Identification of a Hydrogen-Sulfide-Releasing Isochroman-4-One Hybrid as a Cardioprotective Candidate for the Treatment of Cardiac Hypertrophy
Abstract
:1. Introduction
2. Results
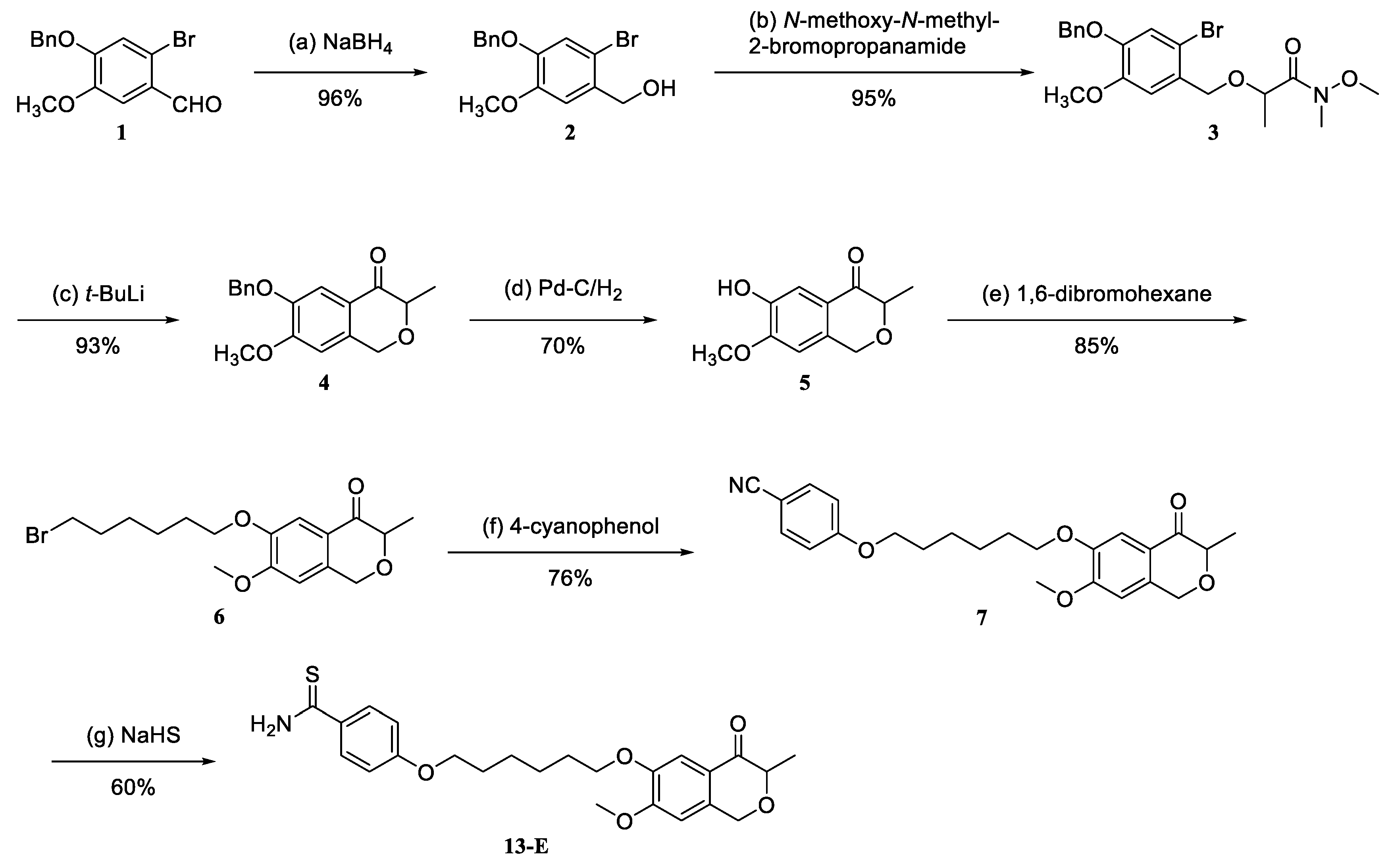
2.1. Synthesis and Characterization of Hybrid 13-E
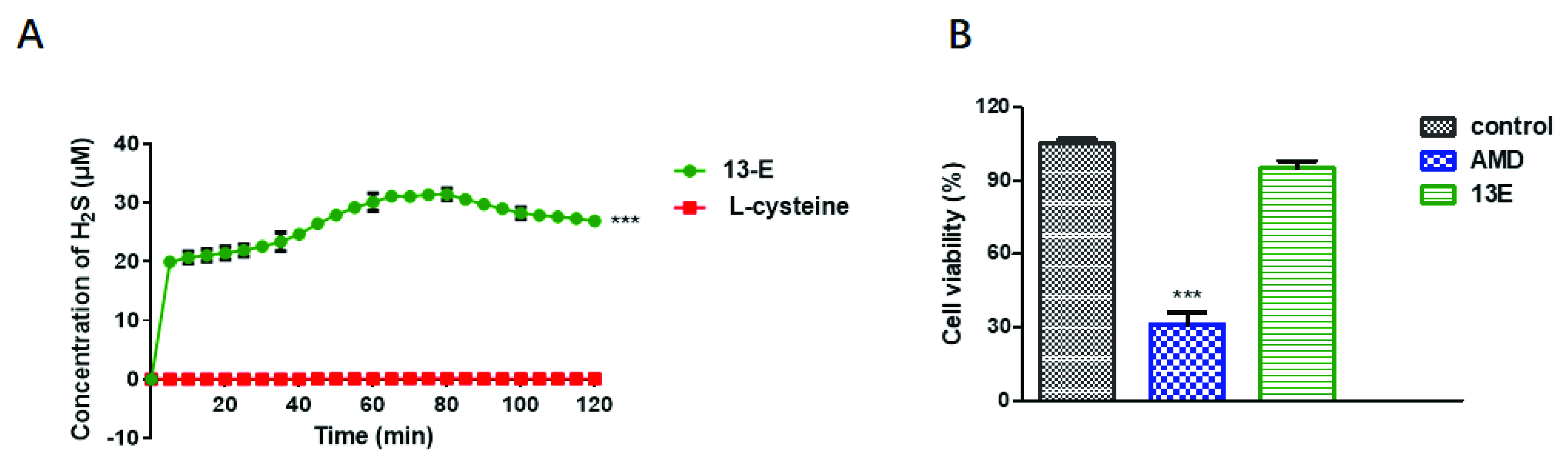
2.2. H2S-Releasing Capability and Safety of 13-E
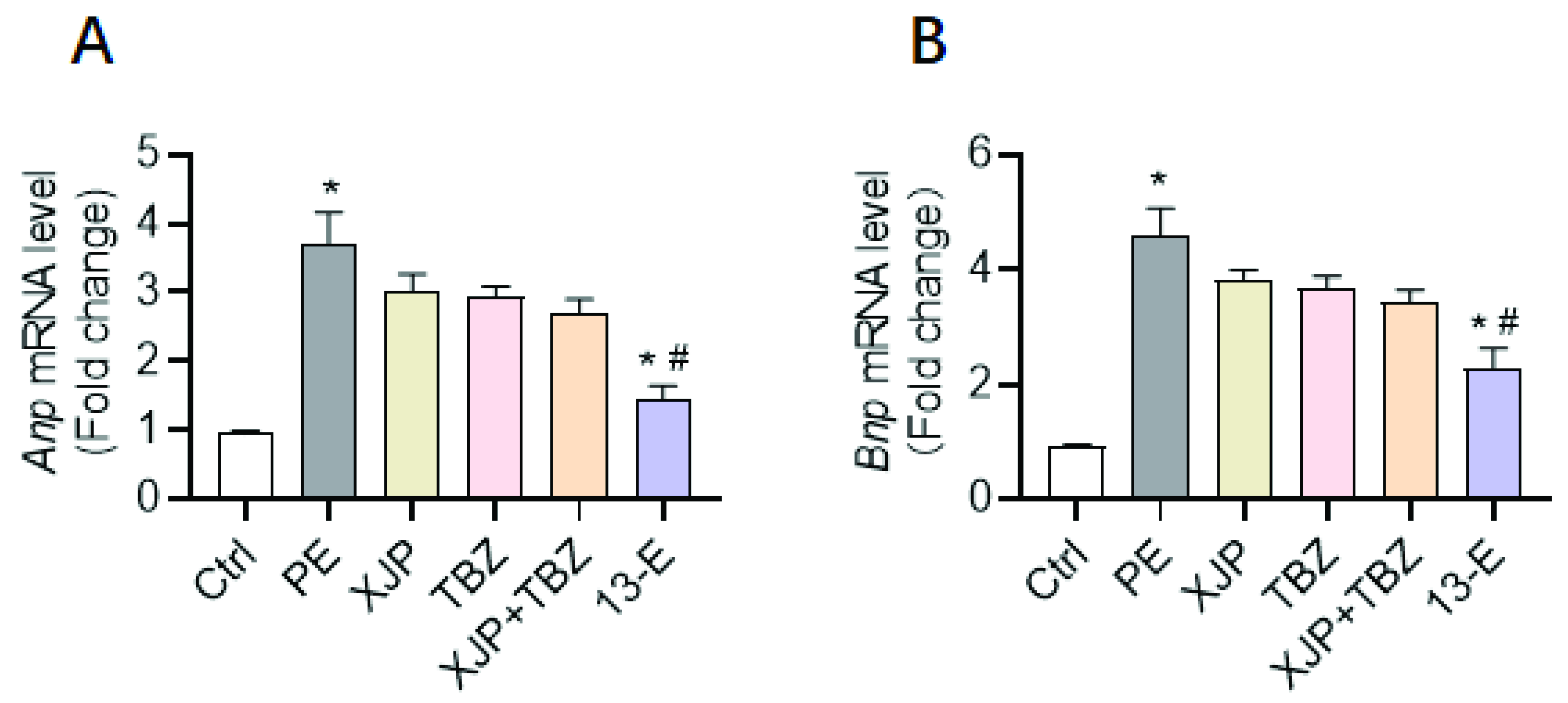
2.3. 13-E Protects against Cardiomyocyte Hypertrophy In Vitro
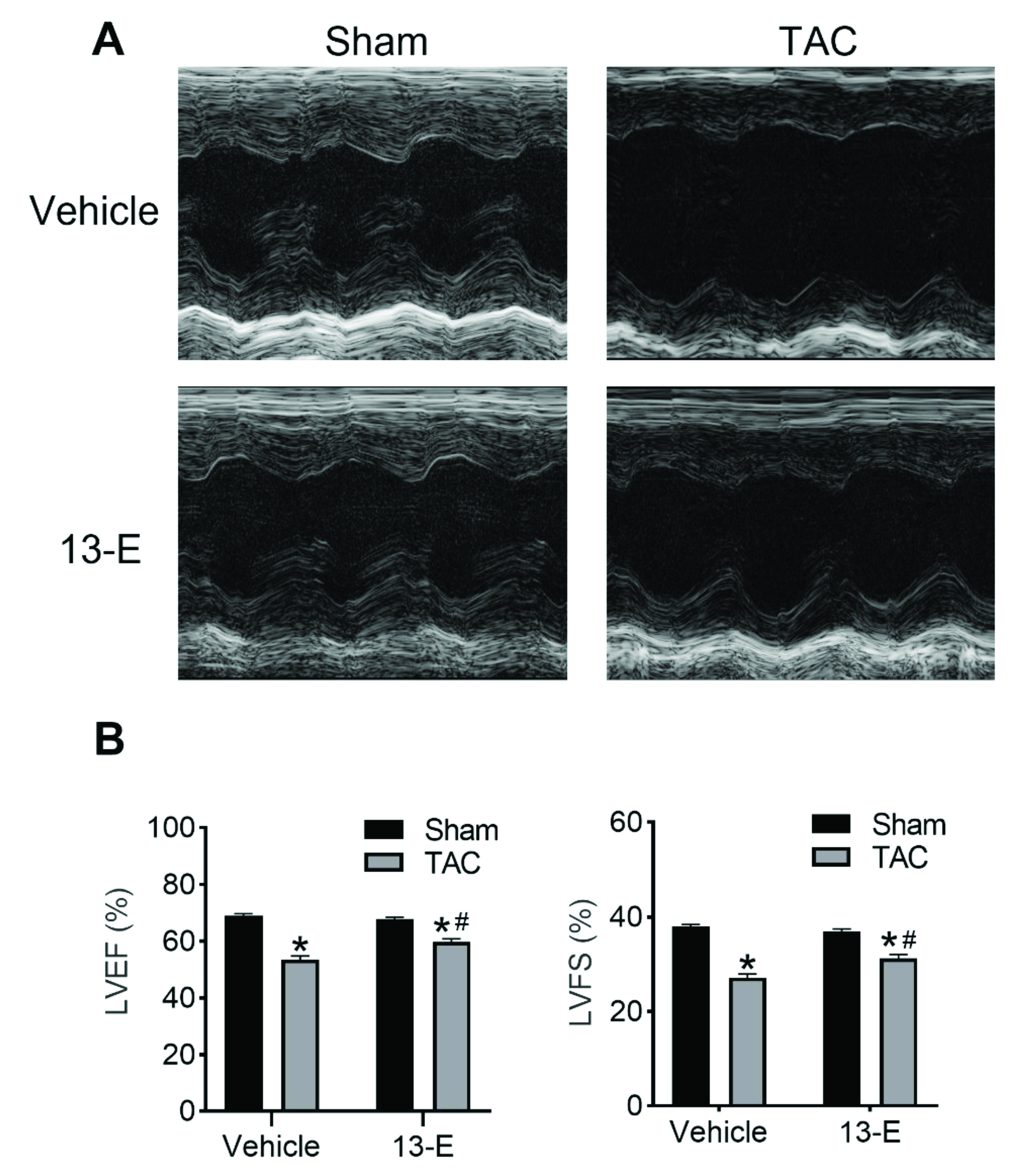
2.4. 13-E Restores Cardiac Function
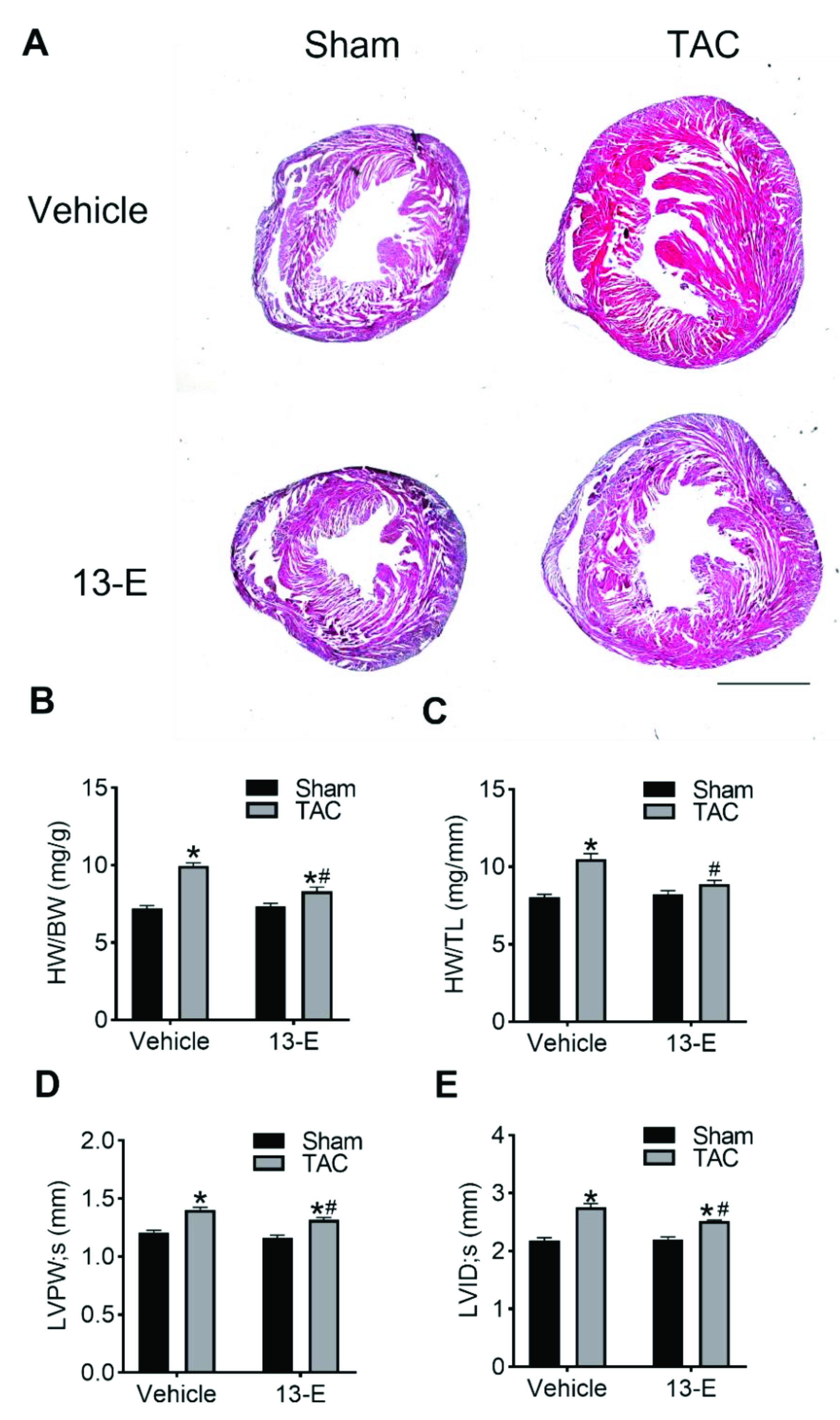
2.5. 13-E Reduces TAC-Induced Cardiac Hypertrophy
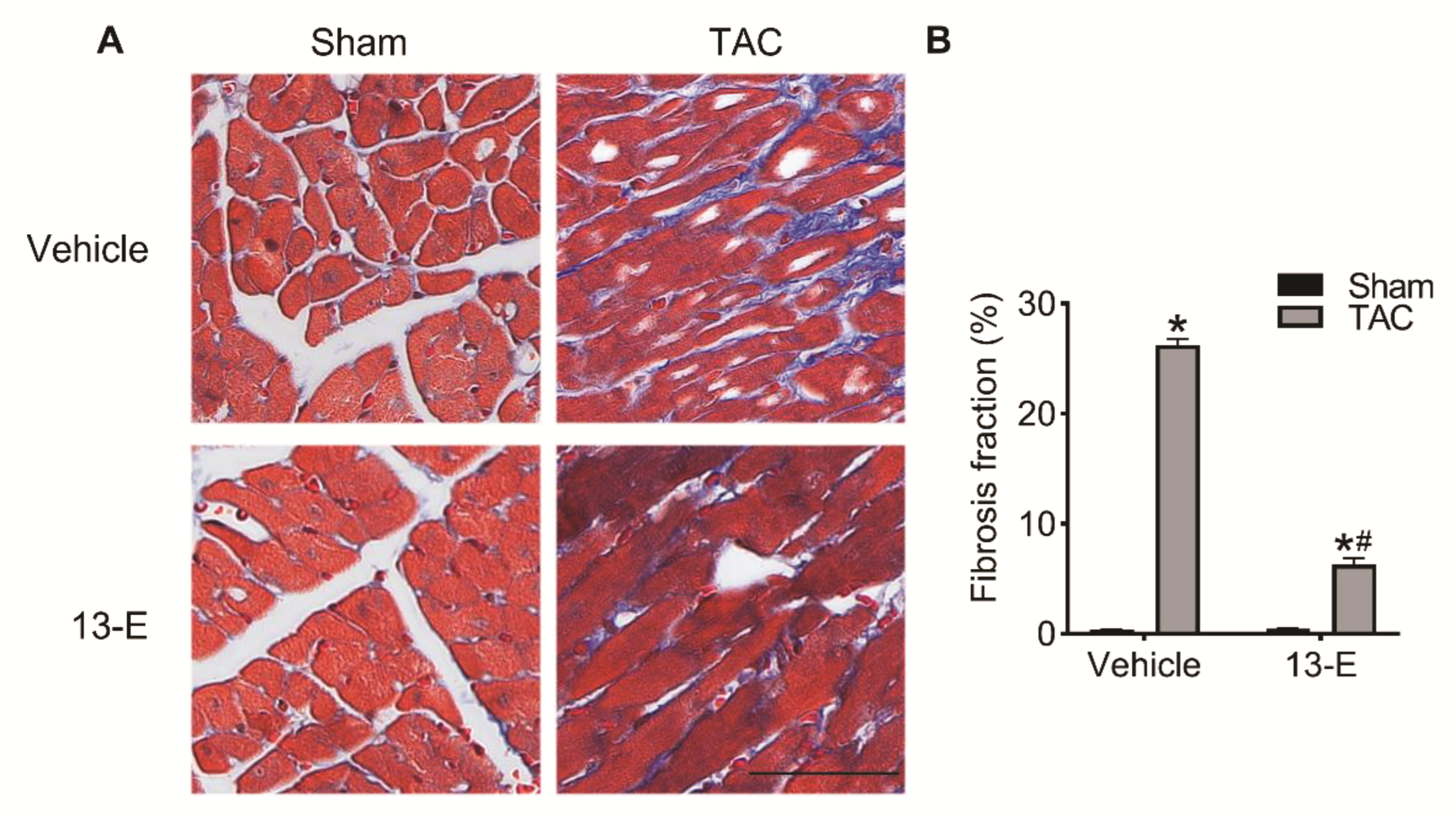
2.6. 13-E Alleviates Cardiac Interstitial Fibrosis
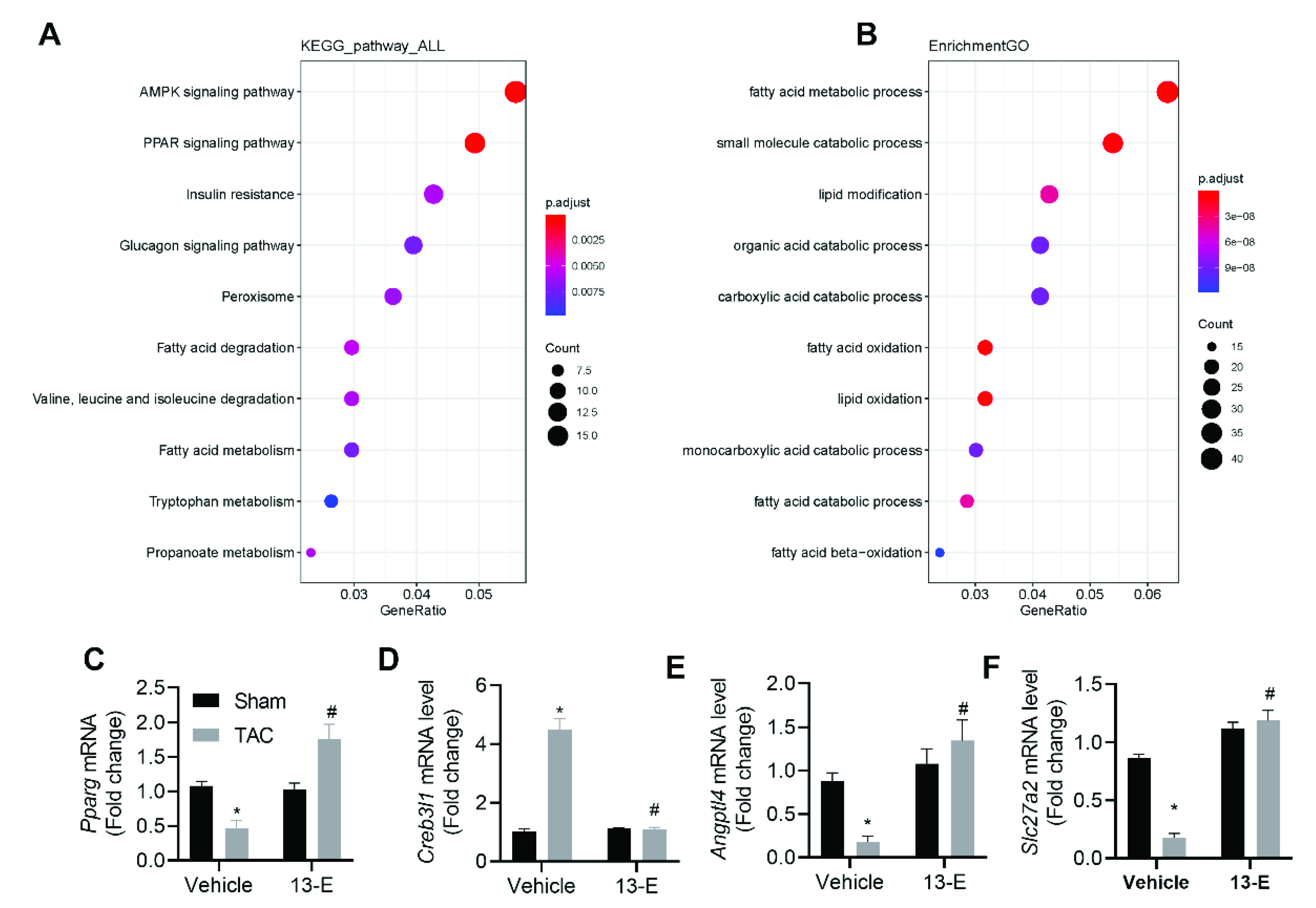
2.7. 13-E Regulates the AMPK Signaling Pathway and Influences Fatty Acid Metabolic Processes
3. Discussion and Conclusions
4. Experimental Section
4.1. Chemistry
4.1.1. 6-(Benzyloxy)-7-methoxy-3-methylisochroman-4-one (4)
4.1.2. 6-Hydroxy-7-methoxy-3-methylisochroman-4-one (5)
4.1.3. 6-((6-Bromohexyl)oxy)-7-methoxy-3-methylisochroman-4-one (6)
4.1.4. 4-((6-((7-Methoxy-3-methyl-4-oxoisochroman-6-yl)oxy)hexyl)oxy)benzonitrile (7)
4.1.5. 4-((6-((7-Methoxy-3-methyl-4-oxoisochroman-6-yl)oxy)hexyl)oxy)benzothioamide (13-E)
4.2. H2S Release Experiment
4.3. Determination of Cytotoxicity
4.4. Animals
4.5. Neonatal Rat Cardiomyocyte Isolation, Culture and Treatment
4.6. Echocardiography
4.7. Histological Staining
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Nakamura, M.; Sadoshima, J. Mechanisms of physiological and pathological cardiac hypertrophy. Nat. Rev. Cardiol. 2018, 15, 387–407. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Hu, J.; Sun, K.; Xu, D. The role and molecular mechanism of epigenetics in cardiac hypertrophy. Heart Fail. Rev. 2021, 26, 1505–1514. [Google Scholar] [CrossRef] [PubMed]
- Tham, Y.K.; Bernardo, B.C.; Ooi, J.Y.; Weeks, K.L.; McMullen, J.R. Pathophysiology of cardiac hypertrophy and heart failure: Signaling pathways and novel therapeutic targets. Arch. Toxicol. 2015, 89, 1401–1438. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Z.; Yu, X.Y.; Xu, S.; Luo, J. Autophagy and cardiac diseases: Therapeutic potential of natural products. Med. Res. Rev. 2021, 41, 314–341. [Google Scholar] [CrossRef]
- Khan, J.; Deb, P.K.; Priya, S.; Medina, K.D.; Devi, R.; Walode, S.G.; Rudrapal, M. Dietary flavonoids: Cardioprotective potential with antioxidant effects and their pharmacokinetic, toxicological and therapeutic concerns. Molecules 2021, 26, 4021. [Google Scholar] [CrossRef]
- Fu, R.; Chen, Z.; Wang, Q.; Guo, Q.; Xu, J.; Wu, X. XJP-1, a novel ACEI, with anti-inflammatory properties in HUVECs. Atherosclerosis 2011, 219, 40–48. [Google Scholar] [CrossRef]
- Bai, R.; Liu, J.; Zhu, Y.; Yang, X.; Yang, C.; Kong, L.; Wang, X.; Zhang, H.; Yao, H.; Shen, M.; et al. Chiral separation, configurational identification and antihypertensive evaluation of (±)-7,8-dihydroxy-3-methyl-isochromanone-4. Bioorg. Med. Chem. Lett. 2012, 22, 6490–6493. [Google Scholar] [CrossRef]
- Xie, S.; Li, X.; Yu, H.; Zhang, P.; Wang, J.; Wang, C.; Xu, S.; Wu, Z.; Liu, J.; Zhu, Z.; et al. Design, synthesis and biological evaluation of isochroman-4-one hybrids bearing piperazine moiety as antihypertensive agent candidates. Bioorg. Med. Chem. 2019, 27, 2764–2770. [Google Scholar] [CrossRef]
- Fu, R.; Wang, Q.; Guo, Q.; Xu, J.; Wu, X. XJP-1 protects endothelial cells from oxidized low-density lipoprotein-induced apoptosis by inhibiting NADPH oxidase subunit expression and modulating the PI3K/Akt/eNOS pathway. Vasc. Pharmacol. 2013, 58, 78–86. [Google Scholar] [CrossRef]
- Uras, G.; Manca, A.; Zhang, P.; Markus, Z.; Mack, N.; Allen, S.; Bo, M.; Xu, S.; Xu, J.; Georgiou, M.; et al. In Vivo evaluation of a newly synthesized acetylcholinesterase inhibitor in a transgenic Drosophila model of Alzheimer’s disease. Front. Neurosci. 2021, 15, 691222. [Google Scholar] [CrossRef]
- Abe, K.; Kimura, H. The possible role of hydrogen sulfide as an endogenous neuromodulator. J. Neurosci. 1996, 16, 1066–1071. [Google Scholar] [CrossRef] [Green Version]
- Wang, R. Two’s company, three’s a crowd: Can H2S be the third endogenous gaseous transmitter? FASEB J. 2002, 16, 1792–1798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, B.; Bhattacharya, R.; Mukherjee, P. Hydrogen sulfide signaling in mitochondria and disease. FASEB J. 2019, 33, 13098–13125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Citi, V.; Piragine, E.; Testai, L.; Breschi, M.C.; Calderone, V.; Martelli, A. The role of hydrogen sulfide and H2S-donors in myocardial protection against ischemia/reperfusion injury. Curr. Med. Chem. 2018, 25, 4380–4401. [Google Scholar] [CrossRef] [PubMed]
- Xie, L.; Gu, Y.; Wen, M.; Zhao, S.; Wang, W.; Ma, Y.; Meng, G.; Han, Y.; Wang, Y.; Liu, G.; et al. Hydrogen sulfide induces Keap1 S-sulfhydration and suppresses diabetes-accelerated atherosclerosis via Nrf2 activation. Diabetes 2016, 65, 3171–3184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, G.; Xiao, Y.; Ma, Y.; Tang, X.; Xie, L.; Liu, J.; Gu, Y.; Yu, Y.; Park, C.M.; Xian, M.; et al. Hydrogen sulfide regulates krüppel-like factor 5 transcription activity via specificity protein 1 S-sulfhydration at Cys664 to prevent myocardial hypertrophy. J. Am. Heart Assoc. 2016, 5, e004160. [Google Scholar] [CrossRef]
- Yao, H.; Luo, S.; Liu, J.; Xie, S.; Liu, Y.; Xu, J.; Zhu, Z.; Xu, S. Controllable thioester-based hydrogen sulfide slow-releasing donors as cardioprotective agents. Chem. Commun. 2019, 55, 6193–6196. [Google Scholar] [CrossRef]
- Luo, S.; Gu, X.; Ma, F.; Liu, C.; Shen, Y.; Ge, R.; Zhu, Y. ZYZ451 protects cardiomyocytes from hypoxia-induced apoptosis via enhancing MnSOD and STAT3 interaction. Free Radic. Biol. Med. 2016, 92, 1–14. [Google Scholar] [CrossRef]
- Luo, S.; Hieu, T.B.; Ma, F.; Yu, Y.; Cao, Z.; Wang, M.; Wu, W.; Mao, Y.; Rose, P.; Law, B.Y.; et al. ZYZ-168 alleviates cardiac fibrosis after myocardial infarction through inhibition of ERK1/2-dependent ROCK1 activation. Sci. Rep. 2017, 7, 43242. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Yang, X.; Zhu, Y.; Zhou, Z.; Xie, W.; Yao, H.; Jiang, J.; Liu, J.; Shen, M.; Wu, X.; et al. Novel nitric oxide-releasing isochroman-4-one derivatives: Synthesis and evaluation of antihypertensive activity. Bioorg. Med. Chem. 2012, 20, 6848–6855. [Google Scholar] [CrossRef] [PubMed]
- Do, A.V.; Smith, R.; Tobias, P.; Carlsen, D.; Pham, E.; Bowden, N.B.; Salem, A.K. Sustained release of hydrogen sulfide (H2S) from poly(lactic acid) functionalized 4-hydroxythiobenzamide microparticles to protect against oxidative damage. Ann. Biomed. Eng. 2019, 47, 1691–1700. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ren, H.; Xu, J.; Bai, R.; Yan, Q.; Huang, W.; Wu, X.; Fu, J.; Wang, Q.; Wu, Q.; et al. Total synthesis and antihypertensive activity of (+/-)7,8-dihydroxy-3-methyl-isochromanone-4. Bioorg. Med. Chem. Lett. 2009, 19, 1822–1824. [Google Scholar] [CrossRef]
- Janani, C.; Ranjitha Kumari, B.D. PARP gamma gene—A review. Diabetes Metab. Syndr. 2015, 9, 46–50. [Google Scholar] [CrossRef] [PubMed]
- Sampieri, L.; Giusto, P.D.; Alvarez, C. CREB3 transcription factors: ER-Golgi stress transducers as Hubs for Cellular Homeostasis. Front. Cell Div. Biol. 2019, 7, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernández-Hernando, C.; Suárez, Y. ANGPTL4: A multifunctional protein involved in metabolism and vascular homestasis. Curr. Opin. Hematol. 2020, 27, 206–213. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Gaivin, R.; Abramovich, C.; Boylan, M.; Calles, J.; Schelling, J.R. Fatty acid transport protein-2 regulates glycemic control and diabetic kidney disease progression. JCI Insight 2020, 5, e136845. [Google Scholar] [CrossRef]
- Wang, L.; Li, Z.; Tan, Y.; Li, Q.; Yang, H.; Wang, P.; Lu, J.; Liu, P. PARP1 interacts with STAT3 and retains active phosphorylated-STAT3 in nucleus during pathological myocardial hypertrophy. Mol. Cell Endocrinol. 2018, 474, 137–150. [Google Scholar] [CrossRef]
- Tamura, T.; Said, S.; Harris, J.; Lu, W.; Gerdes, A.M. Reverse remodeling of cardiac myocyte hypertrophy in hypertension and failure by targeting of the renin-angiotensin system. Circulation 2000, 102, 253–259. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Liu, Y.; Wu, H.; Xu, S.; Ma, F. Identification of a Hydrogen-Sulfide-Releasing Isochroman-4-One Hybrid as a Cardioprotective Candidate for the Treatment of Cardiac Hypertrophy. Molecules 2022, 27, 4114. https://doi.org/10.3390/molecules27134114
Wang Y, Liu Y, Wu H, Xu S, Ma F. Identification of a Hydrogen-Sulfide-Releasing Isochroman-4-One Hybrid as a Cardioprotective Candidate for the Treatment of Cardiac Hypertrophy. Molecules. 2022; 27(13):4114. https://doi.org/10.3390/molecules27134114
Chicago/Turabian StyleWang, Yu, Yuechen Liu, Hongyu Wu, Shengtao Xu, and Fenfen Ma. 2022. "Identification of a Hydrogen-Sulfide-Releasing Isochroman-4-One Hybrid as a Cardioprotective Candidate for the Treatment of Cardiac Hypertrophy" Molecules 27, no. 13: 4114. https://doi.org/10.3390/molecules27134114
APA StyleWang, Y., Liu, Y., Wu, H., Xu, S., & Ma, F. (2022). Identification of a Hydrogen-Sulfide-Releasing Isochroman-4-One Hybrid as a Cardioprotective Candidate for the Treatment of Cardiac Hypertrophy. Molecules, 27(13), 4114. https://doi.org/10.3390/molecules27134114




