Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines, Their NMR Study and Computer Evaluation as Material for LED
Abstract
:1. Introduction
2. Results and Discussion
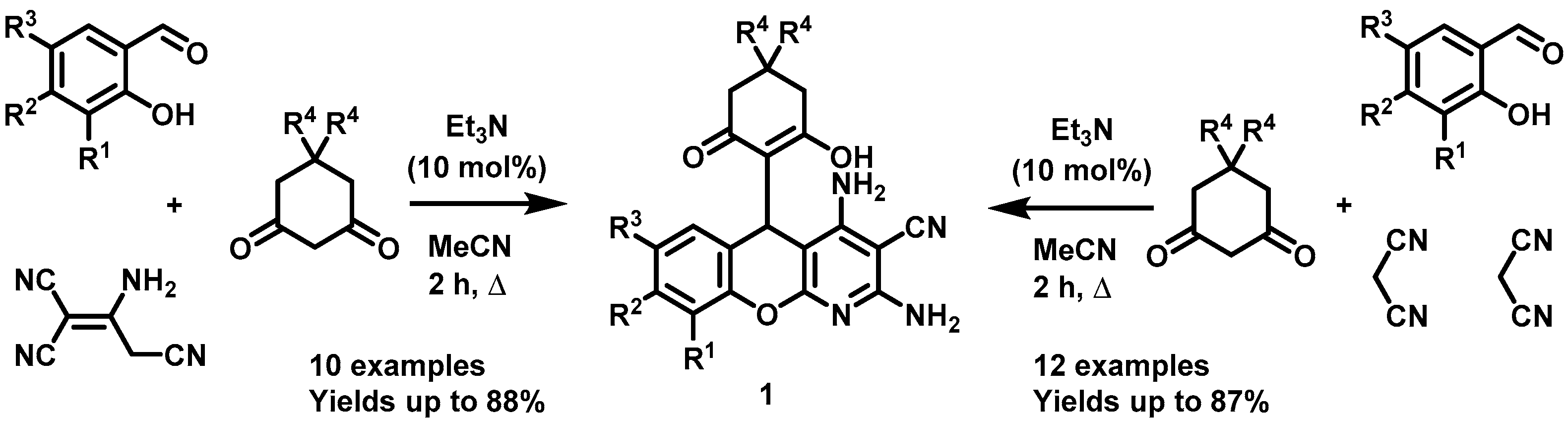
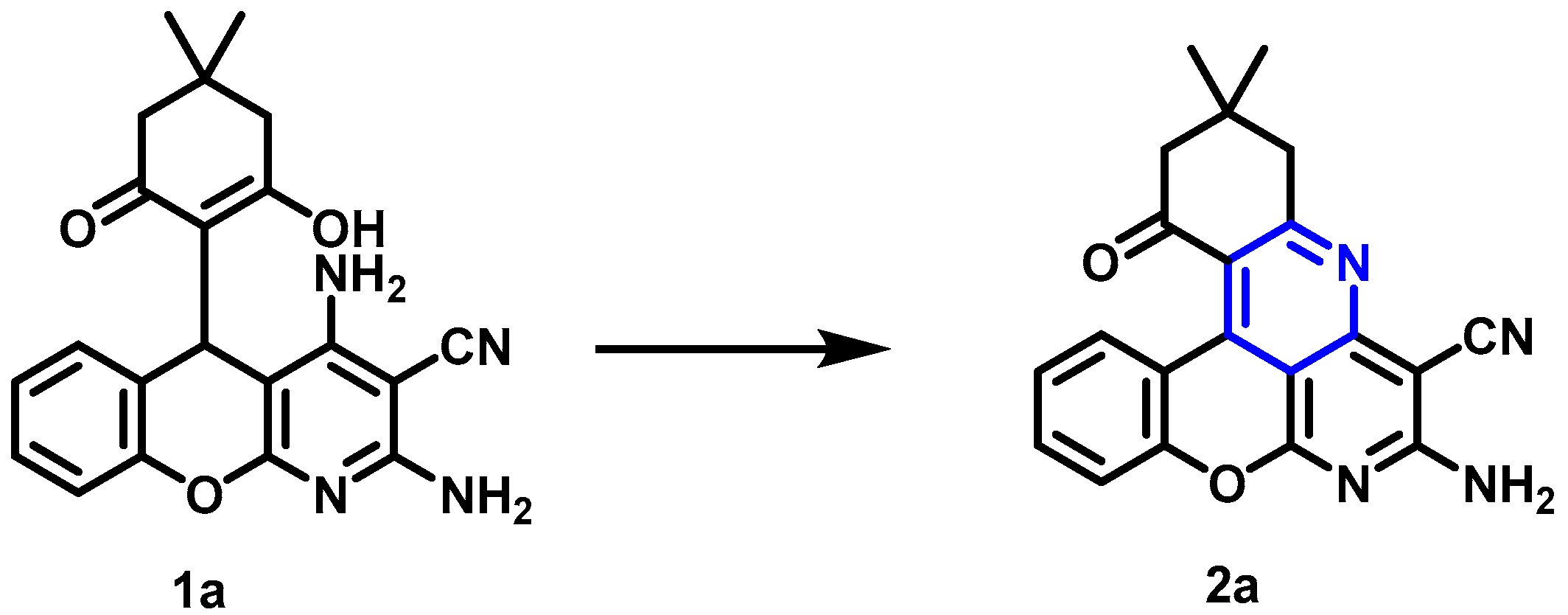
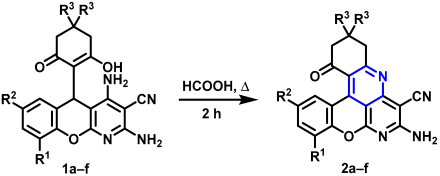
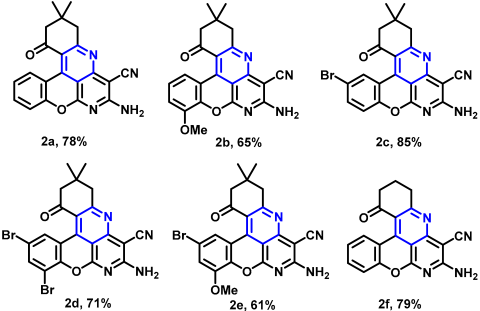
2.1. Intramolecular Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines
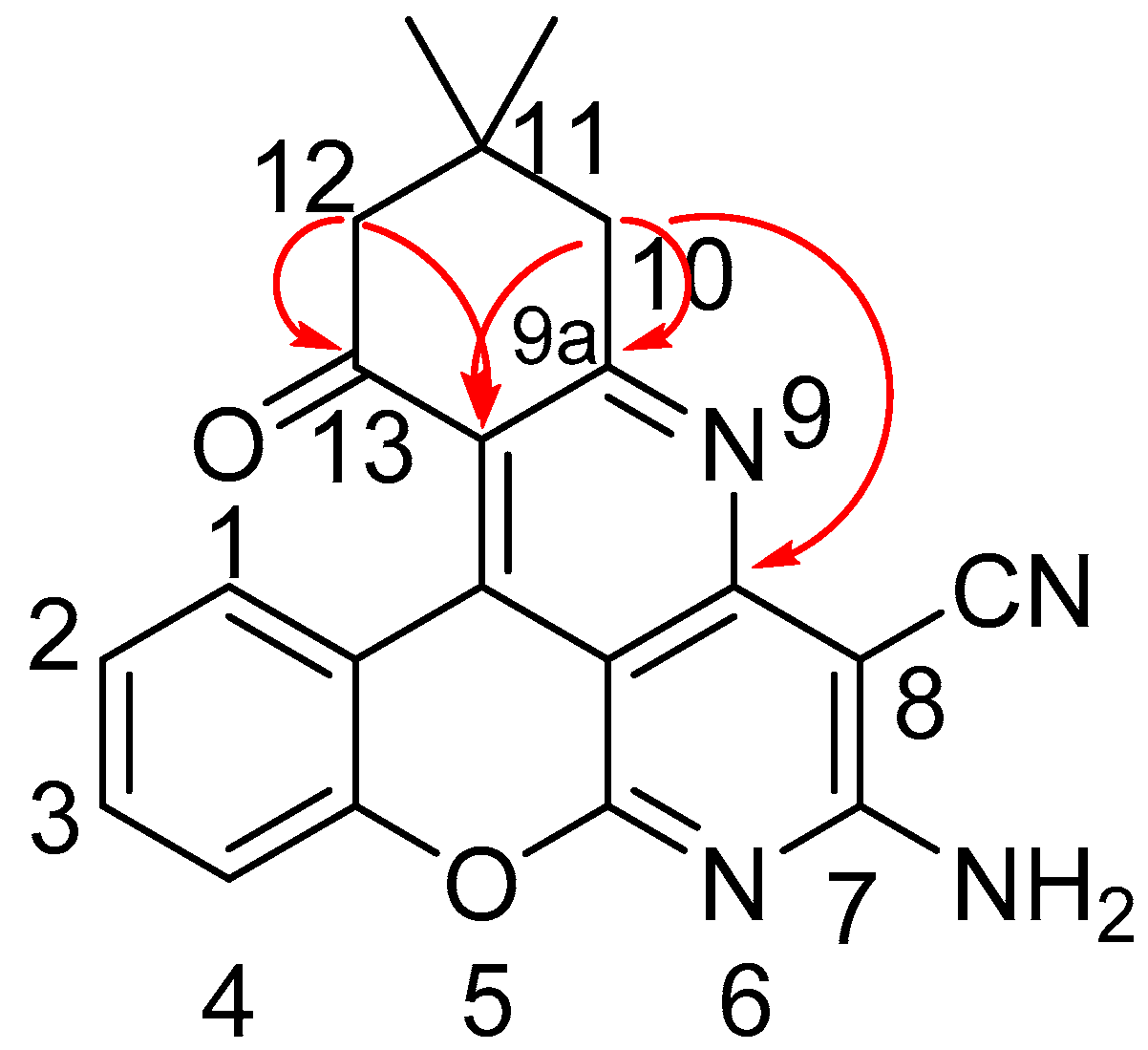
2.2. 2D NMR Study of the Structure of Compound 2a
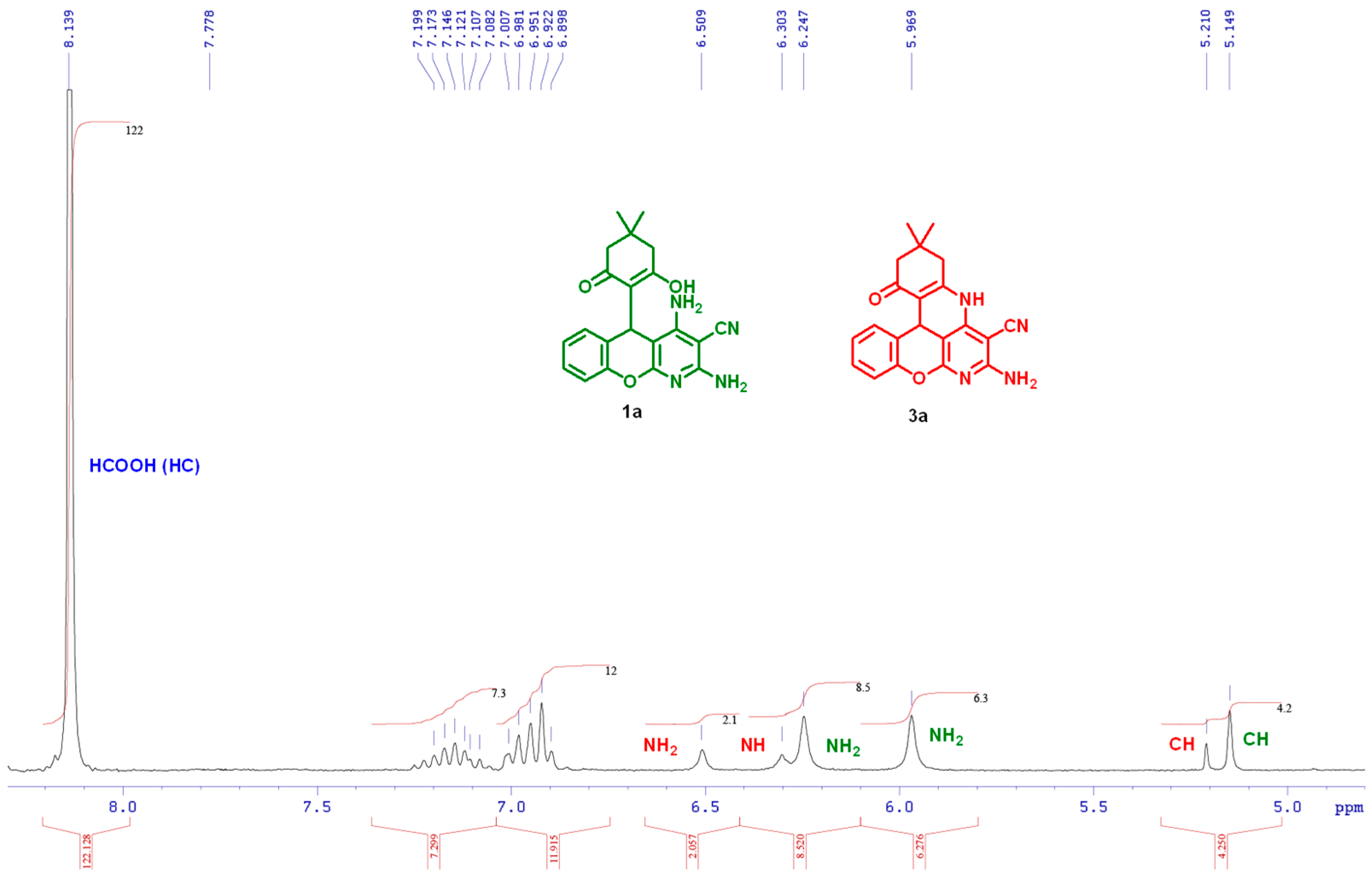
2.3. 1H NMR Reaction Monitoring and Mechanism of the Process
2.4. Computer Evaluation as Material for LED
3. Materials and Methods
3.1. General Information
3.2. Intramolecular Oxidative Cyclization of 5-(2-Hydroxy-6-Oxocyclohexyl)-5H-Chromeno[2,3-b]pyridine-3-Carbonitrile 1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Pola, S.; Subburu, M.; Guja, R.; Muga, V.; Tao, Y.-T. New photocatalyst for allylic aliphatic C–H bond activation and degradation of organic pollutants: Schiff base Ti(IV) complexes. RSC Adv. 2015, 5, 58504–58513. [Google Scholar] [CrossRef]
- Khan, I.; Ibrar, A.; Shehzadi, S.A. Building molecular complexity through transition-metal-catalyzed oxidative annulations/cyclizations: Harnessing the utility of phenols, naphthols and 1,3-dicarbonyl compounds. Coord. Chem. Rev. 2019, 380, 440–470. [Google Scholar] [CrossRef]
- Pericherla, K.; Kaswan, P.; Pandey, K.; Kumar, A. Recent Developments in the Synthesis of Imidazo[1,2-a]pyridines. Synthesis 2015, 47, 887–912. [Google Scholar] [CrossRef]
- Ma, D.; Liu, A.; Li, S.; Lu, C.; Chen, C. TiO2 photocatalysis for C–C bond formation. Catal. Sci. Technol. 2018, 8, 2030–2045. [Google Scholar] [CrossRef]
- Tang, M.-C.; Zou, Y.; Watanabe, K.; Walsh, C.T.; Tang, Y. Oxidative Cyclization in Natural Product Biosynthesis. Chem. Rev. 2017, 117, 5226–5333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ringel, M.T.; Brüser, T. The biosynthesis of pyoverdines. Microb. Cell 2018, 5, 424–437. [Google Scholar] [CrossRef] [PubMed]
- Bao, X.; Jiang, W.; Liang, J.; Huo, C. One-electron oxidative dehydrogenative annulation and cyclization reactions. Org. Chem. Front. 2020, 7, 2107–2144. [Google Scholar] [CrossRef]
- Li, X.-W.; Nay, B. Transition metal-promoted biomimetic steps in total syntheses. Nat. Prod. Rep. 2014, 31, 533–549. [Google Scholar] [CrossRef]
- Rasmussen, S.C. The nomenclature of fused-ring arenes and heterocycles: A guide to an increasingly important dialect of organic chemistry. ChemTexts 2016, 2, 16. [Google Scholar] [CrossRef] [Green Version]
- Devadoss, T.; Sowmya, V.; Bastati, R. Synthesis of 1,6-Naphthyridine and Its Derivatives: A Systematic Review. ChemistrySelect 2021, 6, 3610–3641. [Google Scholar] [CrossRef]
- Wu, H.; Lin, W.; Wan, Y.; Xin, H.; Shi, D.; Shi, Y.; Yuan, R.; Bo, R.; Yin, W. Silica gel-catalyzed one-pot syntheses in water and fluorescence properties studies of 5-amino-2-aryl-3H-chromeno[4,3,2-de][1,6]naphthyridine-4-carbonitriles and 5-amino-2-aryl-3H-quinolino[4,3,2-de][1,6]naphthyridine-4-carbonitriles. J. Comb. Chem. 2010, 12, 31–34. [Google Scholar] [CrossRef]
- Mithula, S.; Nandikolla, A.; Murugesan, S.; Kondapalli, V.G.C.S. 1,8-naphthyridine derivatives: An updated review on recent advancements of their myriad biological activities. Future Med. Chem. 2021, 13, 1591–1618. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.-J.; Shen, Y.; Xie, F.-M.; Chen, J.-D.; Li, Y.-Q.; Tang, J.-X. Recent advances in organic light-emitting diodes: Toward smart lighting and displays. Mater. Chem. Front. 2020, 4, 788–820. [Google Scholar] [CrossRef]
- Kumar, N.; Udayabhanu; Basavaraj, R.B.; Mahadevan, K.M.; Nagaraju, G. Novel aggregation induced emission based 7-(diethylamino)-3-(4-nitrophenyl)-2H-chromen-2-one for forensic and OLEDs applications. Appl. Surf. Sci. 2021, 5, 100095. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Goloveshkin, A.S.; Novikov, R.A.; Egorov, M.P. Synthesis, structural, spectroscopic and docking studies of new 5C-substituted 2,4-diamino-5H-chromeno[2,3-b]pyridine-3-carbonitriles. J. Mol. Struct. 2017, 1146, 766–772. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Novikov, R.A.; Egorov, M.P. PASE Pseudo-Four-Component Synthesis and Docking Studies of New 5-C-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]pyridine-3-Carbonitriles. ChemistrySelect 2017, 2, 4593–4597. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkova, Y.E.; Ryzhkov, F.V. Multicomponent design of chromeno[2,3-b]pyridine systems. Russ. Chem. Rev. 2021, 90, 94–115. [Google Scholar] [CrossRef]
- Gan, J.; Luo, N.; Wu, C.; Wan, X.; Wang, C. Efficient Synthesis of Chromeno[4,3,2-de][1,6]naphthyridine Derivatives via Pseudo Four-Component Reaction. ChemistrySelect 2021, 6, 9032–9037. [Google Scholar] [CrossRef]
- Mohammadi, H.; Shaterian, H.R. γ-Fe2O3@SiO2-γ-aminobutyric acid as a novel superparamagnetic nanocatalyst promoted green synthesis of chromeno[4,3,2-de][1,6]naphthyridine derivatives. Monatsh. Chem. 2019, 150, 327–337. [Google Scholar] [CrossRef]
- Wang, H.-Y.; Shi, J.-J.; Wang, C.; Zhang, X.-X.; Wan, Y.; Wu, H. Novel fluorescence dyes based on entirely new chromeno[4,3,2-de][1,6]naphthyridine framework. Dyes Pigm. 2012, 95, 268–274. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Wang, C.; Zhang, X.; Zhao, L.; Wan, Y.; Wu, H. Synthesis and characteristics of novel fluorescence dyes based on chromeno[4,3,2-de][1,6]naphthyridine framework. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 103, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, F.; Gao, J.; Li, P.; Xiong, W.-W.; Zhao, Y.; Sun, X.W.; Zhang, Q. Synthesis, physical properties and OLED performance of azatetracenes. Dyes Pigm. 2015, 112, 93–98. [Google Scholar] [CrossRef]
- Laudise, R.A.; Kloc, C.; Simpkins, P.G.; Siegrist, T. Physical vapor growth of organic semiconductors. J. Cryst. Growth 1998, 187, 449–454. [Google Scholar] [CrossRef]
- Yoon, J.; Lee, J.S.; Yoon, S.S.; Kim, Y.K. Red Fluorescent Donor-π-Acceptor Type Materials based on Chromene Moiety for Organic Light-Emitting Diodes. Bull. Korean Chem. Soc. 2014, 35, 1670–1674. [Google Scholar] [CrossRef] [Green Version]
- Na, E.J.; Lee, K.H.; Han, H.; Kim, Y.K.; Yoon, S.S. Red fluorescent emitting materials based on di-tert-butyl chromene derivatives for organic light-emitting diodes. J. Nanosci. Nanotechnol. 2013, 13, 554–557. [Google Scholar] [CrossRef]
- Okumoto, K.; Kanno, H.; Hamada, Y.; Takahashi, H.; Shibata, K. High efficiency red organic light-emitting devices using tetraphenyldibenzoperiflanthene-doped rubrene as an emitting layer. Appl. Phys. Lett. 2006, 89, 013502. [Google Scholar] [CrossRef]
- Aprà, E.; Bylaska, E.J.; de Jong, W.A.; Govind, N.; Kowalski, K.; Straatsma, T.P.; Valiev, M.; van Dam, H.J.J.; Alexeev, Y.; Anchell, J.; et al. NWChem: Past, present, and future. J. Chem. Phys. 2020, 152, 184102. [Google Scholar] [CrossRef] [PubMed]

| Entry | Solvent | Catalyst | Time (h) | Temp. (°C) | Yield (%) |
|---|---|---|---|---|---|
| 1 | EtOH | TsOH × H2O | 2 | 78 | – |
| 2 | EtOH | HCl | 2 | 78 | – |
| 3 | EtOH | P2O5 | 2 | 78 | – |
| 4 | HCOOH | – | 2 | 101 | 78 2 |
| 5 | HCONH2 | – | 2 | 100 | 54 2 |
| 6 | HCONH2 | – | 4 | 100 | 58 2 |
| 7 | HCONH2 | – | 2 | 210 | 31 |
| 8 | HCOOH | – | 4 | 101 | 79 2 |
| 9 | HCOOH | – | 6 | 101 | 81 |
 |
 |
| Compound | Total Energy, a.u. | E(HOMO), eV | E(LUMO), eV | ∆E(L-H), eV |
|---|---|---|---|---|
| 2a | −1183.31 | −5.789 | −1.670 | 4.119 |
| 2b | −1298.58 | −5.692 | −1.551 | 4.142 |
| 2c | −3755.00 | −5.751 | −1.675 | 4.076 |
| 2d | −6326.70 | −5.715 | −1.645 | 4.071 |
| 2e | −3869.55 | −5.564 | −1.556 | 4.008 |
| 2f | −1104.56 | −5.785 | −1.653 | 4.132 |
| Compound | HOMO | LUMO |
|---|---|---|
| 2a | 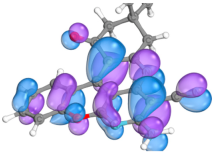 | 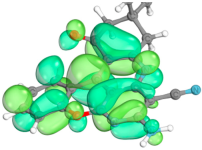 |
| 2b |  |  |
| 2c |  | 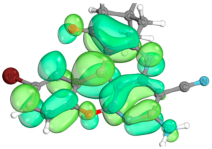 |
| 2d |  | 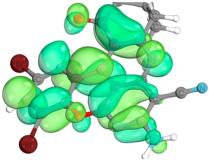 |
| 2e | 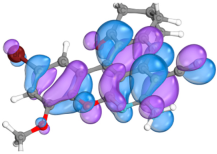 | 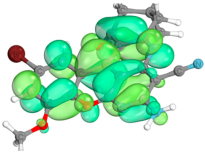 |
| 2f | 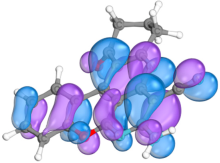 |  |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkova, Y.E.; Ryzhkov, F.V.; Fakhrutdinov, A.N.; Elinson, M.N. Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines, Their NMR Study and Computer Evaluation as Material for LED. Molecules 2022, 27, 4156. https://doi.org/10.3390/molecules27134156
Ryzhkova YE, Ryzhkov FV, Fakhrutdinov AN, Elinson MN. Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines, Their NMR Study and Computer Evaluation as Material for LED. Molecules. 2022; 27(13):4156. https://doi.org/10.3390/molecules27134156
Chicago/Turabian StyleRyzhkova, Yuliya E., Fedor V. Ryzhkov, Artem N. Fakhrutdinov, and Michail N. Elinson. 2022. "Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines, Their NMR Study and Computer Evaluation as Material for LED" Molecules 27, no. 13: 4156. https://doi.org/10.3390/molecules27134156
APA StyleRyzhkova, Y. E., Ryzhkov, F. V., Fakhrutdinov, A. N., & Elinson, M. N. (2022). Oxidative Cyclization of 5H-Chromeno[2,3-b]pyridines to Benzo[b]chromeno[4,3,2-de][1,6]naphthyridines, Their NMR Study and Computer Evaluation as Material for LED. Molecules, 27(13), 4156. https://doi.org/10.3390/molecules27134156










