Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off–On” High-Sensitivity Detection of Fe3+ and L-Cysteine
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization of the Preparation Conditions of CQDs
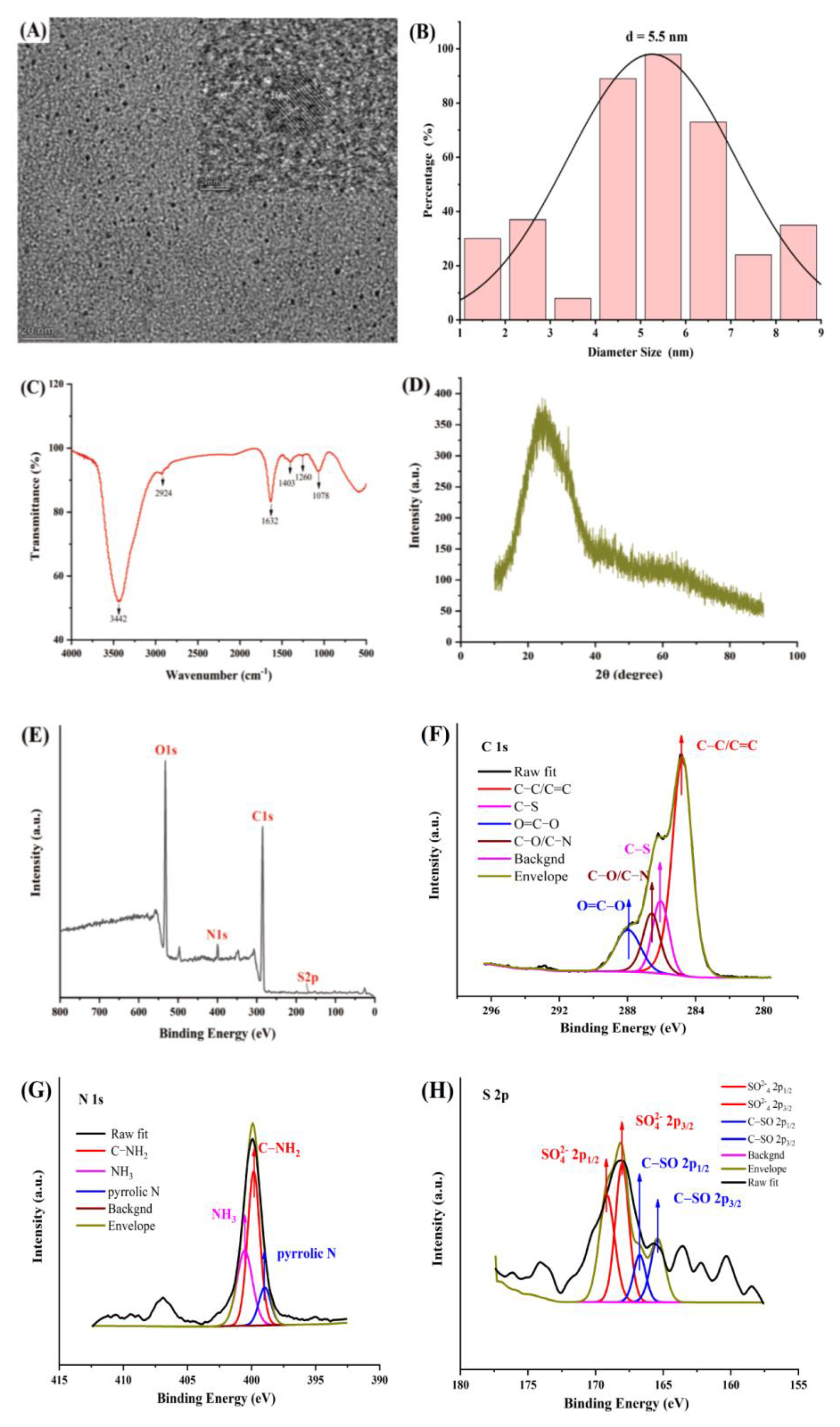
2.2. Characterization
2.3. Optical Properties of CQDs
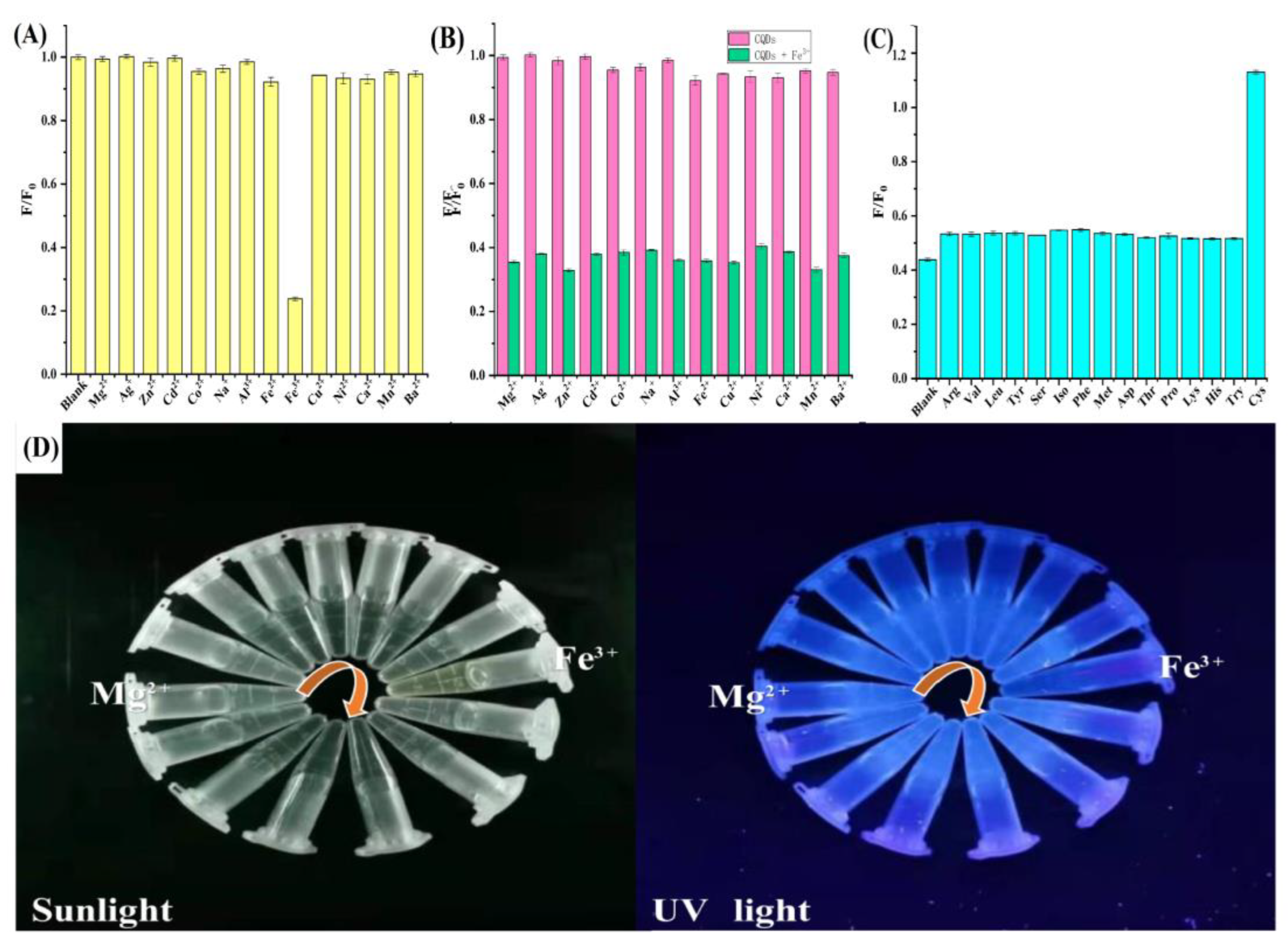
2.4. Selectivity and Sensitivity
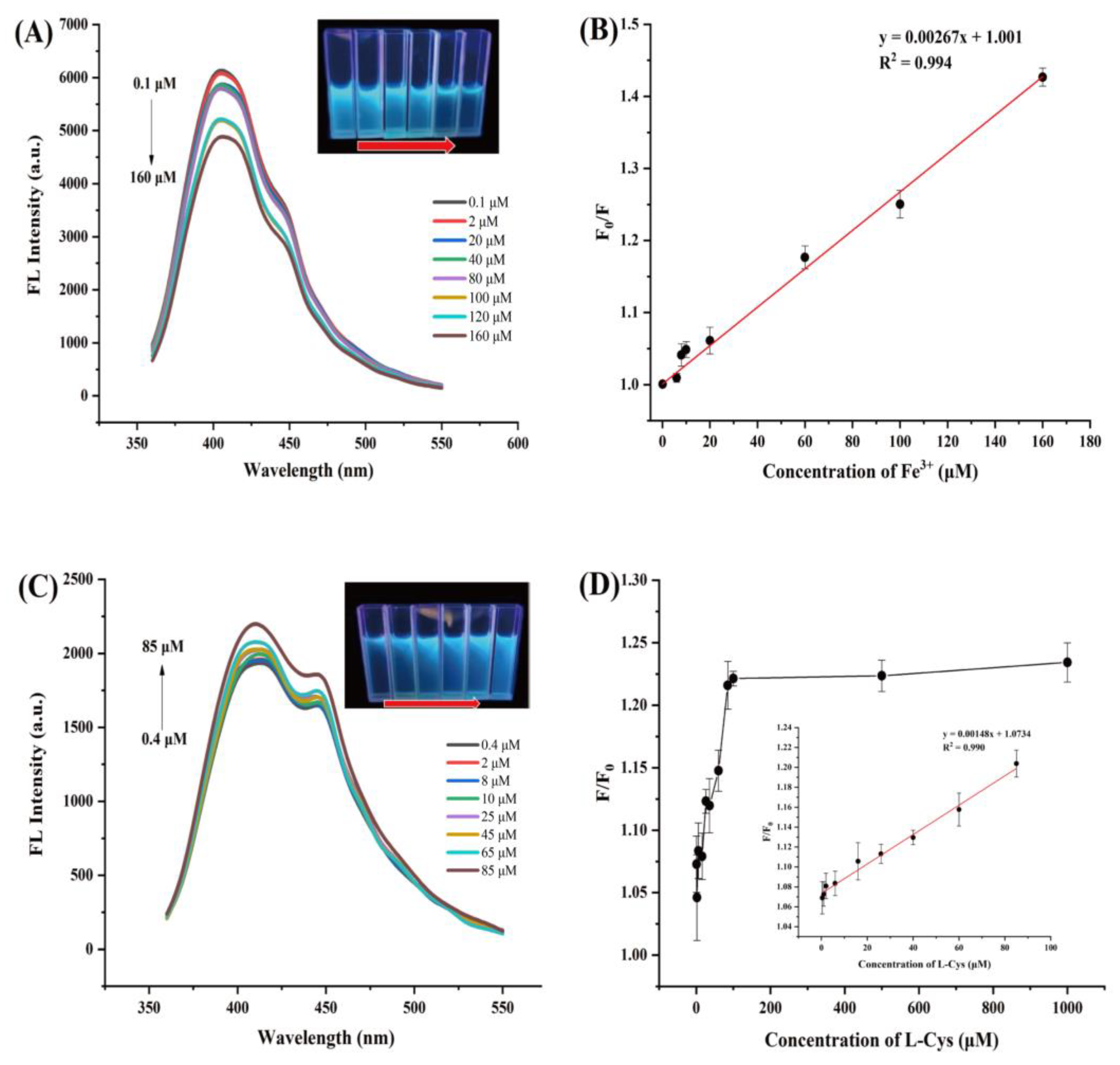
2.5. Optosensing of Fe3+ and L-Cys
2.6. Possible Mechanism of “Turn-Off–On” Mode
2.7. Visualization Cellulose/CQDs Composite Hydrogels and Test Paper
3. Materials and Methods
3.1. Materials
3.2. Instruments
3.3. Synthesis of CQDs
3.4. QY of CQDs
3.5. Fluorescence Stability of CQDs
3.6. Selectivity and Interference Measurements of CQDs
3.7. Sensitive Detection of Fe3+ and L-Cys
3.8. Fe3+ and L-Cys Detection in Real Samples
3.9. Preparation of Cellulose/CQDs Composite Hydrogels
3.10. Preparation of CQD-based Test Papers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Xu, X.; Ray, R.; Gu, Y.; Ploehn, H.J.; Gearheart, L.; Raker, K.; Scrivens, W.A. Electrophoretic analysis and purification of fluorescent single-walled carbon nanotube fragments. J. Am. Chem. Soc. 2004, 126, 12736–12737. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.; Shen, W.; Gao, Z.Q. Carbon quantum dots and their applications. Chem. Soc. Rev. 2015, 44, 362–381. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Zhang, L.; Jiang, K.; Wu, A.; Lin, H. Toward high-efficient red emissive carbon dots: Facile preparation, unique properties, and applications as multifunctional theranostic agents. Chem. Mater. 2016, 28, 8659–8668. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Yu, S.; Jiang, C. Fluorescent carbon dots: Rational synthesis, tunable optical properties and analytical applications. RSC Adv. 2017, 7, 40973–40989. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Yuan, X.; Han, L.; Liu, H.; Sun, B. A smartphone-integrated optosensing platform based on red-emission carbon dots for real-time detection of pyrethroids. Biosens. Bioelectron. 2021, 191, 113460. [Google Scholar] [CrossRef]
- Hutton, G.A.M.; Reuillard, B.; Martindale, B.C.M.; Caputo, C.A.; Lockwood, C.W.J.; Butt, J.N.; Reisner, E. Carbon dots as versatile photosensitizers for solar-driven catalysis with redox enzymes. J. Am. Chem. Soc. 2016, 138, 16722–16730. [Google Scholar] [CrossRef] [Green Version]
- Kumar, P.; Bohidar, H.B. Physical and fluorescent characteristics of non-functionalized carbon nanoparticles from candle soot. J. Nanopart. Res. 2012, 14, 948. [Google Scholar] [CrossRef]
- Zhao, S.; Lan, M.; Zhu, X.; Xue, H.; Ng, T.; Meng, X.; Lee, C.; Wang, P.; Zhang, W. Green synthesis of bifunctional fluorescent carbon dots from garlic for cellular imaging and free radical scavenging. ACS Appl. Mater. Interfaces 2015, 7, 17054–17060. [Google Scholar] [CrossRef]
- Wang, G.; Guo, Q.; Chen, D.; Liu, Z.; Zheng, X.; Xu, A.; Yang, S.; Ding, G. Facile and highly effective synthesis of controllable lattice sulfur-doped graphene quantum dots via hydrothermal treatment of durian. ACS Appl. Mater. Interfaces 2018, 10, 5750–5759. [Google Scholar] [CrossRef]
- Yuan, X.; Jiang, W.; Wang, J.; Liu, H.; Sun, B. High-performance multiporous imprinted microspheres based on N-doped carbon dots exfoliated from covalent organic framework for flonicamid optosensing. ACS Appl. Mater. Interfaces 2020, 12, 25150–25158. [Google Scholar] [CrossRef]
- Huang, S.; Yang, E.; Yao, J.; Liu, Y.; Xiao, Q. Red emission nitrogen, boron, sulfur co-doped carbon dots for “on-off-on” fluorescent mode detection of Ag+ ions and L-cysteine in complex biological fluids and living cells. Anal. Chim. Acta 2018, 1035, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Yan, F.; Shi, D.; Zheng, T.; Yun, K.; Zhou, X.; Chen, L. Carbon dots as nanosensor for sensitive and selective detection of Hg2+ and L-cysteine by means of fluorescence “off-on” switching. Sens. Actuator B Chem. 2016, 224, 926–935. [Google Scholar] [CrossRef]
- Zong, J.; Yang, X.; Trinchi, A.; Hardin, S.; Cole, I.; Zhu, Y.; Li, C.; Muster, T.; Wei, G. Carbon dots as fluorescent probes for “off-on” detection of Cu2+ and L-cysteine in aqueous solution. Biosens. Bioelectron. 2014, 51, 330–335. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.; Hou, C.; Fa, H.; Zhang, L.; Ma, Y.; Dong, L.; Li, D.; Huo, D.; Yang, M. An efficient fluorescent probe for fluazinam using N, S co-doped carbon dots from L-cysteine. Sens. Actuator B-Chem. 2017, 239, 1033–1041. [Google Scholar] [CrossRef]
- Lars, J. Hazards of heavy metal contamination. Br. Med. Bull. 2003, 68, 167–182. [Google Scholar]
- Liu, Y.; Lv, X.; Hou, M.; Shi, Y.; Guo, W. Selective fluorescence detection of cysteine over homocysteine and glutathione based on a cysteine-triggered dual michael addition/retro-aza-aldol cascade reaction. Anal. Chem. 2015, 87, 11475–11483. [Google Scholar] [CrossRef]
- Zhou, Y.; Yoon, J. Recent progress in fluorescent and colorimetric chemosensors for detection of amino acids. Chem. Soc. Rev. 2012, 41, 52–67. [Google Scholar] [CrossRef]
- Mashhadizadeh, M.H.; Azimi, M.S.; Pesteh, M.; Sheikhshoaei, I.; Ardakani, M.M.; Karimi, M.A. Flame atomic absorption spectrometric determination of μg amounts of Fe(III) ions after solid phase extraction using modified octadecyl silica membrane disks. Spectrochim. Acta Part B At. Spectrosc. 2008, 63, 889–892. [Google Scholar] [CrossRef]
- Matusch, A.; Depboylu, C.; Palm, C.; Wu, B.; Hoglinger, G.U.; Schafer, M.K.H.; Becker, J.S. Cerebral bioimaging of Cu, Fe, Zn, and Mn in the MPTP mouse model of Parkinson’s disease using laser ablation inductively coupled plasma mass spectrometry (LA-ICP-MS). J. Am. Soc. Mass Spectrom. 2010, 21, 161–171. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Li, P.; Geng, Q.; Duan, Y.; Guo, M.; Cao, Y. Simultaneous determination of glutathione, cysteine, homocysteine, and cysteinylglycine in biological fluids by ion-pairing high-performance liquid chromatography coupled with precolumn derivatization. J. Agric. Food Chem. 2014, 62, 5845–5852. [Google Scholar] [CrossRef]
- Sun, D.; Liu, T.; Wang, C.; Yang, L.; Yang, S.; Zhuo, K. Hydrothermal synthesis of fluorescent carbon dots from gardenia fruit for sensitive on-off-on detection of Hg2+ and cysteine. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2020, 240, 118598. [Google Scholar] [CrossRef] [PubMed]
- Tian, T.; He, Y.; Ge, Y.; Song, G. One-pot synthesis of boron and nitrogen codoped carbon dots as the fluorescence probe for dopamine based on the redox reaction between Cr(VI) and dopamine. Sens. Actuator B Chem. 2017, 240, 1265–1271. [Google Scholar] [CrossRef]
- Yuan, F.; Wang, Z.; Li, X.; Li, Y.; Tan, Z.; Fan, L.; Yang, S. Bright multicolor bandgap fluorescent carbon quantum dots for electroluminescent light-emitting diodes. Adv. Mater. 2017, 29, 1604436. [Google Scholar] [CrossRef] [PubMed]
- Ensafi, A.A.; Sefat, S.H.; Kazemifard, N.; Rezaei, B.; Moradi, F. A novel one-step and green synthesis of highly fluorescent carbon dots from saffron for cell imaging and sensing of prilocaine. Sens. Actuator B Chem. 2017, 253, 451–460. [Google Scholar] [CrossRef]
- Yu, J.; Song, N.; Zhang, Y.; Zhong, S.; Wang, A.; Chen, J. Green preparation of carbon dots by Jinhua bergamot for sensitive and selective fluorescent detection of Hg2+ and Fe3+. Sens. Actuator B Chem. 2015, 214, 29–35. [Google Scholar] [CrossRef]
- Yang, R.; Guo, X.; Jia, L.; Zhang, Y.; Zhao, Z.; Lonshakov, F. Green preparation of carbon dots with mangosteen pulp for the selective detection of Fe3+ ions and cell imaging. Appl. Surf. Sci. 2017, 423, 426–432. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Hu, S.; Huang, Q.; Wei, C.; Zhang, W.; Kang, L.; Huang, Z.; Hao, A. Fluorescent probes for “off-on” sensitive and selective detection of mercury ions and L-cysteine based on graphitic carbon nitride nanosheets. J. Mater. Chem. C 2015, 3, 2093–2100. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, Y.; Wang, P.; Yang, Y.; Wang, Y.; Xu, J.; Wang, Y.; Yu, W. Synthesis of nitrogen and sulfur co-doped carbon dots from garlic for selective detection of Fe3+. Nanoscale Res. Lett. 2016, 11, 110. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, B.; You, T. Nitrogen and sulfur co-doped carbon dots for highly selective and sensitive detection of Hg(Ⅱ) ions. Biosens. Bioelectron. 2015, 74, 263–269. [Google Scholar] [CrossRef]
- Lan, M.; Zhang, J.; Chui, Y.; Wang, H.; Yang, Q.; Zhu, X.; Wei, H.; Liu, W.; Ge, J.; Wang, P.; et al. A recyclable carbon nanoparticle-based fluorescent probe for highly selective and sensitive detection of mercapto biomolecules. J. Mater. Chem. B 2015, 3, 127–134. [Google Scholar] [CrossRef]
- Jiao, Y.; Gao, Y.; Meng, Y.; Lu, W.; Liu, Y.; Han, H.; Shuang, S.; Li, L.; Dong, C. One-step synthesis of label-free ratiometric fluorescence carbon dots for the detection of silver ions and glutathione and cellular imaging applications. ACS Appl. Mater. Interfaces 2019, 11, 16822–16829. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Lu, Q.; Hou, Y.; Liu, Y.; Zhang, Y. High fluorescence S, N co-doped carbon dots as an ultra-sensitive fluorescent probe for the determination of uric acid. Talanta 2016, 155, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Zhang, R.; He, S.; Chen, W. Nitrogen-doped graphene quantum dots-based fluorescent probe for the sensitive turn-on detection of glutathione and its cellular imaging. RSC Adv. 2014, 4, 52583–52589. [Google Scholar] [CrossRef]
- Venkateswarlu, S.; Viswanath, B.; Reddy, A.S.; Yoon, M.Y. Fungus-derived photoluminescent carbon nanodots for ultrasensitive detection of Hg2+ ions and photoinduced bactericidal activity. Sens. Actuator B Chem. 2018, 258, 172–183. [Google Scholar] [CrossRef]
- Wang, W.; Kim, T.; Yan, Z.; Zhu, G.; Cole, I.; Nam-Trung, N.; Li, Q. Carbondots functionalized by organosilane with double-sided anchoring for nanomolar Hg2+ detection. J. Colloid Interface Sci. 2015, 437, 28–34. [Google Scholar] [CrossRef]
- Lu, M.; Duan, Y.; Song, Y.; Tan, J.; Zhou, L. Green preparation of versatile nitrogen-doped carbon quantum dots from watermelon juice for cell imaging, detection of Fe3+ ions and cysteine, and optical thermometry. J. Mol. Liq. 2018, 269, 766–774. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, Y.; Zhang, D.; Zhu, X.; Liu, H.; Sun, B. Efficient and robust dual modes of fluorescence sensing and smartphone readout for the detection of pyrethroids using artificial receptors bound inside a covalent organic framework. Biosens. Bioelectron. 2021, 194, 113582. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Zhang, F.; Liao, Y.; Wang, F.; Liu, H. Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off–On” High-Sensitivity Detection of Fe3+ and L-Cysteine. Molecules 2022, 27, 4099. https://doi.org/10.3390/molecules27134099
Zhang D, Zhang F, Liao Y, Wang F, Liu H. Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off–On” High-Sensitivity Detection of Fe3+ and L-Cysteine. Molecules. 2022; 27(13):4099. https://doi.org/10.3390/molecules27134099
Chicago/Turabian StyleZhang, Dianwei, Furui Zhang, Yonghong Liao, Fenghuan Wang, and Huilin Liu. 2022. "Carbon Quantum Dots from Pomelo Peel as Fluorescence Probes for “Turn-Off–On” High-Sensitivity Detection of Fe3+ and L-Cysteine" Molecules 27, no. 13: 4099. https://doi.org/10.3390/molecules27134099





