Are Organic Certified Carrots Richer in Health-Promoting Phenolics and Carotenoids than the Conventionally Grown Ones?
Abstract
:1. Introduction
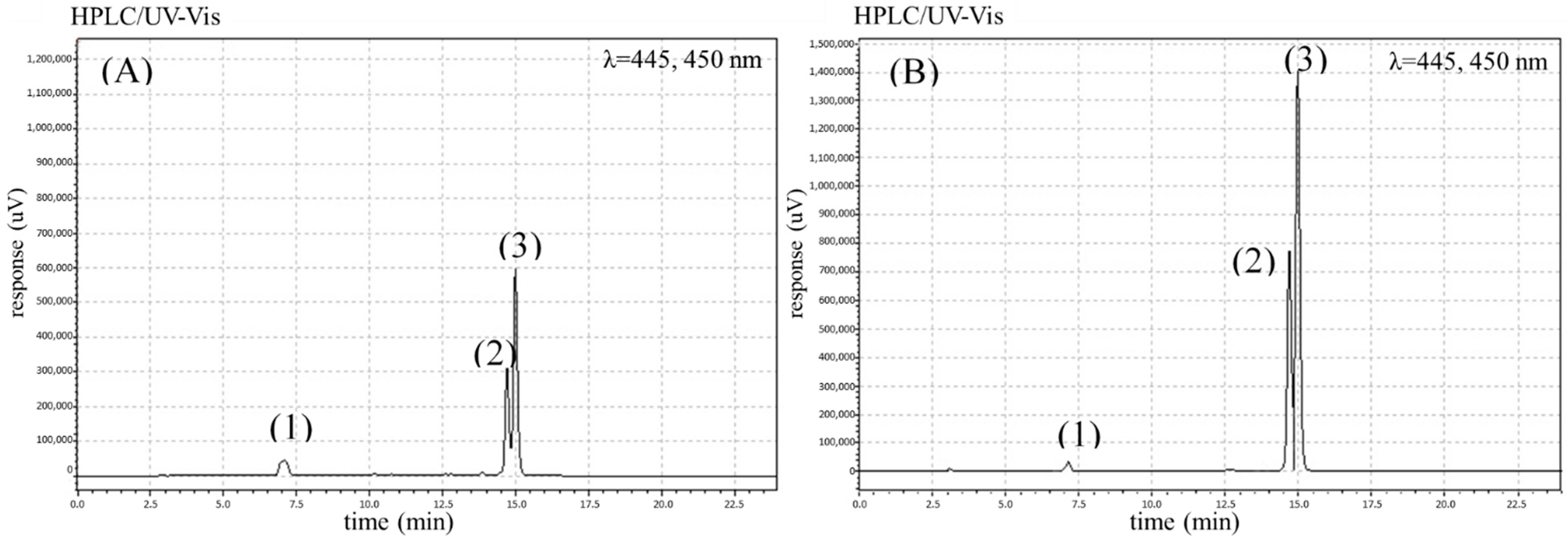
2. Results and Discussion
3. Materials and Methods
3.1. Study Design and Plant Material
3.2. Laboratory Analyses
3.2.1. Dry Matter Content
3.2.2. Phenolic Compounds Extraction and Determination
3.2.3. Carotenoids Extraction and Determination
3.3. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Willer, H.; Trávníček, J.; Meier, C.; Bernhard, S. (Eds.) The World of Organic Agriculture. Statistics and Emerging Trends 2022; Research Institute of Organic Agriculture FiBL, Frick, and IFOAM–Organics International: Bonn, Germany, 2022. [Google Scholar]
- Azzurra, A.; Massimiliano, A.; Angela, M. Measuring sustainable food consumption: A case study on organic food. Sustain. Prod. Consum. 2019, 17, 95–107. [Google Scholar] [CrossRef]
- Mørk, T.; Bech-Larsen, T.; Grunert, K.G.; Tsalis, G. Determinants of citizen acceptance of environmental policy regulating consumption in public settings: Organic food in public institutions. J. Clean. Prod. 2017, 148, 407–414. [Google Scholar] [CrossRef]
- European Parliament; European Council. Regulation (EU) 2018/848 of the European Parliament and of the Council of 30 May 2018 on Organic Production and Labelling of Organic Products and Repealing Council Regulation (EC) No 834/2007; The Official Journal of the European Union, Publications Office of the EU: Luxembourg, 2018.
- Smith, L.G.; Kirk, G.J.D.; Jones, P.J.; Williams, A.G. The greenhouse gas impacts of converting food production in England and Wales to organic methods. Nat. Commun. 2019, 10, 4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diacono, M.; Persiani, A.; Testani, E.; Montemurro, F. Sustainability of agro-ecological practices in organic horticulture: Yield, energy-use and carbon footprint. Agroecol. Sustain. Food Syst. 2020, 44, 726–746. [Google Scholar] [CrossRef]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [Green Version]
- Rempelos, L.; Wang, J.; Barański, M.; Watson, A.; Volakakis, N.; Hoppe, H.-W.; Kühn-Velten, W.N.; Hadall, C.; Hasanaliyeva, G.; Chatzidimitriou, E.; et al. Diet and food type affect urinary pesticide residue excretion profiles in healthy individuals: Results of a randomized controlled dietary intervention trial. Am. J. Clin. Nutr. 2022, 115, 364–377. [Google Scholar] [CrossRef]
- Rempelos, L.; Cooper, J.; Wilcockson, S.; Eyre, M.; Shotton, P.; Volakakis, N.; Orr, C.H.; Leifert, C.; Gatehouse, A.M.R.; Tétard-Jones, C. Quantitative proteomics to study the response of potato to contrasting fertilisation regimes. Mol. Breed. 2013, 31, 363–378. [Google Scholar] [CrossRef]
- van Dijk, J.P.; Cankar, K.; Scheffer, S.J.; Beenen, H.G.; Shepherd, L.V.T.; Stewart, D.; Davies, H.V.; Wilkockson, S.J.; Leifert, C.; Gruden, K.; et al. Transcriptome analysis of potato tubers-effects of different agricultural practices. J. Agric. Food Chem. 2009, 57, 1612–1623. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Siłakiewicz, A.; Hallmann, E.; Srednicka-Tober, D.; Rembiałkowska, E. Chemical composition of selected beetroot juices in relation to beetroot production system and processing technology. Not. Bot. Horti Agrobot. Cluj-Napoca 2016, 44, 491–498. [Google Scholar] [CrossRef] [Green Version]
- Brandt, K.; Srednicka-Tober, D.; Barański, M.; Sanderson, R.; Leifert, C.; Seal, C. Methods for comparing data across differently designed agronomic studies: Examples of different meta-analysis methods used to compare relative composition of plant foods grown using organic or conventional production methods and a protocol for a systemati. J. Agric. Food Chem. 2013, 61, 7173–7180. [Google Scholar] [CrossRef] [Green Version]
- Średnicka-Tober, D.; Barański, M.; Kazimierczak, R.; Ponder, A.; Kopczyńska, K.; Hallmann, E. Selected antioxidants in organic vs. conventionally grown apple fruits. Appl. Sci. 2020, 10, 2997. [Google Scholar] [CrossRef]
- Caprile, A.; Rossi, R. 2021 International Year of Fruits and Vegetables. Available online: https://www.europarl.europa.eu/RegData/etudes/ATAG/2021/689367/EPRS_ATA(2021)689367_EN.pdf (accessed on 10 May 2022).
- Wang, D.D.; Li, Y.; Bhupathiraju, S.N.; Rosner, B.A.; Sun, Q.; Giovannucci, E.L.; Rimm, E.B.; Manson, J.E.; Willett, W.C.; Stampfer, M.J.; et al. Fruit and vegetable intake and mortality: Results from 2 prospective cohort studies of US men and women and a meta-analysis of 26 cohort studies. Circulation 2021, 143, 1642–1654. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization of the United Nations (FAO); World Health Organization (WHO). Fruit and Vegetables for Health Initiative; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017.
- Kalmpourtzidou, A.; Eilander, A.; Talsma, E.F. Global vegetable intake and supply compared to recommendations: A systematic review. Nutrients 2020, 12, 1558. [Google Scholar] [CrossRef] [PubMed]
- Paoletti, F.; Raffo, A.; Kristensen, H.L.; Thorup-Kristensen, K.; Seljåsen, R.; Torp, T.; Busscher, N.; Ploeger, A.; Kahl, J. Multi-method comparison of carrot quality from a conventional and three organic cropping systems with increasing levels of nutrient recycling. J. Sci. Food Agric. 2012, 92, 2855–2869. [Google Scholar] [CrossRef] [PubMed]
- FAOSTAT Crop Statistics. Available online: http://www.fao.org/faostat/en/#data (accessed on 10 May 2022).
- Šeregelj, V.; Vulić, J.; Ćetković, G.; Čanadanovć-Brunet, J.; Tumbas Šaponjac, V.; Stajčić, S. Chapter 9-Natural bioactive compounds in carrot waste for food applications and health benefits. In Studies of Natural Products Chemistry. Bioactive Natural Products; Atta-ur-Rahman, Ed.; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 307–344. ISBN 1572-5995. [Google Scholar]
- Ahmad, T.; Cawood, M.; Iqbal, Q.; Ariño, A.; Batool, A.; Tariq, R.M.S.; Azam, M.; Akhtar, S. Phytochemicals in Daucus carota and Their Health Benefits-Review Article. Foods 2019, 8, 424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balić, A.; Mokos, M. Do We Utilize Our Knowledge of the Skin Protective Effects of Carotenoids Enough? Antioxidants 2019, 8, 259. [Google Scholar] [CrossRef] [Green Version]
- Kazimierczak, R.; Hallmann, E.; Rembiałkowska, E. Effects of organic and conventional production systems on the content of bioactive substances in four species of medicinal plants. Biol. Agric. Hortic. 2015, 31, 118–127. [Google Scholar] [CrossRef]
- Chenard, C.H.; Kopsell, D.A.; Kopsell, D.E. Nitrogen concentration affects nutrient and carotenoid accumulation in parsley. J. Plant Nutr. 2005, 28, 285–297. [Google Scholar] [CrossRef]
- Boskovic-Rakocevic, L. Effect of nitrogen fertilization on carrot quality. Afr. J. Agric. Res. 2012, 7, 2884–2900. [Google Scholar] [CrossRef] [Green Version]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid pigments in kale are influenced by nitrogen concentration and form. J. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- Dean, P.R.; Hurd, C.L. Seasonal growth, erosion rates, and nitrogen and photosynthetic ecophysiology of Undaria pinnatifida (Heterokontophyta) in southern New Zealand. J. Phycol. 2007, 43, 1138–1148. [Google Scholar] [CrossRef]
- Fu, Y.; Li, H.; Yu, J.; Liu, H.; Cao, Z.; Manukovsky, N.S.; Liu, H. Interaction effects of light intensity and nitrogen concentration on growth, photosynthetic characteristics and quality of lettuce (Lactuca sativa L. Var. youmaicai). Sci. Hortic. 2017, 214, 51–57. [Google Scholar] [CrossRef]
- Kaack, K.V.; Nielsen, M.P.; Christensen, L.; Thorup-Kristensen, K. Nutritionally important chemical constituents and yield of carrot (Daucus carota L.) roots grown organically using ten levels of green manure. Acta Agric. Scand. B Sect. B-Soil Plant Sci. 2001, 51, 125–136. [Google Scholar] [CrossRef]
- Scalbert, A.; Johnson, I.T.; Saltmarsh, M. Polyphenols: Antioxidants and beyond. Am. J. Clin. Nutr. 2005, 81, 215–217. [Google Scholar] [CrossRef]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The Role of Polyphenols in Human Health and Food Systems: A Mini-Review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, S.; Ge, S.; Lin, S. Review of Distribution, Extraction Methods, and Health Benefits of Bound Phenolics in Food Plants. J. Agric. Food Chem. 2020, 68, 3330–3343. [Google Scholar] [CrossRef] [PubMed]
- Bloksma, J.; Northolt, M.; Huber, M.; van der Burgt, G.-J.; van de Vijver, L. A new food quality concept based on life processes. In Handbook of Organic Food Safety and Quality; Cooper, J., Niggli, U., Leifert, C., Eds.; Woodhead Publishing, Sawton: Cambridge, UK, 2007; pp. 53–73. ISBN 978-0-8493-9154-5. [Google Scholar]
- Rempelos, L.; Almuayrifi, A.M.; Baranski, M.; Tetard-Jones, C.; Eyre, M.; Shotton, P.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; et al. Effects of agronomic management and climate on leaf phenolic profiles, disease severity, and grain yield in organic and conventional wheat production systems. J. Agric. Food Chem. 2018, 66, 10369–10379. [Google Scholar] [CrossRef]
- Rempelos, L.; Almuayrifi, M.S.B.; Baranski, M.; Tetard-Jones, C.; Barkla, B.; Cakmak, I.; Ozturk, L.; Cooper, J.; Volakakis, N.; Hall, G.; et al. The effect of agronomic factors on crop health and performance of winter wheat varieties bred for the conventional and the low input farming sector. Field Crops Res. 2020, 254, 107822. [Google Scholar] [CrossRef]
- Kazimierczak, R.; Srednicka-Tober, D.; Hallmann, E.; Kopczynska, K.; Zarzynska, K. The impact of organic vs. conventional agricultural practices on selected quality features of eight potato cultivars. Agronomy 2019, 9, 799. [Google Scholar] [CrossRef] [Green Version]
- Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Pakos, P.; Seal, C.; Rosa, E.A.S.; Markellou, E.; Iversen, P.O.; et al. Effect of organic and conventional production methods on fruit yield and nutritional quality parameters in three traditional cretan grape varieties: Results from a farm survey. Foods 2021, 10, 476. [Google Scholar] [CrossRef]
- Hasanaliyeva, G.; Chatzidimitrou, E.; Wang, J.; Baranski, M.; Volakakis, N.; Seal, C.; Rosa, E.A.S.; Iversen, P.O.; Vigar, V.; Barkla, B.; et al. Effects of production region, production systems and grape type/variety on nutritional quality parameters of table grapes; results from a UK retail survey. Foods 2020, 9, 1874. [Google Scholar] [CrossRef]
- Wegener, C.; Jansen, G.; Jurgens, H.-U. Influence of drought and wounding stress on soluble phenols and proteins in potato tubers. Sustain. Agric. Res. 2014, 3, 1. [Google Scholar] [CrossRef] [Green Version]
- Flakelar, C.L.; Luckett, D.J.; Howitt, J.A.; Doran, G.; Prenzler, P.D. Canola (Brassica napus) oil from Australian cultivars shows promising levels of tocopherols and carotenoids, along with good oxidative stability. J. Food Compos. Anal. 2015, 42, 179–186. [Google Scholar] [CrossRef]
- Mou, B. Genetic Variation of Beta-carotene and Lutein Contents in Lettuce. J. Am. Soc. Hortic. Sci. Jashs 2005, 130, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Lee, S.Y.; Chu, S.M.; Lim, S.H.; Suh, S.-C.; Lee, Y.-T.; Cho, H.S.; Ha, S.-H. Variation and Correlation Analysis of Flavonoids and Carotenoids in Korean Pigmented Rice (Oryza sativa L.) Cultivars. J. Agric. Food Chem. 2010, 58, 12804–12809. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, J.; Peng, Z.; Shi, M.; Liu, X.; Wen, H.; Jiang, Y.; Cheng, Y.; Xu, J.; Zhang, H. Integrated Transcriptomic and Metabolomic analysis reveals a transcriptional regulation network for the biosynthesis of carotenoids and flavonoids in ‘Cara cara’ navel Orange. BMC Plant Biol. 2021, 21, 29. [Google Scholar] [CrossRef]
- Ohene, I.; Maalekuu, B.K. Effect of some postharvest treatments on the quality and shelf life of three cultivars of carrot (Daucus carota L.) during storage at room temperature. Am. J. Food Nutr. 2013, 3, 64–72. [Google Scholar]
- Hallmann, E. The influence of organic and conventional cultivation systems on the nutritional value and content of bioactive compounds in selected tomato types. J. Sci. Food Agric. 2012, 92, 2840–2848. [Google Scholar] [CrossRef]
- Collera-Zuniga, O.; Jimenez, F.G.; Gordillo, R.M. Comparative study of carotenoid composition in three Mexican varieties of Capsicum annuum L. Food Chem. 2005, 90, 109–114. [Google Scholar] [CrossRef]
- Hallmann, E.; Rembialkowska, E. Characterisation of antioxidant compounds in sweet bell pepper (Capsicum annuum L.) under organic and conventional growing systems. J. Sci. Food Agric. 2012, 92, 2409–2415. [Google Scholar] [CrossRef]
- Harris, R. An Introduction to R. Quant. Geogr. Basics 2018, 3, 250–286. [Google Scholar]



| cv. Flacoro | cv. Nantejska | ANOVA p-Values | |||||
|---|---|---|---|---|---|---|---|
| Conventional | Organic | Conventional | Organic | System (S) | Cultivar (C) | S × C | |
| Dry matter | 12.7 ± 2.2 | 13.5 ± 1.9 | 13.1 ± 0.8 | 13.9 ± 1.5 | 0.086 | 0.453 | 0.968 |
| Polyphenols (sum) | 7.69 ± 2.78 | 8.85 ± 3.13 | 9.35 ± 2.27 | 9.74 ± 3.21 | 0.392 | 0.272 | 0.638 |
| Phenolic acids (sum) | 3.91 ± 0.91 | 4.09 ± 1.27 | 4.60 ± 1.87 | 4.88 ± 1.38 | 0.580 | 0.175 | 0.904 |
| Chlorogenic acid | 1.49 ± 0.91 | 1.12 ± 0.67 | 1.17 ± 0.90 | 0.96 ± 0.74 | 0.193 | 0.402 | 0.705 |
| Gallic acid | 1.45 ± 0.24 bc | 1.79 ± 0.54 b | 1.14 ± 0.19 c | 2.38 ± 1.00 a | 0.000 | 0.250 | 0.019 |
| Caffeic acid | 0.26 ± 0.26 | 0.26 ± 0.23 | 0.32 ± 0.17 | 0.24 ± 0.21 | 0.427 | 0.873 | 0.518 |
| p-Coumaric acid | 0.71 ± 0.24 b | 0.93 ± 0.70 b | 1.97 ± 0.83 a | 1.30 ± 1.42 ab | 0.275 | 0.123 | 0.110 |
| Flavonoids (sum) | 3.78 ± 2.21 | 4.76 ± 2.13 | 4.75 ± 0.64 | 4.86 ± 2.23 | 0.374 | 0.516 | 0.431 |
| Quercetin-3-O-rutinoside | 0.56 ± 0.57 | 0.58 ± 0.43 | 0.58 ± 0.14 | 0.54 ± 0.30 | 0.943 | 0.854 | 0.790 |
| Quercetin-3-O-glycoside | 0.92 ± 0.52 | 0.88 ± 0.63 | 0.98 ± 0.39 | 0.72 ± 0.50 | 0.259 | 0.588 | 0.440 |
| Kaempferol-3-O-glycoside | 0.23 ± 0.19 | 0.52 ± 0.43 | 0.43 ± 0.62 | 0.44 ± 0.49 | 0.313 | 0.941 | 0.282 |
| Luteolin | 0.28 ± 0.18 b | 0.34 ± 0.20 b | 0.61 ± 0.4 a | 0.29 ± 0.22 b | 0.029 | 0.327 | 0.010 |
| Apigenin | 0.88 ± 0.41 | 1.39 ± 0.93 | 1.17 ± 0.68 | 1.66 ± 1.07 | 0.045 | 0.345 | 0.960 |
| Keampferol | 0.91 ± 0.57 | 1.05 ± 0.68 | 0.98 ± 0.56 | 1.21 ± 0.62 | 0.281 | 0.499 | 0.775 |
| Carotenoids (sum) | 4.22 ± 0.78 ab | 4.36 ± 0.70 ab | 5.00 ± 0.82 a | 3.84 ± 0.71 b | 0.005 | 0.751 | 0.002 |
| Lutein | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.09 ± 0.01 | 0.215 | 0.458 | 0.953 |
| α-carotene | 0.39 ± 0.14 ab | 0.37 ± 0.03 b | 0.41 ± 0.07 ab | 0.43 ± 0.08 a | 0.917 | 0.131 | 0.329 |
| β-carotene | 3.75 ± 0.63 bc | 3.90 ± 0.66 ab | 4.50 ± 0.76 a | 3.31 ± 0.63 c | 0.002 | 0.555 | 0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Średnicka-Tober, D.; Kopczyńska, K.; Góralska-Walczak, R.; Hallmann, E.; Barański, M.; Marszałek, K.; Kazimierczak, R. Are Organic Certified Carrots Richer in Health-Promoting Phenolics and Carotenoids than the Conventionally Grown Ones? Molecules 2022, 27, 4184. https://doi.org/10.3390/molecules27134184
Średnicka-Tober D, Kopczyńska K, Góralska-Walczak R, Hallmann E, Barański M, Marszałek K, Kazimierczak R. Are Organic Certified Carrots Richer in Health-Promoting Phenolics and Carotenoids than the Conventionally Grown Ones? Molecules. 2022; 27(13):4184. https://doi.org/10.3390/molecules27134184
Chicago/Turabian StyleŚrednicka-Tober, Dominika, Klaudia Kopczyńska, Rita Góralska-Walczak, Ewelina Hallmann, Marcin Barański, Krystian Marszałek, and Renata Kazimierczak. 2022. "Are Organic Certified Carrots Richer in Health-Promoting Phenolics and Carotenoids than the Conventionally Grown Ones?" Molecules 27, no. 13: 4184. https://doi.org/10.3390/molecules27134184
APA StyleŚrednicka-Tober, D., Kopczyńska, K., Góralska-Walczak, R., Hallmann, E., Barański, M., Marszałek, K., & Kazimierczak, R. (2022). Are Organic Certified Carrots Richer in Health-Promoting Phenolics and Carotenoids than the Conventionally Grown Ones? Molecules, 27(13), 4184. https://doi.org/10.3390/molecules27134184










