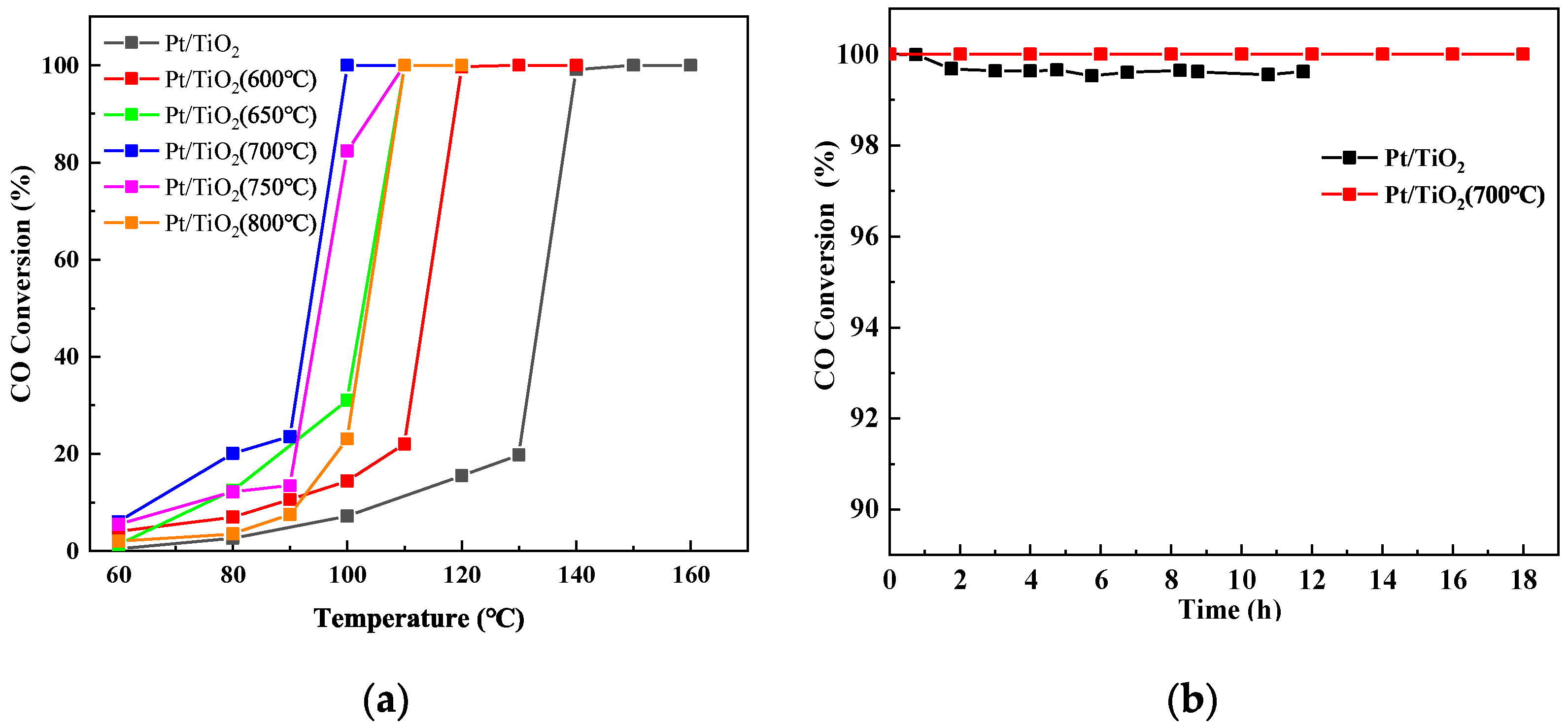
3.1. Catalytic Performance
3.2. Catalyst Characterization
3.2.1. BET
3.2.2. XRD
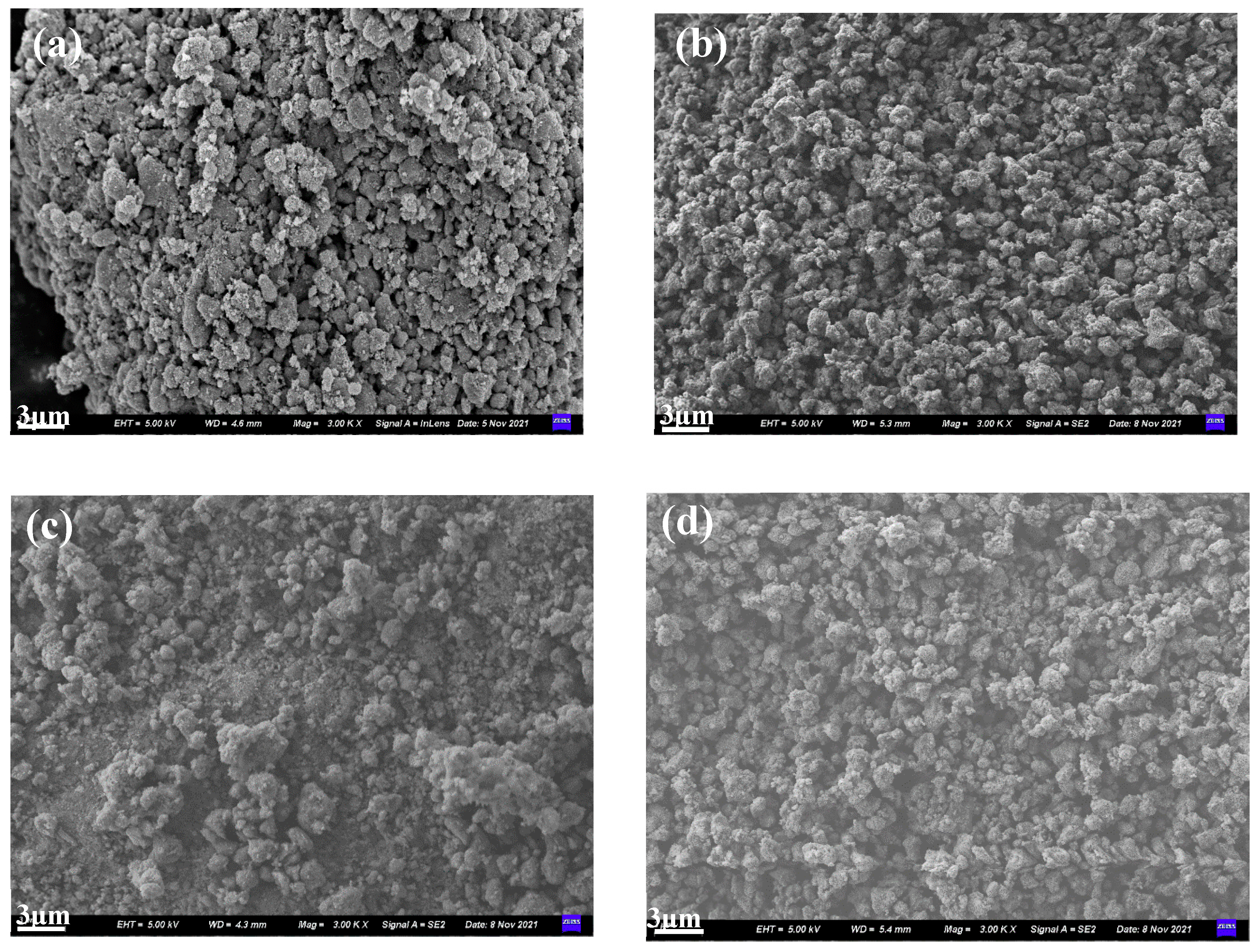
3.2.3. SEM
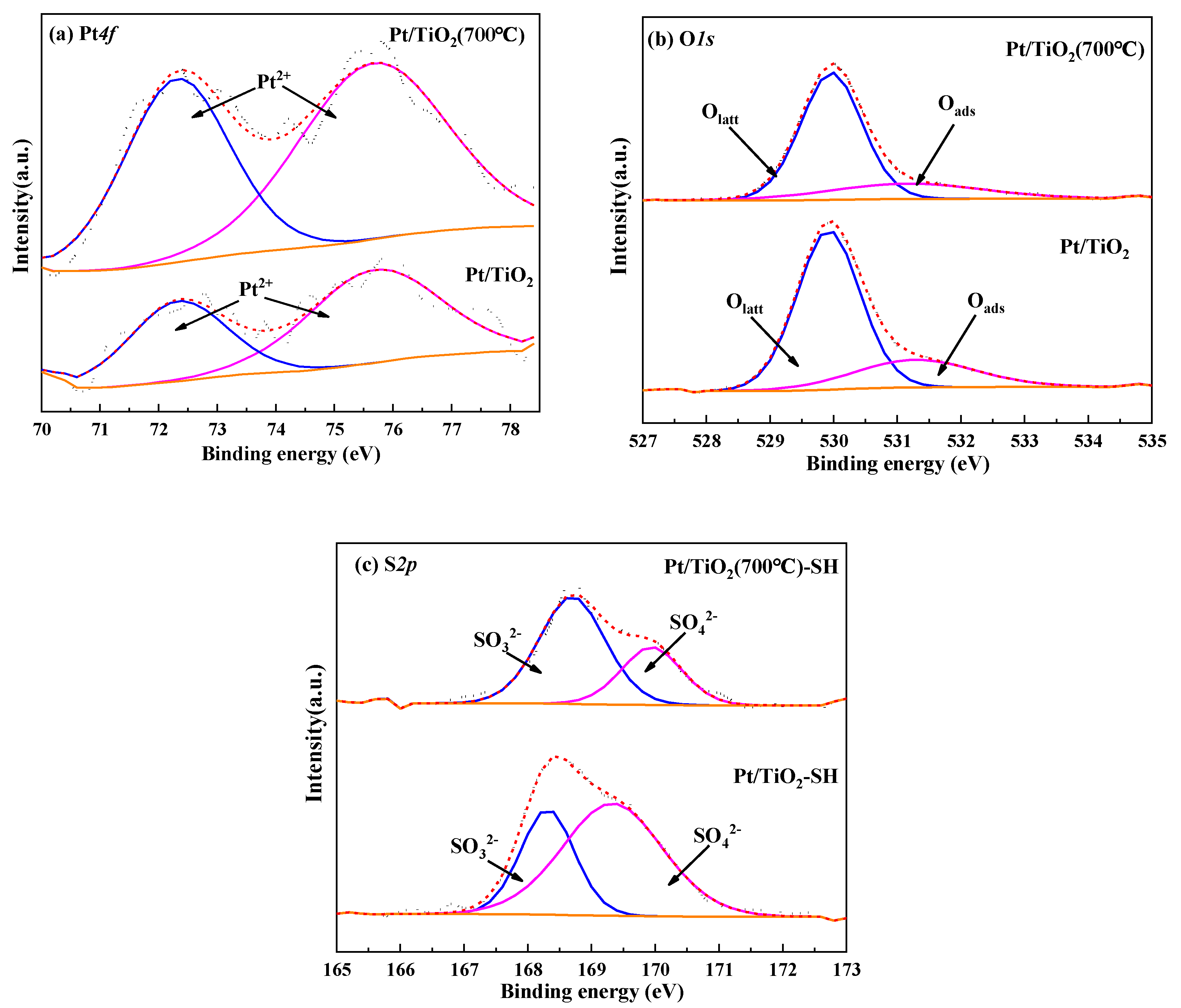
3.2.4. XPS
3.2.5. CO Chemisorption Experiments
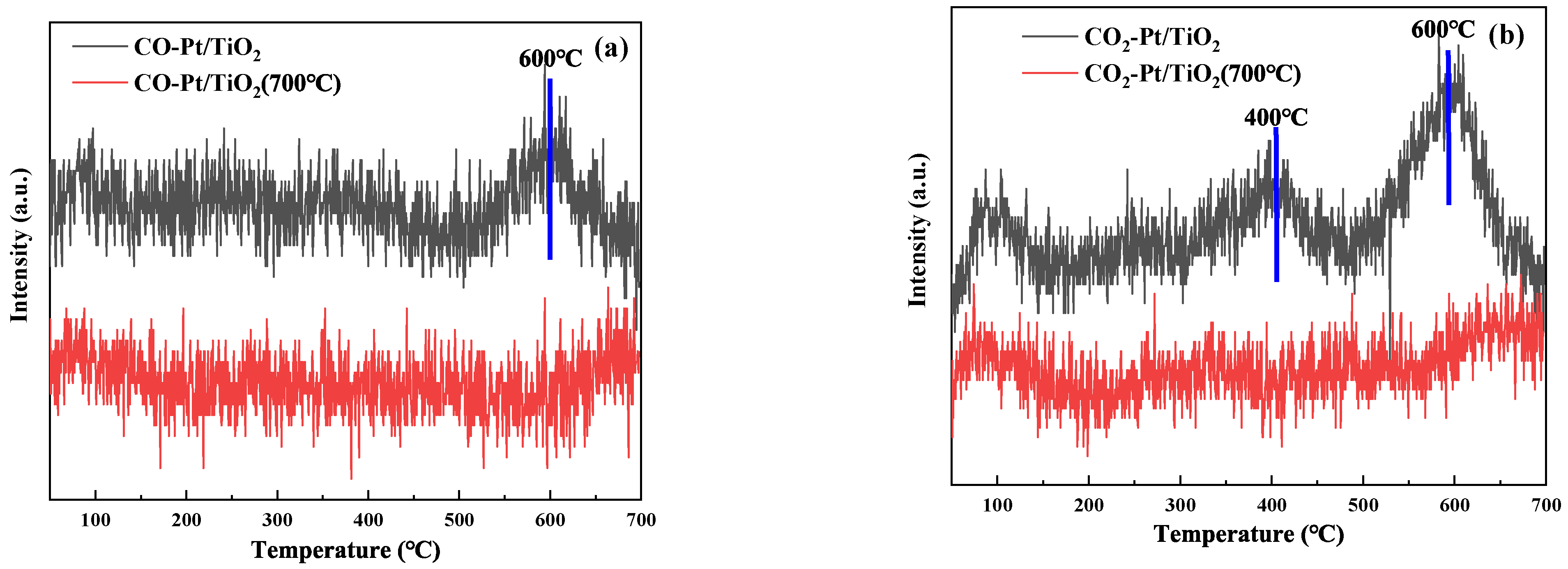
3.2.6. CO-TPD
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Twigg, M.V. Progress and future challenges in controlling automotive exhaust gas emissions. Appl. Catal. B Environ. 2007, 70, 2–15. [Google Scholar] [CrossRef]
- Liu, K.; Wang, A.Q.; Zhang, T. Recent Advances in Preferential Oxidation of CO Reaction over Platinum Group Metal Catalysts. ACS Catal. 2012, 2, 1165–1178. [Google Scholar] [CrossRef]
- Freund, H.J.; Meijre, G.; Scheffler, M.; Schlögl, R.; Wolf, M. CO Oxidation as a Prototypical Reaction for Heterogeneous Processes. Angew. Chem. Int. Ed. 2011, 50, 10064–10094. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.S. Toward understanding the nature of the active sites and structure-activity relationships of heterogeneous catalysts by model catalysis studies. Acta Phys. Chim. Sin. 2017, 33, 2424–2437. [Google Scholar]
- Zhang, B.; Asakura, H.; Yan, N. Atomically dispersed rhodium on self-assembled phosphotungstic acid: Structural features and catalytic CO oxidation properties. Ind. Eng. Chem. Res. 2017, 56, 3578–3587. [Google Scholar] [CrossRef]
- Hülsey, M.J.; Zhang, B.; Ma, Z.R.; Asakura, H.; Do, D.A.; Chen, W.; Tanaka, T.; Zhang, P.; Wu, Z.L.; Yan, N. In situ spectroscopy-guided engineering of rhodium single-atom catalysts for CO oxidation. Nat. Commun. 2019, 10, 1330. [Google Scholar] [CrossRef]
- Liu, J.X.; Qiao, B.T.; Song, Y.; Huang, Y.D.; Jimmy, J.Y. Hetero-epitaxially anchoring Au nanoparticles onto ZnO nanowires for CO oxidation. Chem. Commun. 2015, 51, 15332–15335. [Google Scholar] [CrossRef]
- Li, C.Q.; Yang, Y.; Ren, W.; Wang, J.; Zhu, T.Y.; Xu, W.Q. Effect of Ce doping on catalytic performance of Cu/TiO2 for CO oxidation. Catal. Lett. 2020, 150, 2045–2055. [Google Scholar] [CrossRef]
- Jin, X.; Feng, X.L.; Liu, D.P.; Su, Y.T.; Zhang, Z.; Zhang, Y. Auto-redox Strategy for the Synthesis of Co3O4/CeO2 Nanocomposites and Their Structural Optimization towards catalytic CO oxidation. Chem. J. Chin. Univ. 2020, 41, 652–660. [Google Scholar]
- Wang, C.; Guo, L.H.; Li, X.G.; Ma, K.; Ding, T.; Wang, X.L.; Cheng, Q.P.; Tian, Y. CuO Catalyst Supported on CeO2 Prepared by Oxalate Thermal Decomposition Method for Preferential Oxidation of CO. Chem. J. Chin. Univ. 2017, 38, 2296–2305. [Google Scholar]
- Ma, C.Y.; Mu, Z.; Li, J.J.; Jin, Y.G.; Cheng, J.; Lu, G.Q.; Hao, Z.P.; Qiao, S.Z. Mesoporous Co3O4 and Au/Co3O4 Catalysts for Low-Temperature Oxidation of Trace Ethylene. J. Am. Chem. Soc. 2010, 132, 2608–2613. [Google Scholar] [CrossRef] [PubMed]
- Langmuir, I. The mechanism of the catalytic action of platinum in the reactions 2CO + O2 = 2CO2 and 2H2 + O2 = 2H2O. Trans. Faraday Soc. 1922, 17, 621–654. [Google Scholar] [CrossRef]
- Therrien, A.J.; Hensley, A.J.R.; Marcinkowski, M.D.; Zhang, R.Q.; Lucci, F.R.; Coughlin, B.; Schilling, A.C.; McEwen, J.S.; Sykes, E.C.H. An atomic-scale view of single-site Pt catalysis for low-temperature CO oxidation. Nat. Catal. 2018, 1, 192–198. [Google Scholar] [CrossRef]
- Vasilchenko, D.; Topchiyan, P.; Berdyugin, S.; Filatov, E.; Tkachev, S.; Baidina, I.; Komarov, V.; Slavinskaya, E.; Stadnichenko, A.; Gerasimov, E. Tetraalkylammonium salts of platinum nitrato complexes: Isolation, structure, and relevance to the preparation of PtOx/CeO2 catalysts for low-temperature CO oxidation. Inorg. Chem. 2019, 58, 6075–6087. [Google Scholar] [CrossRef]
- Gili, A.; Schlicker, L.; Bekheet, M.F.; Görke, O.; Penner, S.; Grünbacher, M.; Götsch, T.; Littlewood, P.; Marks, T.J.; Stair, P.C.; et al. Surface carbon as a reactive intermediate in dry reforming of methane to syngas on a 5% Ni/MnO catalyst. ACS Catal. 2018, 8, 8739–8750. [Google Scholar] [CrossRef]
- Lamoth, M.; Plodinec, M.; Scharfenberg, L.; Wrabetz, S.; Girgsdies, F.; Jones, T.; Rosowski, F.; Horn, R.; Schlögl, R.; Frei, E. Supported Ag nanoparticles and clusters for CO oxidation: Size effects and influence of the silver–oxygen interactions. ACS Appl. Nano Mater. 2019, 2, 2909–2920. [Google Scholar] [CrossRef]
- Cai, J.Y.; Yu, Z.H.; Li, J. Effect of Preparation Methods on the Performance of Pt/TiO2 Catalysts for the Catalytic Oxidation of Carbon Monoxide in Simulated Sintering Flue Gas. Catalysts 2021, 11, 804. [Google Scholar] [CrossRef]
- Li, C.; Li, L.; Chen, J.H.; Zhang, X.H.; Xu, J.; Li, Y.B.; Wei, J. Preparation and electrocatalytic performance for methanol oxidation of Pt-CeO2 /sodium-4-styrenesulfonate functionalized carbon nanotube composites. Chem. J. Chin. Univ. 2018, 39, 157–165. [Google Scholar]
- Czupryn, K.; Kocemba, I.; Rynkowski, J. Photocatalytic CO oxidation with water over Pt/TiO2 catalysts. Mech. Catal. 2018, 124, 187–201. [Google Scholar] [CrossRef] [Green Version]
- Derita, L.; Dai, S.; Zepeda, K.l.; Pham, N.; Graham, G.W.; Pan, X.Q.; Christopher, P. Catalyst Architecture for Stable Single Atom Dispersion Enables Site-Specific Spectroscopic and Reactivity Measurements of CO Adsorbed to Pt Atoms, Oxidized Pt Clusters, and Metallic Pt Clusters on TiO2. J. Am. Chem. Soc. 2017, 139, 14150–14165. [Google Scholar] [CrossRef]
- Zhang, W.L. The Synthesis and the Reaction of CO Oxidation over the Supported Pt/Fe2O3 Catalyst. Ph.D. Thesis, Jilin University, Changchun, China, 2012. [Google Scholar]
- Cargnello, M.; Doan-nguyen, V.V.T.; Gordon, T.R.; Diaz, R.E.; Stach, E.A.; Gorte, R.J.; Fornasiero, P.; Murray, C.B. Control of metal nanocrystal size reveals metal-support interface role for ceria catalysts. Science 2013, 341, 771–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, J.; Wang, X.D.; Zhang, T. Recent progress in CO oxidation over Pt-group-metal catalysts at low temperatures. Chin. J. Catal. 2016, 37, 1805–1813. [Google Scholar] [CrossRef]
- Bera, P.; Priolkar, K.R.; Gayen, A.; Sarode, P.R.; Hegde, M.S.; Emura, S.; Kumashiro, R.; Jayaram, V.; Subbanna, G.N. Ionic Dispersion of Pt over CeO2 by the Combustion Method: Structural Investigation by XRD, TEM, XPS, and EXAFS. Chem. Mater. 2003, 15, 2049–2060. [Google Scholar] [CrossRef]
- Rosso, I.; Galletti, C.; Fiorot, S.; Saracco, G.; Garrone, E.; Specchia, V. Preferential CO oxidation over Pt/3A zeolite catalysts in H2-rich gas for fuel cell application. J. Porous Mater. 2007, 14, 245–250. [Google Scholar] [CrossRef]
- An, N.H.; Zhang, W.L.; Yuan, X.L.; Pan, B.; Liu, G.; Jian, M.J.; Yan, W.F.; Zhang, W.X. Catalytic oxidation of formaldehyde over different silica supported platinum catalysts. Chem. Eng. J. 2013, 215/216, 1–6. [Google Scholar] [CrossRef]
- Igarashi, H.; Uchida, H.; Suzuki, M.; Sasaki, Y.; Watanabe, M. Removal of carbon monoxide from hydrogen-rich fuels by selective oxidation over platinum catalyst supported on zeolite. Appl. Catal. A-Gen. 1997, 159, 159–169. [Google Scholar] [CrossRef]
- Ko, E.Y.; Park, E.D.; Seo, K.W.; Lee, H.C.; Lee, D.; Kim, S. Pt–Ni/γ-Al2O3 catalyst for the preferential CO oxidation in the hydrogen stream. Catal. Lett. 2006, 110, 275–279. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, C.Y.; Liang, H.; Liu, Y. Macroporous monolithic Pt/γ-Al2O3 and K–Pt/γ-Al2O3 catalysts used for preferential oxidation of CO. Catal. Lett. 2009, 127, 339–347. [Google Scholar] [CrossRef]
- Jo, M.C.; Kwon, G.H.; Li, W.; Lane, A.M. Preparation and characteristics of pretreated Pt/alumina catalysts for the preferential oxidation of carbon monoxide. J. Ind. Eng. Chem. 2009, 15, 336–341. [Google Scholar] [CrossRef]
- Mergler, Y.J.; Vanaalst, A.; Vandelft, J.; Nieuwenhuys, B.E. CO oxidation over promoted Pt catalysts. Appl. Catal. B Environ. 1996, 10, 245–261. [Google Scholar] [CrossRef]
- Bi, W.; Hu, Y.J.; Jiang, H.; Yu, H.; Li, W.G.; Li, C.Z. In-situ synthesized surface N-doped Pt/TiO2 via flame spray pyrolysis with enhanced thermal stability for CO catalytic oxidation. Appl. Surf. Sci. 2019, 481, 360–368. [Google Scholar] [CrossRef]
- Jiang, J.C.; Lei, J.; Hu, Y.J.; Bi, W.; Xu, N.; Li, Y.F.; Chen, X.L.; Jiang, H.; Li, C.Z. Electron transfer effect from Au to Pt in Au-Pt/TiO2 towards efficient catalytic activity in CO oxidation at low temperature. Appl. Surf. Sci. 2020, 521, 146447. [Google Scholar] [CrossRef]
- Mohamed, Z.; Dasireddy, V.D.B.C.; Singh, S.; Friedrich, H.B. Comparative studies for CO oxidation and hydrogenation over supported Pt catalysts prepared by different synthesis methods. Renew. Energy 2020, 148, 1041–1053. [Google Scholar] [CrossRef]
- Choi, H.; Carboni, M.; Kim, Y.K.; Jung, C.H.; Moon, S.Y.; Koebel, M.M.; Park, J.Y. Synthesis of High Surface Area TiO2 Aerogel Support with Pt Nanoparticle Catalyst and CO Oxidation Study. Catal. Lett. 2018, 148, 1504–1513. [Google Scholar] [CrossRef]
- Xie, S.H.; Liu, Y.X.; Deng, J.G.; Zhao, X.T.; Yang, J.; Zhang, K.F.; Han, Z.; Dai, H.X. Three-dimensionally ordered macroporous CeO2-supported Pd@Co nanoparticles: Highly active catalysts for methane oxidation. J. Catal. 2016, 342, 17–26. [Google Scholar] [CrossRef]
- Taira, K.; Einaga, H. The Effect of SO2 and H2O on the Interaction Between Pt and TiO2(P-25) During Catalytic CO Oxidation. Catal. Lett. 2019, 149, 965–973. [Google Scholar] [CrossRef]
- Feng, C.L.; Liu, X.L.; Zhu, T.Y.; Hu, Y.T. Catalytic oxidation of CO over Pt/TiO2 with low Pt loading: The effect of H2O and SO2. Appl. Catal. A Gen. 2021, 622, 118218. [Google Scholar] [CrossRef]
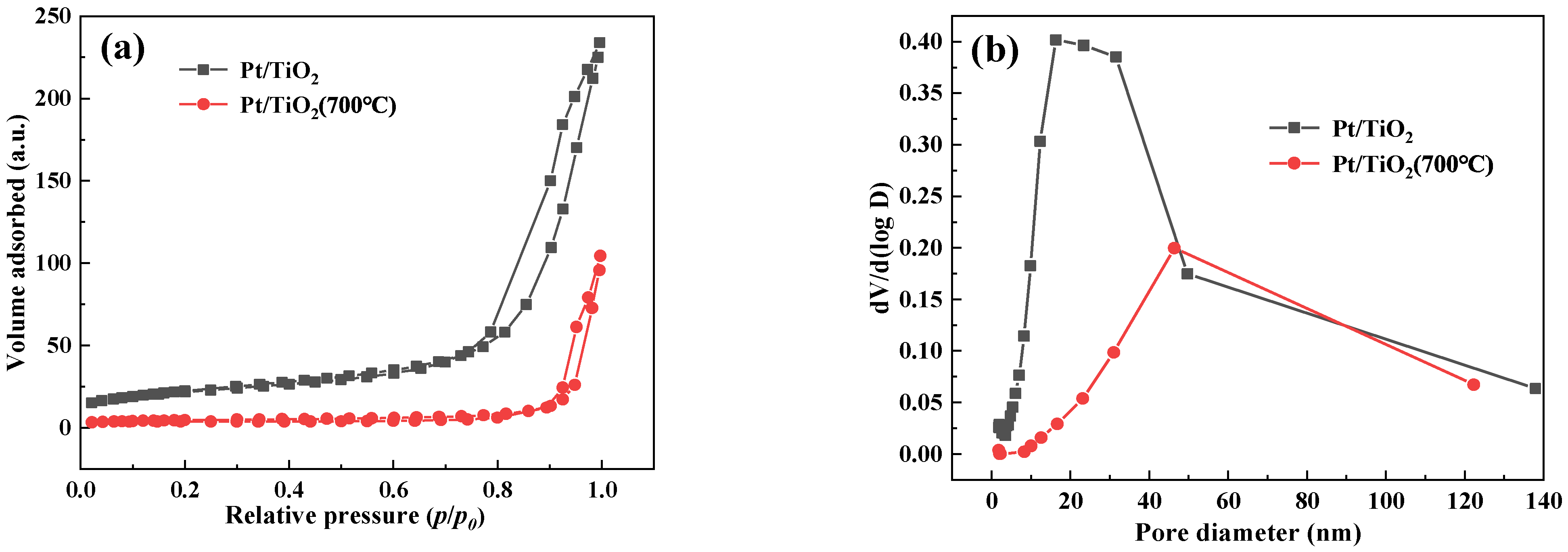

| Catalyst | BET Surface Area m2·g−1 | Pore Volume cm3·g−1 | Pore Size nm |
|---|---|---|---|
| Pt/TiO2 | 78.78 | 0.363 | 17.0 |
| Pt/TiO2 (700 °C) | 15.76 | 0.155 | 42.9 |
| Pt/TiO2-SH | 76.50 | 0.354 | 15.1 |
| Pt/TiO2 (700 °C)-SH | 15.21 | 0.151 | 41.9 |
| Catalyst | ||
|---|---|---|
| Pt/TiO2 | 0.206 | / |
| Pt/TiO2 (700 °C) | 0.295 | / |
| Pt/TiO2-SH | / | 0.369 |
| Pt/TiO2 (700 °C)-SH | / | 0.669 |
| Catalyst | Platinum Dispersion % | Platinum Particle Size nm | Platinum Surface Area m2·g−1 |
|---|---|---|---|
| Pt/TiO2 | 52.44 | 18.01 | 0.26 |
| Pt/TiO2 (700 °C) | 60.27 | 15.67 | 0.92 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, J.; Yu, Z.; Fan, X.; Li, J. Effect of TiO2 Calcination Pretreatment on the Performance of Pt/TiO2 Catalyst for CO Oxidation. Molecules 2022, 27, 3875. https://doi.org/10.3390/molecules27123875
Cai J, Yu Z, Fan X, Li J. Effect of TiO2 Calcination Pretreatment on the Performance of Pt/TiO2 Catalyst for CO Oxidation. Molecules. 2022; 27(12):3875. https://doi.org/10.3390/molecules27123875
Chicago/Turabian StyleCai, Jianyu, Zehui Yu, Xing Fan, and Jian Li. 2022. "Effect of TiO2 Calcination Pretreatment on the Performance of Pt/TiO2 Catalyst for CO Oxidation" Molecules 27, no. 12: 3875. https://doi.org/10.3390/molecules27123875
APA StyleCai, J., Yu, Z., Fan, X., & Li, J. (2022). Effect of TiO2 Calcination Pretreatment on the Performance of Pt/TiO2 Catalyst for CO Oxidation. Molecules, 27(12), 3875. https://doi.org/10.3390/molecules27123875





