Influence of Test Specimen Geometry and Water Soaking on the In Vitro Cytotoxicity of Orthocryl®, Orthocryl® LC, Loctite® EA 9483 and Polypropylene
Abstract
1. Introduction
2. Results
2.1. Experimental Setup
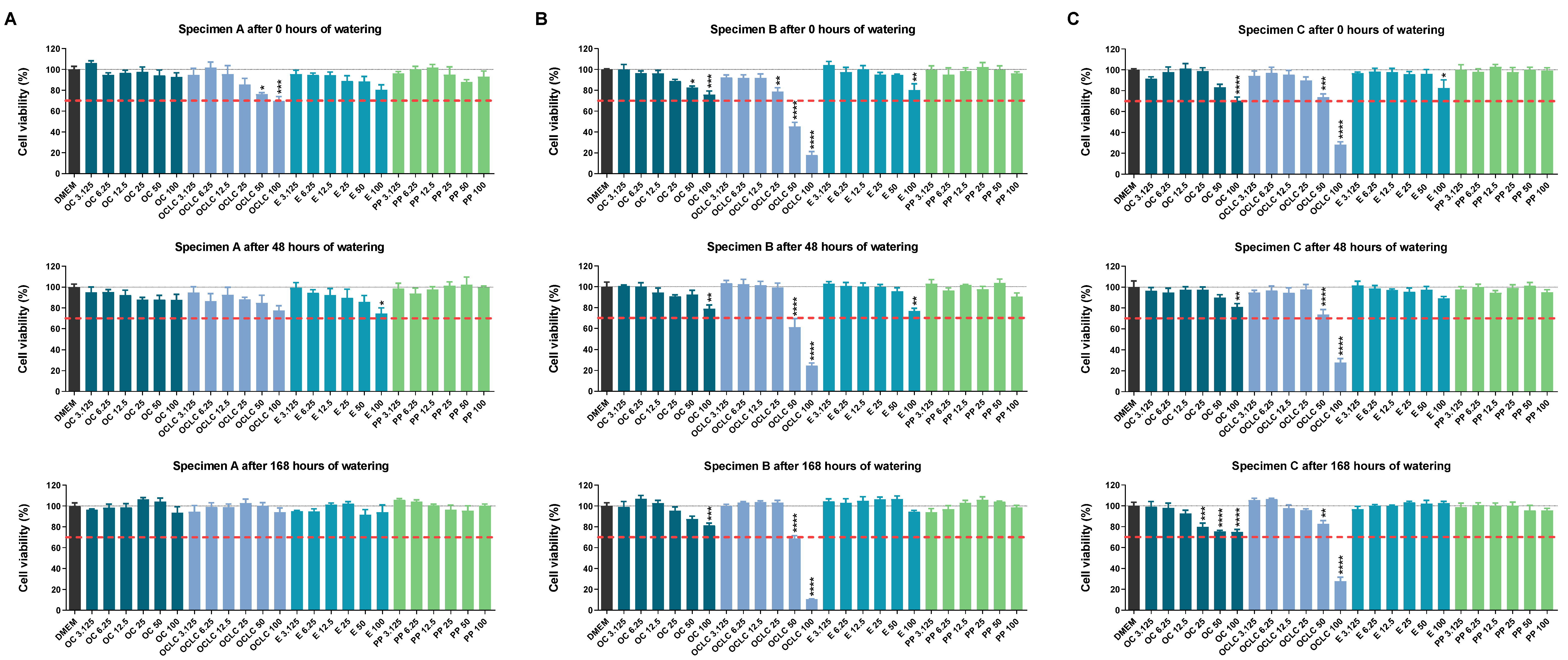
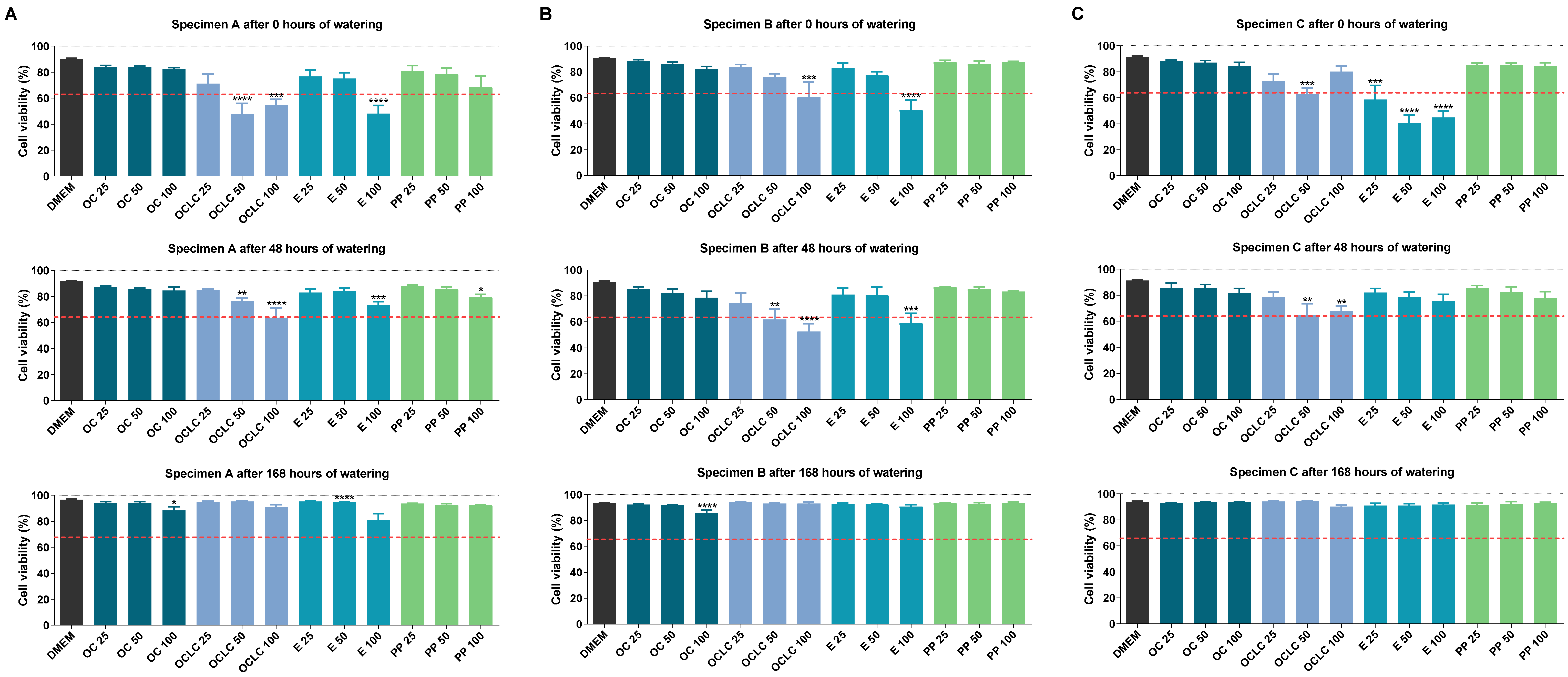
2.2. Influence of Eluates from Test Specimens on Cellular Viability According to the WST-1 Assay
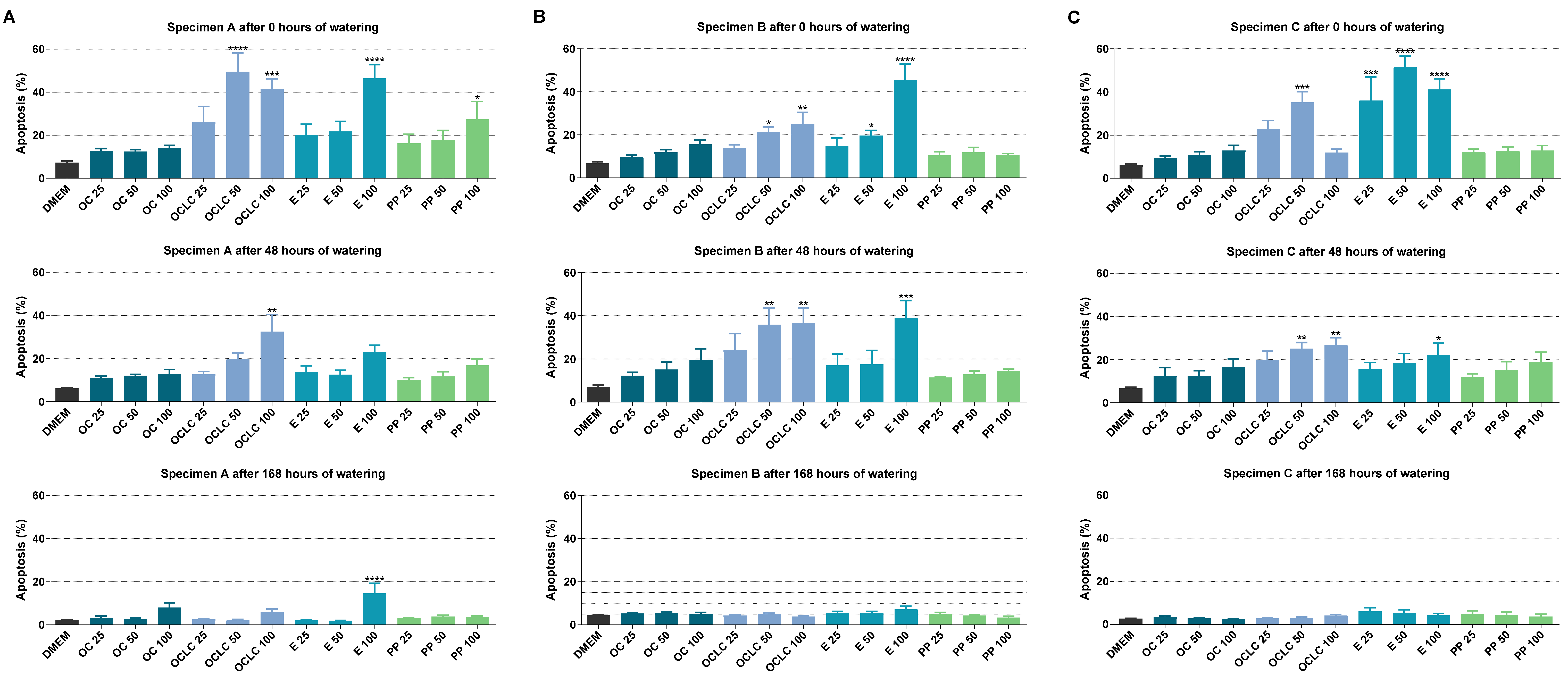
2.3. Influence of Eluates from Test Specimens on Cellular Viability and Apoptosis According to the Annexin V-FITC-PI Assay
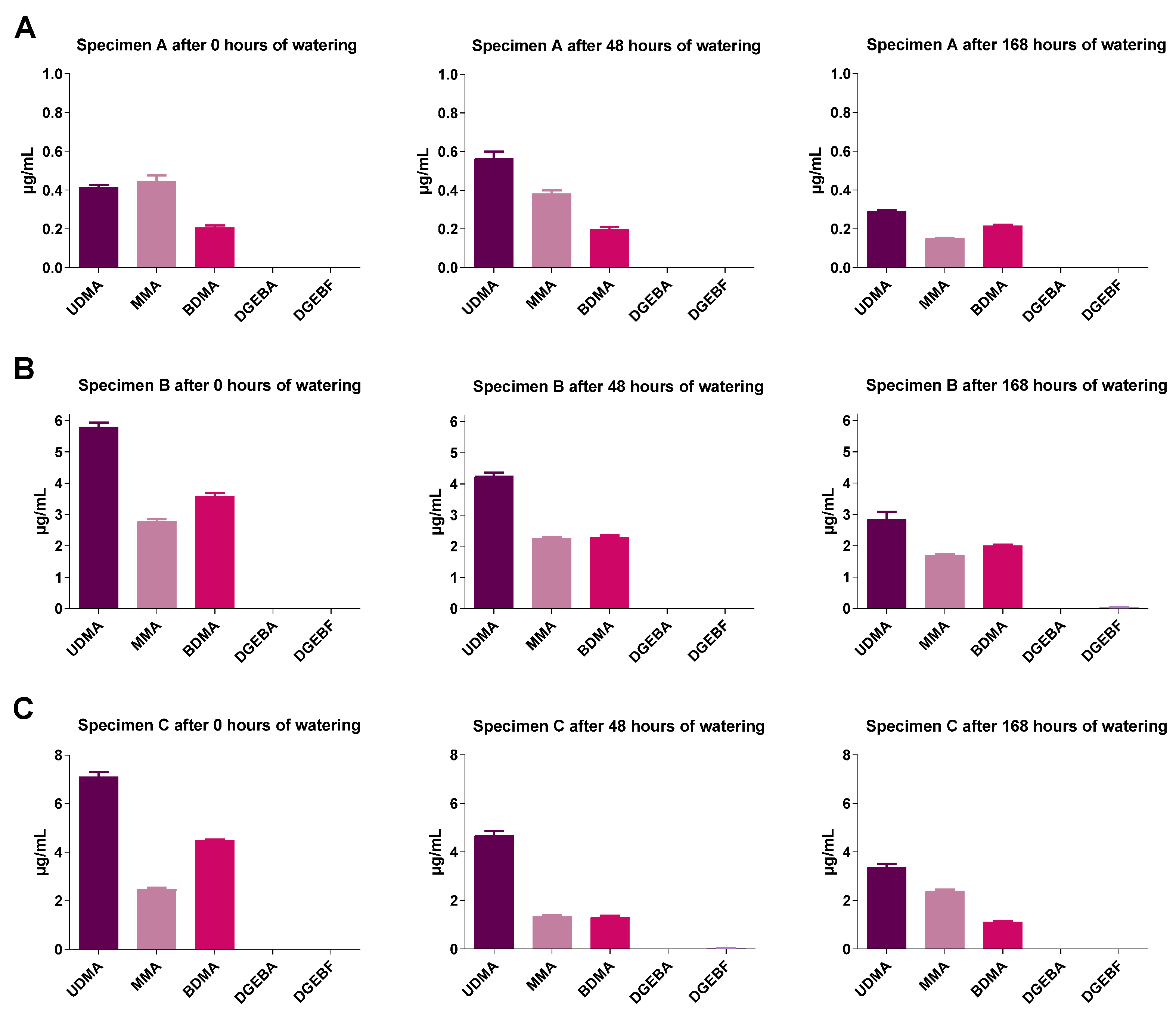
2.4. GC-MS Analysis of Monomer Release from Test Specimens
3. Discussion
4. Materials and Methods
4.1. Specimen Geometries and Polymerization Conditions
4.2. Storage of the Specimens and Extraction of the Eluates
4.3. Cell Culture
4.4. Eluate Exposure, Cell Viability Assay (WST-1) and Annexin V-FITC-PI Assay
4.5. Quantification of Eluted Monomers by Gas Chromatography-Mass Spectrometry (GC-MS)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Leggat, P.A.; Kedjarune, U. Toxicity of methyl methacrylate in dentistry. Int. Dent. J. 2003, 53, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung. Methylmethacrylat; Institut für Arbeitsschutz der Deutschen Gesetzlichen Unfallversicherung: Sankt Augustin, Germany, 2015. [Google Scholar]
- Nik, T.H.; Shahroudi, A.S.; Eraghihzadeh, Z.; Aghajani, F. Comparison of residual monomer loss from cold-cure orthodontic acrylic resins processed by different polymerization techniques. J. Orthod. 2014, 41, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Auzerie, V.; Mahé, E.; Marck, Y.; Auffret, N.; Descamps, V.; Crickx, B. Oral lichenoid eruption due to methacrylate allergy. Contact Dermat. 2001, 45, 241. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Brook, I.M.; Van Noort, R. Biocompatibility of Resin-based Dental Materials. Materials 2009, 2, 514–548. [Google Scholar] [CrossRef]
- Barclay, S.C.; Forsyth, A.; Felix, D.H.; Watson, I.B. Case report—Hypersensitivity to denture materials. Br. Dent. J. 1999, 187, 350–352. [Google Scholar] [CrossRef]
- Gonçalves, T.S.; Morganti, M.A.; Campos, L.C.; Rizzatto, S.M.; Menezes, L.M. Allergy to auto-polymerized acrylic resin in an orthodontic patient. Am. J. Orthod. Dentofacial. Orthop. 2006, 129, 431–435. [Google Scholar] [CrossRef]
- Rose, E.C.; Bumann, J.; Jonas, I.E.; Kappert, H.F. Contribution to the biological assessment of orthodontic acrylic materials. Measurement of their residual monomer output and cytotoxicity. J. Orofac. Orthop. 2000, 61, 246–257. [Google Scholar] [CrossRef]
- Kansu, G.; Kalyoncuoğlu, T.; Uyar, P.; Uzun, E. Cell death induced by eluates from hypoallergenic denture base acrylic resins in NIH-3T3 fibroblast cells. J. Dent. Sci. 2014, 9, 381–387. [Google Scholar] [CrossRef][Green Version]
- Gebhart, M.; Geier, J. Evaluation of patch test results with denture material series. Contact Dermat. 1996, 34, 191–195. [Google Scholar] [CrossRef]
- Schweikl, H.; Spagnuolo, G.; Schmalz, G. Genetic and cellular toxicology of dental resin monomers. J. Dent. Res. 2006, 85, 870–877. [Google Scholar] [CrossRef]
- Kim, R.H.; Williams, D.W.; Bae, S.; Lee, R.S.; Oh, J.E.; Mehrazarin, S.; Kim, T.; Shin, K.H.; Park, N.H.; Kang, M.K. Camphorquinone inhibits odontogenic differentiation of dental pulp cells and triggers release of inflammatory cytokines. J. Endod. 2013, 39, 57–61. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Zentner, A.; Sergl, H.G.; Kretschmer, A. Eine In-vitro-Untersuchung zweier in der Kieferorthopädie verwendeter Kunststoffe auf Zelltoxizität. Fortschr. Kieferorthop. 1994, 55, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Schendel, K.U.; Lenhardt, M.; Fusenig, N.E.; Komposch, G. Testung der Toxizität von in der Kieferorthopädie verwendeten Kunststoffen [The testing of the toxicity of the plastics used in orthodontics]. Fortschr. Kieferorthop. 1992, 53, 263–272, Erratum in Fortschr. Kieferorthop. 1992, 53, 354. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, C.A.; Knoernschild, K.L.; Schuster, G.S. Cytotoxicity of eluates from light-polymerized denture base resins. J. Prosthet. Dent. 1994, 72, 644–650. [Google Scholar] [CrossRef]
- O’Boyle, N.M.; Niklasson, I.B.; Tehrani-Bagha, A.R.; Delaine, T.; Holmberg, K.; Luthman, K.; Karlberg, A.T. Epoxy resin monomers with reduced skin sensitizing potency. Chem. Res. Toxicol. 2014, 27, 1002–1010. [Google Scholar] [CrossRef]
- Aquilina, O. A brief history of cardiac pacing. Images Paediatr. Cardiol. 2006, 8, 17–81. [Google Scholar]
- Elsner, P.; Eyerer, P.; Hirth, T. Kunststoffe: Eigenschaften und Anwendung, 7th ed.; Springer: Berlin/Heidelberg, Germany, 2008; pp. 261–275. [Google Scholar]
- Kelly, M.; Macdougall, K.; Olabisi, O.; McGuire, N. In vivo response to polypropylene following implantation in animal models: A review of biocompatibility. Int. Urogynecol. J. 2017, 28, 171–180. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for in Vitro Cytotoxicity. German version EN ISO 10993-5:2009; International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 10993-12:2021; Biological Evaluation of Medical Devices—Part 12: Sample Preparation and Reference Materials. German version EN ISO 10993-12:2021; International Organization for Standardization: Geneva, Switzerland, 2021.
- Cimpan, M.R.; Cressey, L.I.; Skaug, N.; Halstensen, A.; Lie, S.A.; Gjertsen, B.T.; Matre, R. Patterns of cell death induced by eluates from denture base acrylic resins in U-937 human monoblastoid cells. Eur. J. Oral Sci. 2000, 108, 59–69. [Google Scholar] [CrossRef]
- Schendel, K.U.; Erdinger, L.; Komposch, G.; Sonntag, H.G. Untersuchung kieferorthopädischer Materialien im HET-CAM-Test auf schleimhautreizende Wirkung [Orthodontic materials studied in the HET-CAM test for mucosa-irritating effects]. Fortschr. Kieferorthop. 1994, 55, 28–35. [Google Scholar] [CrossRef]
- Cimpan, M.R.; Matre, R.; Cressey, L.I.; Tysnes, B.; Lie, S.A.; Gjertsen, B.T.; Skaug, N. The effect of heat- and auto-polymerized denture base polymers on clonogenicity, apoptosis, and necrosis in fibroblasts: Denture base polymers induce apoptosis and necrosis. Acta Odontol. Scand. 2000, 58, 217–228. [Google Scholar]
- Thonemann, B.; Schmalz, G.; Hiller, K.A.; Schweikl, H. Responses of L929 mouse fibroblasts, primary and immortalized bovine dental papilla-derived cell lines to dental resin components. Dent. Mater. 2002, 18, 318–323. [Google Scholar] [CrossRef]
- Tsuchiya, H.; Hoshino, Y.; Tajima, K.; Takagi, N. Leaching and cytotoxicity of formaldehyde and methyl methacrylate from acrylic resin denture base materials. J. Prosthet. Dent. 1994, 71, 618–624. [Google Scholar] [CrossRef]
- Dahl, O.E.; Garvik, L.J.; Lyberg, T. Toxic effects of methylmethacrylate monomer on leukocytes and endothelial cells in vitro. Acta Orthop. Scand. 1994, 65, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Emmler, J.; Seiss, M.; Kreppel, H.; Reichl, F.X.; Hickel, R.; Kehe, K. Cytotoxicity of the dental composite component TEGDMA and selected metabolic by-products in human pulmonary cells. Dent. Mater. 2008, 24, 1670–1675. [Google Scholar] [CrossRef]
- Pawlowska, E.; Poplawski, T.; Ksiazek, D.; Szczepanska, J.; Blasiak, J. Genotoxicity and cytotoxicity of 2-hydroxyethyl methacrylate. Mutat. Res. 2010, 696, 122–129. [Google Scholar] [CrossRef]
- Reichl, F.X.; Löhle, J.; Seiss, M.; Furche, S.; Shehata, M.M.; Hickel, R.; Müller, M.; Dränert, M.; Durner, J. Elution of TEGDMA and HEMA from polymerized resin-based bonding systems. Dent. Mater. 2012, 28, 1120–1125. [Google Scholar] [CrossRef]
- Boeckler, A.F.; Morton, D.; Poser, S.; Dette, K.E. Release of dibenzoyl peroxide from polymethyl methacrylate denture base resins: An in vitro evaluation. Dent. Mater. 2008, 24, 1602–1607. [Google Scholar] [CrossRef]
- Charasseangpaisarn, T.; Wiwatwarrapan, C. The effect of various frequencies of ultrasonic cleaner in reducing residual monomer in acrylic resin. Ultrasonics 2015, 63, 163–167. [Google Scholar] [CrossRef]
- Löbler, M.; Behrend, D.; Maletz, R.; Schmitz, K.-P. Sample Geometry Influences the Outcome of in vitro Cytotoxicity Tests. Adv. Eng. Mater. 2008, 10, B72–B76. [Google Scholar] [CrossRef]
- Merry, S.; Courtney, E.R.; Fetherston, C.A.; Kaye, S.B.; Freshney, R.I. Circumvention of drug resistance in human non-small cell lung cancer in vitro by verapamil. Br. J. Cancer. 1987, 56, 401–405. [Google Scholar] [CrossRef]
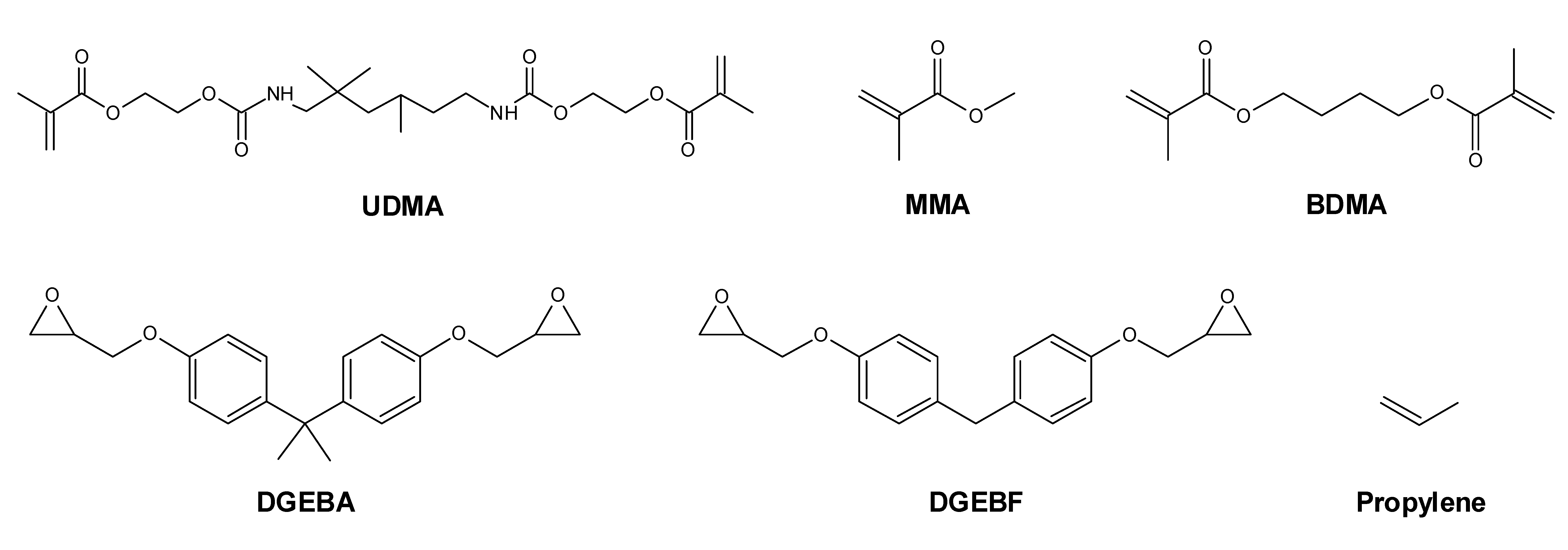



| Monomer | Masses | Lowest Calibrator |
|---|---|---|
| Urethane dimethacrylate (UDMA) | 69, 87 | 0.03 µg/mL |
| Methyl methacrylate (MMA) | 69, 113 | 0.1 µg/mL |
| 1,4-Butanediol dimethacrylate (BDMA) | 69, 87, 140 | 0.04 µg/mL |
| Bisphenol A diglycidyl ether (DGEBA) | 325, 340 | 0.03 µg/mL |
| Bisphenol F diglycidyl ether (DGEBF) | 181, 197 | 0.03 µg/mL |
| Caffeine (internal standard) | 194, 109 | Not determined |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Behnke, R.; Stahl, F.; Duske, K.; Warkentin, M.; Schwartz, M.; Hinz, B.; Walther, U. Influence of Test Specimen Geometry and Water Soaking on the In Vitro Cytotoxicity of Orthocryl®, Orthocryl® LC, Loctite® EA 9483 and Polypropylene. Molecules 2022, 27, 3949. https://doi.org/10.3390/molecules27123949
Behnke R, Stahl F, Duske K, Warkentin M, Schwartz M, Hinz B, Walther U. Influence of Test Specimen Geometry and Water Soaking on the In Vitro Cytotoxicity of Orthocryl®, Orthocryl® LC, Loctite® EA 9483 and Polypropylene. Molecules. 2022; 27(12):3949. https://doi.org/10.3390/molecules27123949
Chicago/Turabian StyleBehnke, Richard, Franka Stahl, Kathrin Duske, Mareike Warkentin, Margit Schwartz, Burkhard Hinz, and Udo Walther. 2022. "Influence of Test Specimen Geometry and Water Soaking on the In Vitro Cytotoxicity of Orthocryl®, Orthocryl® LC, Loctite® EA 9483 and Polypropylene" Molecules 27, no. 12: 3949. https://doi.org/10.3390/molecules27123949
APA StyleBehnke, R., Stahl, F., Duske, K., Warkentin, M., Schwartz, M., Hinz, B., & Walther, U. (2022). Influence of Test Specimen Geometry and Water Soaking on the In Vitro Cytotoxicity of Orthocryl®, Orthocryl® LC, Loctite® EA 9483 and Polypropylene. Molecules, 27(12), 3949. https://doi.org/10.3390/molecules27123949






