Selective Cytotoxicity of Portuguese Propolis Ethyl Acetate Fraction towards Renal Cancer Cells
Abstract
1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Propolis Sample
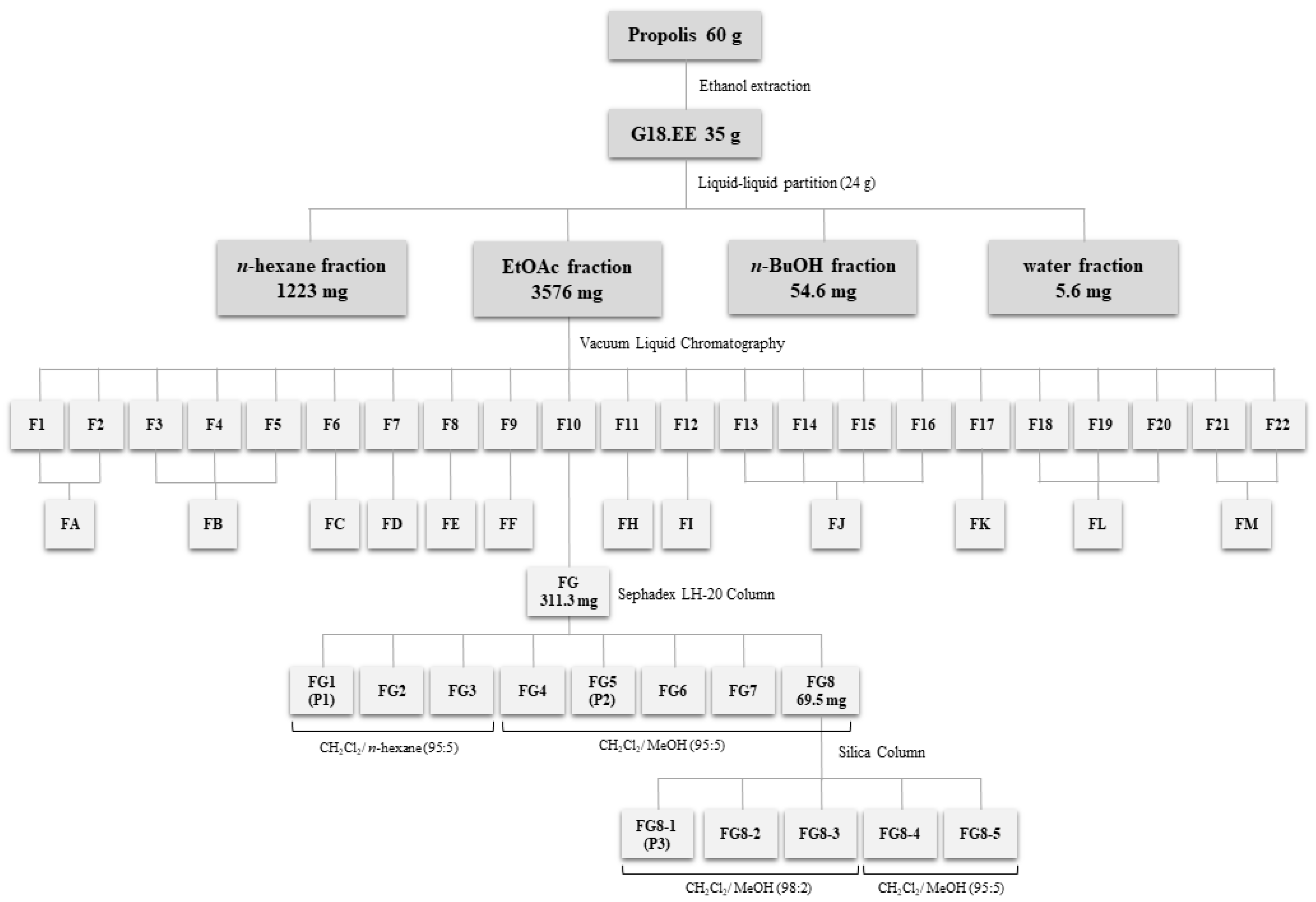
3.3. Extraction and Fractionation of Propolis
Isolation of Compounds from the G18.EE-EtOAc Fraction
3.4. Chemical Analysis of the Subfractions
3.4.1. UPLC-DAD-ESI/MSn Analysis
3.4.2. NMR Analysis
3.5. Cell Lines, Media and Growth Conditions
3.6. Cytotoxicity Assay and Selectivity Index (SI) Calculation
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Capitanio, U.; Montorsi, F. Renal cancer. Lancet 2016, 387, 894–906. [Google Scholar] [CrossRef]
- Yadav, M.H.; Reddy, B.K.K.; Akhileswar, V. Review on nutraceuticals. Int. J. Pharmakeia 2015, 1, 1–12. [Google Scholar]
- Fokt, H.; Pereira, A.; Ferreira, A.M.; Cunha, A.; Aguiar, C. How do bees prevent hive infections? The antimicrobial properties of propolis. Curr. Res. Technol. Educ. Top. Appl. Microbiol. Microb. Biotechnol. 2010, 1, 481–493. [Google Scholar]
- Silva-Carvalho, R.; Baltazar, F.; Almeida-Aguiar, C. Propolis: A complex natural product with a plethora of biological activities that can be explored for drug development. Evid. Based Complement. Alternat. Med. 2015, 2015, 206439. [Google Scholar] [CrossRef]
- Havsteen, B.H. The biochemistry and medical significance of the flavonoids. Pharmacol. Ther. 2002, 96, 67–202. [Google Scholar] [CrossRef]
- Bankova, V.; Castro, S.L.; Marcucci, M.C. Propolis: Recent advances in chemistry and plant origin. Apidologie 2000, 31, 3–15. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, C.P.; Wang, K.; Li, G.Q.; Hu, F.L. Recent advances in the chemical composition of propolis. Molecules 2014, 19, 19610–19632. [Google Scholar] [CrossRef]
- Freitas, A.S.; Cunha, A.; Cardoso, S.M.; Oliveira, R.; Almeida-Aguiar, C. Constancy of the bioactivities of propolis samples collected on the same apiary over four years. Food Res. Int. 2019, 119, 622–633. [Google Scholar] [CrossRef]
- Kamiya, T.; Nishihara, H.; Hara, H.; Adachi, T. Ethanol extract of Brazilian red propolis induces apoptosis in human breast cancer MCF-7 cells through endoplasmic reticulum stress. J. Agric. Food Chem. 2012, 60, 11065–11070. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Kapur, A.; Yang, J.X.; Srivastava, S.; McLeod, D.G.; Paredes-Guzman, J.F.; Daugsch, A.; Park, Y.; Rhim, J.S. Antiproliferation of human prostate cancer cells by ethanolic extracts of Brazilian propolis and its botanical origin. Int. J. Oncol. 2007, 31, 601–606. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, M.; Naoi, K.; Hashita, M.; Itoh, Y.; Suzui, M. Growth inhibitory activity of ethanol extracts of Chinese and Brazilian propolis in four human colon carcinoma cell lines. Oncol. Rep. 2009, 22, 349–354. [Google Scholar] [PubMed]
- Valença, I.; Morais-Santos, F.; Miranda-Gonçalves, V.; Ferreira, A.M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese propolis disturbs glycolytic metabolism of human colorectal cancer in vitro. BMC Complement. Altern. Med. 2013, 13, 184. [Google Scholar] [CrossRef] [PubMed]
- Kubina, R.; Kabała-Dzik, A.; Dziedzic, A.; Bielec, B.; Wojtyczka, R.D.; Bułdak, R.J.; Wyszyńska, M.; Stawiarska-Pięta, B.; Szaflarska-Stojko, E. The ethanol extract of polish propolis exhibits anti-proliferative and/or pro-apoptotic effect on HCT 116 colon cancer and Me45 Malignant melanoma cells in vitro conditions. Adv. Clin. Exp. Med. 2015, 24, 203–212. [Google Scholar] [CrossRef]
- Silva-Carvalho, R.; Miranda-Gonçalves, V.; Ferreira, A.M.; Cardoso, S.M.; Sobral, A.J.; Almeida-Aguiar, C.; Baltazar, F. Antitumoural and antiangiogenic activity of Portuguese propolis in in vitro and in vivo models. J. Funct. Foods 2014, 11, 160–171. [Google Scholar] [CrossRef]
- Calhelha, R.C.; Falcão, S.; Queiroz, M.J.R.; Vilas-Boas, M.; Ferreira, I.C. Cytotoxicity of Portuguese propolis: The proximity of the in vitro doses for tumor and normal cell lines. BioMed Res. Int. 2014, 2014, 897361. [Google Scholar] [CrossRef]
- Daleprane, J.B.; Schmid, T.; Dehne, N.; Rudnicki, M.; Menrad, H.; Geis, T.; Ikegaki, M.; Ong, T.P.; Brüne, B.; Abdalla, D.S. Suppression of hypoxia-inducible factor-1α contributes to the antiangiogenic activity of red propolis polyphenols in human endothelial cells. J. Nutr. 2012, 142, 441–447. [Google Scholar] [CrossRef][Green Version]
- Valente, M.J.; Baltazar, A.F.; Henrique, R.; Estevinho, L.; Carvalho, M. Biological activities of Portuguese propolis: Protection against free radical-induced erythrocyte damage and inhibition of human renal cancer cell growth in vitro. Food Chem. Toxicol. 2011, 49, 86–92. [Google Scholar] [CrossRef]
- Szliszka, E.; Sokół-Łętowska, A.; Kucharska, A.Z.; Jaworska, D.; Czuba, Z.P.; Król, W. Ethanolic extract of Polish propolis: Chemical composition and TRAIL-R2 death receptor targeting apoptotic activity against prostate cancer cells. Evid. Based Complement. Alternat. Med. 2013, 2013, 757628. [Google Scholar] [CrossRef]
- Salim, E.I.; Abd El-Magid, A.D.; Farara, K.M.; Maria, D.S. Antitumoral and antioxidant potential of Egyptian propolis against the PC3 prostate cancer cell line. Asian Pac. J. Cancer Prev. 2015, 16, 7641–7651. [Google Scholar] [CrossRef] [PubMed]
- Taira, N.; Nguyen, B.C.Q.; Be Tu, P.T.; Tawata, S. Effect of Okinawa propolis on PAK1 activity, Caenorhabditis elegans longevity, melanogenesis, and growth of cancer cells. J. Agric. Food Chem. 2016, 64, 5484–5489. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Wu, Y.; Chen, X.; Jiang, X.; Wang, K.; Hu, F. Chinese propolis exerts anti-proliferation effects in human melanoma cells by targeting NLRP1 inflammatory pathway, inducing apoptosis, cell cycle arrest, and autophagy. Nutrients 2018, 10, 1170. [Google Scholar] [CrossRef]
- Umthong, S.; Puthong, S.; Chanchao, C. Trigona laeviceps propolis from Thailand: Antimicrobial, antiproliferative and cytotoxic activities. Am. J. Chin. Med. 2009, 37, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Umthong, S.; Phuwapraisirisan, P.; Puthong, S.; Chanchao, C. In vitro antiproliferative activity of partially purified Trigona laeviceps propolis from Thailand on human cancer cell lines. BMC Complement. Altern. Med. 2011, 11, 37. [Google Scholar] [CrossRef]
- Oliveira, R.D.; Celeiro, S.P.; Barbosa-Matos, C.; Freitas, A.S.; Cardoso, S.M.; Viana-Pereira, M.; Almeida-Aguiar, C.; Baltazar, F. Portuguese Propolis Antitumoral Activity in Melanoma Involves ROS Production and Induction of Apoptosis. Molecules 2022, 27, 3533. [Google Scholar] [CrossRef]
- Lim, H.; Son, K.H.; Chang, H.W.; Bae, K.; Kang, S.S.; Kim, H.P. Anti-inflammatory activity of pectolinarigenin and pectolinarin isolated from Cirsium chanroenicum. Biol. Pharm. Bull. 2008, 31, 2063–2067. [Google Scholar] [CrossRef]
- Garedew, A.; Schmolz, E.; Lamprecht, I. Microbiological and calorimetric investigations on the antimicrobial actions of different propolis extracts: An in vitro approach. Thermochim. Acta 2004, 422, 115–124. [Google Scholar] [CrossRef]
- Bankova, V.; Bertelli, D.; Borba, R.; Conti, B.J.; da Silva Cunha, I.B.; Danert, C.; Eberlin, M.N.; Falcão, S.I.; Isla, M.I.; Moreno, M.I.N.; et al. Standard methods for Apis mellifera propolis research. J. Apic. Res. 2016, 58, 1–49. [Google Scholar] [CrossRef]
- Dezmirean, D.S.; Paşca, C.; Moise, A.R.; Bobiş, O. Plant Sources Responsible for the Chemical Composition and Main Bioactive Properties of Poplar-Type Propolis. Plants 2021, 10, 22. [Google Scholar] [CrossRef]
- Jaganathan, S.K.; Mandal, M. Antiproliferative effects of honey and of its polyphenols: A review. J. Biomed. Biotechnol. 2009, 2009, 830616. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Emerging adjuvant therapy for cancer: Propolis and its constituents. J. Diet. Suppl. 2016, 13, 245–268. [Google Scholar] [CrossRef] [PubMed]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural compounds and combination therapy in colorectal cancer treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef] [PubMed]
- Aumeeruddy, M.Z.; Mahomoodally, M.F. Combating breast cancer using combination therapy with 3 phytochemicals: Piperine, sulforaphane, and thymoquinone. Cancer 2019, 125, 1600–1611. [Google Scholar] [CrossRef]
- Cheriet, T.; Ben-Bachir, B.; Thamri, O.; Seghiri, R.; Mancini, I. Isolation and biological properties of the natural flavonoids pectolinarin and pectolinarigenin—A review. Antibiotics 2020, 9, 417. [Google Scholar] [CrossRef]
- Singh, S.; Gupta, P.; Meena, A.; Luqman, S. Acacetin, a flavone with diverse therapeutic potential in cancer, inflammation, infections and other metabolic disorders. Food Chem. Toxicol. 2020, 145, 111708. [Google Scholar] [CrossRef]
- Somwong, P.; Suttisri, R. Cytotoxic activity of the chemical constituents of Clerodendrum indicum and Clerodendrum villosum roots. J. Integr. Med. 2018, 16, 57–61. [Google Scholar] [CrossRef]
- Lee, H.J.; Venkatarame Gowda Saralamma, V.; Kim, S.M.; Ha, S.E.; Raha, S.; Lee, W.S.; Kim, E.H.; Lee, S.J.; Kim, G.S. Pectolinarigenin induced cell cycle arrest, autophagy, and apoptosis in gastric cancer cell via PI3K/AKT/mTOR signaling pathway. Nutrients 2018, 10, 1043. [Google Scholar] [CrossRef]
- Wu, T.; Dong, X.; Yu, D.; Shen, Z.; Yu, J.; Yan, S. Natural product pectolinarigenin inhibits proliferation, induces apoptosis, and causes G2/M phase arrest of HCC via PI3K/AKT/mTOR/ERK signaling pathway. Onco Targets Ther. 2018, 11, 8633. [Google Scholar] [CrossRef]
- Xu, F.; Gao, X.; Pan, H. Pectolinarigenin inhibits non-small cell lung cancer progression by regulating the PTEN/PI3K/AKT signaling pathway. Oncol. Rep. 2018, 40, 3458–3468. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, J.; Yang, H.; Zhang, Q.; Chen, M. Pectolinarigenin flavonoid exhibits selective anti-proliferative activity in cisplatin-resistant hepatocellular carcinoma, autophagy activation, inhibiting cell migration and invasion. G2/M phase cell cycle arrest and targeting ERK1/2 MAP kinases. J. BUON 2020, 25, 415–420. [Google Scholar] [PubMed]
- Bonesi, M.; Tundis, R.; Deguin, B.; Loizzo, M.R.; Menichini, F.; Tillequin, F.; Menichini, F. In vitro biological evaluation of novel 7-O-dialkylaminoalkyl cytotoxic pectolinarigenin derivatives against a panel of human cancer cell lines. Bioorg. Med. Chem. Lett. 2008, 18, 5431–5434. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.W.; Hu, J.J.; Fu, R.Q.; Liu, X.; Zhang, Y.H.; Li, J.; Liu, L.; Li, Y.N.; Deng, Q.; Luo, Q.S.; et al. Flavonoids inhibit cell proliferation and induce apoptosis and autophagy through downregulation of PI3Kγ mediated PI3K/AKT/mTOR/p70S6K/ULK signaling pathway in human breast cancer cells. Sci. Rep. 2018, 8, 11255. [Google Scholar] [CrossRef]
- Kim, H.R.; Park, C.G.; Jung, J.Y. Acacetin (5,7-dihydroxy-4′-methoxyflavone) exhibits in vitro and in vivo anticancer activity through the suppression of NF-κB/Akt signaling in prostate cancer cells. Int. J. Mol. Med. 2014, 33, 317–324. [Google Scholar] [CrossRef]
- Ouhtit, A.; Gaur, R.L.; Abdraboh, M.; Ireland, S.K.; Rao, P.N.; Raj, S.G.; Al-Riyami, H.; Shanmuganathan, S.; Gupta, I.; Murthy, S.N.; et al. Simultaneous inhibition of cell-cycle, proliferation, survival, metastatic pathways and induction of apoptosis in breast cancer cells by a phytochemical super-cocktail: Genes that underpin its mode of action. J. Cancer 2013, 4, 703. [Google Scholar] [CrossRef] [PubMed]
- Righi, N.; Boumerfeg, S.; Fernandes, P.A.; Deghima, A.; Baali, F.; Coelho, E.; Cardoso, S.M.; Coimbra, M.A.; Baghiani, A. Thymus algeriensis Bioss & Reut: Relationship of phenolic compounds composition with in vitro/in vivo antioxidant and antibacterial activity. Food Res. Int. 2020, 136, 109500. [Google Scholar]
- Pontes, O.; Costa, M.; Santos, F.; Sampaio-Marques, B.; Dias, T.; Ludovico, P.; Baltazar, F.; Proenca, F. Exploitation of new chalcones and 4H-chromenes as agents for cancer treatment. Eur. J. Med. Chem. 2018, 157, 101–114. [Google Scholar] [CrossRef]

| Propolis Fractions | IC50 ± SD (µg mL−1) * | SI α (vs. HK2) | |||||
|---|---|---|---|---|---|---|---|
| Caki-2 | A498 | 786-O | HK2 (Non-Neoplastic) | Caki-2 | A498 | 786-O | |
| n-hexane | >30 | >30 | >30 | >30 | 0 | 0 | 0 |
| EtOAc | >30 | 0.162 ± 0.000 c,d | 0.271 ± 0.005 c,d | >30 | 0 | >184.2 | >109.7 |
| n-BuOH | >30 | 0.239 ± 0.001 c,d | 0.341 ± 0.003 c,d | >30 | 0 | >124.5 | >87.0 |
| water | 0.573 ± 0.030 c | 0.085 ± 0.001 d | 0.199 ± 0.013 c,d | 0.229 ± 0.000 c,d | −0.6 | 1.7 | 0.2 |
| G18.EE (propolis extract) | 0.765 ± 0.041 c | 0.153 ± 0.004 c,d | 87.170 ± 0.707 a | 49.185 ± 0.106 b | 63.3 | 320.5 | −0.4 |
| Subfractions | Compound Code | tR (min) | λmax (nm) | [M-H]− m/z | Main Fragments | Compound Detected |
|---|---|---|---|---|---|---|
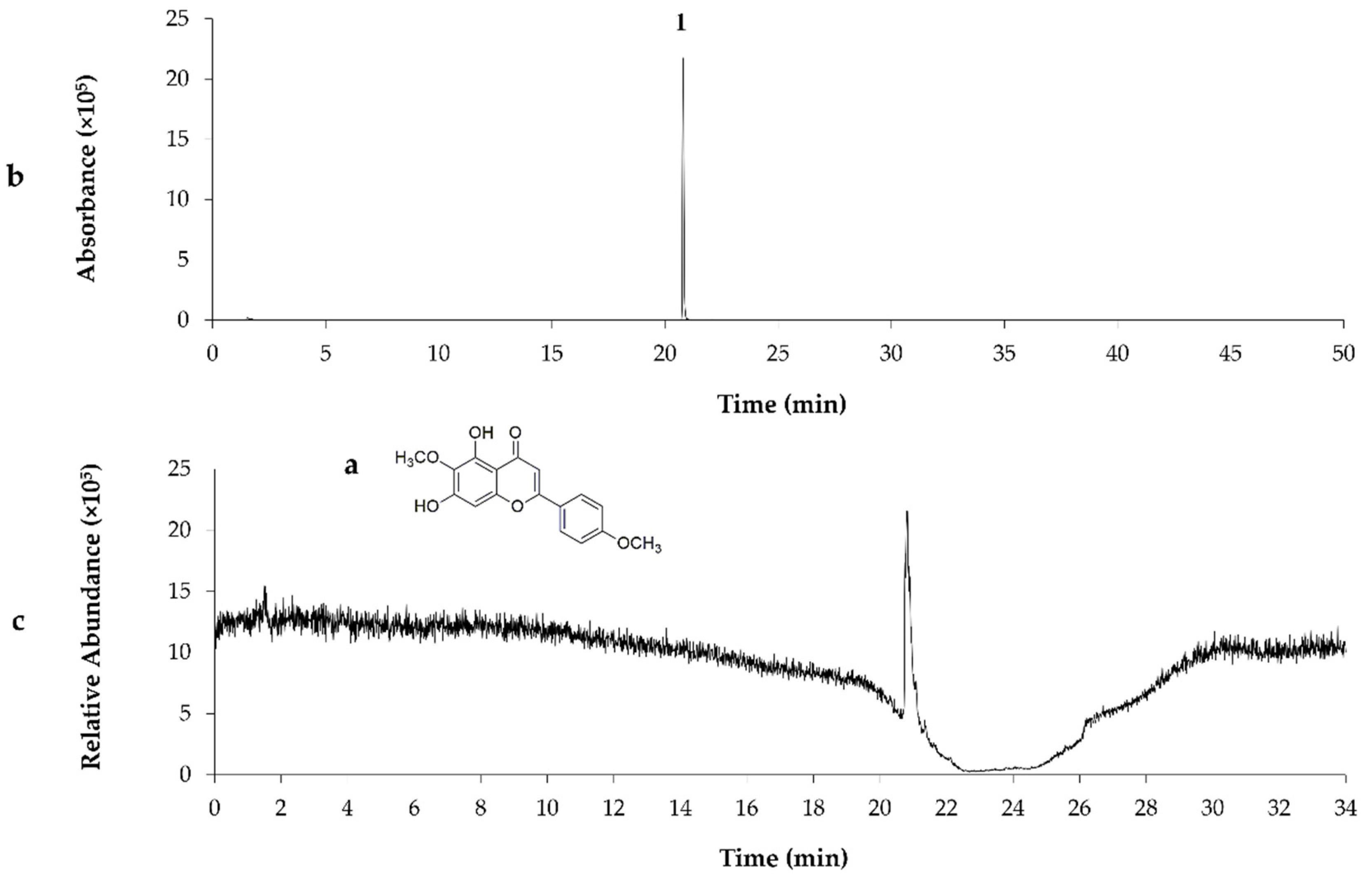
| P1 | 1 | 20.8 | 275, 330 | 313 | - | Pectolinarigenin |
| P2 | 1 | 11.2 | 309 | 163 | 119, 145, 108 | p-Coumaric acid |
| 2 | 17.9 | 281, 334 | 299 | - | unknown | |
| 3 | 19.1 | 291 | 271 | 253, 225 | Pinobanksin | |
| 4 | 20.1 | 259, 368 | 315 | 300 | Isorhamnetin | |
| 5 | 20.2 | 254, 368 | 329 | 314 | Quercetin-dimethyl ether | |
| 6 | 20.8 | 268, 329 | 283 | 269 | Acacetin | |
| P3 | 1 | 20.2 | 253, 368 | 329 | 314 | Quercetin-dimethyl ether |
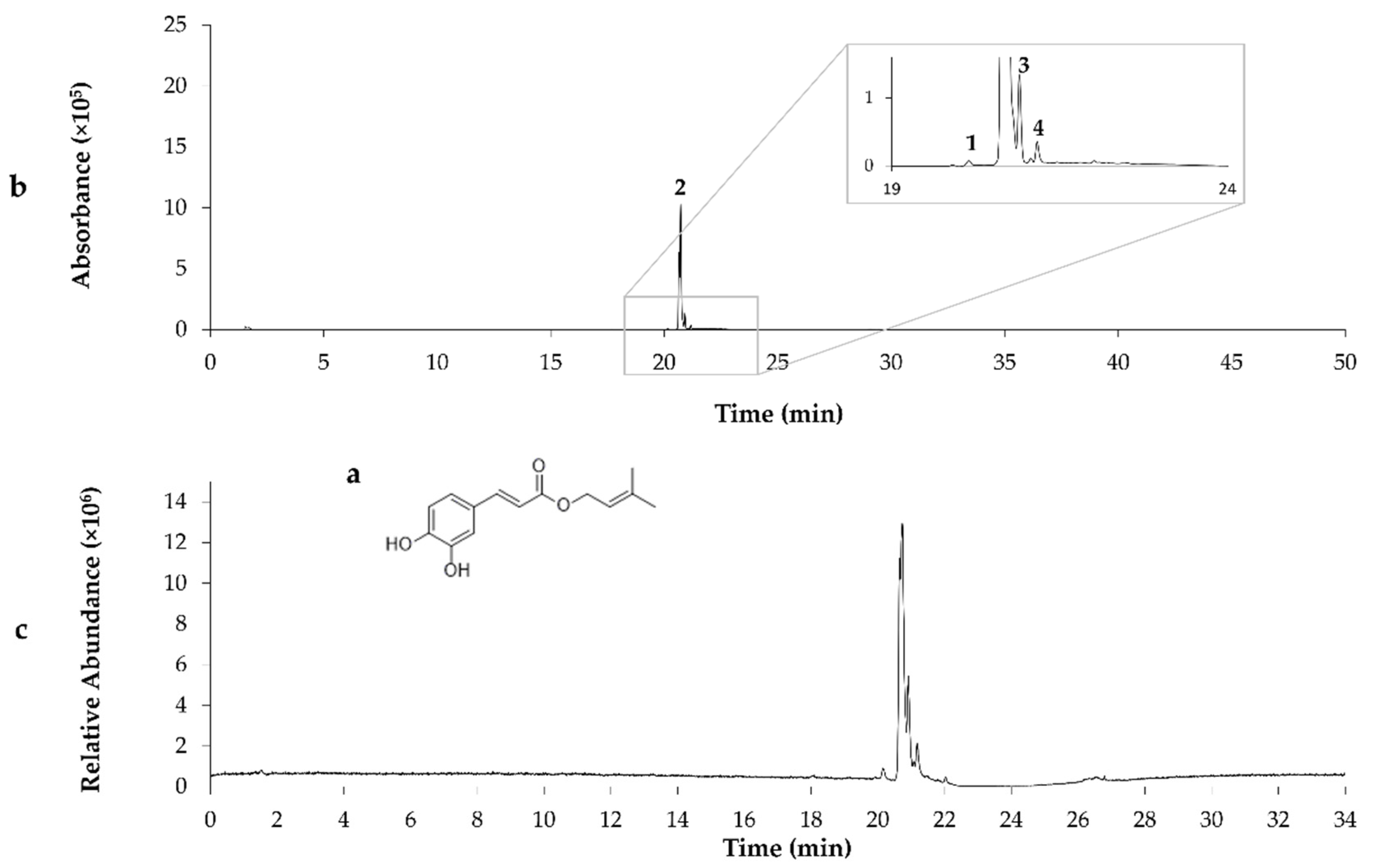
| 2 | 20.7 | 298, 325 | 247 | 179, 135 | Caffeic acid isoprenyl ester | |
| 3 | 20.9 | 298, 325 | 283 | 179, 135 | Caffeic acid phenylethyl ester | |
| 4 | 21.2 | 295, 325 | 295 | 178, 134, 251, 211 | Caffeic acid cinnamyl ester |
| Subfractions | IC50 ± SD (µg mL−1/ µM) * | SI α (vs. HK2) | |||
|---|---|---|---|---|---|
| 786-O | A498 | HK2 (Non-Neoplastic) | 786-O | A498 | |
| P1 (pectolinarigenin) | 3.8 ± 0.2/ 12.1 ± 0.6 e | 11.8 ± 0.04/ 37.8 ± 0.1 d | 13.2 ± 0.09/ 42.2 ± 0.3 c,d | 2.5 | 0.1 |
| P2 | >30 | >16 | 29.7 ± 0.3 a | <0 | <0.9 |
| P3 | 3.1 ± 0.01 e | 11.4 ± 0.1 d | 24.9 ± 0.4 b | 7.1 | 1.2 |
| Mixtures | IC50 ± SD (µg mL−1) * | SI α (vs. HK2) | |
|---|---|---|---|
| 786-O | HK2 (Non-Neoplastic) | 786-O | |
| P1 + P2 + P3 | 8.6 ± 0.06 c | 20.0 ± 0.08 a | 1.3 |
| P1 + P3 | 2.5 ± 0.03 d | 15.7 ± 0.11 b | 5.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Freitas, A.S.; Costa, M.; Pontes, O.; Seidel, V.; Proença, F.; Cardoso, S.M.; Oliveira, R.; Baltazar, F.; Almeida-Aguiar, C. Selective Cytotoxicity of Portuguese Propolis Ethyl Acetate Fraction towards Renal Cancer Cells. Molecules 2022, 27, 4001. https://doi.org/10.3390/molecules27134001
Freitas AS, Costa M, Pontes O, Seidel V, Proença F, Cardoso SM, Oliveira R, Baltazar F, Almeida-Aguiar C. Selective Cytotoxicity of Portuguese Propolis Ethyl Acetate Fraction towards Renal Cancer Cells. Molecules. 2022; 27(13):4001. https://doi.org/10.3390/molecules27134001
Chicago/Turabian StyleFreitas, Ana Sofia, Marta Costa, Olívia Pontes, Veronique Seidel, Fernanda Proença, Susana M. Cardoso, Rui Oliveira, Fátima Baltazar, and Cristina Almeida-Aguiar. 2022. "Selective Cytotoxicity of Portuguese Propolis Ethyl Acetate Fraction towards Renal Cancer Cells" Molecules 27, no. 13: 4001. https://doi.org/10.3390/molecules27134001
APA StyleFreitas, A. S., Costa, M., Pontes, O., Seidel, V., Proença, F., Cardoso, S. M., Oliveira, R., Baltazar, F., & Almeida-Aguiar, C. (2022). Selective Cytotoxicity of Portuguese Propolis Ethyl Acetate Fraction towards Renal Cancer Cells. Molecules, 27(13), 4001. https://doi.org/10.3390/molecules27134001









