Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes
Abstract
:1. Introduction
2. Results
2.1. Preparation and Characterization of Liposomes
2.2. Phytochemical Analysis
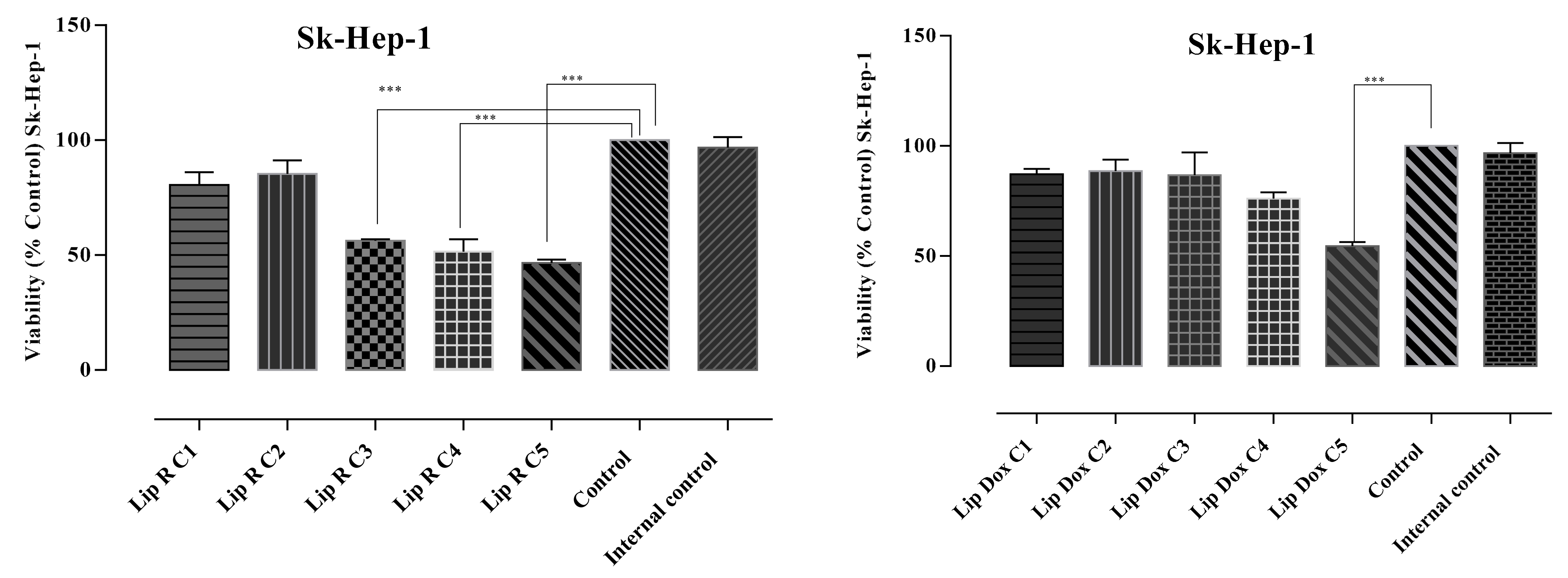
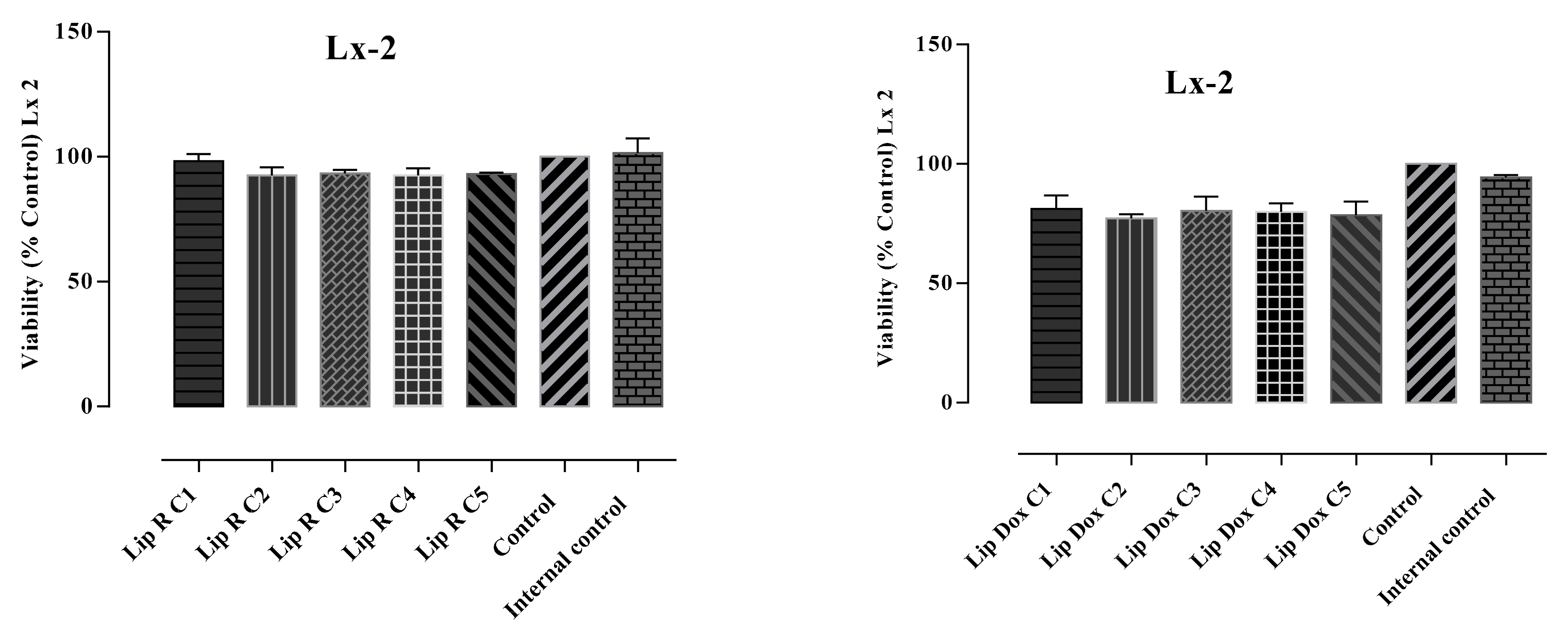
2.3. Cytotoxicity Assays
2.4. Antimicrobial Activity Assays
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of Extracts
4.3. Preparation of Liposomes
4.3.1. Liposomes Loaded with R. officinalis Extract
4.3.2. Liposomes Loaded with DOX
4.4. Characterization of Liposomes
4.4.1. Total Polyphenolic Content (TPC)
4.4.2. DOX Content
4.4.3. Encapsulation Efficiency
4.4.4. Particle Size, Polydispersity Index, Zeta Potential
4.5. LC/MS Analysis
LC/MS Apparatus
4.6. Cell Culture
4.7. Cytotoxicity Assay
4.8. Apoptosis Assay
4.9. Antimicrobial Activity Assays
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andrade, J.M.; Faustino, C.; García, C.; Ladeiras, D.; Reis, C.P.; Rijo, P. Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. Future Sci. 2016, 4, FSO283. [Google Scholar] [CrossRef] [Green Version]
- Amaral, G.P.; Mizdal, C.R.; Stefanello, S.T.; Mendez, A.S.L.; Puntel, R.L.; de Campos, M.M.A.; Soares, F.A.A.; Fachinetto, R. Antibacterial and Antioxidant Effects of Rosmarinus officinalis L. Extract and Its Fractions. J. Tradit. Complement. Med. 2019, 9, 383–392. [Google Scholar] [CrossRef]
- Săvulescu, T. Flora Republicii Populare Române; Editura Academiei Republicii Populare Române: Bucharest, Romania, 1955. [Google Scholar]
- Akshay, K.; Swathi, K.; Bakshi, V.; Boggula, N. Rosmarinus officinalis L.: An Update Review of Its Phytochemistry and Biological Activity. J. Drug Deliv. Ther. 2019, 9, 323–330. [Google Scholar]
- De Oliveira, J.R.; Esteves Afonso Camargo, S.; de Oliveira, L.D. Rosmarinus officinalis L. (Rosemary) as Therapeutic and Prophylactic Agent. J. Biomed. Sci. 2019, 26, 5. [Google Scholar] [CrossRef]
- Begum, A.; Sandhya, S.; Ali, S.S.; Vinod, K.R.; Reddy, S.; Banji, D. An In-Depth Review on the Medicinal Flora Rosmarinus officinalis (Lamiaceae). Acta Sci. Pol. Technol. Aliment. 2013, 12, 61–73. [Google Scholar]
- De Macedo, L.M.; dos Santos, É.M.; Militao, L.; Tundisi, L.L.; Ataide, J.A.; Souto, E.B.; Mazzola, P.G. Rosemary (Rosmarinus officinalis L., Syn Salvia rosmarinus Spenn.) and Its Topical Applications: A Review. Plants 2020, 9, 651. [Google Scholar] [CrossRef]
- Neves, J.A.; Neves, J.A.; de Cassia Meneses Oliveira, R. Pharmacological and Biotechnological Advances with Rosmarinus officinalis L. Expert Opin. Ther. Pat. 2018, 28, 399–413. [Google Scholar] [CrossRef]
- Borrás-Linares, I.; Stojanović, Z.; Quirantes-Piné, R.; Arráez-Román, D.; Švarc-Gajić, J.; Fernández-Gutiérrez, A.; Segura-Carretero, A. Rosmarinus officinalis Leaves as a Natural Source of Bioactive Compounds. Int. J. Mol. Sci. 2014, 15, 20585–20606. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Chatha, S.A.S.; Jabbar, A.; Mahboob, S.; Nigam, P.S. Rosmarinus officinalis Essential Oil: Antiproliferative, Antioxidant and Antibacterial Activities. Braz. J. Microbiol. 2010, 41, 1070–1078. [Google Scholar] [CrossRef] [Green Version]
- Borges, R.S.; Ortiz, B.L.S.; Pereira, A.C.M.; Keita, H.; Carvalho, J.C.T. Rosmarinus officinalis Essential Oil: A Review of Its Phytochemistry, Anti-Inflammatory Activity, and Mechanisms of Action Involved. J. Ethnopharmacol. 2019, 229, 29–45. [Google Scholar] [CrossRef]
- Bai, N.; He, K.; Roller, M.; Lai, C.S.; Shao, X.; Pan, M.H.; Ho, C.T. Flavonoids and Phenolic Compounds from Rosmarinus officinalis. J. Agric. Food Chem. 2010, 58, 5363–5367. [Google Scholar] [CrossRef]
- Ielciu, I.; Sevastre, B.; Olah, N.-K.; Turdean, A.; Chişe, E.; Marica, R.; Oniga, I.; Uifălean, A.; Sevastre-Berghian, A.C.; Niculae, M.; et al. Evaluation of Hepatoprotective Activity and Oxidative Stress Reduction of Rosmarinus officinalis L. Shoots Tincture in Rats with Experimentally Induced Hepatotoxicity. Molecules 2021, 26, 1737. [Google Scholar] [CrossRef]
- EMA. Community Herbal Monograph on Rosmarinus officinalis L., Folium. Eur. Med. Agency 2011, 44, 1–6. [Google Scholar]
- Nieto, G.; Huvaere, K.; Skibsted, L.H. Antioxidant Activity of Rosemary and Thyme By-Products and Synergism with Added Antioxidant in a Liposome System. Eur. Food Res. Technol. 2011, 233, 11–18. [Google Scholar] [CrossRef]
- Bozin, B.; Mimica-Dukic, N.; Samojlik, I.; Jovin, E. Antimicrobial and Antioxidant Properties of Rosemary and Sage (Rosmarinus officinalis L. and Salvia officinalis L., Lamiaceae) Essential Oils. J. Agric. Food Chem. 2007, 55, 7879–7885. [Google Scholar] [CrossRef]
- Cattaneo, L.; Cicconi, R.; Mignogna, G.; Giorgi, A.; Mattei, M.; Graziani, G.; Ferracane, R.; Grosso, A.; Aducci, P.; Schininà, M.E.; et al. Anti-Proliferative Effect of Rosmarinus officinalis L. Extract on Human Melanoma A375 Cells. PLoS ONE 2015, 10, e0132439. [Google Scholar] [CrossRef] [Green Version]
- González-Vallinas, M.; Reglero, G.; De Molina, A.R. Rosemary (Rosmarinus officinalis L.) Extract as a Potential Complementary Agent in Anticancer Therapy. Nutr. Cancer 2015, 67, 1223–1231. [Google Scholar] [CrossRef]
- Chiș, M.-S.; Muste, S.; Păucean, A.; Man, S.; Mureșan, V.; Călian (Ianoș), D.I. A Comprehensive Review About Anticancer and Antimicrobial Activities of Rosemary Oil (Rosmarinus officinalis L.). Hop. Med. Plants 2017, 25, 28–37. [Google Scholar]
- Risaliti, L.; Kehagia, A.; Daoultzi, E.; Lazari, D.; Bergonzi, M.C.; Vergkizi-Nikolakaki, S.; Hadjipavlou-Litina, D.; Bilia, A.R. Liposomes Loaded with Salvia Triloba and Rosmarinus officinalis Essential Oils: In Vitro Assessment of Antioxidant, Antiinflammatory and Antibacterial Activities. J. Drug Deliv. Sci. Technol. 2019, 51, 493–498. [Google Scholar] [CrossRef]
- Nieto, G.; Ros, G.; Castillo, J. Antioxidant and Antimicrobial Properties of Rosemary (Rosmarinus officinalis, L.): A Review. Medicines 2018, 5, 98. [Google Scholar] [CrossRef] [Green Version]
- Petiwala, S.M.; Puthenveetil, A.G.; Johnson, J.J. Polyphenols from the Mediterranean Herb Rosemary (Rosmarinus officinalis) for Prostate Cancer. Front. Pharmacol. 2013, 4, 29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aslan, İ.; Kurt, A.A. In-Vitro Comparison Release Study of Novel Liposome and Conventional Formulation Containing Rosmarinus officinalis Extract. Curr. Perspect. Med. Aromat. Plants 2021, 4, 13–21. [Google Scholar] [CrossRef]
- Mureşan, M.; Olteanu, D.; Filip, G.A.; Clichici, S.; Baldea, I.; Jurca, T.; Pallag, A.; Marian, E.; Frum, A.; Gligor, F.G.; et al. Comparative Study of the Pharmacological Properties and Biological Effects of Polygonum Aviculare L. Herba Extract-Entrapped Liposomes versus Quercetin-Entrapped Liposomes on Doxorubicin-Induced Toxicity on Huvecs. Pharmaceutics 2021, 13, 1418. [Google Scholar] [CrossRef] [PubMed]
- Jahangir, M.A.; Zafar, A.; Khan, S.; Kala, C.; Muheem, A.; Taleuzzaman, M. Phytonutrients and Technological Development in Formulations. J. Pharm. Res. Sci. Technol. 2022, 6, 38–66. [Google Scholar] [CrossRef]
- Kyriakoudi, A.; Spanidi, E.; Mourtzinos, I.; Gardikis, K. Innovative Delivery Systems Loaded with Plant Bioactive Ingredients: Formulation Approaches and Applications. Plants 2021, 10, 1238. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Gortzi, O.; Rovoli, M.; Katsoulis, K.; Graikou, K.; Karagkini, D.A.; Stagos, D.; Kouretas, D.; Tsaknis, J.; Chinou, I. Study of Stability, Cytotoxic and Antimicrobial Activity of Chios Mastic Gum Fractions (Neutral, Acidic) after Encapsulation in Liposomes. Foods 2022, 11, 271. [Google Scholar] [CrossRef]
- Arabi, M.H.; Hora, C.; Mirzapour, A.; Ardestani, M.S.; Saffari, M. Preparation of Nanoliposomes Containing Rosmarinus offi Cinalis L. Essential Oil: A Comparative Study. Biosci. Biotechnol. Res. Commun. 2017, 10, 103–108. [Google Scholar] [CrossRef]
- Alikhani-Koupaei, M. Liposome-Entrapped Essential Oils on in Vitro and in Vivo Antioxidant Activity in Leafy Vegetables. Qual. Assur. Saf. Crop. Foods 2015, 7, 369–373. [Google Scholar] [CrossRef]
- Bankole, V.O.; Osungunna, M.O.; Souza, C.R.F.; Salvador, S.L.; Oliveira, W.P. Spray-Dried Proliposomes: An Innovative Method for Encapsulation of Rosmarinus officinalis L. Polyphenols. AAPS PharmSciTech 2020, 21, 143. [Google Scholar] [CrossRef]
- Sogut, O.; Sezer, U.A.; Sezer, S. Liposomal Delivery Systems for Herbal Extracts. J. Drug Deliv. Sci. Technol. 2021, 61, 102147. [Google Scholar] [CrossRef]
- Porfire, A.; Achim, M.; Barbalata, C.; Rus, I.; Tomuta, I.; Cristea, C. Pharmaceutical Development of Liposomes Using the QbD Approach. Liposomes-Advances and Perspectives. 2019, pp. 1–20. Available online: https://doi.org/10.5772/intechopen.85374 (accessed on 1 May 2022).
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbălată, C.I.; Porfire, A.S.; Sesarman, A.; Rauca, V.F.; Banciu, M.; Muntean, D.; Știufiuc, R.; Moldovan, A.; Moldovan, C.; Tomuță, I. A Screening Study for the Development of Simvastatin-Doxorubicin Liposomes, a Co-Formulation with Future Perspectives in Colon Cancer Therapy. Pharmaceutics 2021, 13, 1526. [Google Scholar] [CrossRef] [PubMed]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M.R. Entrapment of Rosemary Extract by Liposomes Formulated by Mozafari Method: Physicochemical Characterization and Optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef]
- Karadağ, A.E.; Demirci, B.; Çaşkurlu, A.; Demirci, F.; Okur, M.E.; Orak, D.; Sipahi, H.; Başer, K.H.C. In Vitro Antibacterial, Antioxidant, Anti-Inflammatory and Analgesic Evaluation of Rosmarinus officinalis L. Flower Extract Fractions. S. Afr. J. Bot. 2019, 125, 214–220. [Google Scholar] [CrossRef]
- Hcini, K.; Lozano-Perez, A.A.; Cenis, J.L.; Quilez, M.; Jordan, M.J. Extraction and Encapsulation of Phenolic Compounds of Tunisian Rosemary (Rosmarinus officinalis L.) Extracts in Silk Fibroin Nanoparticles. Plants 2021, 10, 2312. [Google Scholar] [CrossRef]
- Pérez-Sánchez, A.; Borrás-Linares, I.; Barrajón-Catalán, E.; Arráez-Román, D.; González-Álvarez, I.; Ibáñez, E.; Segura-Carretero, A.; Bermejo, M.; Micol, V. Evaluation of the Intestinal Permeability of Rosemary (Rosmarinus officinalis L.) Extract Polyphenols and Terpenoids in Caco-2 Cell Monolayers. PLoS ONE 2017, 12, e0172063. [Google Scholar] [CrossRef] [Green Version]
- Birtić, S.; Dussort, P.; Pierre, F.X.; Bily, A.C.; Roller, M. Carnosic Acid. Phytochemistry 2015, 115, 9–19. [Google Scholar] [CrossRef] [Green Version]
- Moreno, S.; Scheyer, T.; Romano, C.S.; Vojnov, A.A. Antioxidant and Antimicrobial Activities of Rosemary Extracts Linked to Their Polyphenol Composition. Free Radic. Res. 2006, 40, 223–231. [Google Scholar] [CrossRef]
- Santomauro, F.; Sacco, C.; Donato, R.; Bellumori, M.; Innocenti, M.; Mulinacci, N. The Antimicrobial Effects of Three Phenolic Extracts from Rosmarinus officinalis L., Vitis vinifera L. and Polygonum cuspidatum L. on Food Pathogens. Nat. Prod. Res. 2018, 32, 2639–2645. [Google Scholar] [CrossRef]
- Ekambaram, S.P.; Perumal, S.S.; Balakrishnan, A.; Marappan, N.; Gajendran, S.S.; Viswanathan, V. Antibacterial Synergy between Rosmarinic Acid and Antibiotics against Methicillin-Resistant Staphylococcus Aureus. J. Intercult. Ethnopharmacol. 2016, 5, 358–363. [Google Scholar] [CrossRef] [PubMed]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- European Pharmacopoeia, 10th ed.; European Directorate for the Quality of Medicines & Health Care: Strasbourg, France, 2022.
- Machado, A.R.; Pinheiro, A.C.; Vicente, A.A.; Souza-Soares, L.A.; Cerqueira, M.A. Liposomes Loaded with Phenolic Extracts of Spirulina LEB-18: Physicochemical Characterization and Behavior under Simulated Gastrointestinal Conditions. Food Res. Int. 2019, 120, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Cheng, Y.; Zhao, P.; Zhang, S.; Li, M.; He, C.; Zhang, X.; Yang, T.; Yan, R.; Ye, P.; et al. Co-Delivery of Doxorubicin and Imatinib by PH Sensitive Cleavable PEGylated Nanoliposomes with Folate-Mediated Targeting to Overcome Multidrug Resistance. Int. J. Pharm. 2018, 542, 266–279. [Google Scholar] [CrossRef]
- Dash, T.K.; Konkimalla, V.B. Formulation and Optimization of Doxorubicin and Biochanin A Combinational Liposomes for Reversal of Chemoresistance. AAPS PharmSciTech 2017, 18, 1116–1124. [Google Scholar] [CrossRef]
- Postescu, I.D.; Tatomir, C.; Chereches, G.; Brie, I.; Damian, G.; Petrisor, D.; Hosu, A.M.; Miclaus, V.; Pop, A. Spectroscopic Characterization of Some Grape Extracts with Potential Role in Tumor Growth Inhibition. J. Optoelectron. Adv. Mater. 2007, 9, 564–567. [Google Scholar]
- Heffelfinger, S.C.; Hawkins, H.H.; Barrish, J.; Taylor, L.; Darlington, G.J. SK HEP-1: A Human Cell Line of Endothelial Origin. Vitr. Cell. Dev. Biol. 2016, 28, 136–142. [Google Scholar] [CrossRef]
- Xu, L.; Hui, A.Y.; Albanis, E.; Arthur, M.J.; O’Byrne, S.M.; Blaner, W.S.; Mukherjee, P.; Friedman, S.L.; Eng, F.J. Human Hepatic Stellate Cell Lines, LX-1 and LX-2: New Tools for Analysis of Hepatic Fibrosis. Gut 2005, 54, 142–151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanganu, D.; Niculae, M.; Ielciu, I.; Olah, N.-K.; Munteanu, M.; Burtescu, R.; Ştefan, R.; Olar, L.; Pall, E.; Andrei, S.; et al. Chemical Profile, Cytotoxic Activity and Oxidative Stress Reduction of Different Syringa vulgaris L. Extracts. Molecules 2021, 26, 3104. [Google Scholar] [CrossRef]
- Păltinean, R.; Ielciu, I.; Hanganu, D.; Niculae, M.; Pall, E.; Angenot, L.; Tits, M.; Mocan, A.; Babotă, M.; Frumuzachi, O.; et al. Biological Activities of Some Isoquinoline Alkaloids from Fumaria schleicheri Soy. Will. Plants 2022, 11, 1202. [Google Scholar] [CrossRef]
- Marian, E.; Duteanu, N.; Vicas, L.; Rusu, G.; Jurca, T.; Muresan, M.; Micle, O.; Hangan, A.C.; Stan, R.L.; Ionescu, C.; et al. Synthesis, Characterization of Inclusion Compounds of Amygdalin with β-Cyclodextrin and Sod-like Activity and Cytotoxicity on Hela Tumor Cells. Arab. J. Chem. 2020, 13, 6828–6837. [Google Scholar] [CrossRef]
- European Committee on Antimicrobial Susceptibility Testing, (EUCAST). Routine and Extended Internal Quality Control for MIC Determination and Disk Diffusion as Recommended by EUCAST, Version 12.0; 2022. Available online: http://www.eucast.org (accessed on 1 May 2022).
- Niculae, M.; Hanganu, D.; Oniga, I.; Benedec, D.; Ielciu, I.; Giupana, R.; Sandru, C.D.; Ciocârlan, N.; Spinu, M. Phytochemical Profile and Antimicrobial Potential of Extracts Obtained from Thymus marschallianus Willd. Molecules 2019, 24, 3101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Quality Attribute | Drug Content (μmol GAE/mL) | Encapsulation Efficiency (EE%) | Particle Size (nm) | Polydispersity Index (PdI) | Zeta Potential (mV) |
|---|---|---|---|---|---|
| Liposomes | |||||
| Empty liposomes (L-E) | - | - | 308.9 | 0.207 | −58.6 |
| Liposomes loaded with DOX (L-DOX) | 0.32 | 45.2 | 208.46 | 0.127 | −4.67 |
| Liposomes loaded with R. officinalis (L-R) | 4.52 | 52.31 | 190.3 | 0.216 | −26.5 |
| Compound | Retention Time (min) | m/z and Main Transition | Concentration (μg/mL) | ||
|---|---|---|---|---|---|
| Reference | Separated Compound | Reference | Separated Compound | ||
| Caffeic acid | 13.8 | 13.6 | 179.0 > 135.0 | 179.0 > 135.0 | 81.07 ± 0.76 |
| Chlorogenic acid | 12.0 | 12.0 | 353.0 > 191.0 | 353.0 > 191.0 | 14.10 ± 0.12 |
| Apigenin | 28.2 | 28.1 | 269.0 > 117.0 | 269.0 > 117.0 | 2.26 ± 0.04 |
| Chrysin | 29.7 | 30.0 | 253.0 > 143.0 | 253.0 > 143.0 | 1.89 ± 0.02 |
| Luteolin | 26.9 | 26.8 | 287.0 > 153.0 | 287.0 > 153.0 | 0.85 ± 0.02 |
| Luteolin-7-O-glucoside | 19.9 | 19.8 | 447.0 > 284.9 | 447.0 > 284.9 | 7.42 ± 0.04 |
| Quercetin | 25.7 | 27.0 | 300.9 > 151.0 | 300.9 > 151.0 | 0.61 ± 0.02 |
| Rutoside | 20.3 | 20.3 | 609.0 > 300.0 | 609.0 > 300.0 | 9.86 ± 0.06 |
| Naringenin | 26.3 | 26.9 | 271.0 > 119.0 | 271.0 > 119.0 | 0.42 ± 0.02 |
| Hesperetin | 27.1 | 27.0 | 301.0 > 164.0 | 301.0 > 164.0 | 9.89 ± 0.12 |
| Carnosic acid | 32.0 | 32.0 | 331.2 > 285.1 | 331.2 > 285.1 | 20.03 ± 0.16 |
| Rosmarinic acid | 21.4 | 21.6 | 358.9 > 161.0 | 358.9 > 161.0 | 39.81 ± 0.35 |
| Ellagic acid | 27.3 | 27.3 | 301.0 > 185.0 | 301.0 > 185.0 | 880.02 ± 0.14 |
| Carnosol | 30.6 | 31.0 | 329.1 > 285.1 | 329.1 > 285.1 | 2.69 ± 0.04 |
| Hyperoside | 20.3 | 20.2 | 463.1 > 300.0 | 463.1 > 300.0 | 14.21 ± 0.18 |
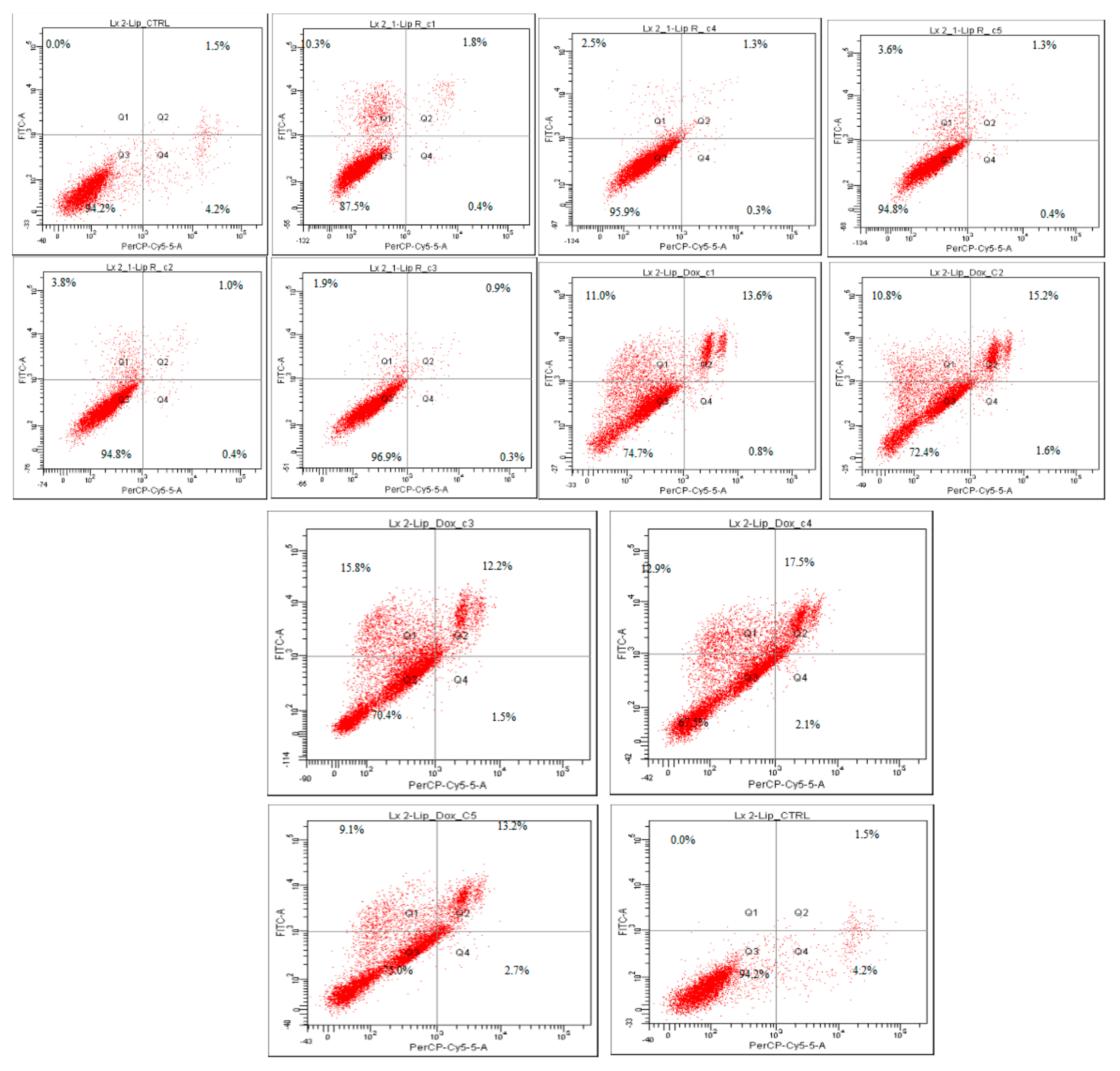
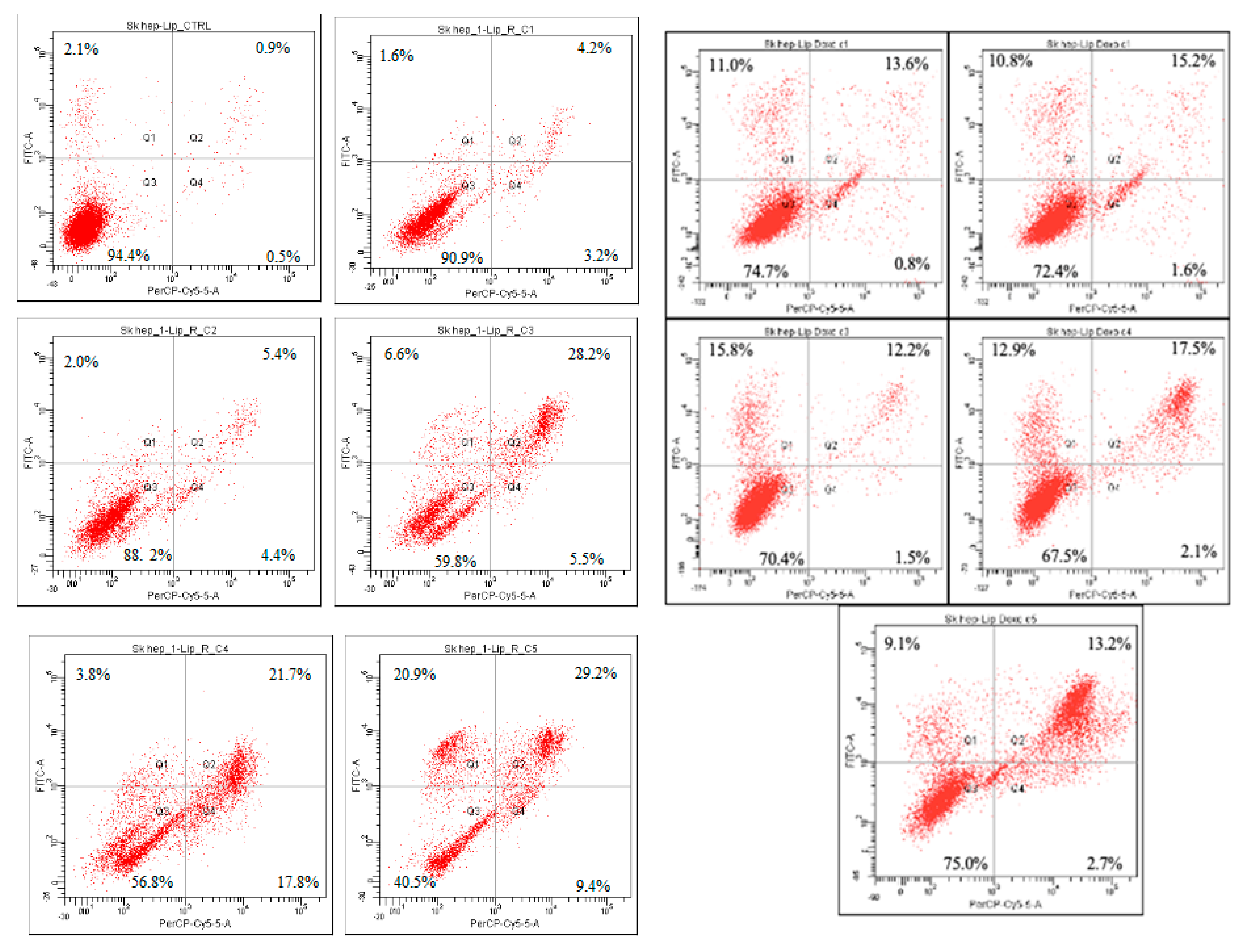
| Treatment | Viable Cells (%) | Apoptotic Cells (%) | Late Apoptotic Cells (%) | Necrotic Cells (%) |
|---|---|---|---|---|
| LX-2 L-E | 94.2 | 0.0 | 1.5 | 4.2 |
| LX-2 L-R-C1 | 87.5 | 10.3 | 1.8 | 0.4 |
| LX-2 L-R-C2 | 94.8 | 3.8 | 1.0 | 0.4 |
| LX-2 L-R-C3 | 96.9 | 1.9 | 0.9 | 0.3 |
| LX-2 L-R-C4 | 95.9 | 2.5 | 1.3 | 0.3 |
| LX-2 L-R-C5 | 94.8 | 3.6 | 1.3 | 0.4 |
| LX-2 L-DOX-C1 | 74.7 | 11.0 | 13.6 | 0.8 |
| LX-2 L-DOX-C2 | 72.4 | 10.8 | 15.2 | 1.6 |
| LX-2 L-DOX-C3 | 70.4 | 15.8 | 12.2 | 1.5 |
| LX-2 L-DOX-C4 | 67.5 | 12.9 | 17.5 | 2.1 |
| LX-2 L-DOX-C5 | 75.0 | 9.1 | 13.2 | 2.7 |
| SK-Hep-1 L-E | 96.4 | 2.1 | 0.9 | 0.5 |
| SK-Hep-1 L-R-C1 | 90.9 | 1.6 | 4.2 | 3.2 |
| SK-Hep-1 L-R-C2 | 88.2 | 2.0 | 5.4 | 4.4 |
| SK-Hep-1 L-R-C3 | 59.8 | 6.6 | 28.2 | 5.5 |
| SK-Hep-1 L-R-C4 | 56.8 | 3.8 | 21.7 | 17.8 |
| SK-Hep-1 L-R-C5 | 40.5 | 20.9 | 29.2 | 9.4 |
| SK-Hep-1 L- DOX-C1 | 82.6 | 6.7 | 4.5 | 6.3 |
| SK-Hep-1 L-DOX-C2 | 88.7 | 8.7 | 1.8 | 0.8 |
| SK-Hep-1 L-DOX-C3 | 87.1 | 8.1 | 3.9 | 0.9 |
| SK-Hep-1 L-DOX-C4 | 74.2 | 9.5 | 14.7 | 1.6 |
| SK-Hep-1 L-DOX-C5 | 46.3 | 7.6 | 40.1 | 6.1 |
| Zone of Inhibition (mm) | ||
|---|---|---|
| Bacterial Species | L-R | Gentamicin |
| MSSA | 25.33 ± 0.47 | 18 ± 0.00 a |
| MRSA | 20.67 ± 0.47 | 17 ± 0.00 b |
| Bacillus cereus | 27.33 ± 0.94 | 21 ± 0.00 b |
| Enterococcus faecalis | 18.33 ± 0.94 | 17 ± 0.00 |
| Salmonella enterica serovar Enteriditis | 16.33 ± 0.47 | 18 ± 0.00 |
| Salmonella enterica serovar Typhimurium | 16.33 ± 0.47 | 17 ± 0.00 |
| Escherichia coli | 17.33 ± 0.47 | 17 ± 0.00 |
| Bacterial Species | MIC Index MBC (μmol GAE/mL)/ MIC (μmol GAE/mL) |
|---|---|
| MSSA | 2 0.28/0.14 |
| MRSA | 2 0.28/0.14 |
| Bacillus cereus | 1 0.14/0.14 |
| Enterococcus faecalis | 2 0.56/0.28 |
| Salmonella enterica serovar Enteriditis | 1 1.13/1.13 |
| Salmonella enterica serovar Typhimurium | 1 1.13/1.13 |
| Escherichia coli | 1 1.13/1.13 |
| Time (min) | % Methanol | % Water | % of 2% Formic Acid in Water |
|---|---|---|---|
| 0.00 | 5 | 90 | 5 |
| 3.00 | 15 | 70 | 15 |
| 6.00 | 15 | 70 | 15 |
| 9.00 | 21 | 58 | 21 |
| 13.00 | 21 | 58 | 21 |
| 18.00 | 30 | 41 | 29 |
| 22.00 | 30 | 41 | 29 |
| 26.00 | 50 | 0 | 50 |
| 29.00 | 50 | 0 | 50 |
| 29.01 | 5 | 90 | 5 |
| 35.00 | 5 | 90 | 5 |
| Reference | Retention Time (min) | m/z and Main Transition | MRM | Other Transitions |
|---|---|---|---|---|
| Caffeic acid | 13.8 | 179.0 > 135.0 | Negative | 179.0 > 134.0 179.0 > 89.0 |
| trans-p-coumaric acid | 17.5 | 163.0 > 119.0 | Negative | 163.0 > 93.0 |
| Chlorogenic acid | 12.0 | 353.0 > 191.0 | Negative | |
| Apigenin | 28.2 | 269.0 > 117.0 | Negative | |
| Chrysin | 29.7 | 253.0 > 143.0 | Negative | 253.0 > 119.0 253.0 > 107.0 |
| Luteolin | 26.9 | 287.0 > 153.0 | Positive | |
| Luteolin-7-O-glucosid | 19.9 | 447.0 > 284.9 | Negative | |
| Quercetin | 25.7 | 300.9 > 151.0 | Negative | 300.9 > 121.0 |
| Rutoside | 20.3 | 609.0 > 300.0 | Negative | 609.0 > 301.0 609.0 > 271.0 |
| Naringenin | 26.3 | 271.0 > 119.0 | Negative | 271.0 > 107.0 |
| Hesperetin | 27.1 | 301.0 > 164.0 | Negative | 301.0 > 136.0 301.0 > 108.0 |
| Carnosic acid | 32.0 | 331.2 > 285.1 | Negative | |
| Ellagic acid | 27.2 | 301.0 > 185.0 | Negative | 301.0 > 257.0 |
| Carnosol | 30.7 | 329.1 > 285.1 | Negative | |
| Kaempferol | 28.0 | 285.0 > 187.0 | Negative | 285.0 > 151.0 285.0 > 133.0 |
| Vitexin | 18.4 | 431.0 > 311.0 | Negative | |
| Rosmarinic acid | 21.4 | 358.9 > 161.0 | Negative | 358.9 > 133.0 |
| Myricetin | 13.6 | 317.0 > 179.0 | Negative | 317.0 > 151.0 317.0 > 137.0 |
| Hyperoside | 20.3 | 463.1 > 300.0 | Negative | 463.1 > 301.0 |
| Quercitrin | 18.4 | 447.0 > 229.9 | Negative | |
| Isoquercitrin | 17.9 | 353.1 > 173.2 | Negative | |
| Ferulic acid | 18.4 | 193.0 > 134.0 | Negative | 193.0 > 178.0 |
| Sinapic acid | 18.4 | 223.0 > 207.9 | Negative | |
| Gallic acid | 7.0 | 168.9 > 125.0 | Negative |
| Reference | Concentration Range (mg/mL) | Calibration Curve Equation | Correlation Factor | Detection Limit (μg/mL) | Quantification Limit (μg/mL) |
|---|---|---|---|---|---|
| Caffeic acid | 0.11–1.10 | A = 4 × 107 × C − 319,689 | 0.9998 | 3.20 | 4.80 |
| trans-p-coumaric acid | 0.16–1.60 | A = 3 × 107 × C + 291,065 | 0.9993 | 1.90 | 3.90 |
| Chlorogenic acid | 0.13–1.30 | A = 2 × 108 × C − 269,699 | 0.9997 | 5.00 | 8.00 |
| Apigenin | 0.10–0.98 | A = 2 × 108 × C + 15,916 | 0.9999 | 0.20 | 0.30 |
| Chrysin | 0.10–1.00 | A = 1 × 108 × C − 82,818 | 0.9997 | 3.00 | 5.00 |
| Luteolin | 0.01–0.10 | A = 2 × 108 × C − 2295.4 | 0.9977 | 0.05 | 0.07 |
| Luteolin- 7-O-glucosid | 0.07–0.70 | A = 1 × 109 × C − 700,317 | 0.9990 | 3.00 | 4.00 |
| Quercetin | 0.09–0.91 | A = 5 × 107 × C − 9556 | 0.9964 | 0.80 | 1.10 |
| Rutoside | 0.17–1.70 | A = 2 × 108 × C − 191,937 | 0.9996 | 4.00 | 6.00 |
| Naringenin | 0.16–1.60 | A = 3 × 108 × C − 43,443 | 0.9999 | 0.60 | 0.90 |
| Hesperetin | 0.10–1.00 | A = 6 × 107 × C − 49,247 | 0.9974 | 3.00 | 5.00 |
| Carnosic acid | 0.28–2.80 | A = 107 × C − 99,360 | 0.9994 | 4.00 | 6.00 |
| Ellagic acid | 0.107–1.070 | A = 14,987 × C − 138.52 | 0.9982 | 3.70 | 5.50 |
| Carnosol | 0.022–0.220 | A = 109 × C − 253,279 | 0.9997 | 1.00 | 2.00 |
| Kaempferol | 0.10–1.00 | A = 107 × C − 20,574 | 0.9996 | 0.80 | 1.20 |
| Rosmarinic acid | 0.028–0.278 | A = 2 × 108 × C − 6664.7 | 0.9996 | 0.10 | 0.20 |
| Myricetin | 0.140–1.400 | A = 26,499 × C − 41.803 | 0.9997 | 0.60 | 0.90 |
| Hyperoside | 0.012–0.107 | A = 4 × 108 × C − 567,182 | 0.9986 | 0.60 | 0.90 |
| Isoquercitrin | 0.140–1.400 | A = 4727 × C + 68.172 | 0.9973 | 2.90 | 5.80 |
| Ferulic acid | 0.100–1.000 | A = 5 × 106 × C − 50,000 | 0.9992 | 4.00 | 6.00 |
| Gallic acid | 0.107–1.070 | A = 8 × 106 × C − 37,131 | 0.9999 | 1.90 | 2.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ielciu, I.; Niculae, M.; Pall, E.; Barbălată, C.; Tomuţă, I.; Olah, N.-K.; Burtescu, R.F.; Benedec, D.; Oniga, I.; Hanganu, D. Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes. Molecules 2022, 27, 3988. https://doi.org/10.3390/molecules27133988
Ielciu I, Niculae M, Pall E, Barbălată C, Tomuţă I, Olah N-K, Burtescu RF, Benedec D, Oniga I, Hanganu D. Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes. Molecules. 2022; 27(13):3988. https://doi.org/10.3390/molecules27133988
Chicago/Turabian StyleIelciu, Irina, Mihaela Niculae, Emoke Pall, Cristina Barbălată, Ioan Tomuţă, Neli-Kinga Olah, Ramona Flavia Burtescu, Daniela Benedec, Ilioara Oniga, and Daniela Hanganu. 2022. "Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes" Molecules 27, no. 13: 3988. https://doi.org/10.3390/molecules27133988
APA StyleIelciu, I., Niculae, M., Pall, E., Barbălată, C., Tomuţă, I., Olah, N.-K., Burtescu, R. F., Benedec, D., Oniga, I., & Hanganu, D. (2022). Antiproliferative and Antimicrobial Effects of Rosmarinus officinalis L. Loaded Liposomes. Molecules, 27(13), 3988. https://doi.org/10.3390/molecules27133988











