Low-Cost Detection of Methane Gas in Rice Cultivation by Gas Chromatography-Flame Ionization Detector Based on Manual Injection and Split Pattern
Abstract
:1. Introduction
2. Results and Discussion
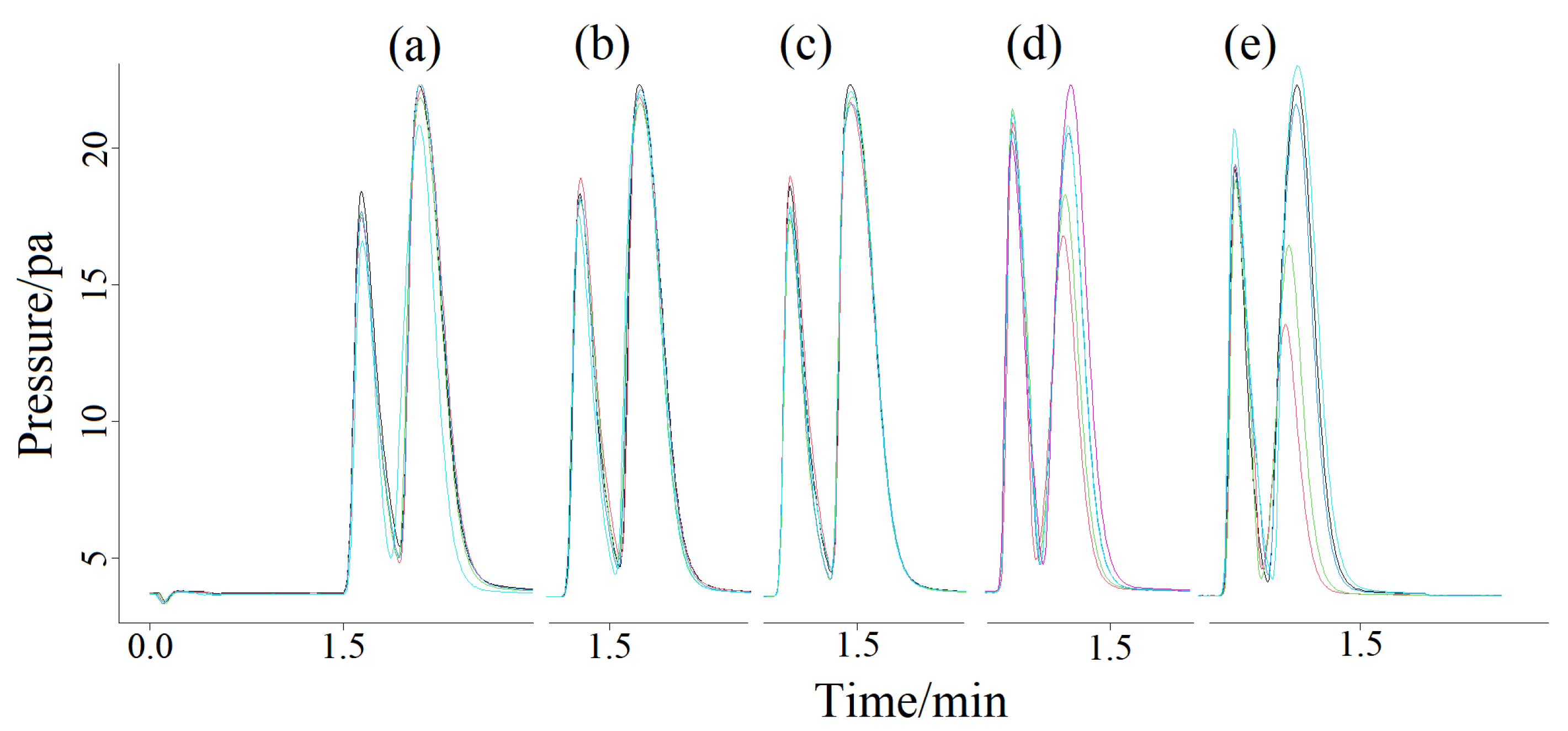
2.1. Qualitative Analysis of Splitless Mode
2.2. Qualitative Analysis of Split Mode
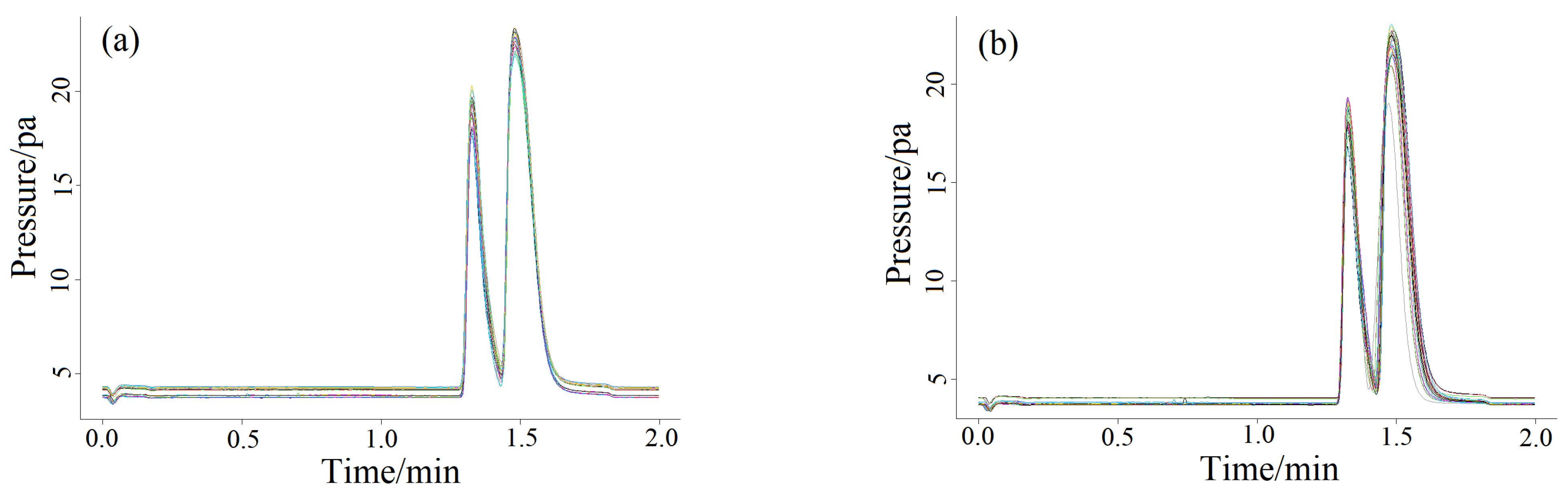
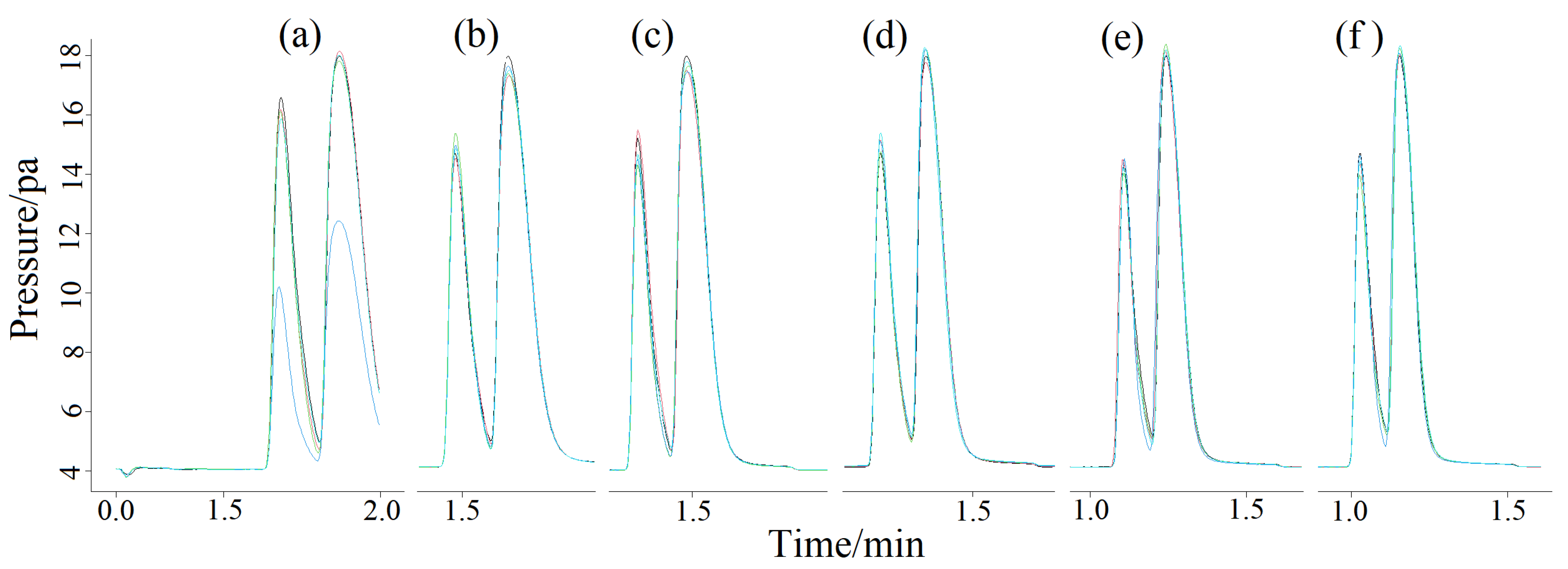
2.3. Quantitative Analysis of Split Mode
2.4. Method Calibration and Validation
2.4.1. Accuracy and Recycle Rate
2.4.2. Method Detection Limit
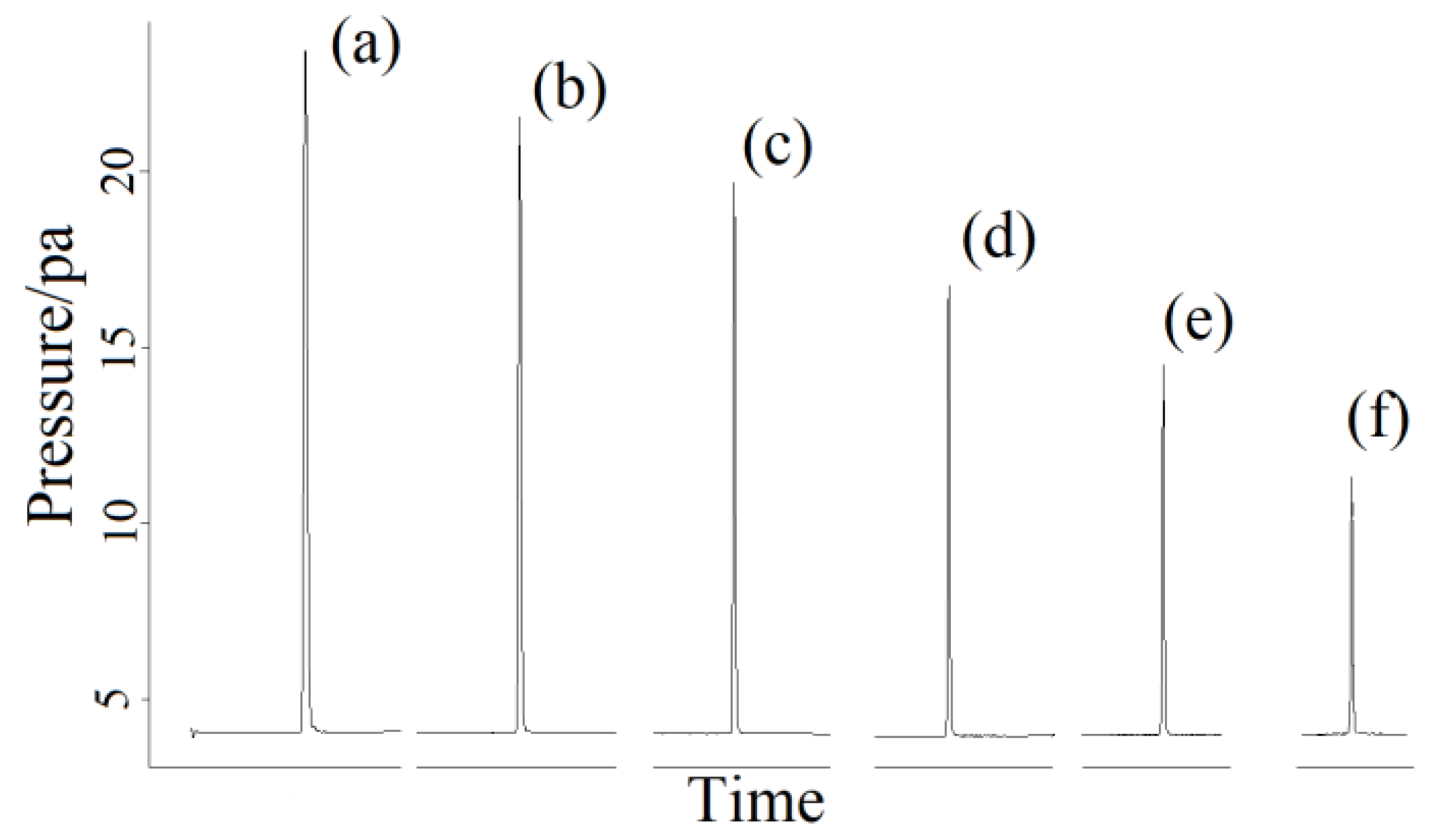
2.5. Actual Gas Samples Detection
3. Materials and Methods
3.1. Materials
3.2. Experiment Methods for Apparatus and GC Analysis
3.3. Preparation of Standard Gas
3.4. Exploring the Conditions in the Splitless Mode
3.5. Exploring the Conditions in the Split Mode
3.6. Calibration and Validation Methods
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jonathan, A.F.; Navin, R.; Kate, A.B.; Emily, S.C.; James, S.G.; Matt, J.; Nathaniel, D.M.; Christine, O.C.; Deepak, K.R.; Paul, C.W.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar]
- Church, J.A.; Clark, P.U.; Cazenave, A.; Gregory, J.M.; Jevrejeva, S.; Levermann, A.; Merrifield, M.A.; MilneG, A.; Nerem, R.S.; Nunn, P.D.; et al. Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Parry, M.L.; Canziani, O.F.; Palutikof, J.P. Contribution of working group II to the fourth assessment report of the intergovernmental panel on climate change. Encycl. Lang. Linguist. 2007, 12, 171–175. [Google Scholar]
- Ghosh, S.; Majumdar, D.; Jain, M.C. Methane and nitrous oxide emissions from an irrigated rice of North India. Chemosphere 2003, 51, 181–195. [Google Scholar] [CrossRef]
- Rodhe, H. A comparison of the contribution of various gases to the greenhouse effect. Science 1990, 248, 1217–1219. [Google Scholar] [CrossRef] [PubMed]
- Schulze, E.D.; Luyssaert, S.; Ciais, P.; Freibauer, A.; Janssens, I.A.; Soussana, J.F.; Smith, P.; Grace, J.; Levin, I.; Thiruchittampalam, B.; et al. Importance of methane and nitrous oxide for Europe’s terrestrial green-house-gas balance. Nat. Geosci. 2009, 2, 842–850. [Google Scholar] [CrossRef]
- Yue, X.L.; Gao, Q.X. Contributions of natural systems and human activity to greenhouse gas emissions. Adv. Clim Chang. Res. 2018, 9, 243–252. [Google Scholar] [CrossRef]
- Stocker, T.F.; Qin, D.; Plattner, G.K. (Eds.) Climate Change 2013: The Physical Science Basis; Cambridge University Press: Cambridge, UK, 2013; pp. 507–509. [Google Scholar]
- Liu, Y.C.; Whitman, W.B. Metabolic, phylogenetic, and ecological diversity of the methanogenic archaea. Ann. N. Y. Acad. Sci. 2008, 1125, 171–189. [Google Scholar] [CrossRef] [PubMed]
- Zou, J.W.; Huang, Y.; Qin, Y.M.; Liu, S.W.; Shen, Q.R.; Pan, G.X.; Lu, Y.Y.; Liu, Q.H. Changes in fertilizer-induced direct N2O emissions from paddy fields during rice-growing season in China between 1950s and 1990s. Glob. Chang. Biol. 2009, 15, 229–242. [Google Scholar] [CrossRef]
- Edenhofer, O. Climate Change 2014: Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2015; pp. 811–922. [Google Scholar]
- Wang, F.; Li, Y.H.; Zhao, T.C.; Chen, C. Ningxia CO2, CH4, N2O greenhouse gas emission estimation and emission reduction measures. Resour. Environ. Arid Region. 2008, 22, 73–77. [Google Scholar]
- Gregory, K.; Grbler, A.; Tae Yong, J.; Kram, T.; La Rovere, E.; Michaelis, L.; Dadi, Z. Special Report on Emissions Scenarios, Working Group III, Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013. [Google Scholar]
- Zhang, H.L.; Lü, W.G.; Zheng, X.Q.; Li, S.X.; Wang, J.Q.; Zhang, J.Q.; He, Q.Y.; Yuan, D.W.; Gu, X.J. Effects of years of straw return to soil on greenhouse gas emission in rice/wheat rotation systems. Chin. J. Eco-Agric. 2015, 23, 302–308. [Google Scholar]
- Balasubramanian, V.; Sie, M.; Hijmans, R.J.; Otsuka, K. Increasing rice production in sub-saharan Africa: Challenges and opportunities. Adv. Agron. 2007, 94, 55–133. [Google Scholar]
- The State of Food Security and Nutrition in the World. 2021. Available online: https://www.fao.org/faostat/en/#home (accessed on 28 July 2021).
- Gao, J.S.; Huang, J.; Dong, C.H.; Xu, M.G.; Zeng, X.B.; Wen, S.L. Effects of long-term combined application of organic and chemical fertilizers on rice yield and soil available nutrients. J. Soil. 2014, 51, 314–324. [Google Scholar]
- Yi, Q.; Fang, Y.W.; Yang, S.H.; Lu, Y.S.; Fu, H.T.; Li, P.; Jiang, R.P.; Tang, S.H. Methane and nitrous oxide emissions in paddy field as influenced by fertilization. Ecol. Environ. Sci. 2013, 22, 1432–1437. [Google Scholar]
- Hogarth, J.R.; Haywood, C.; Whitley, S. Low-Carbon Development in Sub-Saharan Africa: 20 Cross-Sector Transitions; Overseas Development Institute: London, UK, 2015. [Google Scholar]
- Junk, W.J.; An, S.; Finlayson, C.M.; Gopal, B.; Květ, J.; Mitchell, S.A.; Mitsch, W.J.; Robarts, R.D. Current state of knowledge regarding the world’s wetlands and their future under global climate change: A synthesis. Aquat. Sci. 2013, 75, 151–167. [Google Scholar] [CrossRef] [Green Version]
- Mitchell, S.A. The status of wetlands, threats and the predicted effect of global climate change: The situation in Sub-Saharan Africa. Aquat. Sci. 2013, 75, 95–112. [Google Scholar] [CrossRef]
- Van Nguyen, N.; Ferrero, A. Meeting the challenges of global rice production. Paddy Water Environ. 2006, 4, 1–9. [Google Scholar] [CrossRef]
- Zhang, Y.M.; Hu, C.S.; Zhang, J.B.; Ying, W.X.; Wang, Y.Y.; Song, L.N. Research advances on source/sink intensities and greenhouse effects of CO2, CH4and N2O in agricultural soils. Chin. J. Eco-Agric. 2011, 19, 966–975. [Google Scholar] [CrossRef]
- Qi, L.; Ma, Z.L.; Chang, S.X.; Zhou, P.; Huang, R.; Wang, Y.Y.; Wang, Z.F.; Gao, M. Biochar decreases methanogenic archaea abundance and methane emissions in a flooded paddy soil. Sci. Total Environ. 2021, 752, 141958. [Google Scholar] [CrossRef]
- Liu, J.Y.; Qiu, H.S.; Wang, C.; Shen, J.L.; Zhang, W.Z.; Cai, J.M.; Tang, H.; Wu, J.S. Effects of biochar amendment on greenhouse gas emission in two paddy soils with different textures. Paddy Water Environ. 2021, 19, 87–98. [Google Scholar] [CrossRef]
- Hou, P.F.; Feng, Y.F.; Wang, N.; Petropoulos, E.; Li, D.T.; Yu, S.; Xue, L.H.; Yang, L.Z. Win-win: Application of sawdust-derived hydrochar in low fertility soil improves rice yield and reduces greenhouse gas emissions from agricultural ecosystems. Sci. Total Environ. 2020, 748, 142457. [Google Scholar] [CrossRef]
- Feng, Y.F.; Li, D.T.; Sun, H.J.; Xue, L.H.; Zhou, B.B.; Yang, L.Z.; Liu, J.Y.; Xing, B.S. Wood vinegar and biochar co-application mitigates nitrous oxide and methane emissions from rice paddy soil: A two-year experiment. Environ. Pollut. 2020, 267, 115403. [Google Scholar] [CrossRef]
- He, T.H.; Yuan, J.Y.; Lou, J.F.; Lindsey, S.; Xiang, J.; Lin, Y.X.; Liu, D.Y.; Chen, Z.M.; Ding, W.X. Combined application of biochar with urease and nitrification inhibitors have synergistic effects on mitigating CH4 emissions in rice field: A three-year study. Sci. Total Environ. 2020, 743, 140500. [Google Scholar] [CrossRef] [PubMed]
- The Ministry of Environmental Protection. Stationary Source Emission—Determination of Total Hydrocarbons, Methane and Nonmethane Hydrocarbons—Gas Chromatography HJ38-2017; China Environmental Science Press: Beijing, China, 2017. [Google Scholar]
- Ali, H.M.; Alsohaimi, I.H.; Khan, M.R.; Azam, M. Simultaneous Determination of Isothiazolinones and Parabens in Cosmetic Products Using Solid-Phase Extraction and Ultra-High Performance Liquid Chromatography/Diode Array Detector. Pharmaceuticals 2020, 13, 412. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, J.; Ye, T.; Han, Z.Y. Establishment of analysis method for methane detection by gas chromatography. IOP Publ. 2018, 113, 12023. [Google Scholar] [CrossRef]
- Alsohaimi, I.H.; Khan, M.R.; Ali, H.M.; Azam, M.; Alammari, A.M. Solvent extraction and gas chromatography–mass spectrometric determination of probable carcinogen 1, 4-dioxane in cosmetic products. Sci. Rep. 2020, 10, 1–9. [Google Scholar]
- Li, Y.F.; Meng, C.; Liu, C.X. A Review of Research on Standard Gas Preparation Technology. Chem. Equip. Technol. 2021, 42, 12–17. [Google Scholar]
- Chinese Standard. Ethylene for Industrial Use—Determination of Hydrocarbon Impurities–Gas Chromatographic Method GB/T 3391-2002; General Administration of Quality Supervision, Inspection and Quarantine of Chinas: Beijing, China, 2002. [Google Scholar]
- The Ministry of Environmental Protection. Stationary Source Emission-Determination of Chlorobenzenes-Gas Chromatography HJ 1079-2019; China Environmental Science Press: Beijing, China, 2019. [Google Scholar]
- The Ministry of Environmental Protection. Stationary Source Emission—Determination of Acrylonitrile-Gas Chromatography HJ/T 37-1999; China Environmental Science Press: Beijing, China, 1999. [Google Scholar]
- Xu, Z.J.; Sun, H.J. Global methane concentrations are rising. Ecol. Econ. 2022, 38, 5–8. [Google Scholar]

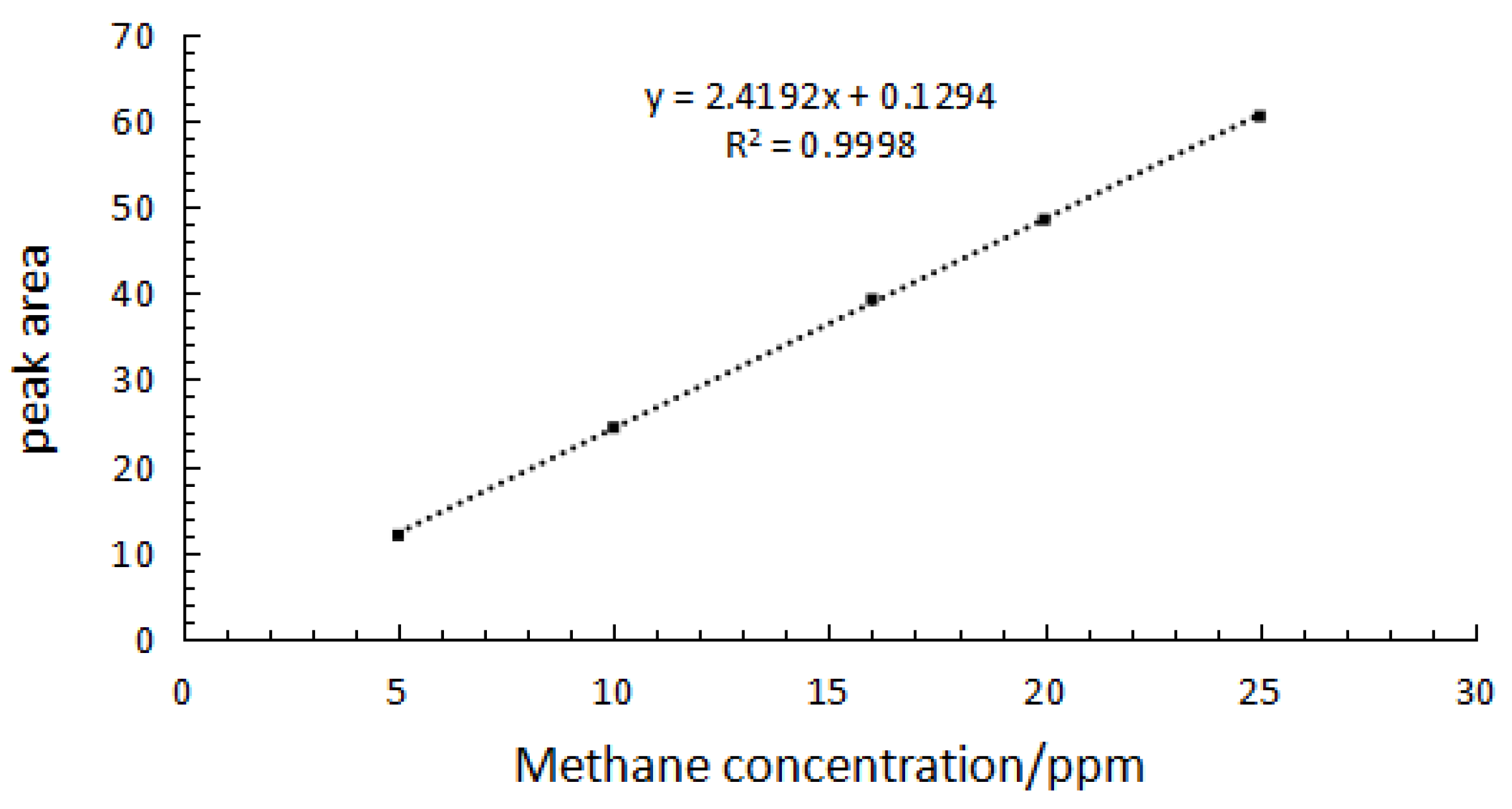
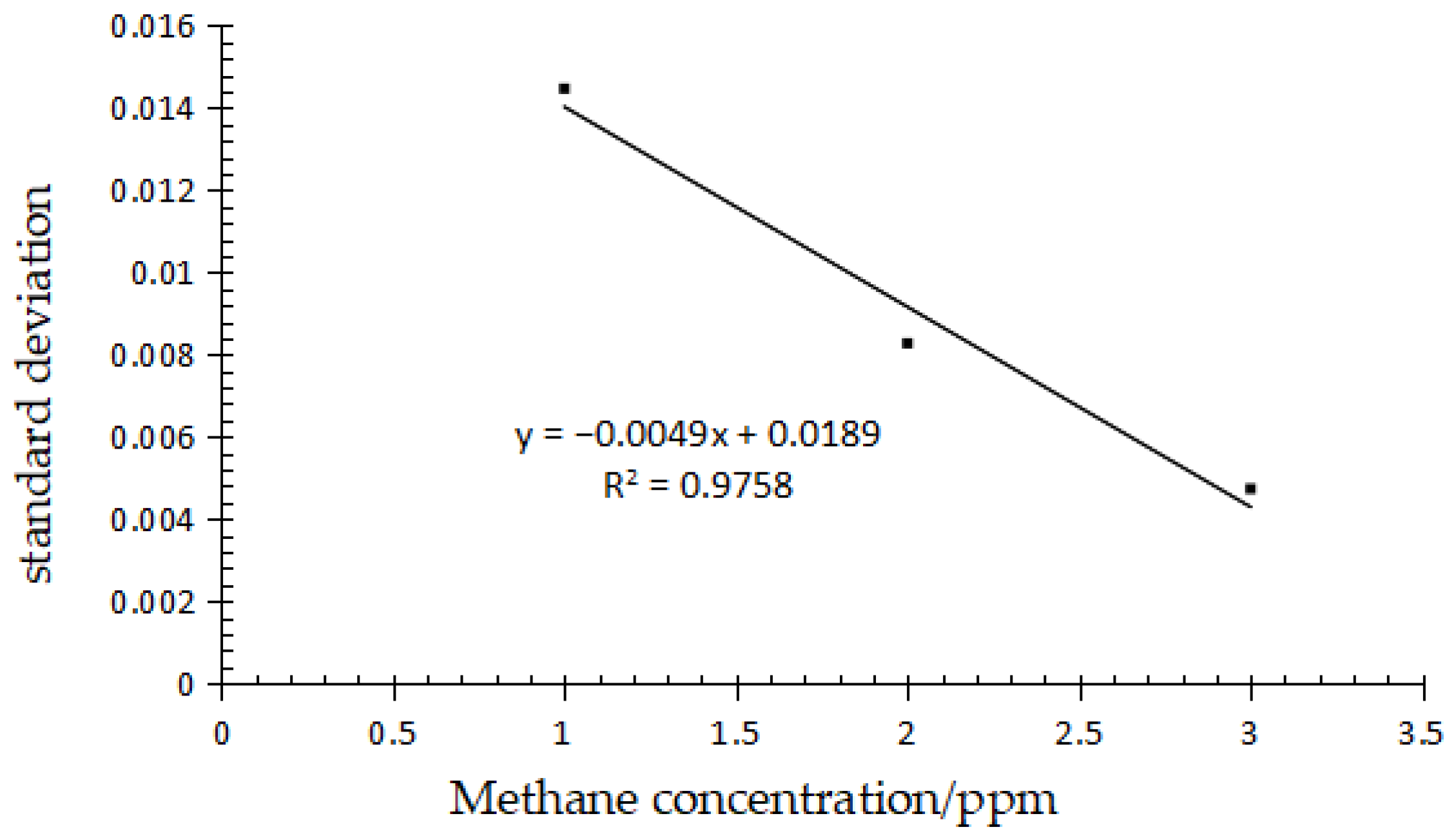
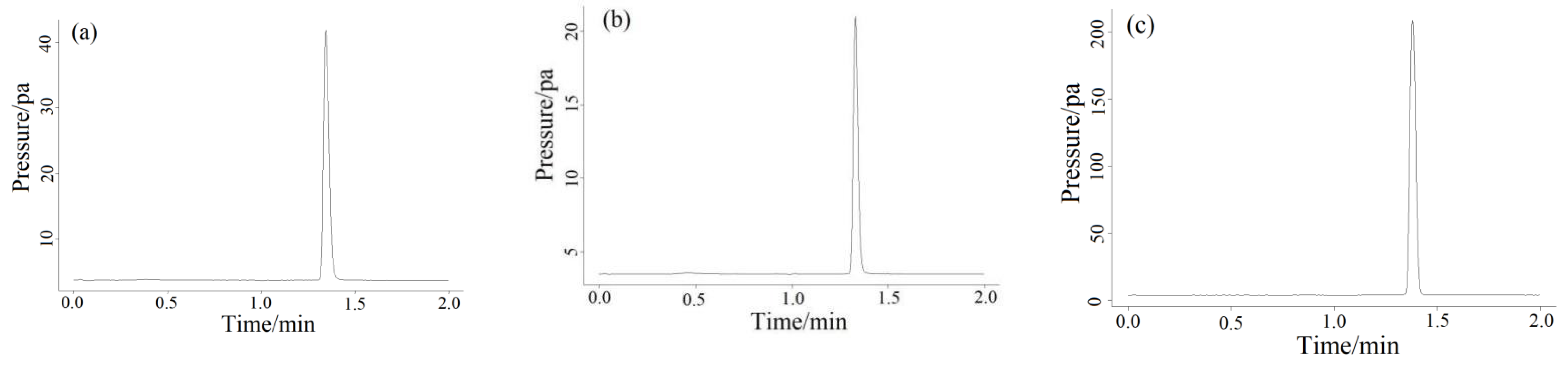
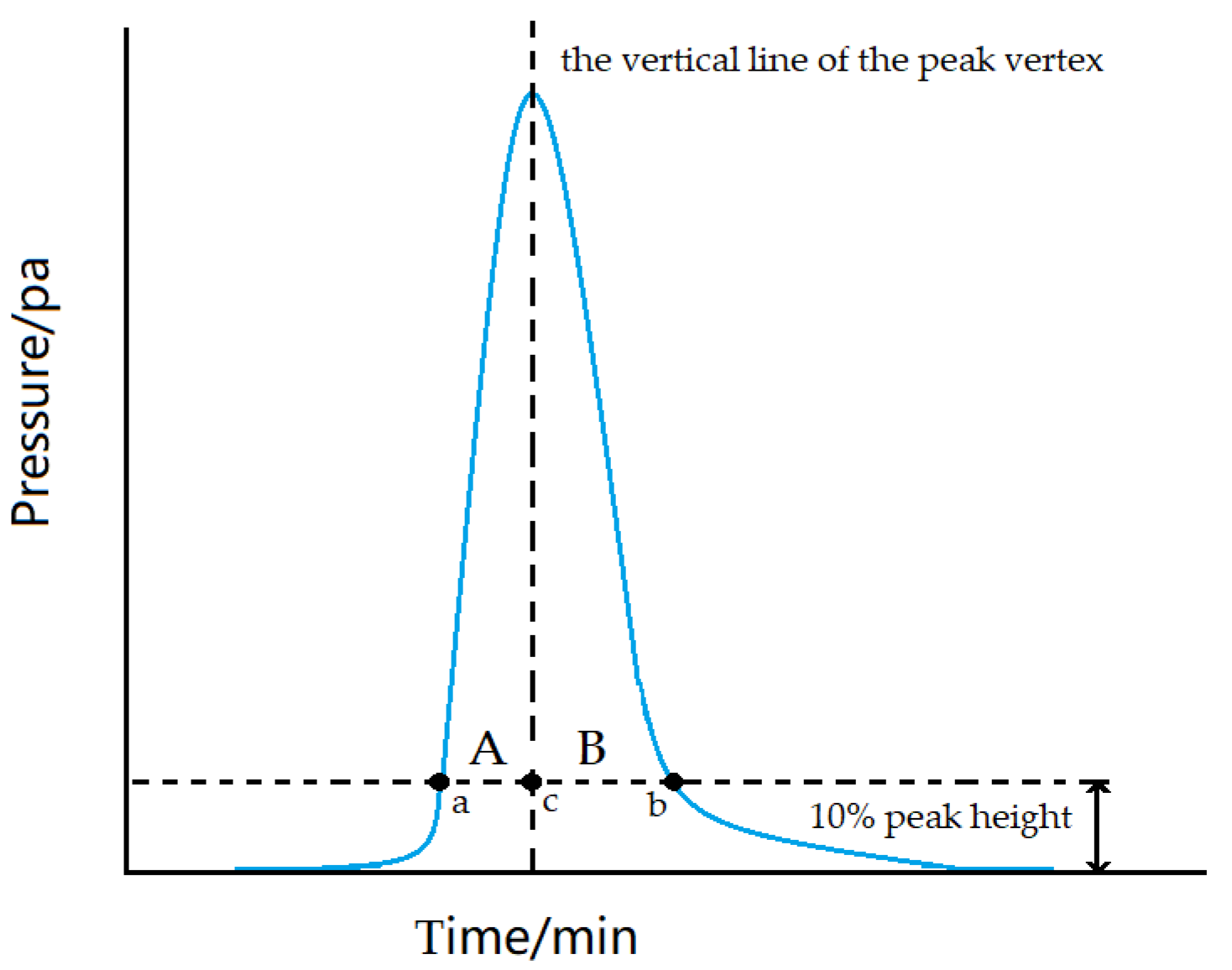
| Standard Content (ppm) | Measured Content (ppm) | RSD (%) | Recycle Rate (%) |
|---|---|---|---|
| 5 | 4.98369 | 2.19% | 99.67 |
| 10 | 10.16944 | 1.60% | 101.69 |
| 16 | 16.32363 | 2.71% | 102.02 |
| 20 | 20.16350 | 2.82% | 100.82 |
| 25 | 25.32475 | 1.01% | 101.30 |
| Gas Concentration (ppm) | Standard Deviation (%) | S0 | 3S0 (LOD) | 10S0 (LOQ) |
|---|---|---|---|---|
| 1 | 1.44690% | 0.0189 | 0.0567 | 0.189 |
| 2 | 0.82748% | |||
| 3 | 0.47354 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Ji, Q.; Fu, X.; Yu, X.; Ye, Z.; Zhang, M.; Sun, C.; Qiu, Y. Low-Cost Detection of Methane Gas in Rice Cultivation by Gas Chromatography-Flame Ionization Detector Based on Manual Injection and Split Pattern. Molecules 2022, 27, 3968. https://doi.org/10.3390/molecules27133968
Li C, Ji Q, Fu X, Yu X, Ye Z, Zhang M, Sun C, Qiu Y. Low-Cost Detection of Methane Gas in Rice Cultivation by Gas Chromatography-Flame Ionization Detector Based on Manual Injection and Split Pattern. Molecules. 2022; 27(13):3968. https://doi.org/10.3390/molecules27133968
Chicago/Turabian StyleLi, Chaofeng, Qingge Ji, Xianshu Fu, Xiaoping Yu, Zihong Ye, Mingzhou Zhang, Chuanxin Sun, and Yulou Qiu. 2022. "Low-Cost Detection of Methane Gas in Rice Cultivation by Gas Chromatography-Flame Ionization Detector Based on Manual Injection and Split Pattern" Molecules 27, no. 13: 3968. https://doi.org/10.3390/molecules27133968







