Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives
Abstract
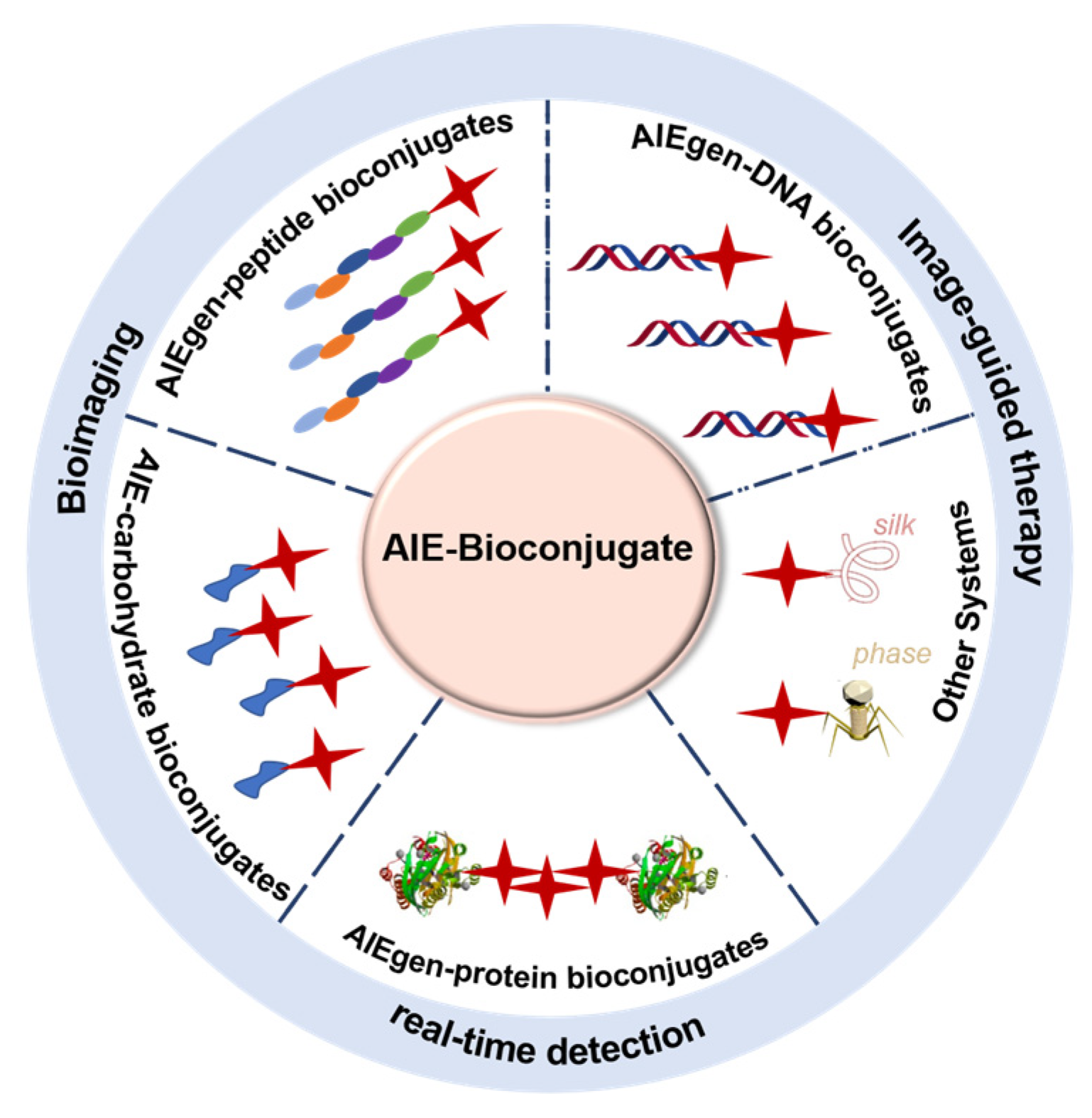
:1. Introduction
2. AIE-Carbohydrate Bioconjugates
3. AIEgen-Peptide Bioconjugates
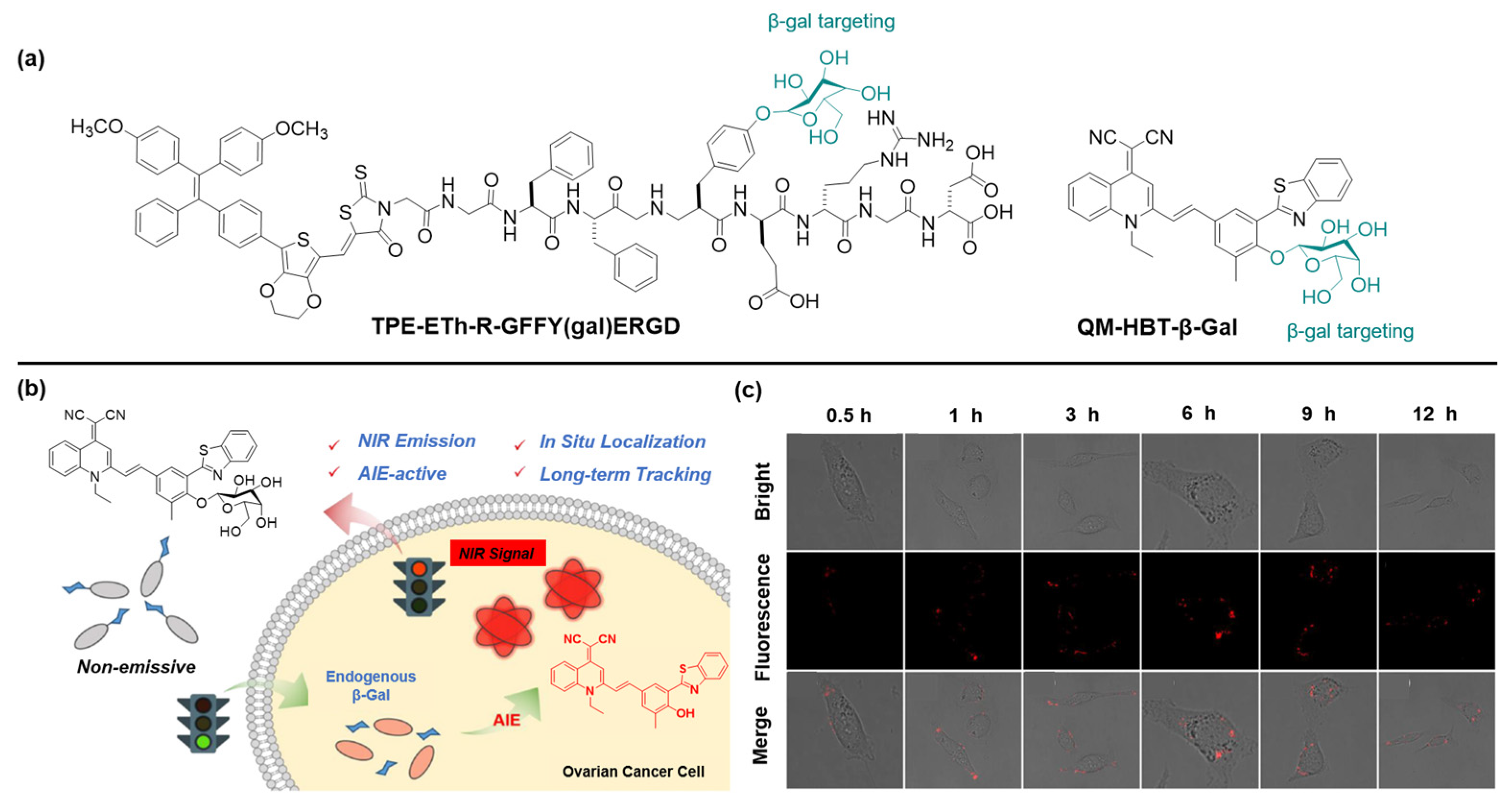
3.1. Activatable AIEgen-Peptide Bioconjugates
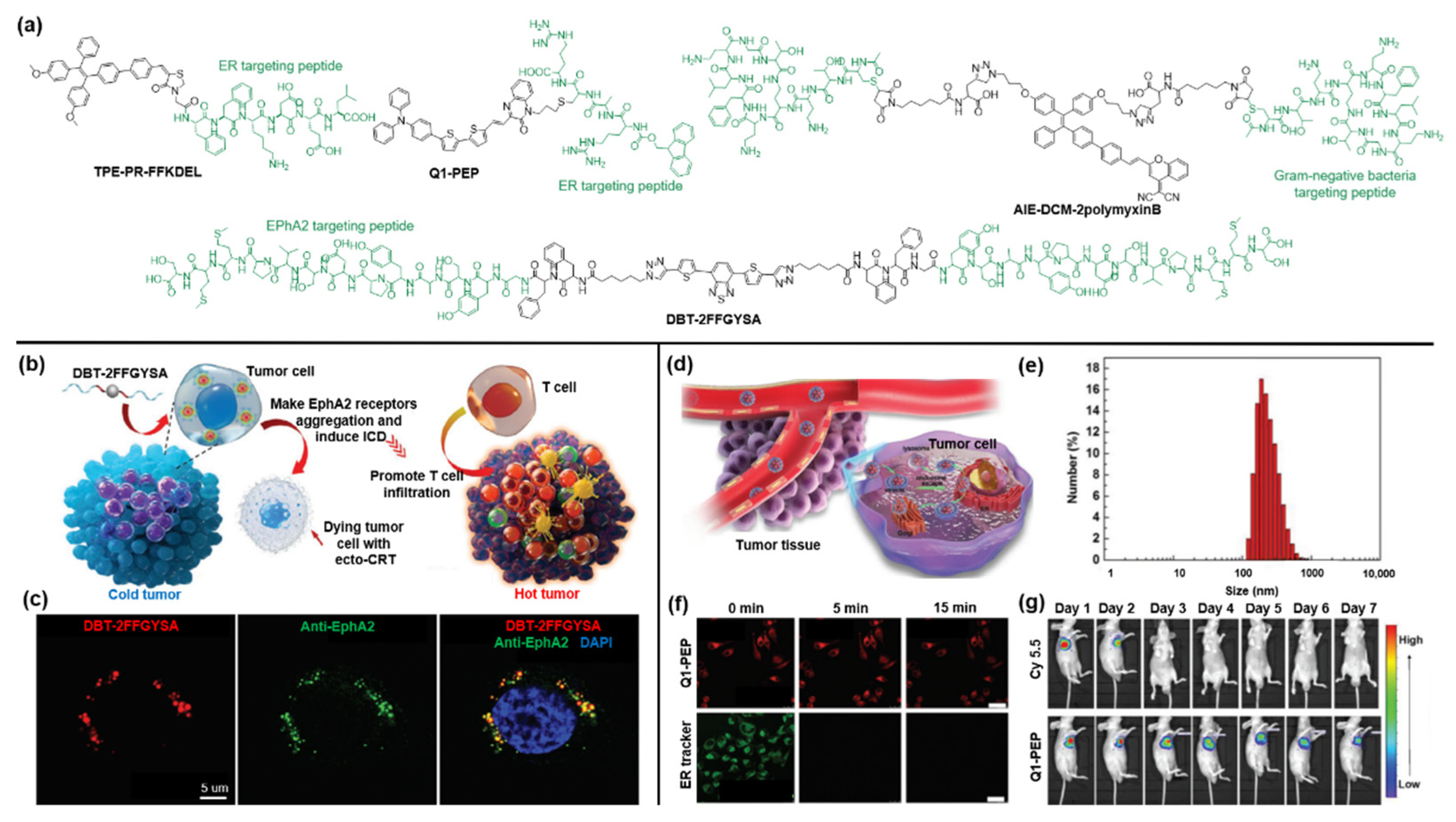
3.2. Targetable AIEgen-Peptide Bioconjugates
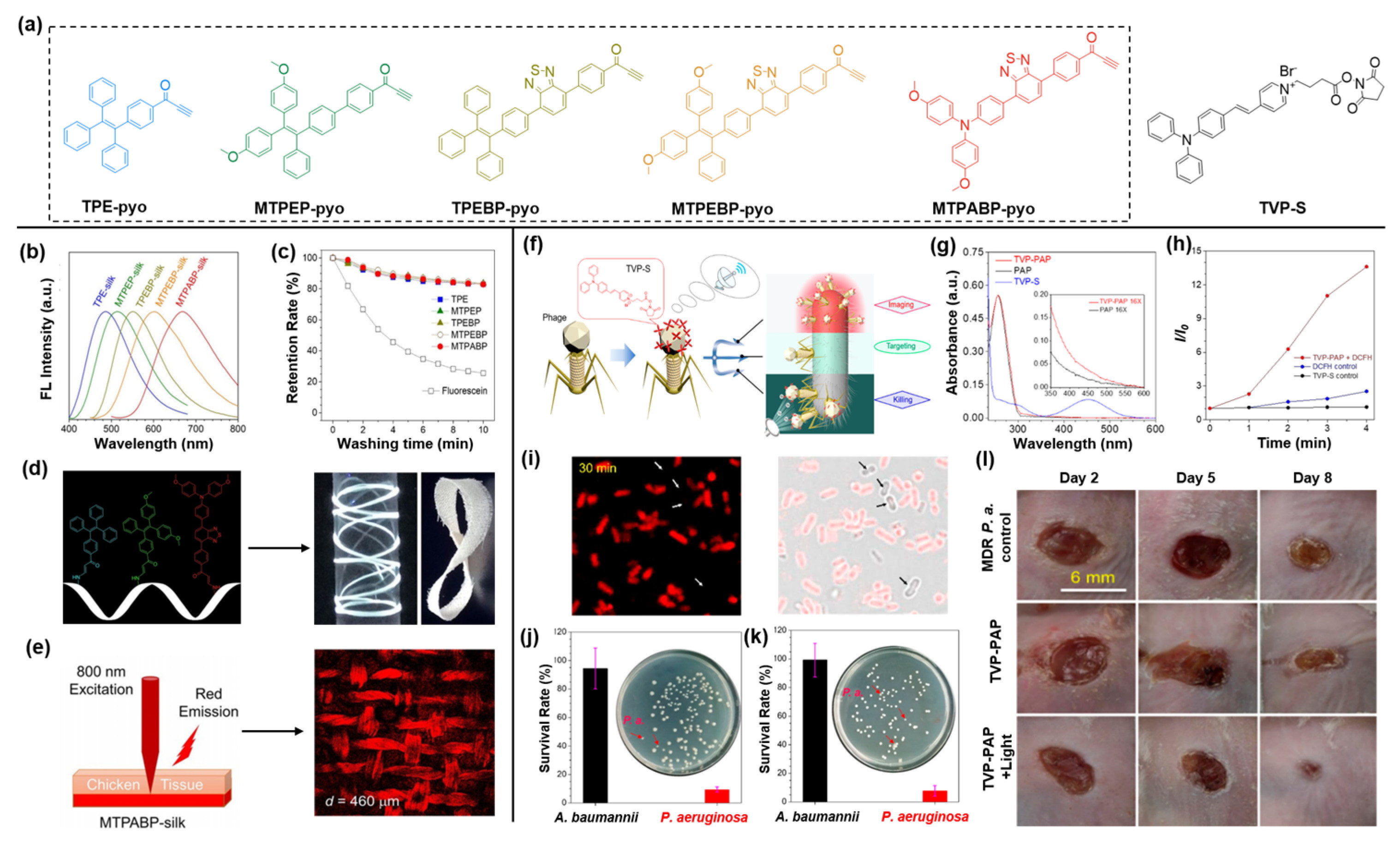
4. AIEgen-Protein Bioconjugates
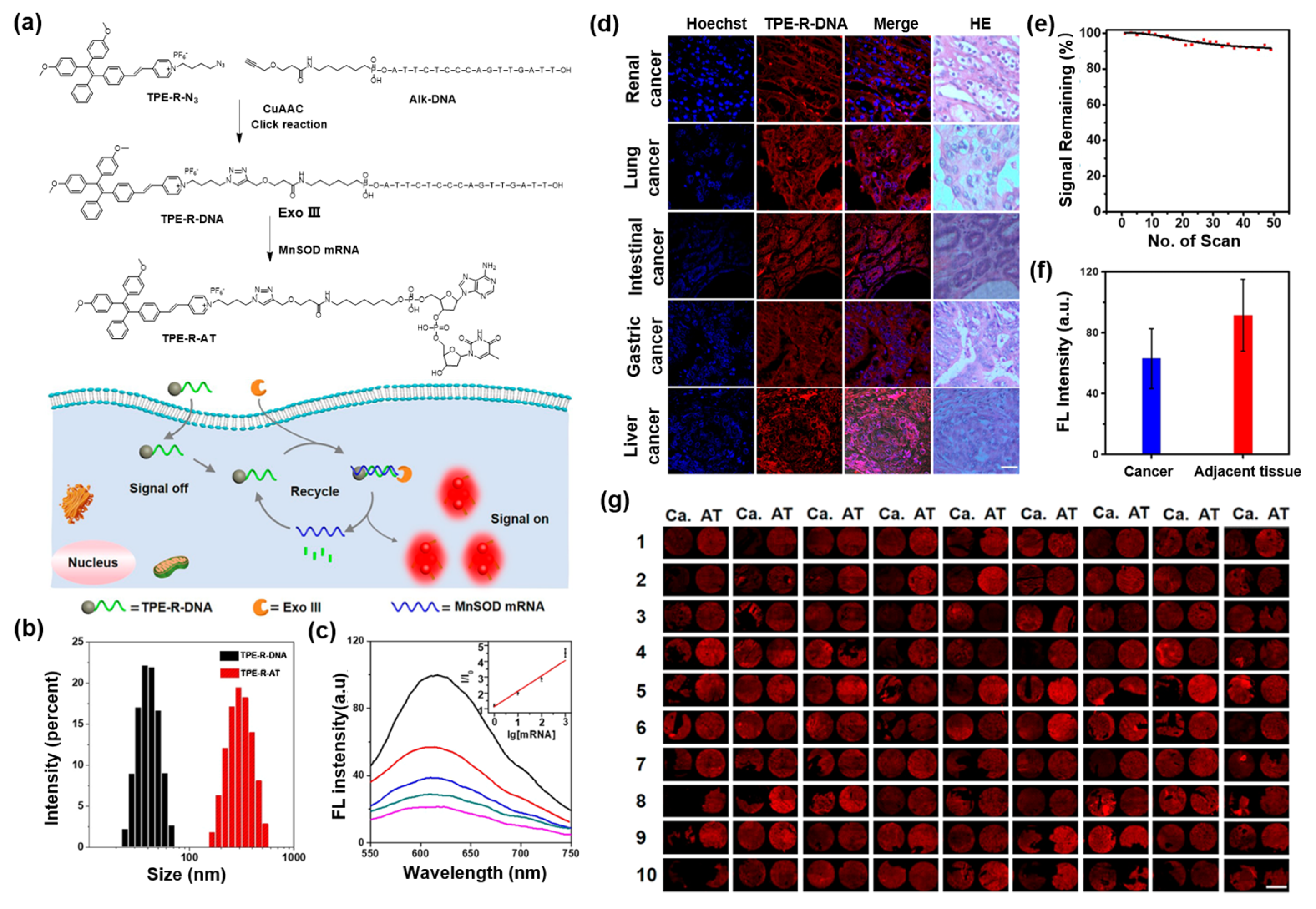
5. AIEgen-DNA Bioconjugates
6. Other Systems
7. Summary and Perspective
Author Contributions
Funding
Conflicts of Interest
References
- Giepmans, B.N.G.; Adams, S.R.; Ellisman, M.H.; Tsien, R.Y. The Fluorescent Toolbox for Assessing Protein Location and Function. Science 2006, 312, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, M.H.; Kim, J.S.; Sessler, J.L. Small molecule-based ratiometric fluorescence probes for cations, anions, and biomolecules. Chem. Soc. Rev. 2015, 44, 4185–4191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Etzioni, R.; Urban, N.; Ramsey, S.; McIntosh, M.; Schwartz, S.; Reid, B.; Radich, J.; Anderson, G.; Hartwell, L. The case for early detection. Nat. Rev. Cancer 2003, 3, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Beatriz, S.P.; Luis, N.; Leonor, C.; Laura, M.; Elena, M.; Yolanda, F.N. Imaging Techniques and Scanning Electron Microscopy as Tools for Characterizing a Si-Based Material Used in Air Monitoring Applications. Materials 2016, 9, 109. [Google Scholar] [CrossRef] [Green Version]
- Garg, P.K.; Singh, S.K.; Prakash, G.; Jakhetiya, A.; Pandey, D. Role of positron emission tomography-computed tomography in non-small cell lung cancer. World J. Methodol. 2016, 6, 105–111. [Google Scholar] [CrossRef]
- Kolokythas, O.; Gauthier, T.; Fernandez, A.T.; Xie, H.; Timm, B.A.; Cuevas, C.; Dighe, M.K.; Mitsumori, L.M.; Bruce, M.F.; Herzka, D.A.; et al. Ultrasound-Based Elastography: A Novel Approach to Assess Radio Frequency Ablation of Liver Masses Performed With Expandable Ablation Probes. J. Ultrasound Med. 2008, 27, 935–946. [Google Scholar] [CrossRef] [Green Version]
- Lusic, H.; Grinstaff, M.W. X-ray-Computed Tomography Contrast Agents. Chem. Rev. 2013, 113, 1641–1666. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef] [Green Version]
- Cormode, D.P.; Jarzyna, P.A.; Mulder, W.J.M.; Fayad, Z.A. Modified natural nanoparticles as contrast agents for medical imaging. Adv. Drug Deliv. Rev. 2010, 62, 329–338. [Google Scholar] [CrossRef] [Green Version]
- Wiederschain, G.Y. The Molecular Probes handbook. A guide to fluorescent probes and labeling technologies. Biochemistry 2011, 76, 1276. [Google Scholar] [CrossRef] [Green Version]
- Tsien, R.Y. Constructing and Exploiting the Fluorescent Protein Paintbox (Nobel Lecture). Angew. Chem. Int. Ed. 2009, 48, 5612–5626. [Google Scholar] [CrossRef]
- He, L.; Yang, X.; Xu, K.; Kong, X.; Lin, W. A multi-signal fluorescent probe for simultaneously distinguishing and sequentially sensing cysteine/homocysteine, glutathione, and hydrogen sulfide in living cells. Chem. Sci. 2017, 8, 6257–6265. [Google Scholar] [CrossRef] [Green Version]
- Chinen, A.B.; Guan, C.M.; Ferrer, J.R.; Barnaby, S.N.; Merkel, T.J.; Mirkin, C.A. Nanoparticle Probes for the Detection of Cancer Biomarkers, Cells, and Tissues by Fluorescence. Chem. Rev. 2015, 115, 10530–10574. [Google Scholar] [CrossRef] [Green Version]
- Gnach, A.; Lipinski, T.; Bednarkiewicz, A.; Rybka, J.; Capobianco, J.A. Upconverting nanoparticles: Assessing the toxicity. Chem. Soc. Rev. 2015, 44, 1561–1584. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Wang, D.; Tang, B.Z. NIR-II AIEgens: A Win-Win Integration towards Bioapplications. Angew. Chem. Int. Ed. 2021, 60, 7476–7487. [Google Scholar] [CrossRef]
- Yan, D.; Wang, M.; Wu, Q.; Niu, N.; Li, M.; Song, R.; Rao, J.; Kang, M.; Zhang, Z.; Zhou, F.; et al. Multimodal Imaging-Guided Photothermal Immunotherapy Based on a Versatile NIR-II Aggregation-Induced Emission Luminogen. Angew. Chem. 2022. [Google Scholar] [CrossRef]
- Wang, D.; Lee, M.M.S.; Shan, G.; Kwok, R.T.K.; Lam, J.W.Y.; Su, H.; Cai, Y.; Tang, B.Z. Highly Efficient Photosensitizers with Far-Red/Near-Infrared Aggregation-Induced Emission for In Vitro and In Vivo Cancer Theranostics. Adv. Mater. 2018, 30, e1802105. [Google Scholar] [CrossRef]
- Yan, D.; Xie, W.; Zhang, J.; Wang, L.; Wang, D.; Tang, B.Z. Donor/π-Bridge Manipulation for Constructing a Stable NIR-II Aggregation-Induced Emission Luminogen with Balanced Phototheranostic Performance. Angew. Chem. Int. Ed. 2021, 60, 26769–26776. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Huang, Y.; Tian, H. Progress and Trends in AIE-Based Bioprobes: A Brief Overview. ACS Appl. Mater. Interfaces 2018, 10, 12217–12261. [Google Scholar] [CrossRef]
- Luo, J.; Xie, Z.; Lam, J.W.Y.; Cheng, L.; Chen, H.; Qiu, C.; Kwok, H.S.; Zhan, X.; Liu, Y.; Zhu, D.; et al. Aggregation-induced emission of 1-methyl-1,2,3,4,5-pentaphenylsilole. Chem. Commun. 2001, 18, 1740–1741. [Google Scholar] [CrossRef]
- Mei, J.; Leung, N.L.C.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. Aggregation-Induced Emission: Together We Shine, United We Soar! Chem. Rev. 2015, 115, 11718–11940. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Law, C.C.W.; Lam, J.W.Y.; Dong, Y.; Lo, S.M.F.; Williams, I.D.; Zhu, D.; Tang, B.Z. Synthesis, Light Emission, Nanoaggregation, and Restricted Intramolecular Rotation of 1,1-Substituted 2,3,4,5-Tetraphenylsiloles. Chem. Mater. 2003, 15, 1535–1546. [Google Scholar] [CrossRef]
- Leung, N.L.C.; Xie, N.; Yuan, W.; Liu, Y.; Wu, Q.; Peng, Q.; Miao, Q.; Lam, J.W.Y.; Tang, B.Z. Restriction of Intramolecular Motions: The General Mechanism behind Aggregation-Induced Emission. Chem. Eur. J. 2014, 20, 15349–15353. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Hong, Y.; Lam, J.W.Y.; Qin, A.; Tang, Y.; Tang, B.Z. Aggregation-Induced Emission: The Whole Is More Brilliant than the Parts. Adv. Mater. 2014, 26, 5429–5479. [Google Scholar] [CrossRef]
- Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens for Activity-Based Sensing. Acc. Chem. Res. 2019, 52, 2559–2570. [Google Scholar] [CrossRef] [PubMed]
- Kwok, R.T.K.; Leung, C.W.T.; Lam, J.W.Y.; Tang, B.Z. Biosensing by luminogens with aggregation-induced emission characteristics. Chem. Soc. Rev. 2015, 44, 4228–4238. [Google Scholar] [CrossRef]
- Wang, D.; Su, H.; Kwok, R.T.K.; Hu, X.; Zou, H.; Luo, Q.; Lee, M.M.S.; Xu, W.; Lam, J.W.Y.; Tang, B.Z. Rational design of a water-soluble NIR AIEgen, and its application in ultrafast wash-free cellular imaging and photodynamic cancer cell ablation. Chem. Sci. 2018, 9, 3685–3693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Han, T.; Niu, N.; Li, H.; Wang, D.; Tang, B.Z. Facile Multicomponent Polymerizations toward Multifunctional Heterochain Polymers with α,β-Unsaturated Amidines. Macromolecules 2021, 54, 9906–9918. [Google Scholar] [CrossRef]
- Wang, Y.; Yan, D.; Wang, L.; Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens Sensitized Quasi-2D Hybrid Perovskites with Unique Photoluminescence and High Stability for Fabricating White Light-Emitting Diodes. Adv. Sci. 2021, 8, 2100811. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.M.S.; Yan, D.; Chau, J.H.C.; Park, H.; Ma, C.C.H.; Kwok, R.T.K.; Lam, J.W.Y.; Wang, D.; Tang, B.Z. Highly efficient phototheranostics of macrophage-engulfed Gram-positive bacteria using a NIR luminogen with aggregation-induced emission characteristics. Biomaterials 2020, 261, 120340. [Google Scholar] [CrossRef]
- Yan, S.; Sun, P.; Niu, N.; Zhang, Z.; Xu, W.; Zhao, S.; Wang, L.; Wang, D.; Tang, B.Z. Surfactant-Inspired Coassembly Strategy to Integrate Aggregation-Induced Emission Photosensitizer with Organosilica Nanoparticles for Efficient Theranostics. Adv. Funct. Mater. 2022, 32, 2200503. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, W.T.; Mohammadniaei, M.; Zheng, T.; Zhang, Q.C.; Ashley, J.; Liu, S.J.; Sun, Y.; Tang, B.Z. Upregulating Aggregation-Induced-Emission Nanoparticles with Blood–Tumor-Barrier Permeability for Precise Photothermal Eradication of Brain Tumors and Induction of Local Immune Responses. Adv. Mater. 2021, 33, 2008802. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Deng, C.; Tang, L.; Qin, A.; Hu, R.; Sun, J.Z.; Tang, B.Z. Specific Detection of d-Glucose by a Tetraphenylethene-Based Fluorescent Sensor. J. Am. Chem. Soc. 2011, 133, 660–663. [Google Scholar] [CrossRef] [PubMed]
- Ou, X.; Hong, F.; Zhang, Z.; Cheng, Y.; Zhao, Z.; Gao, P.; Lou, X.; Xia, F.; Wang, S. A highly sensitive and facile graphene oxide-based nucleic acid probe: Label-free detection of telomerase activity in cancer patient’s urine using AIEgens. Biosens. Bioelectron. 2017, 89, 417–421. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kwok, R.T.K.; Tang, B.Z.; Liu, B. Specific nucleic acid detection based on fluorescent light-up probe from fluorogens with aggregation-induced emission characteristics. RSC Adv. 2013, 3, 10135–10138. [Google Scholar] [CrossRef]
- Gu, X.; Zhao, E.; Zhao, T.; Kang, M.; Gui, C.; Lam, J.W.Y.; Du, S.; Loy, M.M.T.; Tang, B.Z. A Mitochondrion-Specific Photoactivatable Fluorescence Turn-On AIE-Based Bioprobe for Localization Super-Resolution Microscope. Adv. Mater. 2016, 28, 5064–5071. [Google Scholar] [CrossRef]
- Shi, H.; Liu, J.; Geng, J.; Tang, B.Z.; Liu, B. Specific Detection of Integrin αvβ3 by Light-Up Bioprobe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 9569–9572. [Google Scholar] [CrossRef]
- Shi, H.; Kwok, R.T.K.; Liu, J.; Xing, B.; Tang, B.Z.; Liu, B. Real-Time Monitoring of Cell Apoptosis and Drug Screening Using Fluorescent Light-Up Probe with Aggregation-Induced Emission Characteristics. J. Am. Chem. Soc. 2012, 134, 17972–17981. [Google Scholar] [CrossRef]
- Kang, M.; Zhou, C.; Wu, S.; Yu, B.; Zhang, Z.; Song, N.; Lee, M.M.S.; Xu, W.; Xu, F.-J.; Wang, D.; et al. Evaluation of Structure–Function Relationships of Aggregation-Induced Emission Luminogens for Simultaneous Dual Applications of Specific Discrimination and Efficient Photodynamic Killing of Gram-Positive Bacteria. J. Am. Chem. Soc. 2019, 141, 16781–16789. [Google Scholar] [CrossRef]
- Huang, J.; He, B.; Zhang, Z.; Li, Y.; Kang, M.; Wang, Y.; Li, K.; Wang, D.; Tang, B.Z. Aggregation-Induced Emission Luminogens Married to 2D Black Phosphorus Nanosheets for Highly Efficient Multimodal Theranostics. Adv. Mater. 2020, 32, 2003382. [Google Scholar] [CrossRef]
- Liu, H.; Xiong, L.H.; Kwok, R.T.K.; He, X.; Lam, J.W.Y.; Tang, B.Z. AIE Bioconjugates for Biomedical Applications. Adv. Opt. Mater. 2020, 8, 2000162. [Google Scholar] [CrossRef]
- Aron, A.T.; Ramos-Torres, K.M.; Cotruvo, J.A.; Chang, C.J. Recognition- and Reactivity-Based Fluorescent Probes for Studying Transition Metal Signaling in Living Systems. Acc. Chem. Res. 2015, 48, 2434–2442. [Google Scholar] [CrossRef] [Green Version]
- Ding, D.; Li, K.; Liu, B.; Tang, B.Z. Bioprobes Based on AIE Fluorogens. Acc. Chem. Res. 2013, 46, 2441–2453. [Google Scholar] [CrossRef]
- He, S.; Sharpless, N.E. Senescence in Health and Disease. Cell 2017, 169, 1000–1011. [Google Scholar] [CrossRef]
- Kamiya, M.; Asanuma, D.; Kuranaga, E.; Takeishi, A.; Sakabe, M.; Miura, M.; Nagano, T.; Urano, Y. β-Galactosidase Fluorescence Probe with Improved Cellular Accumulation Based on a Spirocyclized Rhodol Scaffold. J. Am. Chem. Soc. 2011, 133, 12960–12963. [Google Scholar] [CrossRef]
- Spergel, D.J.; Krüth, U.; Shimshek, D.R.; Sprengel, R.; Seeburg, P.H. Using reporter genes to label selected neuronal populations in transgenic mice for gene promoter, anatomical, and physiological studies. Prog. Neurobiol. 2001, 63, 673–686. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, Y.; Yan, C.; Zhu, W.-H.; Guo, Z. Enzyme-activatable fluorescent probes for β-galactosidase: From design to biological applications. Chem. Sci. 2021, 12, 9885–9894. [Google Scholar] [CrossRef]
- Gao, Z.; Gao, H.; Zheng, D.; Xu, T.; Chen, Y.; Liang, C.; Wang, L.; Ding, D.; Yang, Z. β-galactosidase responsive AIE fluorogene for identification and removal of senescent cancer cells. Sci. China Ser. B Chem. 2020, 63, 398–403. [Google Scholar] [CrossRef]
- Fu, W.; Yan, C.; Zhang, Y.; Ma, Y.; Guo, Z.; Zhu, W.-H. Near-Infrared Aggregation-Induced Emission-Active Probe Enables in situ and Long-Term Tracking of Endogenous β-Galactosidase Activity. Front. Chem. 2019, 7, 291. [Google Scholar] [CrossRef]
- Chan, J.; Dodani, S.C.; Chang, C.J. Reaction-based small-molecule fluorescent probes for chemoselective bioimaging. Nat. Chem. 2012, 4, 973–984. [Google Scholar] [CrossRef]
- Yang, J.; Wei, J.; Luo, F.; Dai, J.; Hu, J.-J.; Lou, X.; Xia, F. Enzyme-Responsive Peptide-Based AIE Bioprobes. Top. Curr. Chem. 2020, 378, 47. [Google Scholar] [CrossRef] [PubMed]
- Braun, G.B.; Sugahara, K.N.; Yu, O.M.; Kotamraju, V.R.; Mölder, T.; Lowy, A.M.; Ruoslahti, E.; Teesalu, T. Urokinase-controlled tumor penetrating peptide. J. Control. Release 2016, 232, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Gómez-Sintes, R.; Boya, P. Lysosomal membrane permeabilization and cell death. Traffic 2018, 19, 918–931. [Google Scholar] [CrossRef]
- Hengartner, M.O. The biochemistry of apoptosis. Nature 2000, 407, 770–776. [Google Scholar] [CrossRef]
- Hadler-Olsen, E.; Winberg, J.-O.; Uhlin-Hansen, L. Matrix metalloproteinases in cancer: Their value as diagnostic and prognostic markers and therapeutic targets. Tumor Biol. 2013, 34, 2041–2051. [Google Scholar] [CrossRef]
- Shi, H.; Zhao, N.; Ding, D.; Liang, J.; Tang, B.Z.; Liu, B. Fluorescent light-up probe with aggregation-induced emission characteristics for in vivo imaging of cell apoptosis. Org. Biomol. Chem. 2013, 11, 7289–7296. [Google Scholar] [CrossRef] [Green Version]
- Zhu, Z.; Wang, Q.; Chen, X.; Wang, Q.; Yan, C.; Zhao, X.; Zhao, W.; Zhu, W. An Enzyme-Activatable Aggregation-Induced-Emission Probe: Intraoperative Pathological Fluorescent Diagnosis of Pancreatic Cancer via Specific Cathepsin E. Adv. Mater. 2022, 34, 2107444. [Google Scholar] [CrossRef]
- Lyu, Y.; Chen, X.; Wang, Q.; Li, Q.; Wang, Q.; Li, X.; Zhu, Z.; Yan, C.; Zhao, X.; Zhu, W. Monitoring Autophagy with Atg4B Protease-Activated Aggregation-Induced Emission Probe. Adv. Funct. Mater. 2022, 32, 2108571. [Google Scholar] [CrossRef]
- Dai, J.; Cheng, Y.; Wu, J.; Wang, Q.; Wang, W.; Yang, J.; Zhao, Z.; Lou, X.; Xia, F.; Wang, S.; et al. Modular Peptide Probe for Pre/Intra/Postoperative Therapeutic to Reduce Recurrence in Ovarian Cancer. ACS Nano 2020, 14, 14698–14714. [Google Scholar] [CrossRef]
- Chen, C.; Ni, X.; Jia, S.; Liang, Y.; Wu, X.; Kong, D.; Ding, D. Massively Evoking Immunogenic Cell Death by Focused Mitochondrial Oxidative Stress using an AIE Luminogen with a Twisted Molecular Structure. Adv. Mater. 2019, 31, 1904914. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Wu, J.; Wu, X.; Hu, Q.; Dai, J.; Lou, X. Modular Design of Peptide- or DNA-Modified AIEgen Probes for Biosensing Applications. Acc. Chem. Res. 2019, 52, 3064–3074. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.R.; Kanvinde, P.; King, C.; Pasquale, E.B.; Hristova, K. The EphA2 receptor is activated through induction of distinct, ligand-dependent oligomeric structures. Commun. Biol. 2018, 1, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Fang, Y.; Zhang, Y.; Wang, H.; Yang, Z.; Ding, D. Supramolecular Self-Assembly-Facilitated Aggregation of Tumor-Specific Transmembrane Receptors for Signaling Activation and Converting Immunologically Cold to Hot Tumors. Adv. Mater. 2021, 33, 2008518. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Kepp, O.; Zitvogel, L. Immunogenic Cell Death in Cancer Therapy. Annu. Rev. Immunol. 2013, 31, 51–72. [Google Scholar] [CrossRef]
- Li, J.; Gao, H.; Liu, R.; Chen, C.; Zeng, S.; Liu, Q.; Ding, D. Endoplasmic reticulum targeted AIE bioprobe as a highly efficient inducer of immunogenic cell death. Sci. China Ser. B Chem. 2020, 63, 1428–1434. [Google Scholar] [CrossRef]
- Shi, L.; Gao, X.; Yuan, W.; Xu, L.; Deng, H.; Wu, C.; Yang, J.; Jin, X.; Zhang, C.; Zhu, X. Endoplasmic Reticulum–Targeted Fluorescent Nanodot with Large Stokes Shift for Vesicular Transport Monitoring and Long-Term Bioimaging. Small 2018, 14, 1800223. [Google Scholar] [CrossRef]
- Copolovici, D.M.; Langel, K.; Eriste, E.; Langel, Ü. Cell-Penetrating Peptides: Design, Synthesis, and Applications. ACS Nano 2014, 8, 1972–1994. [Google Scholar] [CrossRef]
- Bao, P.; Li, C.; Ou, H.; Ji, S.; Chen, Y.; Gao, J.; Yue, X.; Shen, J.; Ding, D. A peptide-based aggregation-induced emission bioprobe for selective detection and photodynamic killing of Gram-negative bacteria. Biomater. Sci. 2021, 9, 437–442. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schaffer, A.A.; Zhang, J.H.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Vinogradova, E.V.; Spokoyny, A.M.; Buchwald, S.L.; Pentelute, B.L. Arylation Chemistry for Bioconjugation. Angew. Chem. Int. Ed. 2019, 58, 4810–4839. [Google Scholar] [CrossRef] [Green Version]
- Shi, X.J.; Yu, C.Y.Y.; Su, H.F.; Kwok, R.T.K.; Jiang, M.J.; He, Z.K.; Lam, J.W.Y.; Tang, B.Z. A red-emissive antibody-AIEgen conjugate for turn-on and wash-free imaging of specific cancer cells. Chem. Sci. 2017, 8, 7014–7024. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, R.; Ren, C.P.; Liu, M.; Ge, X.P.; Qu, M.S.; Zhou, X.B.; Liang, M.F.; Liu, Y.; Li, F.Y. Early Detection of SARS-CoV-2 Seroconversion in Humans with Aggregation-Induced Near-Infrared Emission Nanoparticle-Labeled Lateral Flow Immunoassay. ACS Nano 2021, 15, 8996–9004. [Google Scholar] [CrossRef]
- Wu, W.B.; Feng, G.X.; Xu, S.D.; Liu, B. A Photostable Far-Red/Near-Infrared Conjugated Polymer Photosensitizer with Aggregation-Induced Emission for Image-Guided Cancer Cell Ablation. Macromolecules 2016, 49, 5017–5025. [Google Scholar] [CrossRef]
- Soleimaninejad, H.; Chen, M.Z.; Lou, X.D.; Smith, T.A.; Hong, Y.N. Measuring macromolecular crowding in cells through fluorescence anisotropy imaging with an AIE fluorogene. Chem. Commun. 2017, 53, 2874–2877. [Google Scholar] [CrossRef] [Green Version]
- Hu, Q.; Yao, B.C.; Owyong, T.C.; Prashanth, S.; Wang, C.Y.; Zhang, X.Y.; Wong, W.W.H.; Tang, Y.H.; Hong, Y.N. Detection of Urinary Albumin Using a “Turn-on” Fluorescent Probe with Aggregation-Induced Emission Characteristics. Chem. Asian J. 2021, 16, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, H.; Longmire, M.R.; Ogawa, M.; Choyke, P.L. Rational chemical design of the next generation of molecular imaging probes based on physics and biology: Mixing modalities, colors and signals. Chem. Soc. Rev. 2011, 40, 4626–4648. [Google Scholar] [CrossRef] [Green Version]
- Reichert, J.M.; Dhimolea, E. The future of antibodies as cancer drugs. Drug Discov. Today 2012, 17, 954–963. [Google Scholar] [CrossRef]
- Baselga, J.; Arteaga, C.L. Critical update and emerging trends in epidermal growth factor receptor targeting in cancer. J. Clin. Oncol. 2005, 23, 2445–2459. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Stadlbauer, D.; Strohmeier, S.; Nguyen, T.H.O.; Chromikova, V.; McMahon, M.; Jiang, K.J.; Arunkumar, G.A.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Vashist, S.K. In Vitro Diagnostic Assays for COVID-19: Recent Advances and Emerging Trends. Diagnostics 2020, 10, 202. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.; Rho, J.; Messersmith, P.B. Facile Conjugation of Biomolecules onto Surfaces via Mussel Adhesive Protein Inspired Coatings. Adv. Mater. 2009, 21, 431–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shull, S.; Heintz, N.H.; Periasamy, M.; Manohar, M.; Janssen, Y.M.W.; Marsh, J.P.; Mossman, B.T. DIFFERENTIAL REGULATION OF ANTIOXIDANT ENZYMES IN RESPONSE TO OXIDANTS. J. Biol. Chem. 1991, 266, 24398–24403. [Google Scholar] [CrossRef]
- Wang, X.D.; Dai, J.; Min, X.H.; Yu, Z.H.; Cheng, Y.; Huang, K.X.; Yang, J.L.; Yi, X.Q.; Lou, X.D.; Xia, F. DNA-Conjugated Amphiphilic Aggregation-Induced Emission Probe for Cancer Tissue Imaging and Prognosis Analysis. Anal. Chem. 2018, 90, 8162–8169. [Google Scholar] [CrossRef]
- Nayerossadat, N.; Maedeh, T.; Ali, P.A. Viral and nonviral delivery systems for gene delivery. Adv. Biomed. Res. 2012, 1, 27. [Google Scholar] [CrossRef]
- He, D.; Wagner, E. Defined Polymeric Materials for Gene Delivery. Macromol. Biosci. 2015, 15, 600–612. [Google Scholar] [CrossRef]
- Tang, F.; Liu, J.-Y.; Wu, C.-Y.; Liang, Y.-X.; Lu, Z.-L.; Ding, A.-X.; Xu, M.-D. Two-Photon Near-Infrared AIE Luminogens as Multifunctional Gene Carriers for Cancer Theranostics. ACS Appl. Mater. Interfaces 2021, 13, 23384–23395. [Google Scholar] [CrossRef] [PubMed]
- Altman, G.H.; Diaz, F.; Jakuba, C.; Calabro, T.; Horan, R.L.; Chen, J.S.; Lu, H.; Richmond, J.; Kaplan, D.L. Silk-based biomaterials. Biomaterials 2003, 24, 401–416. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhao, H.P.; Huang, H.M.; Li, B.; Li, R.K.Y.; Feng, X.Q.; Dai, F.Y. Quantum dots-reinforced luminescent silkworm silk with superior mechanical properties and highly stable fluorescence. J. Mater. Sci. 2019, 54, 9945–9957. [Google Scholar] [CrossRef]
- Amirikia, M.; Shariatzadeh, S.M.A.; Jorsaraei, S.G.A.; Mehranjani, M.S. Auto-fluorescence of a silk fibroin-based scaffold and its interference with fluorophores in labeled cells. Eur. Biophys. J. 2018, 47, 573–581. [Google Scholar] [CrossRef]
- Liu, C.C.; Bai, H.T.; He, B.Z.; He, X.W.; Zhang, J.Y.; Chen, C.; Qiu, Y.P.; Hu, R.; Zhao, F.X.; Zhang, Y.X.; et al. Functionalization of Silk by AIEgens through Facile Bioconjugation: Full-Color Fluorescence and Long-Term Bioimaging. Angew. Chem. Int. Ed. 2021, 60, 12424–12430. [Google Scholar] [CrossRef]
- Lin, N.B.; Hu, F.; Sun, Y.L.; Wu, C.X.; Xu, H.Y.; Liu, X.Y. Construction of White-Light-Emitting Silk Protein Hybrid Films by Molecular Recognized Assembly among Hierarchical Structures. Adv. Funct. Mater. 2014, 24, 5284–5290. [Google Scholar] [CrossRef]
- Kardas, P.; Devine, S.; Golembesky, A.; Roberts, C. A systematic review and meta-analysis of misuse of antibiotic therapies in the community. Int. J. Antimicrob. Agents 2005, 26, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Zmora, N.; Zilberman-Schapira, G.; Mor, U.; Dori-Bachash, M.; Bashiardes, S.; Zur, M.; Regev-Lehavi, D.; Brik, R.B.; Federici, S.; et al. Post-Antibiotic Gut Mucosal Microbiome Reconstitution Is Impaired by Probiotics and Improved by Autologous FMT. Cell 2018, 174, 1406–1423.e16. [Google Scholar] [CrossRef] [Green Version]
- Yoshikawa, T.T. Antimicrobial resistance and aging: Beginning of the end of the antibiotic era? J. Am. Geriatr. Soc. 2002, 50, S226–S229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.W.; Yang, Y.J.; Guo, Y.C.; Lu, S.G.; Du, Y.; Li, J.J.; Zhang, X.P.; Leung, N.L.C.; Zhao, Z.; Niu, G.L.; et al. Phage-Guided Targeting, Discriminative Imaging, and Synergistic Killing of Bacteria by AIE Bioconjugates. J. Am. Chem. Soc. 2020, 142, 3959–3969. [Google Scholar] [CrossRef] [PubMed]
- Xiao, P.H.; Shen, Z.P.; Wang, D.L.; Pan, Y.Z.; Li, Y.; Gong, J.Y.; Wang, L.; Wang, D.; Tang, B.Z. Precise Molecular Engineering of Type I Photosensitizers with Near-Infrared Aggregation-Induced Emission for Image-Guided Photodynamic Killing of Multidrug-Resistant Bacteria. Adv. Sci. 2022, 9, 2104079. [Google Scholar] [CrossRef] [PubMed]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Luo, W.; Tan, Y.; Gui, Y.; Yan, D.; Wang, D.; Tang, B.Z. Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives. Molecules 2022, 27, 3914. https://doi.org/10.3390/molecules27123914
Luo W, Tan Y, Gui Y, Yan D, Wang D, Tang BZ. Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives. Molecules. 2022; 27(12):3914. https://doi.org/10.3390/molecules27123914
Chicago/Turabian StyleLuo, Wenshuai, Yonghong Tan, Yixiong Gui, Dingyuan Yan, Dong Wang, and Ben Zhong Tang. 2022. "Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives" Molecules 27, no. 12: 3914. https://doi.org/10.3390/molecules27123914
APA StyleLuo, W., Tan, Y., Gui, Y., Yan, D., Wang, D., & Tang, B. Z. (2022). Near-Infrared-Emissive AIE Bioconjugates: Recent Advances and Perspectives. Molecules, 27(12), 3914. https://doi.org/10.3390/molecules27123914








