Synthesis and Characterization of Catechol-Containing Polyacrylamides with Adhesive Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis and Characterization of Monomers
2.2. Synthesis and Characterization of Polymers
2.2.1. H-NMR Analysis
2.2.2. Molecular Weight
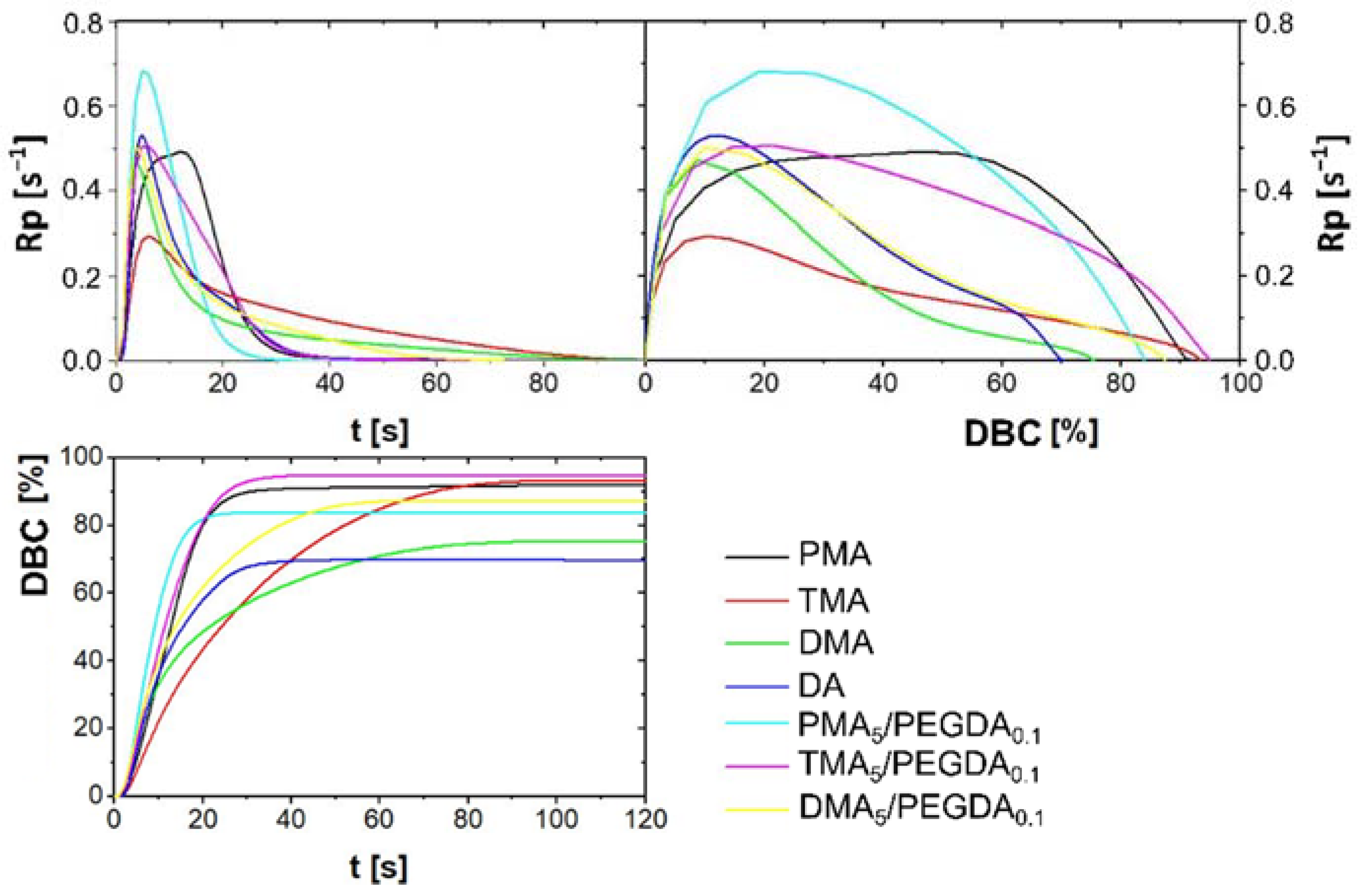
2.2.3. Photo-DSC of Monomers Formulation
2.2.4. Tensile Shear Test
3. Materials and Methods
3.1. Materials
3.2. Characterization
3.2.1. Chromatography
3.2.2. Gel Permeation Chromatography (GPC)
3.2.3. NMR Spectroscopy
3.2.4. Dynamic Differential Calorimetry (DSC)
3.2.5. Adhesion Test
3.3. Synthesis of Monomers
3.3.1. Synthesis for N-(3,4-Dihydroxyphenyethyl) Acrylamide (DMA)
3.3.2. Synthesis for N-(3,4-Dihydroxyphenyethyl) Acrylamide (DA)
3.3.3. Synthesis for N-Phenethyl Methacrylamide (PMA)
3.3.4. Synthesis for N-(4-Hydroxyphenethyl) Methacrylamide (TMA)
3.4. General Procedure for the Synthesis of Polymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Abbreviations
| DA | N-(3,4-dihydroxyphenyethyl) acrylamide |
| DMA | N-(3,4-dihydroxyphenyethyl) meth acrylamide |
| HEAA | N-(2-hydroxyethyl) acrylamide |
| PC | polycarbonate |
| PEGDA | poly(ethylene glycol) diacrylate |
| PMA | N-phenethyl methacrylamide |
| TMA | N-(4-hydroxyphenethyl) methacrylamide |
| XMA | DA, DMA, PMA and TMA |
References
- Young, G.A. Response to, and Selection between, Firm Substrata by Mytilus Edulis. J. Mar. Biol. Ass. 1983, 63, 653–659. [Google Scholar] [CrossRef]
- Waite, J.H. Mussel Adhesion—Essential Footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waite, J.H.; Marvin, L. Tanzer Polyphenolic Substance of Mytilus Edulis: Novel Adhesive Containing L-Dopa and Hydroxyproline. Sci. New Ser. 1981, 212, 1038–1040. [Google Scholar]
- Ye, Q.; Zhou, F.; Liu, W. Bioinspired Catecholic Chemistry for Surface Modification. Chem. Soc. Rev. 2011, 40, 4244. [Google Scholar] [CrossRef]
- Quan, W.-Y.; Hu, Z.; Liu, H.-Z.; Ouyang, Q.-Q.; Zhang, D.-Y.; Li, S.-D.; Li, P.-W.; Yang, Z.-M. Mussel-Inspired Catechol-Functionalized Hydrogels and Their Medical Applications. Molecules 2019, 24, 2586. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.J.; Oh, D.X.; Kim, S.; Seo, J.H.; Hwang, D.S.; Masic, A.; Han, D.K.; Cha, H.J. Mussel-Mimetic Protein-Based Adhesive Hydrogel. Biomacromolecules 2014, 15, 1579–1585. [Google Scholar] [CrossRef]
- Olofsson, K.; Granskog, V.; Cai, Y.; Hult, A.; Malkoch, M. Activated Dopamine Derivatives as Primers for Adhesive-Patch Fixation of Bone Fractures. RSC Adv. 2016, 6, 26398–26405. [Google Scholar] [CrossRef]
- Jo, S.; Sohn, J. Biomimetic Adhesive Materials Containing Cyanoacryl Group for Medical Application. Molecules 2014, 19, 16779–16793. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Li, L.; Wang, Z.; Wang, Y.; Guo, M.; Shi, X.; Yeh, J.-M.; Zhang, P. Mussel-Inspired Conducting Copolymer with Aniline Tetramer as Intelligent Biological Adhesive for Bone Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 634–646. [Google Scholar] [CrossRef]
- Li, K.; Sun, Y.; Tsoi, J.K.H.; Yiu, C.K.Y. The Application of Mussel-Inspired Molecule in Dentin Bonding. J. Dent. 2020, 99, 103404. [Google Scholar] [CrossRef]
- Park, M.; Ju, S.; Linstadt, R.; Ahn, J.; Ahn, K. Dental Adhesion Enhancement on Zirconia Inspired by Mussel’s Priming Strategy Using Catechol. Coatings 2018, 8, 298. [Google Scholar] [CrossRef] [Green Version]
- Han, Y.; Yang, J.; Zhao, W.; Wang, H.; Sun, Y.; Chen, Y.; Luo, J.; Deng, L.; Xu, X.; Cui, W.; et al. Biomimetic Injectable Hydrogel Microspheres with Enhanced Lubrication and Controllable Drug Release for the Treatment of Osteoarthritis. Bioact. Mater. 2021, 6, 3596–3607. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Huang, B.; Wang, Q.; Wu, W.; Coates, P.; Sefat, F.; Lu, C.; Zhang, W.; Zhang, X. A Mussel-Inspired Antibacterial Hydrogel with High Cell Affinity, Toughness, Self-Healing, and Recycling Properties for Wound Healing. ACS Sustain. Chem. Eng. 2021, 9, 3070–3082. [Google Scholar] [CrossRef]
- Li, J.; Ejima, H.; Yoshie, N. Seawater-Assisted Self-Healing of Catechol Polymers via Hydrogen Bonding and Coordination Interactions. ACS Appl. Mater. Interfaces 2016, 8, 19047–19053. [Google Scholar] [CrossRef] [PubMed]
- Faure, E.; Lecomte, P.; Lenoir, S.; Vreuls, C.; Van De Weerdt, C.; Archambeau, C.; Martial, J.; Jerome, C.; Duwez, A.-S.; Detrembleur, C. Sustainable and Bio-Inspired Chemistry for Robust Antibacterial Activity of Stainless Steel. J. Mater. Chem. 2011, 21, 7901–7904. [Google Scholar] [CrossRef]
- Yang, C.; Ding, X.; Ono, R.J.; Lee, H.; Hsu, L.Y.; Tong, Y.W.; Hedrick, J.; Yang, Y.Y. Brush-Like Polycarbonates Containing Dopamine, Cations, and PEG Providing a Broad-Spectrum, Antibacterial, and Antifouling Surface via One-Step Coating. Adv. Mater. 2014, 26, 7346–7351. [Google Scholar] [CrossRef]
- Asha, A.B.; Chen, Y.; Zhang, H.; Ghaemi, S.; Ishihara, K.; Liu, Y.; Narain, R. Rapid Mussel-Inspired Surface Zwitteration for Enhanced Antifouling and Antibacterial Properties. Langmuir 2019, 35, 1621–1630. [Google Scholar] [CrossRef]
- Stepuk, A.; Halter, J.G.; Schaetz, A.; Grass, R.N.; Stark, W.J. Mussel-Inspired Load Bearing Metal–Polymer Glues. Chem. Commun. 2012, 48, 6238. [Google Scholar] [CrossRef]
- Payra, D.; Naito, M.; Fujii, Y.; Yamada, N.L.; Hiromoto, S.; Singh, A. Bioinspired Adhesive Polymer Coatings for Efficient and Versatile Corrosion Resistance. RSC Adv. 2015, 5, 15977–15984. [Google Scholar] [CrossRef]
- Sanz, B.; von Bilderling, C.; Tuninetti, J.S.; Pietrasanta, L.; Mijangos, C.; Longo, G.S.; Azzaroni, O.; Giussi, J.M. Thermally-Induced Softening of PNIPAm-Based Nanopillar Arrays. Soft Matter 2017, 13, 2453–2464. [Google Scholar] [CrossRef]
- García-Peñas, A.; Biswas, C.S.; Liang, W.; Wang, Y.; Yang, P.; Stadler, F.J. Effect of Hydrophobic Interactions on Lower Critical Solution Temperature for Poly(N-Isopropylacrylamide-Co-Dopamine Methacrylamide) Copolymers. Polymers 2019, 11, 991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bratek-Skicki, A. Towards a New Class of Stimuli-Responsive Polymer-Based Materials—Recent Advances and Challenges. Appl. Surf. Sci. Adv. 2021, 4, 100068. [Google Scholar] [CrossRef]
- Flechner, M.; Schaller, J.; Stahl, M.; Achberger, K.; Gerike, S.; Hannappel, Y.; Fu, J.; Jaeger, M.; Hellweg, T.; Duschl, C.; et al. Adhesion, Proliferation, and Detachment of Various Cell Types on Thermoresponsive Microgel Coatings. Biotechnol. Bioeng. 2022, 119, 1728–1739. [Google Scholar] [CrossRef] [PubMed]
- Narumi, A.; Chen, Y.; Sone, M.; Fuchise, K.; Sakai, R.; Satoh, T.; Duan, Q.; Kawaguchi, S.; Kakuchi, T. Poly( N -Hydroxyethylacrylamide) Prepared by Atom Transfer Radical Polymerization as a Nonionic, Water-Soluble, and Hydrolysis-Resistant Polymer and/or Segment of Block Copolymer with a Well-Defined Molecular Weight. Macromol. Chem. Phys. 2009, 210, 349–358. [Google Scholar] [CrossRef]
- Zhao, C.; Zheng, J. Synthesis and Characterization of Poly( N -Hydroxyethylacrylamide) for Long-Term Antifouling Ability. Biomacromolecules 2011, 12, 4071–4079. [Google Scholar] [CrossRef]
- Fu, Y.; Yang, Y.; Xiao, S.; Zhang, L.; Huang, L.; Chen, F.; Fan, P.; Zhong, M.; Tan, J.; Yang, J. Mixed Polymer Brushes with Integrated Antibacterial and Antifouling Properties. Prog. Org. Coat. 2019, 130, 75–82. [Google Scholar] [CrossRef]
- Yabu, H.; Ohshima, H.; Saito, Y. Double-Phase-Functionalized Magnetic Janus Polymer Microparticles Containing TiO2 and Fe2O3 Nanoparticles Encapsulated in Mussel-Inspired Amphiphilic Polymers. ACS Appl. Mater. Interfaces 2014, 6, 18122–18128. [Google Scholar] [CrossRef]
- Zhang, C.; Li, K.; Simonsen, J. A Novel Wood-Binding Domain of a Wood–Plastic Coupling Agent: Development and Characterization. J. Appl. Polym. Sci. 2003, 89, 1078–1084. [Google Scholar] [CrossRef]
- Zhang, F.; Liu, S.; Zhang, Y.; Wei, Y.; Xu, J. Underwater Bonding Strength of Marine Mussel-Inspired Polymers Containing DOPA-like Units with Amino Groups. RSC Adv. 2012, 2, 8919. [Google Scholar] [CrossRef]
- Kurenkov, V.F.; Abramova, L.I. Homogeneous Polymerization of Acrylamide in Solutions. Polym.-Plast. Technol. Eng. 1992, 31, 659–704. [Google Scholar] [CrossRef]
- Kurenkov, V.F.; Myagchenkov, V.A. Effects of Reaction Medium on the Radical Polymerization and Copolymerization of Acrylamide. Eur. Polym. J. 1980, 16, 1229–1239. [Google Scholar] [CrossRef]
- Lartigue-Peyrou, F. The Use of Phenolic Compounds as Free-Radical Polymerization Inhibitors. In Industrial Chemistry Library; Elsevier: Amsterdam, The Netherlands, 1996; Volume 8, pp. 489–505. ISBN 978-0-444-82434-9. [Google Scholar]
- Chen, S.-A.; Tsai, L.-C. Kinetics and Mechanism of Inhibition of an Antioxidant Type Inhibitor in Free Radical Vinyl Copolymerizations. Makromol. Chem. 1986, 187, 653–666. [Google Scholar] [CrossRef]
- Kemmere, M.F.; Mayer, M.J.J.; Meuldijk, J.; Drinkenburg, A.A.H. The Influence of 4-Tert-Butylcatechol on the Emulsion Polymerization of Styrene. J. Appl. Polym. Sci. 1999, 71, 2419–2422. [Google Scholar] [CrossRef]
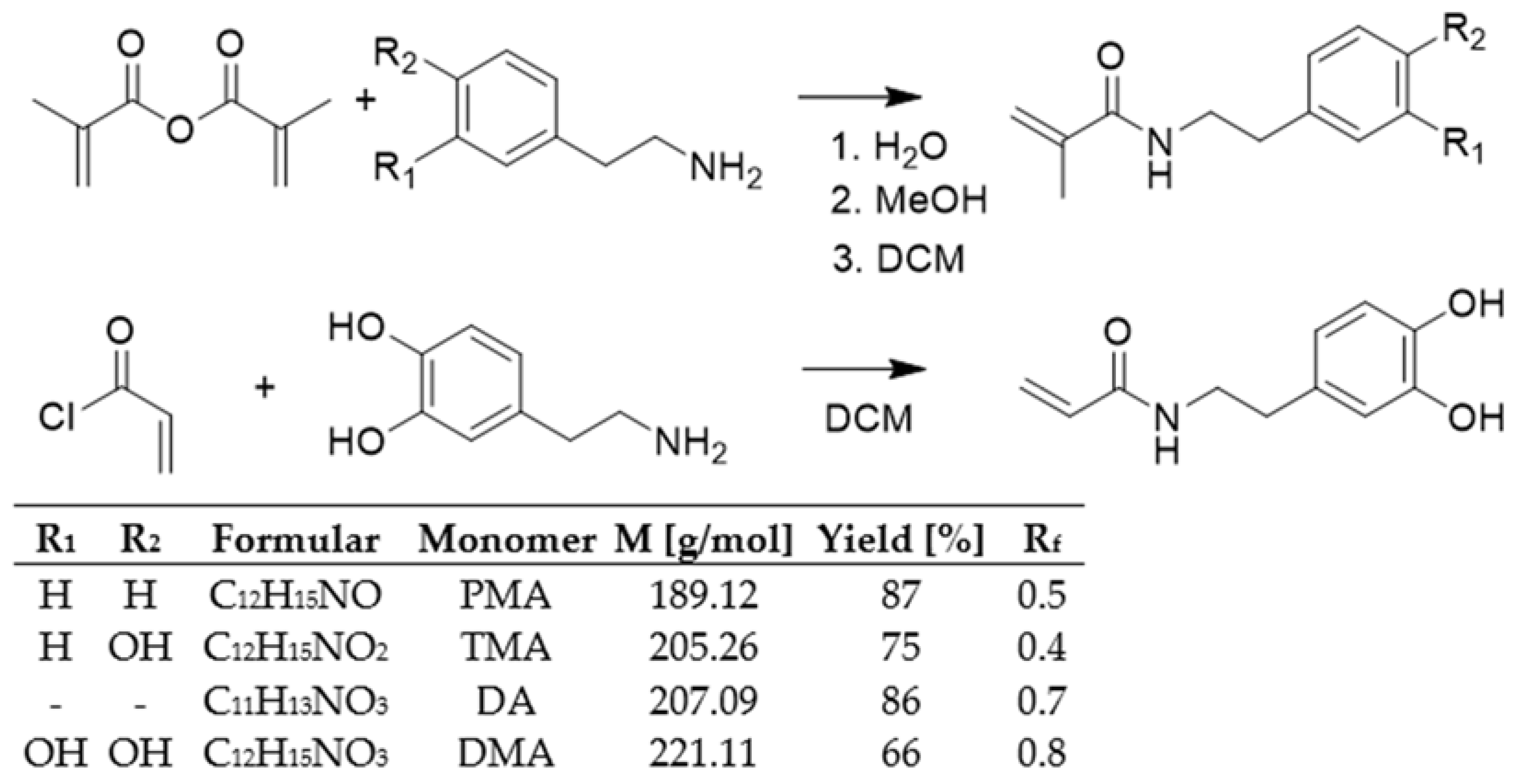

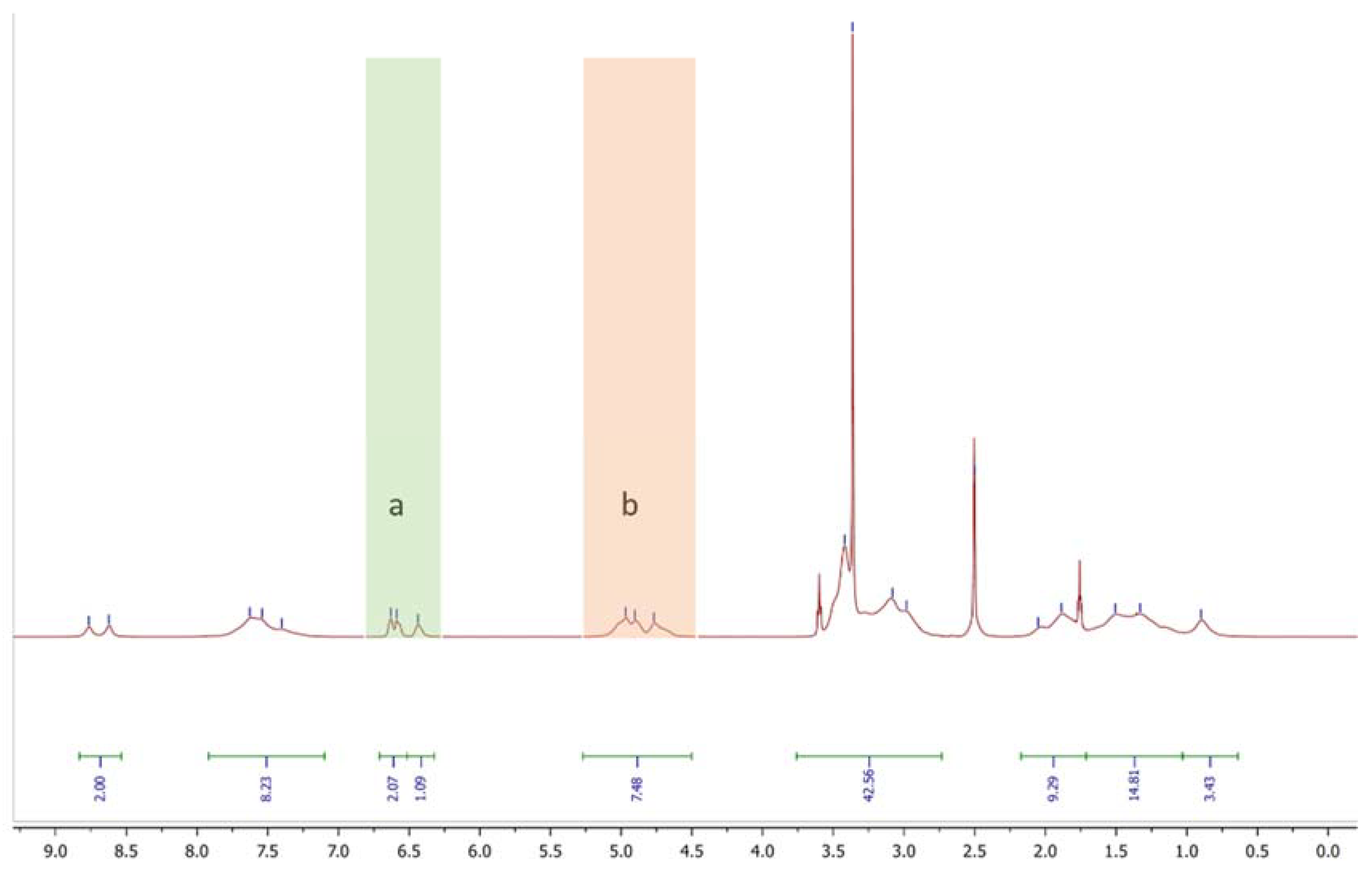
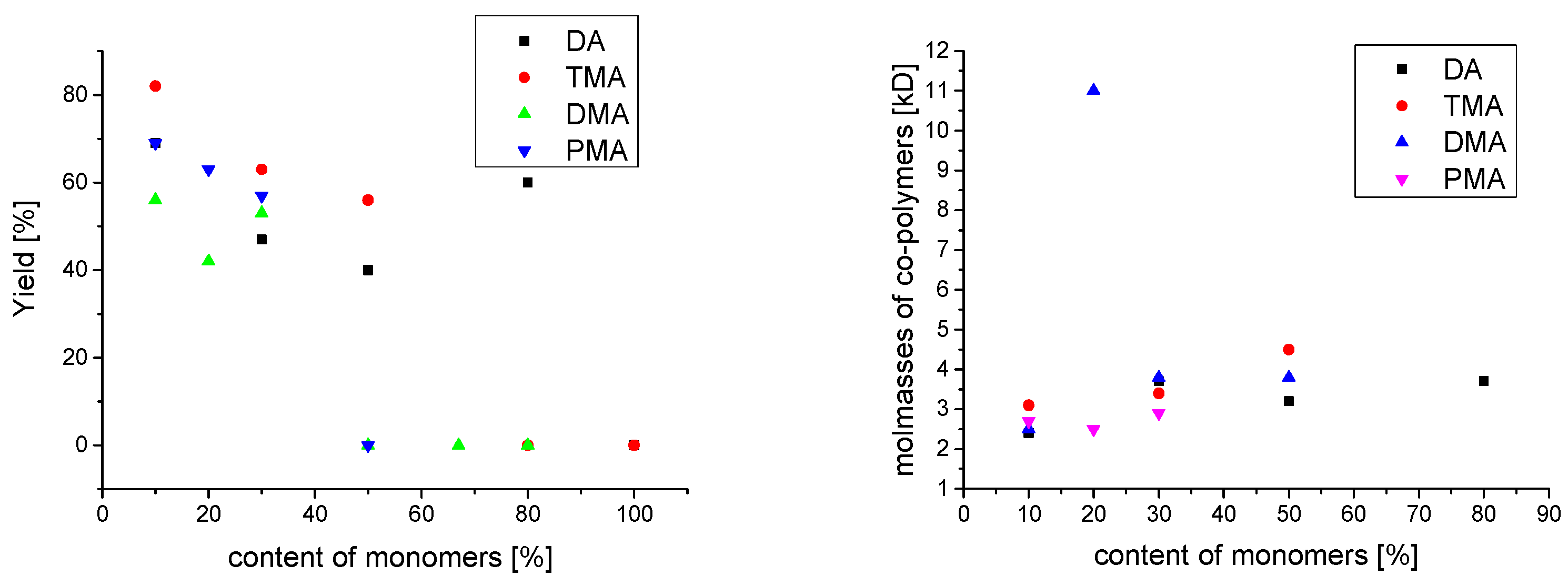

| DA/DMA/TMA/PMA Monomerfeed mol% | DA/DMA/TMA/PMA Monomerfound | Yield % | Mn kg/mol | DP | Mw kg/mol | PDI | Tg °C | Cross-Cut Class | ||
|---|---|---|---|---|---|---|---|---|---|---|
| mol% | wt% | |||||||||
| P(HEAA) | 100 | - | 100 | 51 | 2.5 | 22 | 5.7 | 2.3 | 176.0 | 0 |
| P(HEAA/DA10) | 10 | 9 | 15 | 69 | 2.4 | 19 | 4.8 | 2.0 | n. d. | 0 |
| P(HEAA/DA30) | 30 | 29 | 42 | 47 | 3.7 | 21 | 7.2 | 1.9 | 123.4 | 0 |
| P(HEAA/DA50) | 50 | 50 | 64 | 40 | 3.2 | 20 | 5.4 | 1.7 | 127.9 | 0 |
| P(HEAA/DA80) | 80 | 80 | 88 | 60 | 3.7 | 20 | 7.8 | 2.1 | 128.9 | n. a. |
| P(DA) | 100 | - | - | 0 | no polymerization | - | ||||
| P(HEAA/TMA10) | 10 | 10 | 17 | 82 | 3.1 | 25 | 6.6 | 2.1 | 111.3 | 0 |
| P(HEAA/TMA30) | 30 | 28 | 41 | 63 | 3.4 | 24 | 7.5 | 2.2 | 122.7 | 0 |
| P(HEAA/TMA50) | 50 | 50 | 64 | 56 | 4.5 | 28 | 12.4 | 2.7 | 128.2 | 0 |
| P(HEAA/TMA80) | 80 | - | - | 0 | no polymerization | - | ||||
| P(TMA) | 100 | - | - | 0 | no polymerization | - | ||||
| P(HEAA/DMA10) | 10 | 12 | 21 | 56 | 11.0 | 87 | 30.1 | 2.7 | 131.1 | 0 |
| P(HEAA/DMA20) | 20 | 23 | 36 | 42 | 3.8 | 28 | 10.2 | 2.7 | 126.3 | 0 |
| P(HEAA/DMA30) | 30 | 33 | 49 | 53 | 3.8 | 23 | 10.2 | 2.0 | 126.0 | 0 |
| P(HEAA/DMA50) | 50 | - | - | 0 | no polymerization | - | ||||
| P(HEAA/DMA67) | 67 | - | - | 0 | no polymerization | - | ||||
| P(HEAA/DMA80) | 80 | - | - | 0 | no polymerization | - | ||||
| P(DMA) | 100 | - | - | 0 | no polymerization | - | ||||
| P(HEAA/PMA10) | 10 | 9 | 14 | 69 | 2.7 | 22 | 5.4 | 2.0 | 110.5 | 0 |
| P(HEAA/PMA20) | 20 | 20 | 29 | 63 | 2.5 | 19 | 4.4 | 1.8 | 99.9 | 0 |
| P(HEAA/PMA30) | 30 | 31 | 42 | 57 | 2.9 | 21 | 5.8 | 2.0 | 110.5 | 0 |
| P(HEAA/PMA50) | 50 | - | - | 0 | no polymerization | - | ||||
| Glue Formulation with 5 mol% of | Rpmax s−1 | tmax s | ΔHmax J | DBCmax % | Fmax MPa | Elongationmax % |
|---|---|---|---|---|---|---|
| PMA | 0.49 | 12.0 | −646.2 | 91. 9 | 1.26 | 0.46 |
| TMA | 0.29 | 6.0 | −610.2 | 93.2 | 1.68 | 1.00 |
| DMA | 0.47 | 3.6 | −516.1 | 75.4 | 0.66 | 1.98 |
| DA | 0.53 | 4.8 | −479.6 | 70.0 | n.a | n.a |
| PMA5/PEGDA0.1 | 0.68 | 4.8 | −504.6 | 83.8 | 5.03 | 1.27 |
| TMA5/PEGDA0.1 | 0.51 | 6.0 | −582.8 | 94.8 | 6.02 | 1.57 |
| DMA5/PEGDA0.1 | 0.50 | 3.6 | −524.5 | 87.3 | 6.23 | 1.81 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hennig, K.; Meyer, W. Synthesis and Characterization of Catechol-Containing Polyacrylamides with Adhesive Properties. Molecules 2022, 27, 4027. https://doi.org/10.3390/molecules27134027
Hennig K, Meyer W. Synthesis and Characterization of Catechol-Containing Polyacrylamides with Adhesive Properties. Molecules. 2022; 27(13):4027. https://doi.org/10.3390/molecules27134027
Chicago/Turabian StyleHennig, Kathleen, and Wolfdietrich Meyer. 2022. "Synthesis and Characterization of Catechol-Containing Polyacrylamides with Adhesive Properties" Molecules 27, no. 13: 4027. https://doi.org/10.3390/molecules27134027







