Volatilomic Signatures of AGS and SNU-1 Gastric Cancer Cell Lines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Validation Parameters
2.2. Cell Cultures
2.3. VOCs Signatures of AGS, SNU-1 and GES-1
2.3.1. Volatiles Produced and Metabolized by AGS, SNU-1 and GES-1 Cell Lines
2.3.2. Comparison of Volatilomic Signatures of AGS, SNU-1 and GES-1 Cell Lines
3. Materials and Methods
3.1. Chemicals and Standards
3.2. Cells’ Cultivation
3.3. HS-NTE Sampling Protocol
3.4. GC-MS Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Beauchamp, J.; Davies, C.; Pleil, J.E. Breathborne Biomarkers and the Human Volatilome; Elsevier: Amsterdam, The Netherlands, 2020. [Google Scholar]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and classification of 17 diseases from 1404 subjects via pattern analysis of exhaled molecules. Acs Nano 2017, 11, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldini, C.; Billeci, L.; Sansone, F.; Conte, R.; Domenici, C.; Tonacci, A. Electronic nose as a novel method for diagnosing cancer: A systematic review. Biosensors 2020, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.C.; Balakrishnan, M.; Graham, D.Y. Gastric cancer worldwide except japan. In Gastric Cancer: With Special Focus on Studies from Japan; Shiotani, A., Ed.; Springer: Singapore, 2019; pp. 17–28. [Google Scholar]
- Haddad, G.; Schouwenburg, S.; Altesha, A.; Xu, W.; Liu, G. Using breath analysis as a screening tool to detect gastric cancer: A systematic review. J. Clin. Oncol. 2021, 39, 16013. [Google Scholar] [CrossRef]
- Xu, Z.Q.; Broza, Y.Y.; Ionsecu, R.; Tisch, U.; Ding, L.; Liu, H.; Song, Q.; Pan, Y.Y.; Xiong, F.X.; Gu, K.S.; et al. A nanomaterial-based breath test for distinguishing gastric cancer from benign gastric conditions. Br. J. Cancer 2013, 108, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Broza, Y.Y.; Khatib, S.; Gharra, A.; Krilaviciute, A.; Amal, H.; Polaka, I.; Parshutin, S.; Kikuste, I.; Gasenko, E.; Skapars, R.; et al. Screening for gastric cancer using exhaled breath samples. Br. J. Surg. 2019, 106, 1122–1125. [Google Scholar] [CrossRef]
- Leja, M.; Kortelainen, J.M.; Polaka, I.; Turppa, E.; Mitrovics, J.; Padilla, M.; Mochalski, P.; Shuster, G.; Pohle, R.; Kashanin, D.; et al. Sensing gastric cancer via point-of-care sensor breath analyzer. Cancer 2021, 127, 1286–1292. [Google Scholar] [CrossRef]
- Huber, W. Basic calculations about the limit of detection and its optimal determination. Accred Qual. Assur. 2003, 8, 213–217. [Google Scholar] [CrossRef]
- Chen, M.L.; Carmella, S.G.; Li, Y.P.; Zhao, Y.C.; Hecht, S.S. Resolution and quantitation of mercapturic acids derived from crotonaldehyde, methacrolein, and methyl vinyl ketone in the urine of smokers and nonsmokers. Chem. Res. Toxicol. 2020, 33, 669–677. [Google Scholar] [CrossRef]
- Peterson, L.A. Reactive metabolites in the biotransformation of molecules containing a furan ring. Chem. Res. Toxicol. 2013, 26, 6–25. [Google Scholar] [CrossRef] [Green Version]
- Dekant, W.; Bernauer, U.; Rosner, E.; Amberg, A. Toxicokinetics of ethers used as fuel oxygenates. Toxicol. Lett. 2001, 124, 37–45. [Google Scholar] [CrossRef]
- Kleber, A.; Maurer, F.; Lorenz, D.; Wolf, B.; Albrecht, F.; Shopova, T.; Sessler, D.I.; Volk, T.; Kreuer, S.; Fink, T. Metabolism of 3-pentanone under inflammatory conditions. J. Breath Res. 2016, 10, 47101. [Google Scholar] [CrossRef] [PubMed]
- Filipiak, W.; Mochalski, P.; Filipiak, A.; Ager, C.; Cumeras, R.; Davis, C.E.; Agapiou, A.; Unterkofler, K.; Troppmair, J. A compendium of volatile organic compounds (VOCs) released by human cell lines. Curr. Med. Chem. 2016, 23, 2112–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crabb, D.W.; Matsumoto, M.; Chang, D.; You, M. Overview of the role of alcohol dehydrogenase and aldehyde dehydrogenase and their variants in the genesis of alcohol-related pathology. Proc. Nutr. Soc. 2004, 63, 49–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klyosov, A.A. Kinetics and specificity of human liver aldehyde dehydrogenases toward aliphatic, aromatic, and fused polycyclic aldehydes. Biochemistry 1996, 35, 4457–4467. [Google Scholar] [CrossRef]
- Leiherer, A.; Slefarska, D.; Leja, M.; Heinzle, C.; Mundlein, A.; Kikuste, I.; Mezmale, L.; Drexel, H.; Mayhew, C.A.; Mochalski, P. The volatilomic footprints of human HGC-27 and CLS-145 gastric cancer cell lines. Front. Mol. Biosci. 2021, 7, 607904. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, T.E.; Hanneman, W.H. Physiologically-based pharmacokinetic analysis of benzoic acid in rats, guinea pigs and humans: Implications for dietary exposures and interspecies uncertainty. Comput. Toxicol. 2017, 3, 19–32. [Google Scholar] [CrossRef]
- Ditlow, C.C.; Holmquist, B.; Morelock, M.M.; Vallee, B.L. Physical and enzymatic properties of a class II alcohol dehydrogenase isozyme of human liver: Pi-ADH. Biochemistry 1984, 23, 6363–6368. [Google Scholar] [CrossRef]
- Edwards, J.E.; Rose, R.L.; Hodgson, E. The metabolism of nonane, a JP-8 jet fuel component, by human liver microsomes, P450 isoforms and alcohol dehydrogenase and inhibition of human P450 isoforms by JP-8. Chem.-Biol. Interact. 2005, 151, 203–211. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. Hydrocarbon hydroxylation by cytochrome P450 enzymes. Chem. Rev. 2010, 110, 932–948. [Google Scholar] [CrossRef] [Green Version]
- Walker, R.; Flanagan, R.J.; Lennard, M.S.; Mills, G.A.; Walker, V. Solid-phase microextraction: Investigation of the metabolism of substances that may be abused by inhalation. J. Chromatogr. Sci. 2006, 44, 387–393. [Google Scholar] [CrossRef] [Green Version]
- Walker, V.; Mills, G.A. Urine 4-heptanone: A beta-oxidation product of 2-ethylhexanoic acid from plasticisers. Clin. Chim. Acta 2001, 306, 51–61. [Google Scholar] [CrossRef]
- Walker, V.; Mills, G.A. 2-Pentanone production from hexanoic acid by penicillium roqueforti from Blue Cheese: Is this the pathway used in humans? Sci. World J. 2014, 2014, 215783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mochalski, P.; Sponring, A.; King, J.; Unterkofler, K.; Troppmair, J.; Amann, A. Release and uptake of volatile organic compounds by human hepatocellular carcinoma cells (HepG2) in vitro. Cancer Cell Int. 2013, 13, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipiak, W.; Sponring, A.; Filipiak, A.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. TD-GC-MS analysis of volatile metabolites of human lung cancer and normal cells in vitro. Cancer Epidemiol. Biomark. Prev. 2010, 19, 182–195. [Google Scholar] [CrossRef] [Green Version]
- Mochalski, P.; Diem, E.; Unterkofler, K.; Mundlein, A.; Drexel, H.; Mayhew, C.A.; Leiherer, A. In vitro profiling of volatile organic compounds released by Simpson-Golabi-Behmel syndrome adipocytes. J. Chromatogr. B 2019, 1104, 256–261. [Google Scholar] [CrossRef]
- Zimmermann, D.; Hartmann, M.; Moyer, M.P.; Nolte, J.; Baumbach, J.I. Determination of volatile products of human colon cell line metabolism by GC/MS analysis. Metabolomics 2007, 3, 13–17. [Google Scholar] [CrossRef]
- Rodrigues, D.; Pinto, J.; Araujo, A.M.; Monteiro-Reis, S.; Jeronimo, C.; Henrique, R.; Bastos, M.D.; de Pinho, P.G.; Carvalho, M. Volatile metabolomic signature of bladder cancer cell lines based on gas chromatography-mass spectrometry. Metabolomics 2018, 14, 62. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Y.; Wang, D.; Yu, K.; Wang, L.; Zou, Y.; Zhao, C.; Zhang, X.; Wang, P.; Ying, K. The analysis of volatile organic compounds biomarkers for lung cancer in exhaled breath, tissues and cell lines. Cancer Biomark. 2012, 11, 129–137. [Google Scholar] [CrossRef]
- Yu, J.; Wang, D.; Wang, L.; Wang, P.; Hu, Y.J.; Ying, K.J. Detection of lung cancer with volatile organic biomarkers in exhaled breath and lung cancer cells. Aip. Conf. Proc. 2009, 1137, 198–201. [Google Scholar]
- Lima, A.R.; Araujo, A.M.; Pinto, J.; Jeronimo, C.; Henrique, R.; Bastos, M.D.; Carvalho, M.; de Pinho, P.G. Discrimination between the human prostate normal and cancer cell exometabolome by GC-MS. Sci. Rep. 2018, 8, 5539. [Google Scholar] [CrossRef] [Green Version]
- Mochalski, P.; Leja, M.; Gasenko, E.; Skapars, R.; Santare, D.; Sivins, A.; Aronsson, D.E.; Ager, C.; Jaeschke, C.; Shani, G.; et al. Ex vivo emission of volatile organic compounds from gastric cancer and non-cancerous tissue. J. Breath Res. 2018, 12, 46005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schallschmidt, K.; Becker, R.; Jung, C.; Rolff, J.; Fichtner, I.; Nehls, I. Investigation of cell culture volatilomes using solid phase micro extraction: Options and pitfalls exemplified with adenocarcinoma cell lines. J. Chromatogr. B 2015, 1006, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomas, H.; Camara, J.S. Volatile metabolomic signature of human breast cancer cell lines. Sci. Rep. 2017, 7, 43969. [Google Scholar] [CrossRef] [Green Version]
- Frommer, U.; Ullrich, V.; Stauding, H. Hydroxylation of aliphatic compounds by liver microsomes. 1. Distribution pattern of isomeric alcohols. H-S Z Physiol. Chem. 1970, 351, 903–912. [Google Scholar] [CrossRef] [PubMed]
- Wahl, H.G.; Hong, Q.; Hildenbrand, S.; Risler, T.; Luft, D.; Liebich, H. 4-Heptanone is a metabolite of the plasticizer di(2-ethylhexyl) phthalate (DEHP) in haemodialysis patients. Nephrol. Dial. Transplant. 2004, 19, 2576–2583. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Hong, P.; Tanabe, R.; Nagai, K.; Kato, K. Enzymatic hydrolysis of structurally diverse phthalic acid esters by porcine and bovine pancreatic cholesterol esterases. Chemosphere 2010, 81, 1544–1548. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, H.; Sugihara, K.; Watanabe, Y.; Moriguchi, K.; Uramaru, N.; Sone, T.; Ohta, S.; Kitamura, S. Comparative study of hydrolytic metabolism of dimethyl phthalate, dibutyl phthalate and di(2-ethylhexyl) phthalate by microsomes of various rat tissues. Food Chem. Toxicol. 2017, 100, 217–224. [Google Scholar] [CrossRef]
- Sponring, A.; Filipiak, W.; Mikoviny, T.; Ager, C.; Schubert, J.; Miekisch, W.; Amann, A.; Troppmair, J. Release of volatile organic compounds from the lung cancer cell line NCI-H2087 in vitro. Anticancer Res. 2009, 29, 419–426. [Google Scholar]
- Kwak, J.; Gallagher, M.; Ozdener, M.H.; Wysocki, C.J.; Goldsmith, B.R.; Isamah, A.; Faranda, A.; Fakharzadeh, S.S.; Herlyn, M.; Johnson, A.T.C.; et al. Volatile biomarkers from human melanoma cells. J. Chromatogr. B 2013, 931, 90–96. [Google Scholar] [CrossRef]
- Schallschmidt, K.; Becker, R.; Zwaka, H.; Menzel, R.; Johnen, D.; Fischer-Tenhagen, C.; Rolff, J.; Nehls, I. In vitro cultured lung cancer cells are not suitable for animal-based breath biomarker detection. J. Breath Res. 2015, 9, 27103. [Google Scholar] [CrossRef] [Green Version]
- Furuhashi, T.; Ishii, R.; Onishi, H.; Ota, S. Elucidation of biochemical pathways underlying VOCs production in A549 cells. Front. Mol. Biosci. 2020, 7, 116. [Google Scholar] [CrossRef]
- Mochalski, P.; Theurl, M.; Sponring, A.; Unterkofler, K.; Kirchmair, R.; Amann, A. Analysis of volatile organic compounds liberated and metabolised by human umbilical vein endothelial cells (HUVEC) in vitro. Cell Biochem. Biophys. 2015, 71, 323–329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aghdassi, E.; Allard, J.P. Breath alkanes as a marker of oxidative stress in different clinical conditions. Free Radic. Biol. Med. 2000, 28, 880–886. [Google Scholar] [CrossRef]
- Kneepkens, C.M.; Lepage, G.; Roy, C.C. The potential of the hydrocarbon breath test as a measure of lipid peroxidation. Free Radic. Biol. Med. 1994, 17, 127–160. [Google Scholar] [CrossRef]
- Wu, D.; Mou, Y.P.; Chen, K.; Cai, J.Q.; Zhou, Y.C.; Pan, Y.; Xu, X.W.; Zhou, W.; Gao, J.Q.; Chen, D.W.; et al. Aldehyde dehydrogenase 3A1 is robustly upregulated in gastric cancer stem-like cells and associated with tumorigenesis. Int. J. Oncol. 2016, 49, 611–622. [Google Scholar] [CrossRef] [Green Version]
- Nishikawa, S.; Konno, M.; Hamabe, A.; Hasegawa, S.; Kano, Y.; Ohta, K.; Fukusumi, T.; Sakai, D.; Kudo, T.; Haraguchi, N.; et al. Aldehyde dehydrogenase(high) gastric cancer stem cells are resistant to chemotherapy. Int. J. Oncol. 2013, 42, 1437–1442. [Google Scholar] [CrossRef] [Green Version]
- Jelski, W.; Szmitkowski, M. Alcohol dehydrogenase (ADH) and aldehyde dehydrogenase (ALDH) in the cancer diseases. Clin. Chim. Acta 2008, 395, 1–5. [Google Scholar] [CrossRef]
- Jelski, W.; Chrostek, L.; Zalewski, B.; Szmitkowski, M. Alcohol dehydrogenase (ADH) isoenzymes and aldehyde dehydrogenase (ALDH) activity in the sera of patients with gastric cancer. Digest. Dis. Sci. 2008, 53, 2101–2105. [Google Scholar] [CrossRef]
- Zhang, Y.X.; Gao, G.; Liu, H.J.; Fu, H.L.; Fan, J.; Wang, K.; Chen, Y.S.; Li, B.J.; Zhang, C.L.; Zhi, X.; et al. Identification of volatile biomarkers of gastric cancer cells and ultrasensitive electrochemical detection based on sensing interface of Au-Ag alloy coated MWCNTs. Theranostics 2014, 4, 154–162. [Google Scholar] [CrossRef] [Green Version]
- Skierucha, M.; Milne, A.N.A.; Offerhaus, G.J.A.; Polkowski, W.P.; Maciejewski, R.; Sitarz, R. Molecular alterations in gastric cancer with special reference to the early-onset subtype. World J. Gastroenterol. 2016, 22, 2460–2474. [Google Scholar] [CrossRef] [Green Version]
- Bairoch, A. The Cellosaurus, a Cell-Line Knowledge Resource. J. Biomol. Tech. JBT 2018, 29, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Mitsopoulos, C.; Di Micco, P.; Fernandez, E.V.; Dolciami, D.; Holt, E.; Mica, I.L.; Coker, E.A.; Tym, J.E.; Campbell, J.; Che, K.H.; et al. canSAR: Update to the cancer translational research and drug discovery knowledgebase. Nucleic Acids Res. 2021, 49, D1074–D1082. [Google Scholar] [CrossRef] [PubMed]
- Harada, K.; Baba, Y.; Shigaki, H.; Ishimoto, T.; Miyake, K.; Kosumi, K.; Tokunaga, R.; Izumi, D.; Ohuchi, M.; Nakamura, K.; et al. Prognostic and clinical impact of PIK3CA mutation in gastric cancer: Pyrosequencing technology and literature review. BMC Cancer 2016, 16, 400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Li, Z.; Ding, Y.; Xu, Y.; Zhu, X.; Cao, N.; Huang, C.; Qin, M.; Liu, F.; Zhao, A. TP53 mutation and MET amplification in circulating tumor DNA analysis predict disease progression in patients with advanced gastric cancer. PeerJ 2021, 9, e11146. [Google Scholar] [CrossRef]
- Ozaki, T.; Nakagawara, A. p53: The attractive tumor suppressor in the cancer research field. J. Biomed. Biotechnol. 2011, 2011, 603925. [Google Scholar] [CrossRef] [PubMed]
- Bellini, M.F.; Cadamuro, A.C.; Succi, M.; Proenca, M.A.; Silva, A.E. Alterations of the TP53 gene in gastric and esophageal carcinogenesis. J. Biomed. Biotechnol. 2012, 2012, 891961. [Google Scholar] [CrossRef] [Green Version]
- Hewitt, L.C.; Saito, Y.; Wang, T.; Matsuda, Y.; Oosting, J.; Silva, A.N.S.; Slaney, H.L.; Melotte, V.; Hutchins, G.; Tan, P.; et al. KRAS status is related to histological phenotype in gastric cancer: Results from a large multicentre study. Gastric Cancer 2019, 22, 1193–1203. [Google Scholar] [CrossRef] [Green Version]
- Chiurillo, M.A. Role of the Wnt/beta-catenin pathway in gastric cancer: An in-depth literature review. World J. Exp. Med. 2015, 5, 84–102. [Google Scholar] [CrossRef]
- Mochalski, P.; Agapiou, A.; Statheropoulos, M.; Amann, A. Permeation profiles of potential urine-borne biomarkers of human presence over brick and concrete. Analyst 2012, 137, 3278–3285. [Google Scholar] [CrossRef]
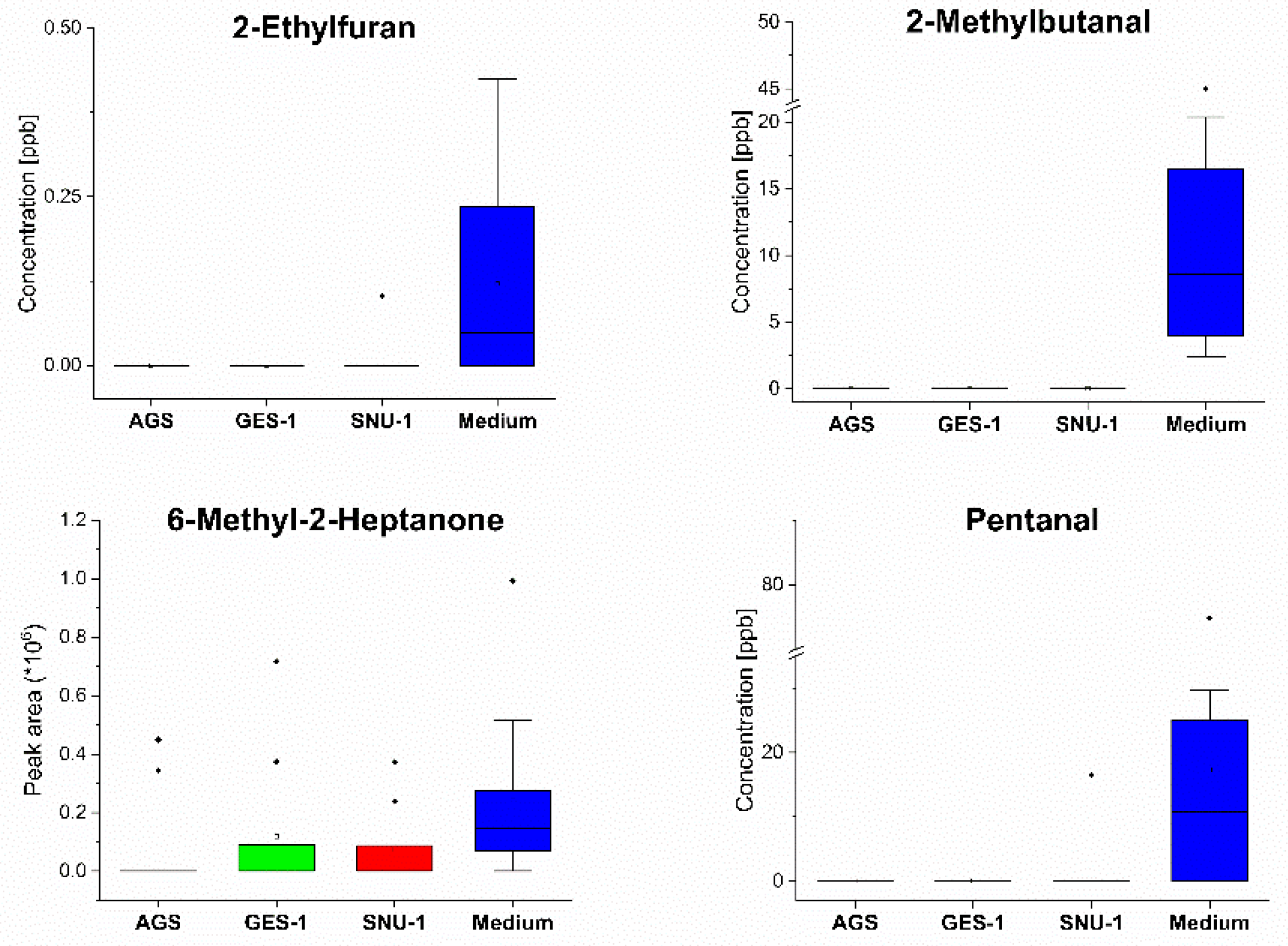
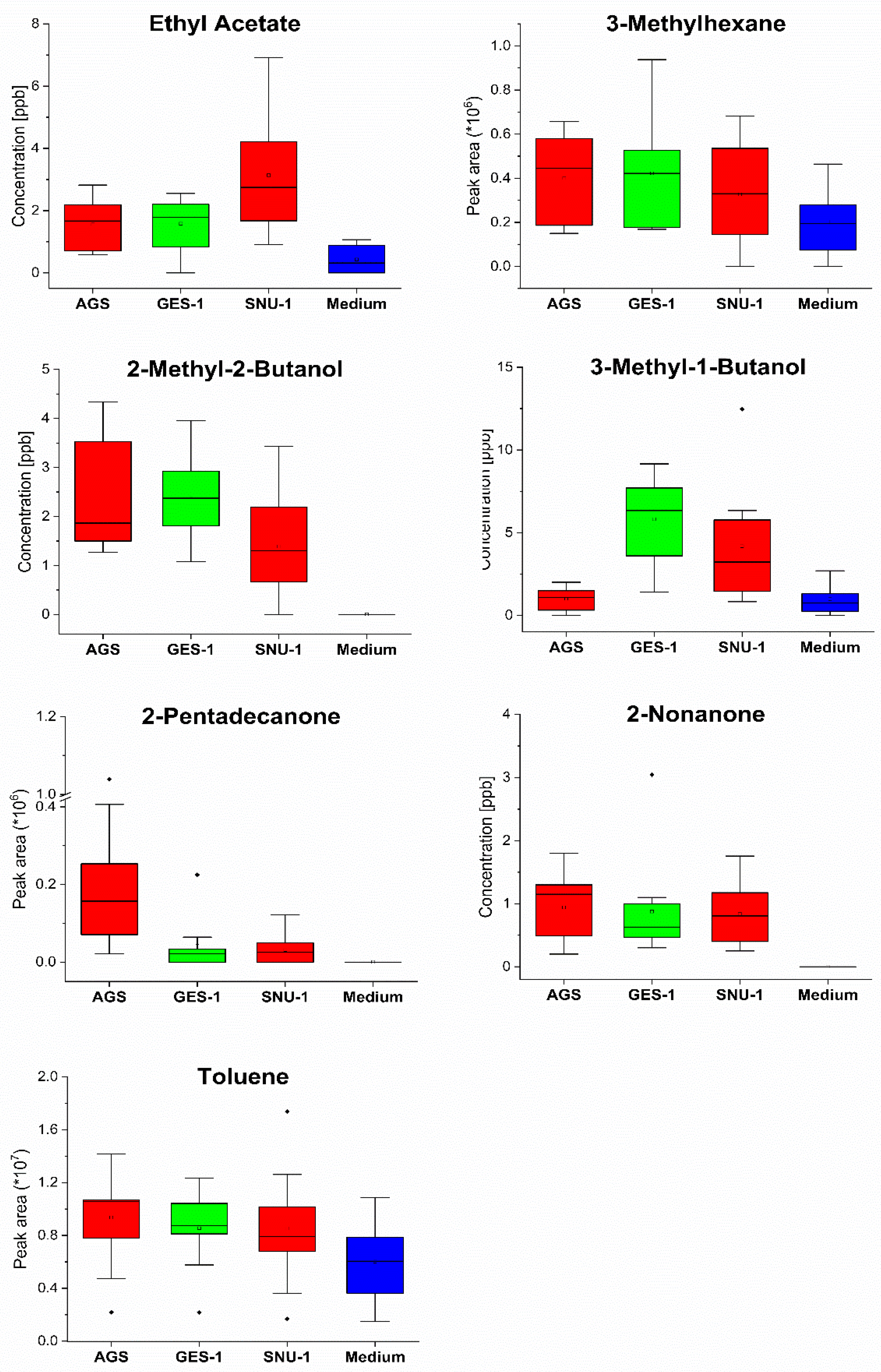
| VOC | CAS | Rt (min) | Quantifier Ion | LOD (ppb) | RSD (%) | R2 | A–D p-Value | Linear Range (ppb) |
|---|---|---|---|---|---|---|---|---|
| 2-Propanol, 2-methyl- | 75-65-0 | 4.17 | 59 | 0.04 | 9.0 | 0.995 | 0.25 | 0.13–20 |
| Propanal, 2-methyl- | 78-84-2 | 4.67 | 72 | 0.04 | 9.0 | 0.981 | 0.12 | 0.13–16 |
| 2-Propenal, 2-methyl- | 78-85-3 | 5.01 | 70 | 0.06 | 10 | 0.990 | 0.27 | 0.18–30 |
| 1-Propanol | 71-23-8 | 5.52 | 59 | 0.09 | 10 | 0.993 | 0.11 | 0.31–37 |
| Propane, 2-ethoxy-2-methyl- | 637-92-3 | 6.13 | 59 | 0.05 | 13 | 0.990 | 0.27 | 0.16–19 |
| 2-Butanone | 78-93-3 | 6.50 | 72 | 0.22 | 10 | 0.989 | 0.27 | 0.73–60 |
| Ethyl acetate | 141-78-6 | 6.71 | 43 | 0.04 | 7.0 | 0.983 | 0.14 | 0.12–13 |
| 1-Propanol, 2-methyl- | 78-83-1 | 9.40 | 43 | 0.13 | 13 | 0.997 | 0.14 | 0.46–20 |
| 2-Butanol, 2-methyl- | 75-85-4 | 9.80 | 59 | 0.09 | 13 | 0.994 | 0.27 | 0.3–19 |
| Butanal, 3-methyl- | 590-86-3 | 9.97 | 58 | 0.06 | 9.0 | 0.981 | 0.27 | 0.17–50 |
| Butanal, 2-methyl- | 96-17-3 | 10.57 | 57 | 0.02 | 9.0 | 0.990 | 0.28 | 0.12–30 |
| Furan, 2-ethyl- | 3208-16-0 | 12.10 | 81 | 0.04 | 10 | 0.990 | 0.22 | 0.12–6 |
| 2-Pentanone | 107-87-9 | 13.30 | 43 | 0.08 | 12 | 0.995 | 0.13 | 0.26–28 |
| n-Pentanal | 110-62-3 | 13.80 | 58 | 2 | 10 | 0.996 | 0.55 | 6–30 |
| 3-Pentanone | 96-22-0 | 14.03 | 57 | 0.03 | 13 | 0.990 | 0.15 | 0.09–20 |
| Ethyl propanoate | 105-37-3 | 14.20 | 57 | 0.04 | 11 | 0.979 | 0.34 | 0.12–13 |
| 2-Pentanone, 4-methyl- | 108-10-1 | 17.30 | 43 | 0.07 | 12 | 0.987 | 0.07 | 0.2–17 |
| 1-Butanol, 3-methyl- | 123-51-3 | 18.20 | 55 | 0.07 | 12 | 0.994 | 0.16 | 0.23–17 |
| Ethyl 2-methylbutyrate | 7452-79-1 | 22.54 | 102 | 0.03 | 11 | 0.982 | 0.36 | 0.09–21 |
| 2-Heptanone | 105-42-0 | 25.17 | 58 | 0.04 | 13 | 0.988 | 0.11 | 0.12–11 |
| Cyclohexanol | 108-93-0 | 25.40 | 57 | 0.02 | 7.0 | 0.990 | 0.39 | 0.07–24 |
| 2-Pentylfuran | 3777-69-3 | 28.19 | 81 | 0.02 | 7.0 | 0.989 | 0.43 | 0.08–3 |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 30.73 | 57 | 0.04 | 7.0 | 0.998 | 0.23 | 0.12–60 |
| 2-Nonanone | 821-55-6 | 32.72 | 58 | 0.03 | 14 | 0.994 | 0.19 | 0.08–9.5 |
| n-Tetradecane | 629-59-4 | 39.20 | 57 | 0.06 | 14 | 0.980 | 0.64 | 0.2–19 |
| 2-Tridecanone | 593-08-8 | 41.60 | 58 | 0.13 | 15 | 0.988 | 0.16 | 0.45–9.5 |
| Line | Total Number of Cells (×106) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | C | D | E | F | G | H | I | J | |
| AGS | 13.5 | 47.5 | 40.4 | 33.3 | 43.1 | 30.1 | 19.8 | 39.1 | 23.8 | 48.7 |
| SNU-1 | 14.7 | 27.2 | 27.7 | 49.5 | 43.0 | 42.0 | 36.5 | 66.5 | 27.3 | 39.2 |
| GES-1 | 20.8 | 37.4 | 19.3 | 16.5 | 38.1 | 22.5 | 30.6 | 48.7 | 35.5 | 45.8 |
| VOC | CAS | AGS | SNU-1 | GES-1 | Medium | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Incidence nd (nq) | Range (Median) (ppb) | Incidence nd (nq) | Range (Median) (ppb) | Incidence nd (nq) | Range (Median) (ppb) | Incidence nd (nq) | Range (Median) (ppb) | |||
| Uptake | Propanal, 2-methyl | 78-84-2 | 0 | - | 1/1 | 0.21 | 1/1 | 0.4 | 8/8 | 0.32–18 (8.7) |
| 2-Propenal, 2-methyl- | 78-85-3 | 0 | - | 0 | - | 0 | - | 8/8 | 0.4–30 (2.4) | |
| Butanal, 3-methyl- | 590-86-3 | 0 | - | 8 | 2.2–10 (2.3) | 1 | 2.3 | 10/10 | 14–110 (70) | |
| Butanal, 2-methyl- | 96-17-3 | 0 | - | 0 | - | 0 | - | 10/10 | 2.4–45 (8.6) | |
| Furan, 2-ethyl- | 3208-16-0 | 0 | - | 1/0 | - | 0 | - | 5/5 | 0.12–0.43 (0.24) | |
| Pentanal | 110-62-3 | 0 | - | 1/1 | 6.5 | 0 | - | 6/6 | 6.2–75 (10.9) | |
| Hexanal | 66-25-1 | 0 | - | 0 | - | 0 | - | 6 | - | |
| 2-Heptanone, 6-methyl- | 928-68-7 | 2 | - | 4 | - | 3 | - | 9 | - | |
| 2-Pentylfuran | 3777-69-3 | 0 | - | 3 | 0.37–1.1 (0.51) | 2/2 | 0.3–1.3 | 7 | 0.43–1.4 (0.74) | |
| Benzoic acid, 2-ethylhexyl ester | 5444-75-7 | 4 | - | 0 | - | 1 | - | 7 | - | |
| Release | n-Pentane | 109-66-0 | 10 | - | 10 | - | 10 | - | 10 | - |
| 2-Propanol, 2-methyl- | 75-65-0 | 10/10 | 1.5–25 (3.21) | 10/10 | 0.3–15 (2.4) | 10/10 | 1.4–24 (2.26) | 9/9 | 0.36–9.0 (0.76) | |
| 1-Propanol | 71-23-8 | 7/7 | 3–258 (7.0) | 8/8 | 3.0–151 (6.0) | 8/8 | 2.5–228 (4.7) | 6/6 | 1.6–167 (4.5) | |
| Propane, 2-ethoxy-2-methyl- | 637-92-3 | 9/9 | 2.4–38 (11) | 10/10 | 2.5–40 (5.8) | 9/9 | 2.2–43 (16.7) | 7/7 | 2.5–22 (4.2) | |
| 2-Butanone | 78-93-3 | 10 | 69–193 (120) | 10 | 37–170 (99) | 10 | 68–194 (127) | 10 | 40–158 (82) | |
| Ethyl acetate | 141-78-6 | 10/10 | 0.6–2.8 (1.7) | 10/10 | 0.9–6.9 (2.7) | 9/9 | 0.75–2.6 (1.9) | 6/6 | 0.3–0.8 (1.1) | |
| Oxetane, 2,2-dimethyl- | 6245-99-4 | 7 | - | 3 | - | 7 | - | 0 | - | |
| Hexane, 3-methyl- | 589-34-4 | 10 | - | 9 | - | 10 | - | 8 | - | |
| Benzene | 71-43-2 | 10 | - | 10 | - | 10 | - | 9 | - | |
| 1-Propanol, 2-methyl- | 78-83-1 | 10/10 | 4.7–15 (6.9) | 9/9 | 1.4–12 (4.9) | 10/10 | 3.8–14 (7.14) | 9/9 | 1.4–4.3 (2.6) | |
| 2-Butanol, 2-methyl- | 75-85-4 | 10/10 | 1.3–4.3 (1.9) | 9/9 | 0.34–3.4 (1.3) | 10/10 | 1.1–4.0 (2.4) | 0 | - | |
| 2-Pentanone | 107-87-9 | 10/10 | 1.9–12 (4.2) | 10/10 | 0.9–9.0 (3.3) | 10/10 | 2–12 (3.6) | 10/10 | 0.5–5.8 (1.8) | |
| Ethyl propanoate | 105-37-3 | 8/6 | 0.3–0.5 (0.36) | 10/9 | 0.18–1.9 (1.0) | 0 | - | 2/0 | - | |
| 3-Pentanone | 96-22-0 | 9/9 | 0.4–0.8 (0.6) | 9/9 | 0.25–0.74 (0.5) | 10/10 | 0.3–0.7 (0.5) | 8/8 | 0.2–0.6 (0.47) | |
| Toluene | 108-88-3 | 10 | - | 10 | - | 10 | - | 10 | - | |
| 3-Pentanone, 2-methyl- | 565-69-5 | 6 | - | 0 | - | 4 | - | 0 | - | |
| 1-Butanol, 3-methyl- | 123-51-3 | 9/9 | 0.22–2 (1.2) | 10/10 | 0.8–12 (3.2) | 10/10 | 1.4–9 (6.34) | 8/8 | 0.25–2.7 (1.0) | |
| Ethane, 1,1-diethoxy- | 105-57-7 | 9 | - | 8 | - | 8 | - | 7 | - | |
| 2-Pentanone, 4-methyl- | 108-10-1 | 9/9 | 0.3–6.4 (0.92) | 9/8 | 0.2–9.4 (0.85) | 10/10 | 0.2–7.8 (0.68) | 9/8 | 0.2–5.3 (0.75) | |
| Ethyl 2-methylbutyrate | 7452-79-1 | 8/6 | 0.15–0.68 (0.33) | 5/2 | 0.04-0.4 | 8/6 | 0.1–0.3 (0.21) | 0 | - | |
| Styrene | 100-42-5 | 10 | - | 10 | - | 10 | - | 10 | - | |
| 2-Heptanone | 105-42-0 | 10/8 | 0.48–0.66 (0.57) | 10/8 | 0.16–1.1 (0.56) | 10/8 | 0.3–1.1 (0.5) | 7/6 | 0.14–0.45 (0.2) | |
| n-Nonane | 111-84-2 | 10 | - | 10 | - | 10 | - | 9 | - | |
| Cyclohexanol | 108-93-0 | 10/10 | 11–37 (20) | 10/10 | 9–32 (16) | 10/10 | 11–36 (18) | 10/10 | 8–34 (16) | |
| Cyclohexanone | 108-94-1 | 10 | - | 10 | - | 10 | - | 9 | - | |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 10/10 | 6–67 (39) | 10/10 | 11–102 (44) | 10/10 | 28–122 (47) | 10/10 | 7–32 (15.7) | |
| 2-Nonanone | 821-55-6 | 10/10 | 0.2–1.8 (1.2) | 10/10 | 0.25–1.8 (0.8) | 10/10 | 0.3–3 (0.6) | 0 | - | |
| n-Dodecane | 112-40-3 | 10 | - | 10 | - | 10 | - | 10 | - | |
| 2-Undecanone | 112-12-9 | 5 | - | 0 | - | 6 | - | 0 | - | |
| n-Tetradecane | 629-59-4 | 10/10 | 9–49 (20.5) | 10/10 | 13–46 (27.6) | 10/10 | 5–39 (17.7) | 10/10 | 5.5–13 (13) | |
| 2-Tridecanone | 593-08-8 | 7/7 | 3.2–8.6 (3.7) | 3/3 | 2.1–5.5 (2.8) | 3/3 | 2.0–11 (2.7) | 0 | - | |
| n-Hexadecane | 544-76-3 | 4 | - | 8 | - | 4 | - | 3 | - | |
| 2-Pentadecanone | 2345-28-0 | 10 | - | 6 | - | 7 | - | 0 | - | |
| 2-Heptadecanone | 2922-51-2 | 8 | - | 0 | - | 0 | - | 0 | - | |
| 1-Hexadecanol, 2-methyl- | 2490-48-4 | 8 | - | 2 | - | 2 | - | 1 | - | |
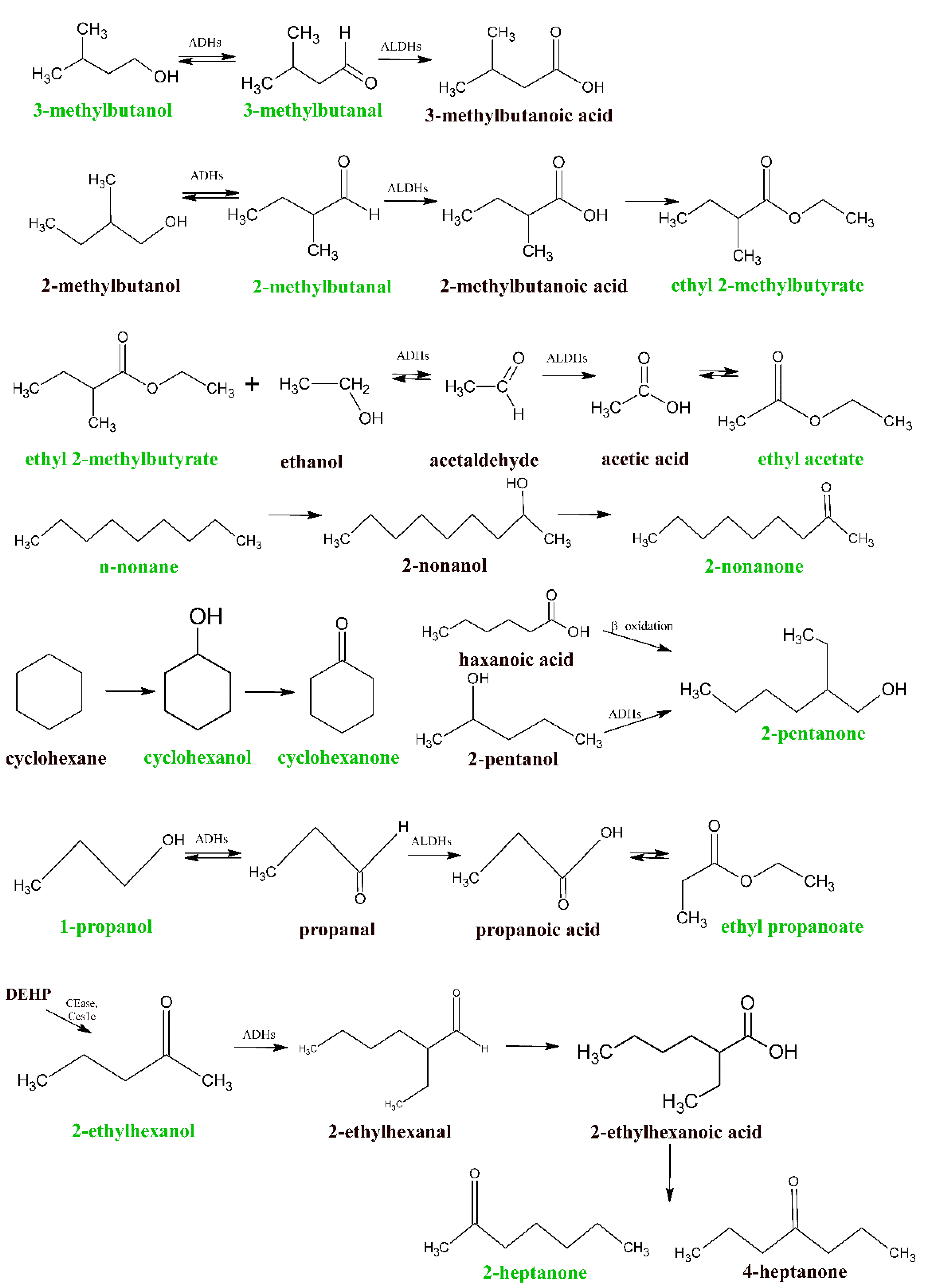
| VOC | CAS | AGS | SNU-1 | GES-1 | Tentative Metabolic Pathways | ||
|---|---|---|---|---|---|---|---|
| p-Value | p-Value | p-Value | Tentative Product(s) | Enzyme/Other | |||
| Uptake | Propanal, 2-methyl | 78-84-2 | 7.1 × 10−3 | 6.4 × 10−3 | 7.1 × 10−3 | I. 2-Methylpropanol, II. 2-Methyl propanic acid | I. ADHs II. ALDHs |
| 2-Propenal, 2-methyl- | 78-85-3 | 7.1 × 10−3 | 7.1 × 10−3 | 7.1 × 10−3 | 3-Hydroxy-2-methylpropyl mercapturic acid [10] | γ-glutamyl transpeptidase, cysteinyl lycinase, N-acetyl transferase, reductasesa | |
| Butanal, 3-methyl- | 590-86-3 | 9.8 × 10−4 | 9.8 × 10−4 | 9.8 × 10−4 | I.3-Methylbutanol, II. 3-Methyl butanoic acid | I. ADHs II. ALDHs | |
| Butanal, 2-methyl- | 96-17-3 | 9.8 × 10−4 | 9.8 × 10−4 | 9.8 × 10−4 | I. 2-Methylbutanol, II. 2-Methyl butanoic acid | I. ADHs II. ALDHs | |
| Furan, 2-ethyl- | 3208-16-0 | 0.03 | 0.05 | 0.03 | association with microsomal proteins and/or DNA [11] | Cytochrome P450 (2E1) | |
| Pentanal | 110-62-3 | 0.02 | 0.04 | 0.02 | I. 1-Pentanol II. Pentanoic acid | I. ADHs II. ALDHs | |
| Hexanal | 66-25-1 | 0.03 | 0.03 | 0.03 | I. 1-Hexanol, II. Hexanoic acid | I. ADHs II. ALDHs | |
| 2-Heptanone, 6-methyl- | 928-68-7 | 0.02 | 0.05 | n.s. | 6-Methyl-2-heptanol | ADHs | |
| 2-Pentylfuran | 3777-69-3 | 0.01 | n.s. | 0.05 | association with microsomal proteins and/or DNA [11] | Cytochrome P450 (2E1) | |
| Benzoic acid, 2-ethylhexyl ester | 5444-75-7 | 0.02 | 0.01 | 0.01 | Benzoic acid and 2-ethyl-1-hexanol | Carboxylesterases | |
| n-Pentane | 109-66-0 | 0.01 | 0.01 | 0.01 | lipids | Oxidative stress, in vivo lipid peroxidation | |
| 2-Propanol, 2-methyl- | 75-65-0 | 9.8 × 10−4 | 0.01 | 0.03 | I. 2-Methoxy-2-methylpropane/ 2-Ethoxy-2-methyl-propane [12] II. 2 Methylpropane | I. monoxygenase e.g., cytochrome P-450 2A6 II. hydroxylation catalyzed by cytochrome p450 isoforms (1A2, 2B6, and 2E1) | |
| 1-Propanol | 71-23-8 | 0.01 | n.s. | 0.01 | Propanal | ADHs | |
| Propane, 2-ethoxy-2-methyl- | 637-92-3 | 0.03 | n.s. | 0.01 | unknown | unknown | |
| 2-Butanone | 78-93-3 | 9.8 × 10−4 | n.s. | 9.8 × 10−4 | I. 2-Butanol II. fatty acids | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| Ethyl acetate | 141-78-6 | 9.8 × 10−4 | 9.8 × 10−4 | 4.6 × 10−3 | Ethanol + acetic acid | esterification | |
| Oxetane, 2,2-dimethyl- | 6245-99-4 | 0.01 | - | 0.01 | unknown | unknown | |
| Hexane, 3-methyl- | 589-34-4 | 0.01 | n.s. | 1.9 × 10−3 | |||
| Benzene | 71-43-2 | 1.9 × 10−3 | 9.8 × 10−4 | 2.9 × 10−3 | unknown | unknown | |
| 1-Propanol, 2-methyl- | 78-83-1 | 9.8 × 10−4 | 6.4 × 10−3 | 9.8 × 10−4 | I. 2-Methyl-propanal | ADHs | |
| 2-Butanol, 2-methyl- | 75-85-4 | 9.8 × 10−4 | 4.6 × 10−3 | 9.8 × 10−4 | I. Tert-amyl methyl ether [12] II. 2-Methylbutane | I. monoxygenase e.g., cytochrome P-450 II. hydroxylation catalyzed by cytochrome p450 isoforms (1A2, 2B6, and 2E1) | |
| 2-Pentanone | 107-87-9 | 9.8 × 10−4 | 4.9 × 10−3 | 9.8 × 10−4 | I. 2-Pentanol II. fatty acids: hexanoic acid | I, ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| Ethyl propanoate | 105-37-3 | 7.8 × 10−4 | 2.0 × 10−3 | n.s. | Ethanol +propanoic acid | esterification | |
| 3-Pentanone | 96-22-0 | 6.4 × 10−3 | 0.03 | 2.9 × 10−3 | 2-Methyl- 3-ketovaleric acid [13] | propionyl-CoA/methylmalonyl-CoA | |
| Toluene | 108-88-3 | 4.8 × 10−3 | 4.8 × 10−3 | 6.8 × 10−3 | unknown | unknown | |
| 3-Pentanone, 2-methyl- | 565-69-5 | 0.02 | n.s. | n.s. | 2-Methyl-3-pentanol | ADHs and/or cytochrome p450 CYP2E1 | |
| 1-Butanol, 3-methyl- | 123-51-3 | n.s. | 1.9 × 10−3 | 1.9 × 10−3 | 3-Methylbutanal | ADHs | |
| Ethane, 1,1-diethoxy- | 105-57-7 | 4.6 × 10−3 | n.s. | 0.02 | unknown | unknown | |
| 2-Pentanone, 4-methyl- | 108-10-1 | 0.03 | n.s. | 0.02 | 4-Methyl2-pentanol | ADHs and/or cytochrome p450 CYP2E1 | |
| Ethyl 2-methylbutyrate | 7452-79-1 | 7.8 × 10−3 | n.s. | 7.8 × 10−3 | Ethanol + 2-methylbutanoic acid | esterification | |
| Styrene | 100-42-5 | 0.01 | 4.9 × 10−3 | 9.8 × 10−4 | unknown | ||
| 2-Heptanone | 105-42-0 | 1.9 × 10−3 | 1.9 × 10−3 | 1.9 × 10−3 | I. 2-Heptanol II. fatty acids: 2-ethylhexanoic acid | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| n-Nonane | 111-84-2 | 0.03 | 0.04 | 0.02 | unknown | ||
| Cyclohexanol | 108-93-0 | 0.02 | n.s. | 0.03 | Cyclohexane (medium) | Hydroxylation by cytochrome P-450 | |
| Cyclohexanone | 108-94-1 | 0.03 | n.s. | 0.03 | Cyclohexanol and cyclohexane (medium) | ADHs | |
| 1-Hexanol, 2-ethyl- | 104-76-7 | 2.9 × 10−3 | 9.8 × 10−4 | 1.9 × 10−3 | I. Di(2-ethylhexyl)phtalate II. 2-Ethyl-hexanal III. 2-Ethylhexyl ester benzoic acid | I. CEase, Ces1e II. ADHs III. cholesterol esterase (CEase), and/or carboxylesterase Ces1e | |
| 2-Nonanone | 821-55-6 | 1.9 × 10−3 | 1.9 × 10−3 | 1.9 × 10−3 | I. 2-Nonanol and n-nonane (medium) II. fatty acids | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| n-Dodecane | 112-40-3 | 4.8 × 10−3 | n.s. | n.s. | unknown | ||
| 2-Undecanone | 112-12-9 | 0.03 | 0.02 | n.s. | I. 2-Undecanol II. fatty acids | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| n-Tetradecane | 629-59-4 | 0.01 | 9.8 × 10−4 | n.s. | unknown | ||
| 2-Tridecanone | 593-08-8 | 0.01 | n.s. | n.s. | I. 2-Tridecanol II. fatty acids | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| n-Hexadecane | 544-76-3 | n.s. | 7.1 × 10−3 | n.s. | unknown | ||
| 2-Pentadecanone | 2345-28-0 | 9.8 × 10−4 | 0.02 | 0.01 | I. 2-Pentadecanol II. fatty acids | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| 2-Heptadecanone | 2922-51-2 | 7.1 × 10−3 | n.s. | n.s. | I. 2-Heptadecanol II. fatty acids | I. ADHs and/or cytochrome p450 CYP2E1 II. β-oxidation | |
| 1-Hexadecanol, 2-methyl- | 2490-48-4 | 0.04 | n.s. | n.s. | unknown | ||
| VOC | CAS | AGS vs. GES-1 p-Value | SNU-1 vs. GES-1 p-Value | AGS vs. SNU-1 p-Value | |
|---|---|---|---|---|---|
| Release | n-Pentane | 109-66-0 | n.s. | n.s. | n.s. |
| 2-Propanol, 2-methyl- | 75-65-0 | n.s. | n.s. | ↑9.8 × 10−4 | |
| 1-Propanol | 71-23-8 | n.s. | n.s. | n.s. | |
| Propane, 2-ethoxy-2-methyl- | 637-92-3 | n.s. | n.s. | n.s. | |
| 2-Butanone | 78-93-3 | n.s. | ↓9.8 × 10−4 | ↑9.8 × 10−4 | |
| Ethyl acetate | 141-78-6 | n.s. | ↑9.8 × 10−4 | ↓9.8 × 10−4 | |
| Oxetane, 2,2-dimethyl- | 6245-99-4 | n.s. | n.s. | n.s. | |
| Hexane, 3-methyl- | 589-34-4 | n.s. | ↓2.9 × 10−3 | ↑0.04 | |
| Benzene | 71-43-2 | n.s. | ↑4.8 × 10−3 | ||
| 1-Propanol, 2-methyl- | 78-83-1 | n.s. | ↓1.9 × 10−3 | ↑4.8 × 10−3 | |
| 2-Butanol, 2-methyl- | 75-85-4 | n.s. | ↓ 9.8 × 10−4 | ↑1.9 × 10−3 | |
| 2-Pentanone | 107-87-9 | n.s. | ↓6.8 × 10−3 | ↑0.03 | |
| Ethyl propanoate | 105-37-3 | ↑3.9 × 10−3 | ↑9.8 × 10−4 | ↓6.8 × 10−3 | |
| 3-Pentanone | 96-22-0 | ↑0.04 | n.s. | ↑0.04 | |
| Toluene | 108-88-3 | n.s. | n.s. | n.s. | |
| 3-Pentanone, 2-methyl- | 565-69-5 | n.s. | n.s. | ↑0.02 | |
| 1-Butanol, 3-methyl- | 123-51-3 | ↓9.8 × 10−4 | ↓0.03 | ↓4.8 × 10−3 | |
| Ethane, 1,1-diethoxy- | 105-57-7 | n.s. | ↓7.8 × 10−3 | ↑1.9 × 10−3 | |
| 2-Pentanone, 4-methyl- | 108-10-1 | ↓0.02 | ↓0.02 | n.s. | |
| Ethyl 2-methylbutyrate | 7452-79-1 | n.s. | n.s. | n.s. | |
| Styrene | 100-42-5 | n.s. | n.s. | n.s. | |
| 2-Heptanone | 105-42-0 | n.s. | n.s. | n.s. | |
| n-Nonane | 111-84-2 | n.s. | n.s. | n.s. | |
| Cyclohexanol | 108-93-0 | n.s. | ↓9.8 × 10−4 | ↑2.9 × 10−3 | |
| Cyclohexanone | 108-94-1 | n.s. | ↓9.8 × 10−4 | ↑9.8 × 10−4 | |
| 1-Hexanol, 2-ethyl- | 104-76-7 | ↓9.7 × 10−3 | n.s. | n.s. | |
| 2-Nonanone | 821-55-6 | n.s. | n.s. | n.s. | |
| n-Dodecane | 112-40-3 | ↓6.8 × 10−3 | n.s. | ↓0.01 | |
| 2-Undecanone | 112-12-9 | n.s. | n.s. | n.s. | |
| n-Tetradecane | 629-59-4 | n.s. | n.s. | n.s. | |
| 2-Tridecanone | 593-08-8 | ↑0.05 | n.s. | ↑0.02 | |
| n-Hexadecane | 544-76-3 | n.s. | ↑7.8 × 10−3 | ↓2.7 × 10−3 | |
| 2-Pentadecanone | 2345-28-0 | ↑9.8 × 10−4 | n.s. | ↑9.8 × 10−4 | |
| 2-Heptadecanone | 2922-51-2 | ↑3.9 × 10−3 | n.s. | ↑3.9 × 10−3 | |
| 1-Hexadecanol, 2-methyl- | 2490-48-4 | n.s. | n.s. | n.s. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ślefarska-Wolak, D.; Heinzle, C.; Leiherer, A.; Ager, C.; Muendlein, A.; Mezmale, L.; Leja, M.; Corvalan, A.H.; Drexel, H.; Królicka, A.; et al. Volatilomic Signatures of AGS and SNU-1 Gastric Cancer Cell Lines. Molecules 2022, 27, 4012. https://doi.org/10.3390/molecules27134012
Ślefarska-Wolak D, Heinzle C, Leiherer A, Ager C, Muendlein A, Mezmale L, Leja M, Corvalan AH, Drexel H, Królicka A, et al. Volatilomic Signatures of AGS and SNU-1 Gastric Cancer Cell Lines. Molecules. 2022; 27(13):4012. https://doi.org/10.3390/molecules27134012
Chicago/Turabian StyleŚlefarska-Wolak, Daria, Christine Heinzle, Andreas Leiherer, Clemens Ager, Axel Muendlein, Linda Mezmale, Marcis Leja, Alejandro H. Corvalan, Heinz Drexel, Agnieszka Królicka, and et al. 2022. "Volatilomic Signatures of AGS and SNU-1 Gastric Cancer Cell Lines" Molecules 27, no. 13: 4012. https://doi.org/10.3390/molecules27134012
APA StyleŚlefarska-Wolak, D., Heinzle, C., Leiherer, A., Ager, C., Muendlein, A., Mezmale, L., Leja, M., Corvalan, A. H., Drexel, H., Królicka, A., Shani, G., Mayhew, C. A., Haick, H., & Mochalski, P. (2022). Volatilomic Signatures of AGS and SNU-1 Gastric Cancer Cell Lines. Molecules, 27(13), 4012. https://doi.org/10.3390/molecules27134012







