Should We ‘Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables
Abstract
1. Introduction
2. Results
2.1. Characteristics of Included Studies
2.2. Bioactive Pigment Interventions
2.2.1. SLRs and MAs
2.2.2. Single RCTs and Cohort Studies
2.3. Health Outcomes and Confidence in the Body of Evidence
2.3.1. SLRs and MAs
2.3.2. Single RCTs and COHORT STUDIES
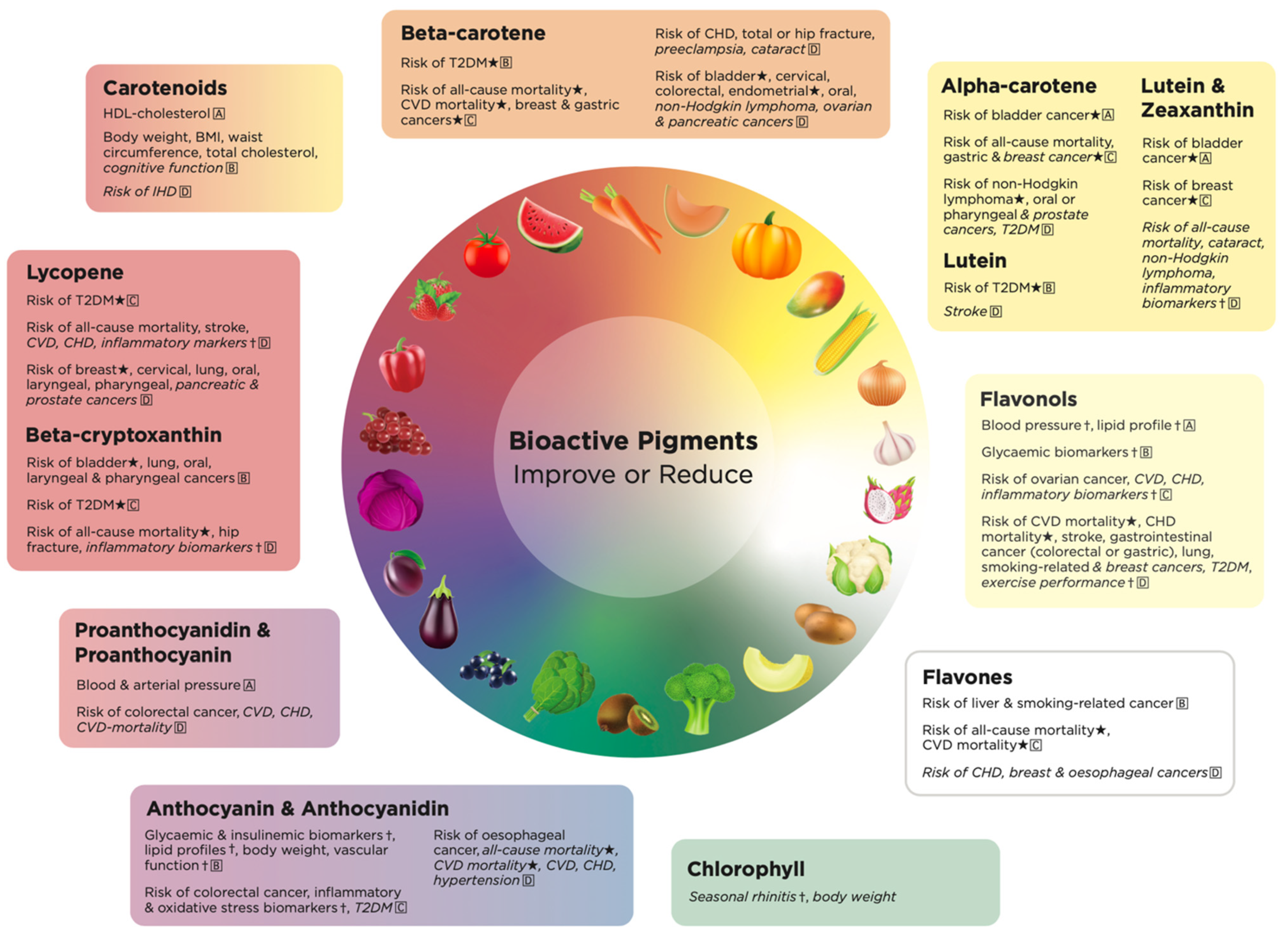
2.4. Health Effects of Total Carotenoid Pigments in Fruits and Vegetables
2.5. Health Effects of Red Pigments in Fruits and Vegetables
2.5.1. Beta-Cryptoxanthin
2.5.2. Lycopene
2.6. Health Effects of Orange Pigments in Fruits and Vegetables
2.7. Health Effects of Yellow Bioactive Pigments in Fruits and Vegetables
2.7.1. Alpha-Carotene
2.7.2. Lutein
2.7.3. Zeaxanthin
2.7.4. Lutein and Zeaxanthin
2.8. Health Effects of Pale-Yellow Bioactive Pigments in Fruits and Vegetables
2.8.1. Flavonols
2.8.2. Kaempferol, Quercetin and Myricetin
2.9. Health Effects of White Bioactive Pigments in Fruits and Vegetables
2.10. Health Effects of Purple/Blue Bioactive Pigments in Fruits and Vegetables
2.10.1. Anthocyanidins
2.10.2. Anthocyanins
2.10.3. Proanthocyanidins
2.10.4. Proanthocyanins
2.11. Health Effects of Green Bioactive Pigments in Fruits and Vegetables
2.12. Health Effects Unique to Each Bioactive Pigment
3. Discussion
3.1. Implications for Future Research and Practice
3.2. Limitations
4. Materials and Methods
4.1. Characterization of Natural Pigments
4.2. Eligibility Criteria
4.3. Search Strategy
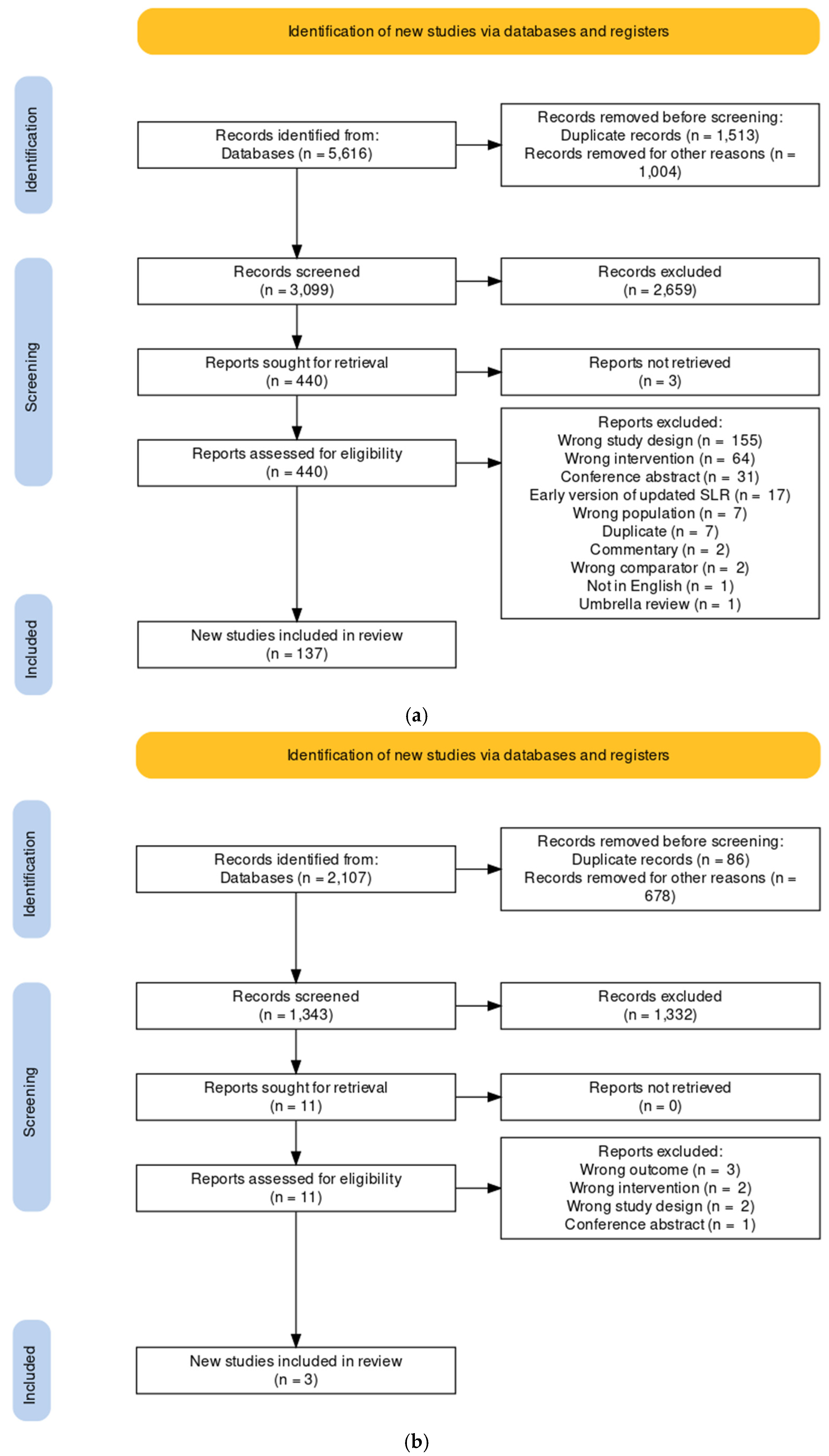
4.4. Selection Process
4.5. Data Extraction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- GBD 2017 Diet Collaborations. Health effects of dietary risks in 195 countries, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2019, 393, 1958–1972. [Google Scholar] [CrossRef]
- Iddir, M.; Brito, A.; Dingeo, G.; Sosa Fernandez Del Campo, S.; Samouda, H.; La Frano, M.R.; Bohn, T. Strengthening the immune system and reducing inflammation and oxidative streess through diet and nutrition: Considerations during the COVID-19 crisis. Nutrients 2020, 12, 1562. [Google Scholar] [CrossRef] [PubMed]
- World Health Organisation. Increasing Fruit and Vegetable Consumption to Reduce the Risk of Noncommunicable Diseases. Available online: https://www.who.int/elena/titles/fruit_vegetables_ncds/en/ (accessed on 29 November 2021).
- Cooper, A.J.; Sharp, S.J.; Lentjes, M.A.; Luben, R.N.; Khaw, K.T.; Wareham, N.J.; Forouhi, N.G. A prospective study of the association between quantity and variety of fruit and vegetable intake and incident type 2 diabetes. Diabetes Care 2012, 35, 1293–1300. [Google Scholar] [CrossRef] [PubMed]
- Büchner, F.L.; Bueno-de-Mesquita, H.B.; Ros, M.M.; Overvad, K.; Dahm, C.C.; Hansen, L.; Tjønneland, A.; Clavel-Chapelon, F.; Boutron-Ruault, M.C.; Touillaud, M.; et al. Variety in fruit and vegetable consumption and the risk of lung cancer in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2010, 19, 2278–2286. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Lee, A.H.; Su, D.; Binns, C.W. Fruit and vegetable consumption associated with reduced risk of epithelial ovarian cancer in southern Chinese women. Gynecol. Oncol. 2014, 132, 241–247. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jeurnink, S.; Büchner, F.; Bueno-de-Mesquita, H.; Siersema, P.; Boshuizen, H.; Numans, M.; Dahm, C.C.; Overvad, K.; Tjønneland, A.; Roswall, N. Variety in vegetable and fruit consumption and the risk of gastric and esophageal cancer in the European Prospective Investigation into Cancer and Nutrition. Int. J. Cancer 2012, 131, E963–E973. [Google Scholar] [CrossRef]
- Tao, L.; Xie, Z.; Huang, T. Dietary diversity and all-cause mortality among Chinese adults aged 65 or older: A community-based cohort study. Asia Pac. J. Clin. Nutr. 2020, 29, 152–160. [Google Scholar] [CrossRef]
- Blekkenhorst, L.C.; Lewis, J.R.; Bondonno, C.P.; Sim, M.; Devine, A.; Zhu, K.; Lim, W.H.; Woodman, R.J.; Beilin, L.J.; Thompson, P.L.; et al. Vegetable diversity in relation with subclinical atherosclerosis and 15-year atherosclerotic vascular disease deaths in older adult women. Eur. J. Nutr. 2020, 59, 217–230. [Google Scholar] [CrossRef]
- Ye, X.; Bhupathiraju, S.N.; Tucker, K.L. Variety in fruit and vegetable intake and cognitive function in middle-aged and older Puerto Rican adults. Br. J. Nutr. 2013, 109, 503–510. [Google Scholar] [CrossRef]
- Yeung, S.S.Y.; Kwok, T.; Woo, J. Higher fruit and vegetable variety associated with lower risk of cognitive impairment in Chinese community-dwelling older men: A 4-year cohort study. Eur. J. Nutr. 2022, 61, 1791–1799. [Google Scholar] [CrossRef]
- Dalwood, P.; Marshall, S.; Burrows, T.L.; McIntosh, A.; Collins, C.E. Diet quality indices and their associations with health-related outcomes in children and adolescents: An updated systematic review. Nutr. J. 2020, 19, 118. [Google Scholar] [CrossRef] [PubMed]
- Gupta, C.; Prakash, D. Phytonutrients as therapeutic agents. J. Complement. Integr. Med. 2014, 11, 151–169. [Google Scholar] [CrossRef]
- Hall, J.N.; Moore, S.; Harper, S.B.; Lynch, J.W. Global variability in fruit and vegetable consumption. Am. J. Prev. Med. 2009, 36, 402–409.e405. [Google Scholar] [CrossRef] [PubMed]
- Minich, D.M. A Review of the Science of Colorful, Plant-Based Food and Practical Strategies for “Eating the Rainbow”. J. Nutr. Metab. 2019, 2019, 2125070. [Google Scholar] [CrossRef] [PubMed]
- Forkmann, G. Flavonoids as flower pigments: The formation of the natural spectrum and its extension by genetic engineering. Plant Breed. 1991, 106, 1–26. [Google Scholar] [CrossRef]
- Nutrilite Health Institute. America’s Phytonutrient Report: Quantifying the Gap; Nutrilite Health Institute: Buena Park, CA, USA, 2009. [Google Scholar]
- Marshall, A.N.; van den Berg, A.; Ranjit, N.; Hoelscher, D.M. A Scoping Review of the Operationalization of Fruit and Vegetable Variety. Nutrients 2020, 12, 2868. [Google Scholar] [CrossRef]
- Marshall, S.; Burrows, T.; Collins, C.E. Systematic review of diet quality indices and their associations with health-related outcomes in children and adolescents. J. Hum. Nutr. Diet. 2014, 27, 577–598. [Google Scholar] [CrossRef]
- Griep, L.M.O.; Verschuren, W.M.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Colors of Fruit and Vegetables and 10-Year Incidence of Stroke. Stroke 2011, 42, 3190–3195. [Google Scholar] [CrossRef]
- Griep, L.M.O.; Verschuren, W.M.; Kromhout, D.; Ocké, M.C.; Geleijnse, J.M. Colours of fruit and vegetables and 10-year incidence of CHD. Br. J. Nutr. 2011, 106, 1562–1569. [Google Scholar] [CrossRef]
- Becerra-Tomás, N.; Paz-Graniel, I.; Tresserra-Rimbau, A.; Martínez-González, M.Á.; Barrubés, L.; Corella, D.; Muñoz-Martínez, J.; Romaguera, D.; Vioque, J.; Alonso-Gómez, Á.M.; et al. Fruit consumption and cardiometabolic risk in the PREDIMED-plus study: A cross-sectional analysis. Nutr. Metab. Cardiovasc. Dis. 2021, 31, 1702–1713. [Google Scholar] [CrossRef]
- Mirmiran, P.; Bahadoran, Z.; Moslehi, N.; Bastan, S.; Azizi, F. Colors of fruits and vegetables and 3-year changes of cardiometabolic risk factors in adults: Tehran lipid and glucose study. Eur. J. Clin. Nutr. 2015, 69, 1215–1219. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Ling, W.; Du, Z.; Chen, Y.; Li, D.; Deng, S.; Liu, Z.; Yang, L. Effects of Anthocyanins on Cardiometabolic Health: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Adv. Nutr. 2017, 8, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.; Fatehi, P.; Jafari, T. Impact of dietary anthocyanins on systemic and vascular inflammation: Systematic review and meta-analysis on randomised clinical trials. Food Chem. Toxicol. 2020, 135, 110922. [Google Scholar] [CrossRef] [PubMed]
- Fallah, A.A.; Sarmast, E.; Jafari, T. Effect of dietary anthocyanins on biomarkers of glycemic control and glucose metabolism: A systematic review and meta-analysis of randomized clinical trials. Food Res. Int. 2020, 137, 109379. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, M.; Lee, M. Effects of Anthocyanin Supplementation on Reduction of Obesity Criteria: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2121. [Google Scholar] [CrossRef] [PubMed]
- Davinelli, S.; Ali, S.; Solfrizzi, V.; Scapagnini, G.; Corbi, G. Carotenoids and Cognitive Outcomes: A Meta-Analysis of Randomized Intervention Trials. Antioxidants 2021, 10, 223. [Google Scholar] [CrossRef]
- Hu, F.; Wang Yi, B.; Zhang, W.; Liang, J.; Lin, C.; Li, D.; Wang, F.; Pang, D.; Zhao, Y. Carotenoids and breast cancer risk: A meta-analysis and meta-regression. Breast Cancer Res. Treat. 2012, 131, 239–253. [Google Scholar] [CrossRef]
- Aune, D.; Keum, N.; Giovannucci, E.; Fadnes, L.T.; Boffetta, P.; Greenwood, D.C.; Tonstad, S.; Vatten, L.J.; Riboli, E.; Norat, T. Dietary intake and blood concentrations of antioxidants and the risk of cardiovascular disease, total cancer, and all-cause mortality: A systematic review and dose-response meta-analysis of prospective studies. Am. J. Clin. Nutr. 2018, 108, 1069–1091. [Google Scholar] [CrossRef]
- Sandoval-Ramírez, B.; Catalán, U.; Llauradó, E.; Valls, R.; Salamanca, P.; Rubió, L.; Yuste, S.; Solà, R. The health benefits of anthocyanins: An umbrella review of systematic reviews and meta-analyses of observational studies and controlled clinical trials. Nutr. Rev. 2021, nuab086. [Google Scholar] [CrossRef]
- Li, N.; Wu, X.; Zhuang, W.; Xia, L.; Chen, Y.; Wu, C.; Rao, Z.; Du, L.; Zhao, R.; Yi, M.; et al. Tomato and lycopene and multiple health outcomes: Umbrella review. Food Chem. 2021, 343, 128396. [Google Scholar] [CrossRef]
- Blumfield, M.; Mayr, H.L.; De Vlieger, N.; Abbott, K.; Starck, C.; Fayet-Moore, F.; Marshall, S. Systematic review data of the health effects of colourful bioactive pigments in fruits and vegetables. Dryad, 2022; under review. [Google Scholar]
- Rowles, J.L., 3rd; Ranard, K.M.; Smith, J.W.; An, R.; Erdman, J.W., Jr. Increased dietary and circulating lycopene are associated with reduced prostate cancer risk: A systematic review and meta-analysis. Prostate Cancer Prostatic Dis. 2017, 20, 361–377. [Google Scholar] [CrossRef] [PubMed]
- Yao, N.; Yan, S.; Guo, Y.; Wang, H.; Li, X.; Wang, L.; Hu, W.; Li, B.; Cui, W. The association between carotenoids and subjects with overweight or obesity: A systematic review and meta-analysis. Food Funct. 2021, 12, 4768–4782. [Google Scholar] [CrossRef] [PubMed]
- Law, M.R.; Morris, J.K. By how much does fruit and vegetable consumption reduce the risk of ischaemic heart disease? Eur. J. Clin. Nutr. 1998, 52, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Hajizadeh-Sharafabad, F.; Zahabi, E.S.; Malekahmadi, M.; Zarrin, R.; Alizadeh, M. Carotenoids supplementation and inflammation: A systematic review and meta-analysis of randomized clinical trials. Crit. Rev. Food Sci. Nutr. 2021, 1–17. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef]
- Kim, S.J.; Anh, N.H.; Diem, N.C.; Park, S.; Cho, Y.H.; Long, N.P.; Hwang, I.G.; Lim, J.; Kwon, S.W. Effects of β-Cryptoxanthin on Improvement in Osteoporosis Risk: A Systematic Review and Meta-Analysis of Observational Studies. Foods 2021, 10, 296. [Google Scholar] [CrossRef]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary Antioxidants, Circulating Antioxidant Concentrations, Total Antioxidant Capacity, and Risk of All-Cause Mortality: A Systematic Review and Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2018, 9, 701–716. [Google Scholar] [CrossRef]
- Leoncini, E.; Nedovic, D.; Panic, N.; Pastorino, R.; Edefonti, V.; Boccia, S. Carotenoid Intake from Natural Sources and Head and Neck Cancer: A Systematic Review and Meta-analysis of Epidemiological Studies. Cancer Epidemiol. Biomark. Prev. 2015, 24, 1003–1011. [Google Scholar] [CrossRef]
- Wu, S.; Liu, Y.; Michalek, J.E.; Mesa, R.A.; Parma, D.L.; Rodriguez, R.; Mansour, A.M.; Svatek, R.; Tucker, T.C.; Ramirez, A.G. Carotenoid Intake and Circulating Carotenoids Are Inversely Associated with the Risk of Bladder Cancer: A Dose-Response Meta-analysis. Adv. Nutr. 2020, 11, 630–643. [Google Scholar] [CrossRef]
- Gallicchio, L.; Boyd, K.; Matanoski, G.; Tao, X.; Chen, L.; Lam, T.K.; Shiels, M.; Hammond, E.; Robinson, K.A.; Caulfield, L.E.; et al. Carotenoids and the risk of developing lung cancer: A systematic review. Am. J. Clin. Nutr. 2008, 88, 372–383. [Google Scholar] [CrossRef]
- Jiang, Y.W.; Sun, Z.H.; Tong, W.W.; Yang, K.; Guo, K.Q.; Liu, G.; Pan, A. Dietary Intake and Circulating Concentrations of Carotenoids and Risk of Type 2 Diabetes: A Dose-Response Meta-Analysis of Prospective Observational Studies. Adv. Nutr. 2021. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Yin, Y.; Wu, C.-R.; Liu, Y.; Guo, F.; Li, M.; Ma, L. Dietary vitamin and carotenoid intake and risk of age-related cataract. Am. J. Clin. Nutr. 2019, 109, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Chong, E.W.; Wong, T.Y.; Kreis, A.J.; Simpson, J.A.; Guymer, R.H. Dietary antioxidants and primary prevention of age related macular degeneration: Systematic review and meta-analysis. BMJ 2007, 335, 755. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Nyssen, O.P.; Syed, A.; Jansen, E.; Bueno-De-Mesquita, B.; Gallo, V. Vitamin A and carotenoids and the risk of parkinson’s disease: A systematic review and meta-analysis. Neuroepidemiology 2013, 42, 25–38. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Hu, J.; Liu, P.; Li, J.; Wei, Z.; Liu, P. Carotenoid intake and risk of non-Hodgkin lymphoma: A systematic review and dose-response meta-analysis of observational studies. Ann. Hematol. 2017, 96, 957–965. [Google Scholar] [CrossRef]
- Panic, N.; Nedovic, D.; Pastorino, R.; Boccia, S.; Leoncini, E. Carotenoid intake from natural sources and colorectal cancer: A systematic review and meta-analysis of epidemiological studies. Eur. J. Cancer Prev. 2017, 26, 27–37. [Google Scholar] [CrossRef]
- Huang, X.; Gao, Y.; Zhi, X.; Ta, N.; Jiang, H.; Zheng, J. Association between vitamin A, retinol and carotenoid intake and pancreatic cancer risk: Evidence from epidemiologic studies. Sci. Rep. 2016, 6, 38936. [Google Scholar] [CrossRef]
- Myung, S.K.; Ju, W.; Kim, S.C.; Kim, H. Vitamin or antioxidant intake (or serum level) and risk of cervical neoplasm: A meta-analysis. BJOG 2011, 118, 1285–1291. [Google Scholar] [CrossRef]
- Song, B.; Liu, K.; Gao, Y.; Zhao, L.; Fang, H.; Li, Y.; Pei, L.; Xu, Y. Lycopene and risk of cardiovascular diseases: A meta-analysis of observational studies. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef]
- Cheng, H.M.; Koutsidis, G.; Lodge, J.K.; Ashor, A.W.; Siervo, M.; Lara, J. Lycopene and tomato and risk of cardiovascular diseases: A systematic review and meta-analysis of epidemiological evidence. Crit. Rev. Food Sci. Nutr. 2019, 59, 141–158. [Google Scholar] [CrossRef]
- Cohen, J.M.; Beddaoui, M.; Kramer, M.S.; Platt, R.W.; Basso, O.; Kahn, S.R. Maternal Antioxidant Levels in Pregnancy and Risk of Preeclampsia and Small for Gestational Age Birth: A Systematic Review and Meta-Analysis. PLoS ONE 2015, 10, e0135192. [Google Scholar] [CrossRef]
- Xu, J.; Song, C.; Song, X.; Zhang, X.; Li, X. Carotenoids and risk of fracture: A meta-analysis of observational studies. Oncotarget 2017, 8, 2391–2399. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, R.; Xiao, Y.; Fang, J.; Xu, Q. Effect of Carotene and Lycopene on the Risk of Prostate Cancer: A Systematic Review and Dose-Response Meta-Analysis of Observational Studies. PLoS ONE 2015, 10, e0137427. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Meng, Q.; Zhai, S. Association of carotenoids with risk of gastric cancer: A meta-analysis. Clin. Nutr. 2016, 35, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Xu, J. Meta-analysis of the association between dietary lycopene intake and ovarian cancer risk in postmenopausal women. Sci. Rep. 2014, 4, 4885. [Google Scholar] [CrossRef]
- Ried, K.; Fakler, P. Protective effect of lycopene on serum cholesterol and blood pressure: Meta-analyses of intervention trials. Maturitas 2011, 68, 299–310. [Google Scholar] [CrossRef]
- Tierney, A.C.; Rumble, C.E.; Billings, L.M.; George, E.S. Effect of dietary and supplemental lycopene on cardiovascular risk factors: A systematic review and meta-analysis. Adv. Nutr. 2020, 11, 1453–1488. [Google Scholar] [CrossRef]
- Ilic, D.; Forbes, K.M.; Hassed, C. Lycopene for the prevention of prostate cancer. Cochrane Database Syst. Rev. 2011, Cd008007. [Google Scholar] [CrossRef]
- Druesne-Pecollo, N.; Latino-Martel, P.; Norat, T.; Barrandon, E.; Bertrais, S.; Galan, P.; Hercberg, S. Beta-carotene supplementation and cancer risk: A systematic review and metaanalysis of randomized controlled trials. Int. J. Cancer 2010, 127, 172–184. [Google Scholar] [CrossRef]
- Huncharek, M.; Klassen, H.; Kupelnick, B. Dietary beta-carotene intake and the risk of epithelial ovarian cancer: A meta-analysis of 3,782 subjects from five observational studies. In Vivo 2001, 15, 339–343. [Google Scholar]
- Bandera, E.V.; Gifkins, D.M.; Moore, D.F.; McCullough, M.L.; Kushi, L.H. Antioxidant vitamins and the risk of endometrial cancer: A dose-response meta-analysis. Cancer Causes Control 2009, 20, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Mente, A.; de Koning, L.; Shannon, H.S.; Anand, S.S. A systematic review of the evidence supporting a causal link between dietary factors and coronary heart disease. Arch. Intern. Med. 2009, 169, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Jayedi, A.; Rashidy-Pour, A.; Parohan, M.; Zargar, M.S.; Shab-Bidar, S. Dietary and circulating vitamin C, vitamin E, β-carotene and risk of total cardiovascular mortality: A systematic review and dose-response meta-analysis of prospective observational studies. Public Health Nutr. 2019, 22, 1872–1887. [Google Scholar] [CrossRef] [PubMed]
- Charkos, T.G.; Liu, Y.; Oumer, K.S.; Vuong, A.M.; Yang, S. Effects of β-carotene intake on the risk of fracture: A Bayesian meta-analysis. BMC Musculoskelet. Disord. 2020, 21, 711. [Google Scholar] [CrossRef]
- Zhang, Y.P.; Chu, R.X.; Liu, H. Vitamin A intake and risk of melanoma: A meta-analysis. PLoS ONE 2014, 9, e102527. [Google Scholar] [CrossRef] [PubMed]
- Seyedrezazadeh, E.; Moghaddam, M.P.; Ansarin, K.; Asghari Jafarabadi, M.; Sharifi, A.; Sharma, S.; Kolahdooz, F. Dietary Factors and Risk of Chronic Obstructive Pulmonary Disease: A Systemic Review and Meta-Analysis. Tanaffos 2019, 18, 294–309. [Google Scholar]
- Zhang, X.; Zhang, R.; Moore, J.B.; Wang, Y.; Yan, H.; Wu, Y.; Tan, A.; Fu, J.; Shen, Z.; Qin, G.; et al. The Effect of Vitamin A on Fracture Risk: A Meta-Analysis of Cohort Studies. Int. J. Environ. Res. Public Health 2017, 14, 1043. [Google Scholar] [CrossRef]
- Li, F.J.; Shen, L. Dietary intakes of vitamin E, vitamin C, and β-carotene and risk of Alzheimer’s disease: A meta-analysis. J. Alzheimer’s Dis. 2012, 31, 253–258. [Google Scholar] [CrossRef]
- Leermakers, E.T.; Darweesh, S.K.; Baena, C.P.; Moreira, E.M.; Melo van Lent, D.; Tielemans, M.J.; Muka, T.; Vitezova, A.; Chowdhury, R.; Bramer, W.M.; et al. The effects of lutein on cardiometabolic health across the life course: A systematic review and meta-analysis. Am. J. Clin. Nutr. 2016, 103, 481–494. [Google Scholar] [CrossRef]
- Grosso, G.; Micek, A.; Godos, J.; Pajak, A.; Sciacca, S.; Galvano, F.; Giovannucci, E.L. Dietary Flavonoid and Lignan Intake and Mortality in Prospective Cohort Studies: Systematic Review and Dose-Response Meta-Analysis. Am. J. Epidemiol. 2017, 185, 1304–1316. [Google Scholar] [CrossRef]
- Fan, Z.K.; Wang, C.; Yang, T.; Li, X.Q.; Guo, X.F.; Li, D. Flavonoid subclasses and coronary heart disease risk: A meta-analysis of prospective cohort studies. Br. J. Nutr. 2021, 1–34. [Google Scholar] [CrossRef] [PubMed]
- Huxley, R.R.; Neil, H.A. The relation between dietary flavonol intake and coronary heart disease mortality: A meta-analysis of prospective cohort studies. Eur. J. Clin. Nutr. 2003, 57, 904–908. [Google Scholar] [CrossRef] [PubMed]
- Micek, A.; Godos, J.; Del Rio, D.; Galvano, F.; Grosso, G. Dietary Flavonoids and Cardiovascular Disease: A Comprehensive Dose-Response Meta-Analysis. Mol. Nutr. Food Res. 2021, 65, e2001019. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Zhao, D.; Nie, Z.L.; Zhao, H.; Zhou, B.; Gao, W.; Wang, L.S.; Yang, Z.J. Flavonol intake and stroke risk: A meta-analysis of cohort studies. Nutrition 2014, 30, 518–523. [Google Scholar] [CrossRef]
- Rienks, J.; Barbaresko, J.; Oluwagbemigun, K.; Schmid, M.; Nöthlings, U. Polyphenol exposure and risk of type 2 diabetes: Dose-response meta-analyses and systematic review of prospective cohort studies. Am. J. Clin. Nutr. 2018, 108, 49–61. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and risk of stomach and colorectal cancer. World J. Gastroenterol. 2013, 19, 1011–1019. [Google Scholar] [CrossRef]
- Woo, H.D.; Kim, J. Dietary flavonoid intake and smoking-related cancer risk: A meta-analysis. PLoS ONE 2013, 8, e75604. [Google Scholar] [CrossRef]
- Xie, Y.; Huang, S.; Su, Y. Dietary Flavonols Intake and Risk of Esophageal and Gastric Cancer: A Meta-Analysis of Epidemiological Studies. Nutrients 2016, 8, 91. [Google Scholar] [CrossRef]
- Hua, X.; Yu, L.; You, R.; Yang, Y.; Liao, J.; Chen, D.; Yu, L. Association among Dietary Flavonoids, Flavonoid Subclasses and Ovarian Cancer Risk: A Meta-Analysis. PLoS ONE 2016, 11, e0151134. [Google Scholar] [CrossRef]
- Hui, C.; Qi, X.; Qianyong, Z.; Xiaoli, P.; Jundong, Z.; Mantian, M. Flavonoids, flavonoid subclasses and breast cancer risk: A meta-analysis of epidemiologic studies. PLoS ONE 2013, 8, e54318. [Google Scholar] [CrossRef]
- Grosso, G.; Godos, J.; Lamuela-Raventos, R.; Ray, S.; Micek, A.; Pajak, A.; Sciacca, S.; D’Orazio, N.; Del Rio, D.; Galvano, F. A comprehensive meta-analysis on dietary flavonoid and lignan intake and cancer risk: Level of evidence and limitations. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.M.; Nie, Z.L.; Zhou, B.; Lian, X.Q.; Zhao, H.; Gao, W.; Wang, Y.S.; Jia, E.Z.; Wang, L.S.; Yang, Z.J. Flavonols intake and the risk of coronary heart disease: A meta-analysis of cohort studies. Atherosclerosis 2012, 222, 270–273. [Google Scholar] [CrossRef]
- Godos, J.; Vitale, M.; Micek, A.; Ray, S.; Martini, D.; Del Rio, D.; Riccardi, G.; Galvano, F.; Grosso, G. Dietary Polyphenol Intake, Blood Pressure, and Hypertension: A Systematic Review and Meta-Analysis of Observational Studies. Antioxidants 2019, 8, 152. [Google Scholar] [CrossRef]
- Cui, L.; Liu, X.; Tian, Y.; Xie, C.; Li, Q.; Cui, H.; Sun, C. Flavonoids, Flavonoid Subclasses, and Esophageal Cancer Risk: A Meta-Analysis of Epidemiologic Studies. Nutrients 2016, 8, 350. [Google Scholar] [CrossRef]
- Guo, K.; Liang, Z.; Liu, L.; Li, F.; Wang, H. Flavonoids intake and risk of prostate cancer: A meta-analysis of observational studies. Andrologia 2016, 48, 1175–1182. [Google Scholar] [CrossRef] [PubMed]
- Menezes, R.; Dumont, J.; Schär, M.; Palma-Duran, S.A.; Combet, E.; Ruskovska, T.; Maksimova, V.; Pinto, P.; Rodriguez-Mateos, A.; Kaltsatou, A.; et al. Impact of Flavonols on Cardiometabolic Biomarkers: A Meta-Analysis of Randomized Controlled Human Trials to Explore the Role of Inter-Individual Variability. Nutrients 2017, 9, 117. [Google Scholar] [CrossRef] [PubMed]
- Akhlaghi, M.; Ghobadi, S.; Mohammad Hosseini, M.; Gholami, Z.; Mohammadian, F. Flavanols are potential anti-obesity agents, a systematic review and meta-analysis of controlled clinical trials. Nutr. Metab. Cardiovasc. Dis. 2018, 28, 675–690. [Google Scholar] [CrossRef]
- Guo, W.; Gong, X.; Li, M. Quercetin Actions on Lipid Profiles in Overweight and Obese Individuals: A Systematic Review and Meta-Analysis. Curr. Pharm. Des. 2019, 25, 3087–3095. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Clinical effectiveness of quercetin supplementation in the management of weight loss: A pooled analysis of randomized controlled trials. Diabetes Metab. Syndr. Obes. 2019, 12, 553–563. [Google Scholar] [CrossRef]
- Huang, H.; Liao, D.; Dong, Y.; Pu, R. Effect of quercetin supplementation on plasma lipid profiles, blood pressure, and glucose levels: A systematic review and meta-analysis. Nutr. Rev. 2020, 78, 615–626. [Google Scholar] [CrossRef]
- Mohammadi-Sartang, M.; Mazloom, Z.; Sherafatmanesh, S.; Ghorbani, M.; Firoozi, D. Effects of supplementation with quercetin on plasma C-reactive protein concentrations: A systematic review and meta-analysis of randomized controlled trials. Eur. J. Clin. Nutr. 2017, 71, 1033–1039. [Google Scholar] [CrossRef] [PubMed]
- Ostadmohammadi, V.; Milajerdi, A.; Ayati, E.; Kolahdooz, F.; Asemi, Z. Effects of quercetin supplementation on glycemic control among patients with metabolic syndrome and related disorders: A systematic review and meta-analysis of randomized controlled trials. Phytother. Res. 2019, 33, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Zheng, Z.; Zhao, Y.; Lin, W. Impact of quercetin on systemic levels of inflammation: A meta-analysis of randomised controlled human trials. Int. J. Food Sci. Nutr. 2020, 71, 152–163. [Google Scholar] [CrossRef]
- Pelletier, D.M.; Lacerte, G.; Goulet, E.D. Effects of quercetin supplementation on endurance performance and maximal oxygen consumption: A meta-analysis. Int. J. Sport Nutr. Exerc. Metab. 2013, 23, 73–82. [Google Scholar] [CrossRef]
- Peluso, I.; Raguzzini, A.; Serafini, M. Effect of flavonoids on circulating levels of TNF-α and IL-6 in humans: A systematic review and meta-analysis. Mol. Nutr. Food Res. 2013, 57, 784–801. [Google Scholar] [CrossRef] [PubMed]
- Somerville, V.; Bringans, C.; Braakhuis, A. Polyphenols and Performance: A Systematic Review and Meta-Analysis. Sports Med. 2017, 47, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Kimble, R.; Keane, K.M.; Lodge, J.K.; Howatson, G. Dietary intake of anthocyanins and risk of cardiovascular disease: A systematic review and meta-analysis of prospective cohort studies. Crit. Rev. Food Sci. Nutr. 2019, 59, 3032–3043. [Google Scholar] [CrossRef]
- Kimble, R.; Jones, K.; Howatson, G. The effect of dietary anthocyanins on biochemical, physiological, and subjective exercise recovery: A systematic review and meta-analysis. Crit. Rev. Food Sci. Nutr. 2021, 1–15. [Google Scholar] [CrossRef]
- Bloedon, T.K.; Braithwaite, R.E.; Carson, I.A.; Klimis-Zacas, D.; Lehnhard, R.A. Impact of anthocyanin-rich whole fruit consumption on exercise-induced oxidative stress and inflammation: A systematic review and meta-analysis. Nutr. Rev. 2019, 77, 630–645. [Google Scholar] [CrossRef]
- Shah, K.; Shah, P. Effect of Anthocyanin Supplementations on Lipid Profile and Inflammatory Markers: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Cholesterol 2018, 2018, 8450793. [Google Scholar] [CrossRef]
- Araki, R.; Yada, A.; Ueda, H.; Tominaga, K.; Isoda, H. Differences in the Effects of Anthocyanin Supplementation on Glucose and Lipid Metabolism According to the Structure of the Main Anthocyanin: A Meta-Analysis of Randomized Controlled Trials. Nutrients 2021, 13, 2003. [Google Scholar] [CrossRef] [PubMed]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [PubMed]
- Sangsefidi, Z.S.; Mozaffari-Khosravi, H.; Sarkhosh-Khorasani, S.; Hosseinzadeh, M. The effect of anthocyanins supplementation on liver enzymes: A systematic review and meta-analysis of randomized clinical trials. Food Sci. Nutr. 2021, 9, 3954–3970. [Google Scholar] [CrossRef] [PubMed]
- Daneshzad, E.; Shab-Bidar, S.; Mohammadpour, Z.; Djafarian, K. Effect of anthocyanin supplementation on cardio-metabolic biomarkers: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. 2019, 38, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; An, J.; Chen, M.; Yang, H.; Ma, Y. Effect of proanthocyanidins on blood pressure: A systematic review and meta-analysis of randomized controlled trials. Pharmacol. Res. 2021, 165, 105329. [Google Scholar] [CrossRef]
- Fujiwara, T.; Nishida, N.; Nota, J.; Kitani, T.; Aoishi, K.; Takahashi, H.; Sugahara, T.; Hato, N. Efficacy of chlorophyll c2 for seasonal allergic rhinitis: Single-center double-blind randomized control trial. Eur. Arch. Oto-Rhino-Laryngol. 2016, 273, 4289–4294. [Google Scholar] [CrossRef]
- Montelius, C.; Erlandsson, D.; Vitija, E.; Stenblom, E.-L.; Egecioglu, E.; Erlanson-Albertsson, C. Body weight loss, reduced urge for palatable food and increased release of GLP-1 through daily supplementation with green-plant membranes for three months in overweight women. Appetite 2014, 81, 295–304. [Google Scholar] [CrossRef]
- Balder, H.F.; Vogel, J.; Jansen, M.C.; Weijenberg, M.P.; van den Brandt, P.A.; Westenbrink, S.; van der Meer, R.; Goldbohm, R.A. Heme and chlorophyll intake and risk of colorectal cancer in the Netherlands cohort study. Cancer Epidemiol. Prev. Biomark. 2006, 15, 717–725. [Google Scholar] [CrossRef]
- Ministry of Health. Eating and Activity Guidelines for New Zealand Adults: Updated 2020; Ministry of Health: Wellington, New Zealand, 2020.
- Public Health England. The Eatwell Guide; PHE Publications: London, UK, 2018.
- Wallace, T.C.; Bailey, R.L.; Blumberg, J.B.; Burton-Freeman, B.; Chen, C.O.; Crowe-White, K.M.; Drewnowski, A.; Hooshmand, S.; Johnson, E.; Lewis, R.; et al. Fruits, vegetables, and health: A comprehensive narrative, umbrella review of the science and recommendations for enhanced public policy to improve intake. Crit. Rev. Food Sci. Nutr. 2020, 60, 2174–2211. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. Food-Based Dietary Guidelines. Available online: https://www.fao.org/nutrition/nutrition-education/food-dietary-guidelines/en/ (accessed on 5 April 2022).
- Johra, F.T.; Bepari, A.K.; Bristy, A.T.; Reza, H.M. A Mechanistic Review of β-Carotene, Lutein, and Zeaxanthin in Eye Health and Disease. Antioxidants 2020, 9, 1046. [Google Scholar] [CrossRef]
- Panche, A.N.; Diwan, A.D.; Chandra, S.R. Flavonoids: An overview. J. Nutr. Sci. 2016, 5, e47. [Google Scholar] [CrossRef] [PubMed]
- Atrahimovich, D.; Avni, D.; Khatib, S. Flavonoids-Macromolecules Interactions in Human Diseases with Focus on Alzheimer, Atherosclerosis and Cancer. Antioxidants 2021, 10, 423. [Google Scholar] [CrossRef] [PubMed]
- Corrêa, T.A.F.; Rogero, M.M.; Hassimotto, N.M.A.; Lajolo, F.M. The Two-Way Polyphenols-Microbiota Interactions and Their Effects on Obesity and Related Metabolic Diseases. Front. Nutr. 2019, 6, 188. [Google Scholar] [CrossRef] [PubMed]
- Pengpid, S.; Peltzer, K. The prevalence and social determinants of fruit and vegetable consumption among adults in Kenya: A cross-sectional national population-based survey, 2015. Pan Afr. Med. J. 2018, 31, 137. [Google Scholar] [CrossRef]
- Singh, J.K.; Acharya, D.; Gautam, S.; Adhikari, M.; Park, J.H.; Yoo, S.J.; Lee, K. Socio-Demographic and Diet-Related Factors Associated with Insufficient Fruit and Vegetable Consumption among Adolescent Girls in Rural Communities of Southern Nepal. Int. J. Environ. Res. Public Health 2019, 16, 2145. [Google Scholar] [CrossRef]
- Marshall, S.; Petocz, P.; Duve, E.; Abbott, K.; Cassettari, T.; Blumfield, M.; Fayet-Moore, F. The Effect of Replacing Refined Grains with Whole Grains on Cardiovascular Risk Factors: A Systematic Review and Meta-Analysis of Randomized Controlled Trials with GRADE Clinical Recommendation. J. Acad. Nutr. Diet 2020, 120, 1859–1883.e1831. [Google Scholar] [CrossRef]
- National Health and Medical Research Council. NHMRC Levels of Evidence and Grades for Recommendations for Developers of Guidelines; National Health and Medical Research Council: Canberra, Australia, 2009.
- Fortmann, S.P.; Burda, B.U.; Senger, C.A.; Lin, J.S.; Whitlock, E.P. Vitamin and mineral supplements in the primary prevention of cardiovascular disease and cancer: An updated systematic evidence review for the U.S. Preventive Services Task Force. Ann. Intern. Med. 2013, 159, 824–834. [Google Scholar] [CrossRef]
- Vivekananthan, D.P.; Penn, M.S.; Sapp, S.K.; Hsu, A.; Topol, E.J. Use of antioxidant vitamins for the prevention of cardiovascular disease: Meta-analysis of randomised trials. Lancet 2003, 361, 2017–2023. [Google Scholar] [CrossRef]
- Black, H.S. Pro-oxidant and anti-oxidant mechanism(s) of BHT and beta-carotene in photocarcinogenesis. Front. Biosci. 2002, 7, d1044–d1055. [Google Scholar] [CrossRef][Green Version]
- McManus, K.D. Phytonutrients: Paint Your Plate with the Colours of the Rainbow; Harvard Health Publishing: Cambridge, MA, USA, 2019. [Google Scholar]
- Davidson, K. Eating the Rainbow—Is It Useful and Should You Try It? 2020. Available online: https://www.bhf.org.uk/ (accessed on 5 May 2022).
- British Heart Foundation. Should You Eat a Rainbow of Fruits and Vegetables? British Heart Foundation: High Street, UK, 2022; Available online: https://www.bhf.org.uk/informationsupport/heart-matters-magazine/nutrition/5-a-day/colourful-foods (accessed on 5 May 2022).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar]
- Clark, J.M.; Sanders, S.; Carter, M.; Honeyman, D.; Cleo, G.; Auld, Y.; Booth, D.; Condron, P.; Dalais, C.; Bateup, S.; et al. Improving the translation of search strategies using the Polyglot Search Translator: A randomized controlled trial. J. Med. Libr. Assoc. 2020, 108, 195–207. [Google Scholar] [CrossRef] [PubMed]
- Pieper, D.; Antoine, S.-L.; Mathes, T.; Neugebauer, E.A.; Eikermann, M. Systematic review finds overlapping reviews were not mentioned in every other overview. J. Clin. Epidemiol. 2014, 67, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Handbook for Grading the Quality of Evidence and the Strength of Recommendations Using the GRADE Approach. Updated October 2013. Available online: https://gdt.gradepro.org/app/ (accessed on 1 September 2021).
- Systematic Reviews Critical Appraisal Sheet; Centre for Evidence-Based Medicine, Unviersity of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools (accessed on 5 May 2022).
- RCT Critical Appraisal Sheet; Centre for Evidence-Based Medicine, Unviersity of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools (accessed on 5 May 2022).
- Prognosis Critical Appraisal Sheet; Centre for Evidence-Based Medicine, Unviersity of Oxford. Available online: https://www.cebm.ox.ac.uk/resources/ebm-tools/critical-appraisal-tools (accessed on 5 May 2022).
| Pigment Class | Pigment Subclass | Pigment Minor Subclass | Typical Colors |
|---|---|---|---|
| Carotenoids | Lycopene | - | Red |
| Beta-cryptoxanthin | |||
| Capsorubin | |||
| Capsanthin | |||
| Beta-carotene | - | Orange | |
| Alpha-carotene | - | Yellow | |
| Lutein | |||
| Zeaxanthin | |||
| Violaxanthin | |||
| Flavonoids | Anthocyanins/ | Cyanidin | Red, purple, blue |
| anthocyanidins | Malvidin | ||
| Peonidin | |||
| Delphinidin | |||
| Pelargonidin | |||
| Petunidin | |||
| Aurones | Kaempferol | Pale yellow | |
| Chalcones | Quercetin | ||
| Flavonols | Myricetin | ||
| Flavones | Apigenin | White | |
| Luteolin | |||
| Isoetin | |||
| Tannins | Proanthocyanidins | Red, purple, blue, brown | |
| Proanthocyanins | |||
| Betalains | Betacyanins | Betanin | Red, violet, orange, yellow |
| Betaxanthin | Indicaxanthan | ||
| Vulgaxanthin | |||
| Chlorophylls | Chlorophyll a and b | - | Green |
| Bioactive Pigment Color | Highly Unique Health Effects a,c | Unique Health Effects b,c |
|---|---|---|
| Red/orange/yellow | ↑ cognitive function (GRADE: medium) ↓ risk of IHD (GRADE: very low) ↑ HDL cholesterol (GRADE: high) ↓ waist circumference (GRADE: low to medium) | |
| Red | ↓ risk of cervical cancer (GRADE: very low) ↓ risk of lung cancer (GRADE: very low) ↓ risk of pancreatic cancer (GRADE: very low) ↓ risk of pharyngeal cancer (GRADE: very low to medium) ↓ risk of hip fracture (GRADE: very low) ↓ risk of laryngeal cancer (GRADE: very low to medium) | |
| Orange | ↓ risk of preeclampsia (GRADE: very low) ↓ risk of total fracture (GRADE: very low) ↓ endometrial cancer (GRADE: very low) | ↓ risk of non-Hodgkin lymphoma (GRADE: very low) ↓ risk of ovarian cancer (GRADE: very low) ↓ risk of cervical cancer (GRADE: very low) ↓ risk of pancreatic cancer (GRADE: very low) ↓ risk of cataract (GRADE: very low) ↓ risk of hip fracture (GRADE: very low) ↓ risk of laryngeal cancer (GRADE: very low to medium) |
| Yellow | ↓ risk of non-Hodgkin lymphoma (GRADE: very low) ↓ risk of cataract (GRADE: very low) ↓ risk of pharyngeal cancer (GRADE: very low) | |
| Pale-yellow | ↑ exercise performance (GRADE: very low) | ↓ risk of ovarian cancer (GRADE: low) ↓ risk of cervical cancer (GRADE: very low) ↓ blood pressure (GRADE: low to high) ↓ glycemic biomarkers (GRADE: medium) ↓ risk of smoking-related cancers (GRADE: very low) |
| White | ↓ risk of liver cancer (GRADE: medium) | ↓ risk of smoking-related cancers (GRADE: medium) ↓ risk of esophageal cancers (GRADE: very low) |
| Blue/purple | ↓ risk of hypertension (GRADE: very low) ↓ oxidative stress biomarkers (GRADE: very low to low) ↓ insulinemic biomarkers (GRADE: medium) ↓ vascular function (GRADE: very low to medium) ↓ arterial pressure (GRADE: high) | ↓ glycemic biomarkers (GRADE: medium) ↓ risk of esophageal cancers (GRADE: very low) ↓ blood pressure (GRADE: high) |
| Green | ↓ seasonal rhinitis (GRADE: N/A) |
| PICOS Elements | Inclusion Criteria | Exclusion Criteria |
|---|---|---|
| Participant/ population | Humans | Animal and in vitro |
| Intervention/ exposure | Natural pigments found in fruits and vegetables that contribute to their visible color (as described in Table 1). The pigment must be: (1) consumed through whole fruit or vegetable; (2) extract from fruits or vegetables; or (3) provided as a supplement derived from fruits or vegetables. SLRs which included a mix of natural and synthetic bioactive pigments, or the derivation of the bioactive pigment was not described, were included. | Pigments within pharmaceuticals or synthetic forms. Pigments sourced from non-fruit or vegetable foods (e.g., nuts, soy, tea). Nutrients or phytonutrients that are not pigments and do not contribute to the visible color of the FV, but may be high in concentration in FV of a particular color (e.g., folate in green fruits and vegetables). Pigments delivered as a co-intervention or administered via non-oral routes (e.g., topical, aromatherapy, moxibustion). |
| Comparator | Placebo, presence of the pigment versus no pigment, or varying levels of the pigment (comparison of high versus low). | No control or comparator group. Alternative intervention. |
| Outcome | Health-related outcomes relevant to population health including the prevention of disease and optimization of disease risk factors, general wellbeing, function (cognitive function, physical function, and exercise performance), growth and development in children, maternal and neonatal health. | Biomarkers of pigment intake, disease treatment (e.g., cancer treatment), in-born errors of metabolism, biomarkers not related to disease prevention. |
| Study design/ source | SLRs with MAs of RCTs and/or cohort studies. RCTs and/or cohort studies if no eligible SLRs available. Case–control studies were included if based on longitudinal data. | SLRs without MAs, cross-sectional studies, single arm interventions, narrative reviews, expert opinion articles, or consensus guidelines. Studies unable to be translated into English via Google Translate or manual translation by multilingual colleagues. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blumfield, M.; Mayr, H.; De Vlieger, N.; Abbott, K.; Starck, C.; Fayet-Moore, F.; Marshall, S. Should We ‘Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables. Molecules 2022, 27, 4061. https://doi.org/10.3390/molecules27134061
Blumfield M, Mayr H, De Vlieger N, Abbott K, Starck C, Fayet-Moore F, Marshall S. Should We ‘Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables. Molecules. 2022; 27(13):4061. https://doi.org/10.3390/molecules27134061
Chicago/Turabian StyleBlumfield, Michelle, Hannah Mayr, Nienke De Vlieger, Kylie Abbott, Carlene Starck, Flavia Fayet-Moore, and Skye Marshall. 2022. "Should We ‘Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables" Molecules 27, no. 13: 4061. https://doi.org/10.3390/molecules27134061
APA StyleBlumfield, M., Mayr, H., De Vlieger, N., Abbott, K., Starck, C., Fayet-Moore, F., & Marshall, S. (2022). Should We ‘Eat a Rainbow’? An Umbrella Review of the Health Effects of Colorful Bioactive Pigments in Fruits and Vegetables. Molecules, 27(13), 4061. https://doi.org/10.3390/molecules27134061






