Emerging Lipids from Arecaceae Palm Fruits in Brazil
Abstract
:1. Introduction
2. Methodology
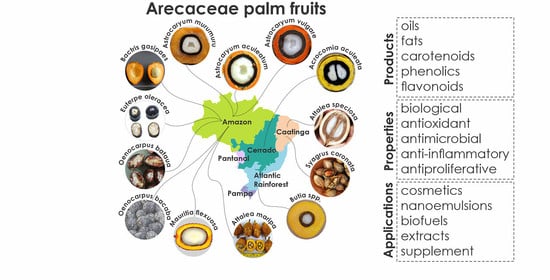
3. Occurrence, Distribution, and Studies on Arecaceae
4. Arecaceae Palms in Brazil
4.1. Acrocomia aculeata
4.2. Astrocaryum aculeatum
4.3. Astrocaryum murumuru
4.4. Astrocaryum vulgare
4.5. Attalea maripa
4.6. Attalea speciosa
4.7. Bactris gasipaes
4.8. Butia spp.
4.9. Euterpe oleracea
4.10. Mauritia flexuosa
4.11. Oenocarpus bataua
4.12. Oenocarpus bacaba
4.13. Syagrus coronata
5. The Oil Content in Arecaceae Palm Fruits
5.1. A. aculeata
5.2. A. aculeatum
5.3. A. murumuru
5.4. A. vulgare
5.5. A. maripa
5.6. A. speciosa
5.7. B. gasipaes
5.8. Butia spp.
5.9. E. oleraceae
5.10. M. flexuosa
5.11. O. bataua
5.12. O. bacaba
5.13. S. coronata
6. Characteristics of Arecaceae Palm Tree Fruit Oils (APTFOs)
6.1. Physicochemical Properties
6.2. Fatty Acids
6.3. Triacylglycerols
6.4. Bioactive Compounds
| Compound | MAP | MAK | MUK | TPP | TPK | INP | INK | BBK | PUP | PUK | BRP | BRK | PTP | PTK | BAP | LIP |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Tocopherols (mg kg−1 oil) | ||||||||||||||||
| α-Tocopherol | 17.1–143.70 | 14.25 | 89.94 | 52.0 | 7–16 | 20.0–114.85 | 2–3 | 42.16 | 47 | 2–3 | 252.1–1125.0 | nd | 1704 | 56.5 | - | - |
| β-Tocopherol | 3.25 | 0.85 | 91.52 | - | - | 25 | 1 | nd | 7 | - | 71.3–761.87 | - | - | 7.8 | - | - |
| γ-Tocopherol | 57.85–145.7 | nd | 10.09 | - | - | 50.97 | 1 | 11.91 | 4 | - | 56.71–1074.0 | 616.9 | 269 | - | - | - |
| δ-Tocopherol | 6.7–24.7 | 7.90 | 5.65 | nd | - | nd | tr | 12.09 | - | - | 44.1–224.18 | 378.82 | - | 7.7 | - | - |
| α-Tocotrienol | - | - | nd | - | 55–59 | 36 | 6–7 | nd | - | 1–2 | 90.80 | - | - | - | - | |
| β-Tocotrienol | - | - | nd | - | - | 12 | 2 | nd | - | - | nd | - | - | - | - | |
| γ-Tocotrienol | - | - | nd | - | - | 10 | 2 | nd | - | - | nd | - | - | - | - | |
| δ-Tocotrienol | - | - | nd | - | - | 10 | tr | nd | - | - | 10.60 | - | - | - | - | |
| Total tocopherols | 126.9–212.95 | 23.10 | 197.2 | - | 12–18 | 26.6–185 | 5–15 | - | 59 | 90–115 | 1129.78 | 995.72 | - | 72.0 | - | - |
| α-TE * | - | - | 136.88 | 52.9 | - | 22.0 | - | 43.71 | - | - | 56.00–842.30 | 73.32 | - | 59.1 | - | - |
| Total vitamin E | - | - | 197.2 | - | - | - | - | 66.16 | - | - | 1511.01 | - | - | - | - | |
| Carotenoids (mg kg−1 oil) | ||||||||||||||||
| β-Carotene | 1366.6–3560.5 | - | - | - | - | 571.4–1409.9 | - | - | 150.19 | - | 295.24–781.6 | - | 2.38 | - | 3.02 | - |
| Total carotenes | 130–300 | 1.82 | - | 1222–2420 | 3 | - | - | - | 357.42 | - | 540–1722.87 | - | 22 | - | 13.53 | 87.0 |
| Phytosterols (mg kg−1 oil) | ||||||||||||||||
| Brassicasterol | - | - | - | - | - | 3.50 | - | - | - | - | 2.50 | nd | ||||
| Campesterol | 28–338.7 | - | - | 13.9–133.2 | 84–87 | 18.8 | - | - | 6.3–10.9 | - | 6.6–16.0 | 8.0 | 7.2 | - | 11.0 | - |
| Campestanol | - | - | - | - | - | - | - | - | - | - | - | - | 6.0 | - | - | - |
| Stigmasterol | 48.1–152.6 | - | - | 8.1–66.1 | 35–40 | 5.4 | - | - | 2.9–4.2 | - | 16.8–38.5 | 6.0 | 19.2 | - | 12.6 | - |
| Δ5,23-Stigmastadienol | - | - | - | - | - | 4.1 | - | - | - | - | - | - | - | - | - | - |
| β-Sitosterol | 173.1–1496.5 | - | - | 76.6–488.2 | 1353–1363 | 65.4 | - | - | 51–3-55.0 | - | 76.6 | 6.0 ** | 34.2 | - | 76.4 | - |
| ∆5-Avenasterol | - | - | - | 1.4 | 638–648 | 2.4 | - | - | 2.7–4.8 | - | - | - | 27.8 | - | - | - |
| Δ5,24-Stigmastadienol | - | - | - | - | - | 2.3 | - | - | - | - | - | - | - | - | - | - |
| Δ7-Stigmastenol | - | - | - | - | - | 0.6 | - | - | - | - | - | - | - | - | - | |
| Squalene | - | - | 58.8 | - | - | - | 16.9 | - | - | - | - | - | - | - | ||
| Total phytosterols | 233.9–1881.7 | - | 0.8 | 1497.2–2708 | 1.1 | 1463 | - | - | 4456 | - | 2332 | - | 0.7–368 | - | 981 | - |
| References | [28,29,72] | [29] | [11,37] | [12,37,164,174] | [37,39] | [12,43,174,177] | [39,43] | [11] | [49,174] | [39] | [11,12,137,172,174,177] | [177] | [37,60] | [12] | [37,137,174] | [161] |
6.5. Similarities in the Lipid Composition among APTFOs
7. Biological Effects Reported for APTFOs
8. Potential Applications of APTFOs
| Oil | Potential Application | Reference |
|---|---|---|
| MUK butter | Nanostructured lipid carriers for β-carotene and α-tocopherol | [209] |
| Soaps and margarine | [38,132] | |
| Biodiesel | [210] | |
| Cosmetic model emulsions | [211] | |
| Interesterified lipid fractions | [86] | |
| Skin care creams, shampoos, moisturizers, soaps, conditioners, moisturizing facial and hair masks, combing cream, hair butter, and leave-in, solid shampoo, serum, hair cleaning cream, and hair balm | This study | |
| TAK oil | Cosmetic nanocapsules | [149,195] |
| Biodiesel | [212] | |
| TPK oil | Biodiesel | [44] |
| BRP oil | Biodiesel | [19,213] |
| LIK oil | Biofuel | [18,68,153,154,214] |
| Moisturizer | [221] | |
| Biofilms for food packaging | [222] | |
| Bio-oil | [214] | |
| BBK oil | Cosmetic model emulsion for skin | [215] |
| Cosmetics and pharmaceutical nanoemulsions | [216] | |
| Skin moisturizers | [217] | |
| Biodiesel and cosmetics | [168,218] | |
| Frying oil | This study | |
| BUK oil | Foodstuffs and animal feed | [52] |
| ACP oil | Extracts with cytotoxicity on HT-29 colon cancer cells | [182] |
| Feed for Nile tilapia (Oreochromis niloticus) | [198] | |
| Polyurethane nanoparticles for controlled and targeted delivery of therapeutics | [219] | |
| Lotion with sun protection factor | [220] |
9. Co-Products from the Oil Extraction of Arecaceae Palm Fruits
| Raw Material | Co-Product | Reference |
|---|---|---|
| Macaúba kernel | Press-cake rich in essential amino acids | [223] |
| Activated carbon | [224] | |
| Tucumã-do-Amazonas seeds | Activated and nanomagnetic activated carbon | [225,226] |
| Tucumã-do-Amazonas peel | Biodiesel | [227] |
| Tucumã-do-Amazonas peel and pulp | Extracts with antifungal and antibacterial activity | [228] |
| Extracts with antioxidant and anti-inflammatory activity | [82,229] | |
| Extracts with no cytotoxic effects in Wistar rats | [230] | |
| Babassu mesocarp | Mesocarp powder | [234] |
| Enzymes (lipase, protease, amylase) production | [235,236] | |
| Ethanol | [237] | |
| Butia spp. kernels | Ingredient for foodstuffs (sweets, bread, cakes, pies, and cookies) | [52] |
| Açaí seeds | Edible flour with potential anti-obesity properties | [143] |
| Extracts with antimicrobial and antioxidant properties | [238] | |
| Dietary fiber and antioxidant-rich extracts | [56] |
10. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Statista Production of Major Vegetable Oils Worldwide from 2012/13 to 2021/2022, by Type. Available online: https://www.statista.com/statistics/263933/production-of-vegetable-oils-worldwide-since-2000/ (accessed on 2 June 2022).
- FAO Crops Processed. Available online: http://www.fao.org/faostat (accessed on 2 June 2022).
- Statista Leading Soybean Producing Countries Worldwide from 2012/13 to 2021/22. Available online: https://www.statista.com/statistics/263926/soybean-production-in-selected-countries-since-1980/ (accessed on 2 June 2022).
- Statista Consumption of Vegetable Oils Worldwide from 2013/14 to 2021/2022, by Oil Type. Available online: https://www.statista.com/statistics/263937/vegetable-oils-global-consumption/ (accessed on 2 June 2022).
- Statista Import Volume of Major Vegetable Oils Worldwide in 2021/22, by Type. Available online: https://www.statista.com/statistics/613191/vegetable-oil-import-volume-worldwide-by-type/ (accessed on 2 June 2022).
- Statista Export Volume of Major Vegetable Oils Worldwide in 2021/22, by Type. Available online: https://www.statista.com/statistics/613218/vegetable-oil-export-volume-worldwide-by-type/ (accessed on 2 June 2022).
- ABIOVE Processing Capacity, Refining and Bottling of Vegetable Oils in Brazil. (In Portuguese). Available online: https://abiove.org.br/en/statistics/ (accessed on 2 June 2022).
- GBIF Global Biodiversity Information Facility Secretariat. Available online: https://www.gbif.org/species/7681 (accessed on 5 May 2022).
- Bezerra, C.V.; Rodrigues, A.M.C.; Oliveira, P.D.; Silva, D.A.; Silva, L.H.M. Technological Properties of Amazonian Oils and Fats and Their Applications in the Food Industry. Food Chem. 2017, 221, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Pereira, E.; Ferreira, M.C.; Sampaio, K.A.; Grimaldi, R.; Meirelles, A.J.A.; Maximo, G.J. Physical Properties of Amazonian Fats and Oils and Their Blends. Food Chem. 2019, 278, 208–215. [Google Scholar] [CrossRef]
- Serra, J.L.; Rodrigues, A.M.C.; Freitas, R.A.; Meirelles, A.J.A.; Darnet, S.H.; da Silva, L.H.M. Alternative Sources of Oils and Fats from Amazonian Plants: Fatty Acids, Methyl Tocols, Total Carotenoids and Chemical Composition. Food Res. Int. 2019, 116, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.M.C.; Darnet, S.; Silva, L.H.M. Fatty Acid Profiles and Tocopherol Contents of Buriti (Mauritia flexuosa), Patawa (Oenocarpus bataua), Tucuma (Astrocaryum vulgare), Mari (Poraqueiba paraensis) and Inaja (Maximiliana maripa) Fruits. J. Braz. Chem. Soc. 2010, 21, 2000–2004. [Google Scholar] [CrossRef]
- Funasaki, M.; Barroso, H.S.; Fernandes, V.L.A.; Menezes, I.S. Amazon Rainforest Cosmetics: Chemical Approach for Quality Control. Quim. Nova 2016, 39, 194–209. [Google Scholar] [CrossRef]
- Burlando, B.; Cornara, L. Revisiting Amazonian Plants for Skin Care and Disease. Cosmetics 2017, 4, 25. [Google Scholar] [CrossRef] [Green Version]
- Dourado, D.; Barreto, C.; Fernandes, R.S.; Blanco, I.M.R.; Oliveira, D.; Pereira, N.; Leite, M.F. Development and Evaluation of Emulsifying Systems of the Material Grease from Brazilian Flora. J. Pharm. Pharmacogn. Res. 2015, 3, 130–140. [Google Scholar]
- Souza, F.G.; Araújo, F.F.; Farias, D.P.; Zanotto, A.W.; Neri-Numa, I.A.; Pastore, G.M. Brazilian Fruits of Arecaceae Family: An Overview of Some Representatives with Promising Food, Therapeutic and Industrial Applications. Food Res. Int. 2020, 138, 109690. [Google Scholar] [CrossRef]
- Lima, N.E.; Carvalho, A.A.; Meerow, A.W.; Manfrin, M.H. A Review of the Palm Genus Acrocomia: Neotropical Green Gold. Org. Divers. Evol. 2018, 18, 151–161. [Google Scholar] [CrossRef]
- Bergmann, J.C.; Tupinambá, D.D.; Costa, O.Y.A.; Almeida, J.R.M.; Barreto, C.C.; Quirino, B.F. Biodiesel Production in Brazil and Alternative Biomass Feedstocks. Renew. Sustain. Energy Rev. 2013, 21, 411–420. [Google Scholar] [CrossRef]
- Lima, R.P.; Luz, P.T.S.; Braga, M.; Batista, P.R.S.; Costa, C.E.F.; Zamian, J.R.; Nascimento, L.A.S.; Rocha Filho, G.N. Murumuru (Astrocaryum murumuru Mart.) Butter and Oils of Buriti (Mauritia flexuosa Mart.) and Pracaxi (Pentaclethra macroloba (Willd.) Kuntze) Can Be Used for Biodiesel Production: Physico-Chemical Properties and Thermal and Kinetic S. Ind. Crops Prod. 2017, 97, 536–544. [Google Scholar] [CrossRef]
- Motoike, S.Y.; Kuki, K.N. The Potential of Macaw Palm (Acrocomia aculeata) as Source of Biodiesel in Brazil. Int. Rev. Chem. Eng. 2009, 1, 632–635. [Google Scholar]
- Bastos, R.R.C.; Corrêa, A.P.L.; Luz, P.T.S.; Rocha Filho, G.N.; Zamian, J.R.; Conceição, L.R.V. Optimization of Biodiesel Production Using Sulfonated Carbon-Based Catalyst from an Amazon Agro-Industrial Waste. Energy Convers. Manag. 2020, 205, 112457. [Google Scholar] [CrossRef]
- Colombo, C.A.; Chorfi Berton, L.H.; Diaz, B.G.; Ferrari, R.A. Macauba: A Promising Tropical Palm for the Production of Vegetable Oil. OCL 2018, 25, D108. [Google Scholar] [CrossRef] [Green Version]
- Nunes, A.M.; Bianchi, V.J.; Fachinello, J.C.; Carvalho, A.Z.; Cardoso, G. Molecular Characterization of Pindo Palm by RAPD Markers. Rev. Bras. Frutic. 2008, 30, 702–707. [Google Scholar] [CrossRef]
- Elsevier Scopus® Database. Available online: https://www.scopus.com/search (accessed on 2 June 2022).
- Clarivate Web of Science Core Collection. Available online: https://www.webofknowledge.com/ (accessed on 2 June 2022).
- Rio de Janeiro Botanical Garden Brazilian Flora 2020. Available online: https://floradobrasil.jbrj.gov.br/reflora/listaBrasil/ConsultaPublicaUC/ConsultaPublicaUC.do#CondicaoTaxonCP (accessed on 2 June 2022).
- Soares, K.P.; Lorenzi, H.; Vianna, S.A.; Leitman, P.M.; Heiden, G.; Moraes, R.M.; Martins, R.C.; Campos-Rocha, A.; Ellert-Pereira, P.E.; Eslabão, M.P. Arecaceae. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB53 (accessed on 17 February 2022).
- Trentini, C.P.; Santos, K.A.; Silva, E.A.; Garcia, V.A.S.; Cardozo-Filho, L.; Silva, C. Oil Extraction from Macauba Pulp Using Compressed Propane. J. Supercrit. Fluids 2017, 126, 72–78. [Google Scholar] [CrossRef]
- Coimbra, M.C.; Jorge, N. Proximate Composition of Guariroba (Syagrus oleracea), Jerivá (Syagrus romanzoffiana) and Macaúba (Acrocomia aculeata) Palm Fruits. Food Res. Int. 2011, 44, 2139–2142. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, K.T.; Tavares, T.S.; Gomes, T.M.C.; Nunes, C.A. Multi-Target Response Surface Optimization of the Aqueous Extraction of Macauba Kernel Oil. Grasas Aceites 2020, 71, 377. [Google Scholar] [CrossRef]
- Del Río, J.C.; Evaristo, A.B.; Marques, G.; Martín-Ramos, P.; Martín-Gil, J.; Gutiérrez, A. Chemical Composition and Thermal Behavior of the Pulp and Kernel Oils from Macauba Palm (Acrocomia aculeata) Fruit. Ind. Crops Prod. 2016, 84, 294–304. [Google Scholar] [CrossRef]
- Yuyama, L.K.O.; Maeda, R.N.; Pantoja, L.; Aguiar, J.P.L.; Marinho, H.A. Processing and Shelf-Life Evaluation of Dehydrated and Pulverized Tucuman (Astrocaryum aculeatum Meyer). Ciência Tecnol. Aliment. 2008, 28, 408–412. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, S.C.M.; Vieira, L.M.; Matsuura, T.; Silva, G.F.; Duvoisin, S.; Albuquerque, P.M. Estudo Da Conservação Das Propriedades Nutricionais Da Polpa de Tucumã (Astrocaryum aculeatum) in Natura Em Embalagens a Vácuo. Brazilian J. Food Technol. 2017, 20, e2016107. [Google Scholar] [CrossRef] [Green Version]
- Linhares, B.M.; Costa, A.M.D.C.; Abreu, H.D.F.; Melo, A.C.G.R.; Ribeiro, P.R.E.; Montero, I.F.; Melo Filho, A.A.; Santos, R.C. Fatty Acids Profile, Physicalchemical Properties and Minerals with Quantify Indicador of Astrocaryum aculeatum Pulp Oil. J. Agric. Sci. 2017, 9, 352. [Google Scholar] [CrossRef] [Green Version]
- Didonet, A.A.; Antoniassi, R.; Back, G.R.; Faria-Machado, A.F.; Wilhelm, A.E.; Ferraz, I.D.K. Characterization of Amount and Quality of Tucuman Kernel Oil as a Potential Biomass. J. Am. Oil Chem. Soc. 2020, 97, 955–962. [Google Scholar] [CrossRef]
- Pereira, S.S.C.; Bezerra, V.S.; Ferreira, L.A.M.; Lucien, V.G.; Carim, M.D.J.V.; Guedes, M.C. Avaliações Físico-Químicas Do Fruto Do Murumuruzeiro (Astrocaryum murumuru Mart.). In Proceedings of the 3° Congresso Brasileiro de Plantas Oleaginosas, Óleos, Gorduras e Biodiesel, Varginha, Brazil, 1–26 July 2006; Universidade Federal de Lavras: Lavras, Brazil, 2006; pp. 576–580. [Google Scholar]
- Mambrin, M.C.; Barrera-Arellano, D. Caracterización de Aeites de Frutos de Palmeras de La Región Amazónica Del Brasil. Grasas Aceites 1997, 48, 154–158. [Google Scholar] [CrossRef] [Green Version]
- Smith, N. Astrocaryum murumuru. In Palms and People in the Amazon. Geobotany Studies (Basics, Methods and Case Studies); Springer: Cham, Switzerland, 2015; pp. 61–72. ISBN 978-3-319-05509-1. [Google Scholar]
- Bereau, D.; Benjelloun-Mlayah, B.; Banoub, J.; Bravo, R. FA and Unsaponifiable Composition of Five Amazonian Palm Kernel Oils. J. Am. Oil Chem. Soc. 2003, 80, 49–53. [Google Scholar] [CrossRef]
- Ferreira, E.S.; Lucien, V.G.; Amaral, A.S.; Silveira, C.S. Caracterização Físico-Química Do Fruto e Do Óleo Extraído de Tucumã (Astrocaryum vulgare Mart). Aliment. Nutr. 2008, 19, 427–433. [Google Scholar]
- Barbi, R.C.T.; Souza, A.R.C.; Melo, A.M.; Teixeira, G.L.; Corazza, M.L.; Ribani, R.H. Fatty Acid Profile and Lipid Quality of Maximiliana maripa Oil Obtained by Supercritical CO2 and Pressurized Ethanol. J. Supercrit. Fluids 2020, 165, 104979. [Google Scholar] [CrossRef]
- Duarte, O.R. A Quantitative Evaluation and Analysis of Biological, Chemical and Physicochemical Parameters of Maximiliana maripa (Aubl.) Drude (Inajá) Fruits as a Subsidy to the Study of the Oil Potential of Promising Populations for The State of Roraima (Brazil). Ph.D. Thesis, Federal University of Amazonas, Manaus, Brazil, 2008. (In Portuguese). [Google Scholar]
- Bereau, D.; Benjelloun-Mlayah, B.; Delmas, M. Maximiliana maripa Drude Mesocarp and Kernel Oils: Fatty Acid and Total Tocopherol Compositions. J. Am. Oil Chem. Soc. 2001, 78, 213–214. [Google Scholar] [CrossRef]
- Lima, J.R.O.; Silva, R.B.; Moura, E.M.; Moura, C.V.R. Biodiesel of Tucum Oil, Synthesized by Methanolic and Ethanolic Routes. Fuel 2008, 87, 1718–1723. [Google Scholar] [CrossRef]
- Oliveira, N.A.; Mazzali, M.R.; Fukumasu, H.; Gonçalves, C.B.; Oliveira, A.L. Composition and Physical Properties of Babassu Seed (Orbignya Phalerata) Oil Obtained by Supercritical CO2 Extraction. J. Supercrit. Fluids 2019, 150, 21–29. [Google Scholar] [CrossRef]
- Yuyama, L.K.O.; Aguiar, J.P.L.; Yuyama, K.; Clement, C.R.; Macedo, S.H.M.; Fávaro, D.I.T.; Afonso, C.; Vasconcellos, M.B.A.; Pimentel, S.A.; Badolato, E.S.G.; et al. Chemical Composition of the Fruit Mesocarp of Three Peach Palm (Bactris gasipaes) Populations Grown in Central Amazonia, Brazil. Int. J. Food Sci. Nutr. 2003, 54, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Santos, O.V.; Soares, S.D.; Dias, P.C.S.; Duarte, S.P.A.; Santos, M.P.L.; Nascimento, F.C.A. Chromatographic Profile and Bioactive Compounds Found in the Composition of Pupunha Oil (Bactris gasipaes Kunth): Implications for Human Health. Rev. Nutr. 2020, 33, e190146. [Google Scholar] [CrossRef]
- Arkcoll, D.B.; Aguiar, J.P.L. Peach Palm (Bactris gasipaes H.B.K.), a New Source of Vegetable Oil from the Wet Tropics. J. Sci. Food Agric. 1984, 35, 520–526. [Google Scholar] [CrossRef]
- Radice, M.; Viafara, D.; Neill, D.; Asanza, M.; Sacchetti, G.; Guerrini, A.; Maietti, S. Chemical Characterization and Antioxidant Activity of Amazonian (Ecuador) Caryodendron orinocense Karst. and Bactris gasipaes Kunth Seed Oils. J. Oleo Sci. 2014, 63, 1243–1250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faria, J.P.; Almeida, F.; Silva, L.C.R.; Vieira, R.F.; Agostini-Costa, T.S. Caracterização Da Polpa Do Coquinho-Azedo (Butia capitata var capitata). Rev. Bras. Frutic. 2008, 30, 827–829. [Google Scholar] [CrossRef] [Green Version]
- Lopes, R.M.; Silva, J.P.; Vieira, R.F.; Silva, D.B.; Gomes, I.S.; Agostini-Costa, T.S. Composição de Ácidos Graxos Em Polpa de Frutas Nativas Do Cerrado. Rev. Bras. Frutic. 2012, 34, 635–640. [Google Scholar] [CrossRef] [Green Version]
- Faria, J.P.; Arellano, D.B.; Grimaldi, R.; Silva, L.C.R.; Vieira, R.F.; Silva, D.B.; Agostini-Costa, T.S. Caracterização Química Da Amêndoa de Coquinho-Azedo (Butia capitata var capitata). Rev. Bras. Frutic. 2008, 30, 549–552. [Google Scholar] [CrossRef]
- Barbosa, M.C.A.; Rosa, Q.S.; Cardoso, L.M.; Gomides, A.F.F.; Barbosa, L.C.A.; Sant’Anna, H.M.P.; Pinheiro, S.S.; Peluzio, M.C.G.; Teixeira, R.D.B.L.; Valente, M.A.S. Composition Proximate, Bioactive Compounds and Antioxidant Capacity of Butia capitata. Food Sci. Technol. 2021, 2061, 1–6. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; Mattietto, R.A.; Friedrich, M.; Matta, V.M.; Marx, F. Chemical Characterization and Evaluation of Antioxidant Properties of Açaí Fruits (Euterpe oleraceae Mart.) during Ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Okada, Y.; Motoya, T.; Tanimoto, S.; Nomura, M. A Study on Fatty Acids in Seeds of Euterpe oleracea Mart Seeds. J. Oleo Sci. 2011, 60, 463–467. [Google Scholar] [CrossRef] [Green Version]
- Melo, P.S.; Selani, M.M.; Gonçalves, R.H.; Paulino, J.O.; Massarioli, A.P.; Alencar, S.M. Açaí Seeds: An Unexplored Agro-Industrial Residue as a Potential Source of Lipids, Fibers, and Antioxidant Phenolic Compounds. Ind. Crops Prod. 2021, 161, 113204. [Google Scholar] [CrossRef]
- Darnet, S.H.; Silva, L.H.M.; Rodrigues, A.M.C.; Lins, R.T. Nutritional Composition, Fatty Acid and Tocopherol Contents of Buriti (Mauritia flexuosa) and Patawa (Oenocarpus bataua) Fruit Pulp from the Amazon Region. Ciência e Tecnol. Aliment. 2011, 31, 488–491. [Google Scholar] [CrossRef] [Green Version]
- Carneiro, T.B.; Carneiro, J.G.M. Frutos e Polpa Desidratada Buriti (Mauritia flexuosa L.): Aspectos Físicos, Químicos e Tecnológicos. Rev. Verde 2011, 6, 105–111. [Google Scholar]
- Vásquez-Ocmín, P.G.; Alvarado, L.F.; Solís, V.S.; Torres, R.P.; Mancini-Filho, J. Chemical Characterization and Oxidative Stability of the Oils from Three Morphotypes of Mauritia flexuosa L.f, from the Peruvian Amazon. Grasas Aceites 2010, 61, 390–397. [Google Scholar] [CrossRef] [Green Version]
- Montúfar, R.; Laffargue, A.; Pintaud, J.C.; Hamon, S.; Avallone, S.; Dussert, S. Oenocarpus bataua Mart. (Arecaceae): Rediscovering a Source of High Oleic Vegetable Oil from Amazonia. J. Am. Oil Chem. Soc. 2010, 87, 167–172. [Google Scholar] [CrossRef]
- Escriche, I.; Restrepo, J.; Serra, J.A.; Herrera, L.R. Composition and Nutritive Value of Amazonian Palm Fruits. Food Nutr. Bull. 1999, 20, 361–365. [Google Scholar] [CrossRef]
- Canuto, G.A.B.; Xavier, A.A.O.; Neves, L.C.; Benassi, M.T. Caracterização Físico-Química de Polpas de Frutos Da Amazônia e Sua Correlação Com a Atividade Anti-Radical Livre. Rev. Bras. Frutic. 2010, 32, 1196–1205. [Google Scholar] [CrossRef] [Green Version]
- Cunha, V.M.B.; Silva, M.P.; Sousa, S.H.B.; Bezerra, P.N.; Menezes, E.G.O.; Silva, N.J.N.; Banna, D.A.D.S.; Araújo, M.E.; Carvalho Junior, R.N. Bacaba-de-Leque (Oenocarpus distichus Mart.) Oil Extraction Using Supercritical CO2 and Bioactive Compounds Determination in the Residual Pulp. J. Supercrit. Fluids 2019, 144, 81–90. [Google Scholar] [CrossRef]
- Pinto, R.H.H.; Sena, C.; Santos, O.V.; Costa, W.A.; Rodrigues, A.M.C.; Carvalho Junior, R.N. Extraction of Bacaba (Oenocarpus bacaba) Oil with Supercritical CO2: Global Yield Isotherms, Fatty Acid Composition, Functional Quality, Oxidative Stability, Spectroscopic Profile and Antioxidant Activity. Grasas Aceites 2018, 69, 246. [Google Scholar] [CrossRef] [Green Version]
- Crepaldi, I.C.; Almeida-Muradian, L.B.; Rios, M.D.G.; Penteado, M.V.C.; Salatino, A. Composição Nutricional Do Fruto de Licuri (Syagrus coronata (Martius) Beccari). Rev. Bras. Botânica 2001, 24, 155–159. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, J.E.P.; Rocha, V.R.; Peiter, A.S. Ouricuri (Syagrus coronata) Oil Extraction Using Mechanical Pressing. Brazilian Appl. Sci. Rev. 2020, 4, 3458–3466. [Google Scholar] [CrossRef]
- De Paula Filho, G.X.; Barreira, T.F.; Rodrigues, V.C.C.; Cardoso, L.M.; Martino, H.S.D.; Pinheiro-Sanťana, H.M. Study of the Physical and Physicochemical Characteristics of Fruits of the Licuri Palm (Syagrus coronata (Mart.) Becc.) Found in the Atlantic Forest of Minas Gerais, Brazil. Food Sci. Technol. 2015, 35, 474–480. [Google Scholar] [CrossRef] [Green Version]
- Iha, O.K.; Alves, F.C.S.C.; Suarez, P.A.Z.; Oliveira, M.B.F.; Meneghetti, S.M.P.; Santos, B.P.T.; Soletti, J.I. Physicochemical Properties of Syagrus coronata and Acrocomia aculeata Oils for Biofuel Production. Ind. Crops Prod. 2014, 62, 318–322. [Google Scholar] [CrossRef]
- Segall, S.D.; Artz, W.E.; Raslan, D.S.; Ferraz, V.P.; Takahashi, J.A. Ouricuri (Syagrus coronata) Triacylglycerol Analysis Using HPLC and Positive Ion Electrospray Tandem MS. J. Am. Oil Chem. Soc. 2004, 81, 143–149. [Google Scholar] [CrossRef]
- Scariot, A.O.; Lleras, E.; Hay, J.D. Reproductive Biology of the Palm Acrocomia aculeata in Central Brazil. Biotropica 1991, 23, 12. [Google Scholar] [CrossRef]
- Vianna, S.A.; Campos-Rocha, A. Acrocomia aculeata (Jacq.) Lodd. Ex Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15663 (accessed on 17 February 2022).
- Tilahun, W.W.; Grossi, J.A.S.; Favaro, S.P.; Sediyama, C.S.; Goulart, S.D.M.; Pimentel, L.D.; Motoike, S.Y. Increase in Oil Content and Changes in Quality of Macauba Mesocarp Oil along Storage. OCL 2019, 26, 20. [Google Scholar] [CrossRef] [Green Version]
- Cruz, G.; Silva, A.V.S.; Silva, J.B.S.; Caldeiras, R.N.; Souza, M.E.P. Biofuels from Oilseed Fruits Using Different Thermochemical Processes: Opportunities and Challenges. Biofuels Bioprod. Biorefin. 2020, 14, 696–719. [Google Scholar] [CrossRef]
- Plath, M.; Moser, C.; Bailis, R.; Brandt, P.; Hirsch, H.; Klein, A.M.; Walmsley, D.; von Wehrden, H. A Novel Bioenergy Feedstock in Latin America? Cultivation Potential of Acrocomia aculeata under Current and Future Climate Conditions. Biomass Bioenergy 2016, 91, 186–195. [Google Scholar] [CrossRef]
- Evaristo, A.B.; Grossi, J.A.S.; Carneiro, A.C.O.; Pimentel, L.D.; Motoike, S.Y.; Kuki, K.N. Actual and Putative Potentials of Macauba Palm as Feedstock for Solid Biofuel Production from Residues. Biomass Bioenergy 2016, 85, 18–24. [Google Scholar] [CrossRef]
- César, A.S.; Almeida, F.A.; Souza, R.P.; Silva, G.C.; Atabani, A.E. The Prospects of Using Acrocomia aculeata (Macaúba) a Non-Edible Biodiesel Feedstock in Brazil. Renew. Sustain. Energy Rev. 2015, 49, 1213–1220. [Google Scholar] [CrossRef]
- Forest Investment Program in Brazil Macaúba Project. Available online: http://fip.mma.gov.br/projeto-macauba/ (accessed on 2 June 2022).
- Costa, J.M.C.; Oliveira, D.M.; Costa, L.E.C. Macauba Palm—Acrocomia aculeata. In Exotic Fruits; Elsevier: Amsterdam, The Netherlands, 2018; pp. 297–304. [Google Scholar]
- Andrade, A.C.; Marinho, J.F.U.; de Souza, A.C.; de Sousa Tavares, T.; Dias, D.R.; Schwan, R.F.; Nunes, C.A.; Bastos, S.C. Prebiotic Potential of Pulp and Kernel Cake from Jerivá (Syagrus romanzoffiana) and Macaúba Palm Fruits (Acrocomia aculeata). Food Res. Int. 2020, 136, 109595. [Google Scholar] [CrossRef] [PubMed]
- Vianna, S.A. Astrocaryum aculeatum G.Mey. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB22080 (accessed on 17 February 2022).
- Kahn, F.; Moussa, F. Economic Importance of Astrocaryum aculeatum (Palmae) in Central Brazilian Amazonia. Acta Bot. Venez. 1999, 22, 237–245. [Google Scholar]
- Cabral, F.L.; Bernardes, V.M.; Passos, D.F.; Oliveira, J.S.; Doleski, P.H.; Silveira, K.L.; Horvarth, M.C.; Bremm, J.M.; Barbisan, F.; Azzolin, V.F.; et al. Astrocaryum aculeatum Fruit Improves Inflammation and Redox Balance in Phytohemagglutinin-Stimulated Macrophages. J. Ethnopharmacol. 2020, 247, 112274. [Google Scholar] [CrossRef] [PubMed]
- Vianna, S.A. Astrocaryum murumuru Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB22086 (accessed on 17 February 2022).
- Pesce, C. Murumuru (Astrocaryum murumuru Mart.). In Oleaginosas da Amazônia; Pesce, C., Ed.; Museu Paraense Emílio Goeldi: Belém, Brazil, 2009; pp. 59–65. [Google Scholar]
- Hovorková, P.; Laloučková, K.; Skřivanová, E. Determination of in vitro Antibacterial Activity of Plant Oils Containing Medium-Chain Fatty Acids against Gram-Positive Pathogenic and Gut Commensal Bacteria. Czech. J. Anim. Sci. 2018, 63, 119–125. [Google Scholar] [CrossRef] [Green Version]
- Speranza, P.; Ribeiro, A.P.B.; Macedo, G.A. Application of Lipases to Regiospecific Interesterification of Exotic Oils from an Amazonian Area. J. Biotechnol. 2016, 218, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Vianna, S.A. Astrocaryum vulgare Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15674 (accessed on 14 October 2021).
- Ribeiro, L.L.O.; Lima, I.; Cunha, L.; Pacheco, E.; Silva, R.T. Biometria dos frutos de tucumã (Astrocaryum vulgare Mart.) no município de Capitão Poço/PA. Enciclopédia Biosf. 2014, 10, 2776–2786. [Google Scholar]
- Soares, K.P. Attalea maripa (Aubl.) Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15682 (accessed on 17 February 2022).
- Smith, N. Attalea maripa. In Geobotany Studies; Springer: Cham, Switzerland, 2015; pp. 91–105. [Google Scholar]
- Cavallari, M.M.; Toledo, M.M. What Is the Name of the Babassu? A Note on the Confusing Use of Scientific Names for This Important Palm Tree. Rodriguesia 2016, 67, 533–538. [Google Scholar] [CrossRef] [Green Version]
- Soares, K.P. Attalea speciosa Mart. Ex Spreng. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15686 (accessed on 17 February 2022).
- Souza, B.V.C.; Moreira-Araujo, R.S.R.; Galvao, L.M.V.; Vale, L.C.; Rocha, L.M.; Cardoso, M.L.S.; Luz, R.G.C.L.P.; Goncalves, M.F.B.; Silva, O.A.; Nunes, L.V.C. Food Products with Mesocarp Babassu: A Review. Curr. Nutr. Food Sci. 2018, 14, 274–279. [Google Scholar] [CrossRef]
- Ferrari, R.A.; Soler, M.P. Obtention and Characterization of Coconut Babassu Derivatives. Sci. Agric. 2015, 72, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, M.A. Babassu-A New Approach for an Ancient Brazilian Biomass. Biomass Bioenergy 2008, 32, 857–864. [Google Scholar] [CrossRef]
- Lorenzi, H. Bactris gasipaes Kunth. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB22106 (accessed on 17 February 2022).
- Santos, M.F.G.; Alves, R.E.; Brito, E.S.; Silva, S.M.; Silveira, M.R.S. Quality Characteristis of Fruits and Oils of Palms Native to the Brazilian Amazon. Rev. Bras. Frutic. 2017, 39. [Google Scholar] [CrossRef] [Green Version]
- Padilha, H.K.M.; Mistura, C.C.; Villela, J.C.B.; Rivas, M.; Heiden, G.; Barbieri, R.L. Avaliação Da Produção de Cachos de Frutas Em Palmeiras de Butiá (Butia odorata (Barb. Rodr.) Noblick & Lorenzi). Magistra 2016, 28, 419–426. [Google Scholar]
- Beskow, G.T.; Hoffmann, J.F.; Teixeira, A.M.; Fachinello, J.C.; Chaves, F.C.; Rombaldi, C.V. Bioactive and Yield Potential of Jelly Palms (Butia odorata Barb. Rodr.). Food Chem. 2015, 172, 699–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heiden, G.; Ellert-Pereira, P.E.; Eslabão, M.P. Butia (Becc.) Becc. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15703 (accessed on 17 February 2022).
- Krolow, A.C.; Fonseca, L.X.; Corrêa, A.P.A. Butiá Em Pó Liofilizado. Available online: https://www.infoteca.cnptia.embrapa.br/infoteca/bitstream/doc/927492/1/Comunicado280.pdf (accessed on 5 May 2021).
- Büttow, M.V.; Barbieri, R.L.; Neitzke, R.S.; Heiden, G. Traditional Knowledge Associated with the Use of Butia Palm (Butia Spp., Arecaceae) in the Southern of Brazil. Rev. Bras. Frutic. 2009, 31, 1069–1075. [Google Scholar] [CrossRef]
- Schwartz, E.; Fachinello, J.C.; Barbieri, R.L.; Silva, J.B.D. Avaliação de Populações de Butia capitata de Santa Vitória Do Palmar. Rev. Bras. Frutic. 2010, 32, 736–745. [Google Scholar] [CrossRef] [Green Version]
- Soares, K.P.; Longhi, S.J.; Neto, L.W.; Assis, L.C. Palms (Arecaceae) from Rio Grande Do Sul, Brazil. Rodriguesia 2014, 65, 113–139. [Google Scholar] [CrossRef]
- Corrêa, L.B.; Barbieri, R.L.; Rossato, M.; Büttow, M.V.; Heiden, G. Caracterização Cariológica de Palmeiras Do Gênero Butia (Arecaceae). Rev. Bras. Frutic. 2009, 31, 1111–1116. [Google Scholar] [CrossRef]
- Nazareno, A.G.; Zucchi, M.I.; Reis, M.S. Microsatellite Markers for Butia eriospatha (Arecaceae), a Vulnerable Palm Species from the Atlantic Rainforest of Brazil. Am. J. Bot. 2011, 98, e198–e200. [Google Scholar] [CrossRef] [Green Version]
- Faria, J.P.; Siqueira, E.M.A.; Vieira, R.F.; Agostini-Costa, T.S. Fruits of Butia capitata (Mart.) Becc as Good Sources of β -Carotene and Provitamina. Rev. Bras. Frutic. 2011, 33, 612–617. [Google Scholar] [CrossRef] [Green Version]
- Moura, R.C.; Lopes, P.S.N.; Brandão Junior, D.S.; Gomes, J.G.; Pereira, M.B. Biometria de Frutos e Sementes de Butia capitata (Mart.) Beccari (Arecaceae), Em Vegetação Natural No Norte de Minas Gerais, Brasil. Biota Neotrop. 2010, 10, 415–419. [Google Scholar] [CrossRef]
- Magalhães, H.M.; Catão, H.C.R.M.; Sales, N.L.P.; Lima, N.F.; Lopes, P.S.N. Health Quality of Butia capitata Seeds in the North of Minas Gerais, Brazil. Cienc. Rural 2008, 38, 2371–2374. [Google Scholar] [CrossRef]
- Kumagai, L.; Hanazaki, N. Ethnobotanical and Ethnoecological Study of Butia catarinensis Noblick & Lorenzi: Contributions to the Conservation of an Endangered Area in Southern Brazil. Acta Bot. Brasilica 2013, 27, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Ressel, K.; Guilherme, F.A.G. Ten Years from Propagule to Mature Plant of Butia purpurascens Glassman (Arecaceae): An Endemic and Endangered Palm of the Brazilian Cerrado. Brazilian J. Biol. 2022, 82, e233941. [Google Scholar] [CrossRef] [PubMed]
- Vianna, S.A. Euterpe oleracea Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15713 (accessed on 17 February 2022).
- Menezes, E.M.S.; Torres, A.T.; Srur, A.U.S. Valor Nutricional Da Polpa de Açaí (Euterpe oleracea Mart) Liofilizada. Acta Amaz. 2008, 38, 311–316. [Google Scholar] [CrossRef]
- Vianna, S.A. Mauritia flexuosa L.F. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15723 (accessed on 17 February 2022).
- Sampaio, M.B. Boas Práticas de Manejo Para o Extrativismo Sustentável Do Buriti, 1st ed.; Instituto Sociedade, População e Natureza: Brasília, Brazil, 2011; ISBN 978-85-63288-07-3. [Google Scholar]
- Cândido, T.L.N.; Silva, M.R.; Agostini-Costa, T.S. Bioactive Compounds and Antioxidant Capacity of Buriti (Mauritia flexuosa L.f.) from the Cerrado and Amazon Biomes. Food Chem. 2015, 177, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.L.S.; Guedes, I.; Alcantara, P., Jr; Moreira, S.G.C. Infrared Absorption Spectra of Buriti (Mauritia flexuosa L.) Oil. Vib. Spectrosc. 2003, 33, 127–131. [Google Scholar] [CrossRef]
- Pinto, M.V.S.; Silva, D.L.; Saraiva, A.C.F. Obtenção e Caracterização de Carvão Ativado de Caroço de Buriti (Mauritia flexuosa L. f.) Para a Avaliação Do Processo de Adsorção de Cobre (II). Acta Amaz. 2013, 43, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Lorenzi, H. Oenocarpus bataua Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB22178 (accessed on 17 February 2022).
- Hidalgo, P.S.P.; Nunomura, R.C.S.; Nunomura, S.M. Amazon Oilseeds: Chemistry and Antioxidant Activity of Patawa (Oenocarpus bataua Mart.). Rev. Virtual Quim. 2016, 8, 130–140. [Google Scholar] [CrossRef]
- Lorenzi, H. Oenocarpus bacaba Mart. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB22174 (accessed on 17 February 2022).
- Mendes, G.G.C.; Gusmão, M.T.A.; Martins, T.G.V.; Rosado, R.D.S.; Sobrinho, R.S.A.; Nunes, A.C.P.; Ribeiro, W.S.; Zanuncio, J.C. Genetic Divergence of Native Palms of Oenocarpus distichus Considering Biometric Fruit Variables. Sci. Rep. 2019, 9, 4943. [Google Scholar] [CrossRef]
- Rufino, M.U.L.; Costa, J.T.M.; Silva, V.A.; Andrade, L.H.C. Conhecimento e Uso Do Ouricuri (Syagrus coronata) e Do Babaçu (Orbignya phalerata) Em Buíque, PE, Brasil. Acta Bot. Brasilica 2008, 22, 1141–1149. [Google Scholar] [CrossRef] [Green Version]
- Soares, K.P. Syagrus coronata (Mart.) Becc. Available online: http://floradobrasil.jbrj.gov.br/reflora/floradobrasil/FB15736 (accessed on 17 February 2022).
- Souza, M.C.P.; Moura, F.; Silva, J.V.; Almeida, C. Phylogeography of the Palm Syagrus coronata (Martius) Beccari (Arecaceae): Distribution in the “Caatinga” and Atlantic Forest Domains. Rev. Bras. Bot. 2018, 41, 849–857. [Google Scholar] [CrossRef]
- Lima, V.V.F.; Scariot, A.; Sevilha, A.C. Predicting the Distribution of Syagrus coronata Palm: Challenges for the Conservation of an Important Resource in Northeastern Brazil. Flora 2020, 269, 151607. [Google Scholar] [CrossRef]
- Belviso, S.; Ghirardello, D.; Giordano, M.; Sousa Ribeiro, G.; de Souza Alves, J.; Parodi, S.; Risso, S.; Zeppa, G. Phenolic Composition, Antioxidant Capacity and Volatile Compounds of Licuri (Syagrus coronata (Martius) Beccari) Fruits as Affected by the Traditional Roasting Process. Food Res. Int. 2013, 51, 39–45. [Google Scholar] [CrossRef]
- Oliveira, B.S.D. Gastroprotective Activity of Almond Oil from Syagrus coronata (Mart.) Becc. Master’s Thesis, Federal University of Pernambuco, Recife, Brazil, 2008. (In Portuguese). [Google Scholar]
- Trentini, C.P.; Oliveira, D.M.; Zanette, C.M.; Silva, C.D. Low-Pressure Solvent Extraction of Oil from Macauba (Acrocomia aculeata) Pulp: Characterization of Oil and Defatted Meal. Ciência Rural 2016, 46, 725–731. [Google Scholar] [CrossRef] [Green Version]
- Trentini, C.P.; Silva, S.B.; Rodrigues, G.M.; Garcia, V.A.S.; Cardozo-Filho, L.; Silva, C. Pressurized Liquid Extraction of Macauba Pulp Oil. Can. J. Chem. Eng. 2017, 95, 1579–1584. [Google Scholar] [CrossRef]
- Zaninetti, R.A.; Moreira, A.; Ferraudo, A.S.; Teixeira, S.T. Variabilidade Populacional Na Produção De Óleo, Lipídios Totais Na Amêndoa E Polpa De Tucumã Coletados No Estado Do Acre. Agrotrópica (Itabuna) 2016, 28, 179–184. [Google Scholar] [CrossRef]
- Bezerra, V.S. Considerações Sobre a Palmeira Murumuruzeiro (Astrocaryum murumuru Mart.); Embrapa Comunicado Técnico 130; Embrapa Amapá: Macapá, Brazil, 2012. [Google Scholar]
- Burnett, C.L.; Fiume, M.M.; Bergfeld, W.F.; Belsito, D.V.; Hill, R.A.; Klaassen, C.D.; Liebler, D.; Marks, J.G.; Shank, R.C.; Slaga, T.J.; et al. Final Report on Plant-Derived Fatty Acid Oils as Used in Cosmetics. Cosmet. Ingred. Rev. 2011, 36, 100. [Google Scholar]
- Costa, B.E.T.; Santos, O.V.; Corrêa, N.C.F.; França, L.F.D. Comparative Study on the Quality of Oil Extracted from Two Tucumã Varieties Using Supercritical Carbon Dioxide. Food Sci. Technol. 2016, 36, 322–328. [Google Scholar] [CrossRef] [Green Version]
- Pardauil, J.J.R.; Molfetta, F.A.; Braga, M.; Souza, L.K.C.; Filho, G.N.R.; Zamian, J.R.; Costa, C.E.F. Characterization, Thermal Properties and Phase Transitions of Amazonian Vegetable Oils. J. Therm. Anal. Calorim. 2017, 127, 1221–1229. [Google Scholar] [CrossRef]
- Barbi, R.C.T.; Souza, A.R.C.; Hamerski, F.; Teixeira, G.L.; Corazza, M.L.; Ribani, R.H. Subcritical Propane Extraction of High-Quality Inajá (Maximiliana maripa) Pulp Oil. J. Supercrit. Fluids 2019, 153, 104576. [Google Scholar] [CrossRef]
- Santos, M.F.G.; Alves, R.E.; Roca, M. Carotenoid Composition in Oils Obtained from Palm Fruits from the Brazilian Amazon. Grasas Aceites 2015, 66, e086. [Google Scholar] [CrossRef] [Green Version]
- Bauer, L.C.; Santos, L.S.; Sampaio, K.A.; Ferrão, S.P.B.; Fontan, R.C.I.; Minim, L.A.; Veloso, C.M.; Bonomo, R.C.F. Physicochemical and Thermal Characterization of Babassu Oils (Orbignya phalerata Mart.) Obtained by Different Extraction Methods. Food Res. Int. 2020, 137, 109474. [Google Scholar] [CrossRef] [PubMed]
- Rufino, M.S.M.; Pérez-Jiménez, J.; Arranz, S.; Alves, R.E.; Brito, E.S.; Oliveira, M.S.P.; Saura-Calixto, F. Açaí (Euterpe oleraceae) ‘BRS Pará’: A Tropical Fruit Source of Antioxidant Dietary Fiber and High Antioxidant Capacity Oil. Food Res. Int. 2011, 44, 2100–2106. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, E.S.; Rogez, H.L.G.; Herman, C.A.N.P. Effect of the Combination of Enzymatic Preparations on the Aqueous Extraction Yield of the Oil from the Pulp of Euterpe Oleracea Fruit. Brazilian J. Chem. Eng. 2018, 35, 1193–1201. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, R.J.S.; Couri, S.; Antoniassi, R.; Freitas, S.P. Composição Em Ácidos Graxos Do Óleo Da Polpa de Açaí Extraído Com Enzimas e Com Hexano. Rev. Bras. Frutic. 2008, 30, 498–502. [Google Scholar] [CrossRef]
- Wycoff, W.; Luo, R.; Schauss, A.G.; Neal-Kababick, J.; Sabaa-Srur, A.U.O.; Maia, J.G.S.; Tran, K.; Richards, K.M.; Smith, R.E. Chemical and Nutritional Analysis of Seeds from Purple and White Açaí (Euterpe oleracea Mart.). J. Food Compos. Anal. 2015, 41, 181–187. [Google Scholar] [CrossRef]
- Silva, R.C.; Batista, A.; Costa, D.C.F.; Moura-Nunes, N.; Koury, J.C.; Costa, C.A.; Resende, Â.C.; Daleprane, J.B. Açai (Euterpe oleracea Mart.) Seed Flour Prevents Obesity-Induced Hepatic Steatosis Regulating Lipid Metabolism by Increasing Cholesterol Excretion in High-Fat Diet-Fed Mice. Food Res. Int. 2018, 111, 408–415. [Google Scholar] [CrossRef]
- Sampaio, M.B.; Carrazza, L.R. Manual Tecnológico de Aproveitamento Integral Do Fruto e Da Folha Do Buriti (Mauritia flexuosa), 1st ed.; Instituto Sociedade, População e Natureza: Brasília, Brazil, 2012; ISBN 9788563288097. [Google Scholar]
- Lisboa, M.C.; Wiltshire, F.M.S.; Fricks, A.T.; Dariva, C.; Carrière, F.; Lima, Á.S.; Soares, C.M.F. Oleochemistry Potential from Brazil Northeastern Exotic Plants. Biochimie 2020, 178, 96–104. [Google Scholar] [CrossRef]
- Tilahun, W.W.; Grossi, J.A.S.; Favaro, S.P. Mesocarp Oil Quality of Macauba Palm Fruit Improved by Gamma Irradiation in Storage. Radiat. Phys. Chem. 2020, 168, 108575. [Google Scholar] [CrossRef]
- Pesce, C. Oleaginosas Da Amazonia, 2nd ed.; Pesce, C., Ed.; Museu Paraense Emílio Goeldi: Belém, Brazil, 2009; ISBN 9788561377069. [Google Scholar]
- Santos, M.F.G.; Marmesat, S.; Brito, E.S.; Alves, R.E.; Dobarganes, M.C. Major Components in Oils Obtained from Amazonian Palm Fruits. Grasas Aceites 2013, 64, 328–334. [Google Scholar] [CrossRef] [Green Version]
- Nascimento, K.; Copetti, P.M.; Fernandes, A.; Klein, B.; Fogaça, A.; Zepka, L.Q.; Wagner, R.; Ourique, A.F.; Sagrillo, M.R.; da Silva, J.E.P. Phytochemical Analysis and Evaluation of the Antioxidant and Antiproliferative Effects of Tucumã Oil Nanocapsules in Breast Adenocarcinoma Cells (MCF-7). Nat. Prod. Res. 2019, 35, 2060–2065. [Google Scholar] [CrossRef] [PubMed]
- Contente, D.M.L.; Pereira, R.R.; Rodrigues, A.M.C.; da Silva, E.O.; Ribeiro-Costa, R.M.; Silva-Júnior, J.O.C. Nanoemulsions of Acai Oil: Physicochemical Characterization for the Topical Delivery of Antifungal Drugs. Chem. Eng. Technol. 2020, 43, 1424–1432. [Google Scholar] [CrossRef]
- Silva, S.M.; Sampaio, K.A.; Taham, T.; Rocco, S.A.; Ceriani, R.; Meirelles, A.J.A. Characterization of Oil Extracted from Buriti Fruit (Mauritia flexuosa) Grown in the Brazilian Amazon Region. J. Am. Oil Chem. Soc. 2009, 86, 611–616. [Google Scholar] [CrossRef]
- Fonseca, H.M.; Santos, C.O.; Cruz, L.P.A.; Arthur, V.; Freitas, B.C.B.; Souza, A.R.M.; Martins, G.A.S. The Effects of Microwave Application on the Physicochemical Properties of Bacaba (Oenocarpus bacaba Mart.) Oil. Acta Sci. Pol. Technol. Aliment. 2021, 20, 189–196. [Google Scholar] [CrossRef] [PubMed]
- La Salles, K.T.; Meneghetti, S.M.P.; La Salles, W.F.; Meneghetti, M.R.; Santos, I.C.F.; Silva, J.P.V.; de Carvalho, S.H.V.; Soletti, J.I. Characterization of Syagrus coronata (Mart.) Becc. Oil and Properties of Methyl Esters for Use as Biodiesel. Ind. Crops Prod. 2010, 32, 518–521. [Google Scholar] [CrossRef]
- Araújo, P.H.M.; Maia, A.S.; Cordeiro, A.M.T.M.; Gondim, A.D.; Santos, N.A. Catalytic Deoxygenation of the Oil and Biodiesel of Licuri (Syagrus coronata) to Obtain n-Alkanes with Chains in the Range of Biojet Fuels. ACS Omega 2019, 4, 15849–15855. [Google Scholar] [CrossRef] [Green Version]
- Fontanel, D. Unsaponifiable Matter in Plant Seed Oils, 1st ed.; Fontanel, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; ISBN 978-3-642-35709-1. [Google Scholar]
- Teixeira, G.L.; Züge, L.C.B.; Silveira, J.L.M.; Scheer, A.P.; Ribani, R.H. The Impact of Polyoxyethylene Sorbitan Surfactants in the Microstructure and Rheological Behaviour of Emulsions Made with Melted Fat from Cupuassu (Theobroma grandiflorum). J. Surfactants Deterg. 2016, 19, 725–738. [Google Scholar] [CrossRef]
- Rodenbush, C.M.; Hsieh, F.H.; Viswanath, D.S. Density and Viscosity of Vegetable Oils. J. Am. Oil Chem. Soc. 1999, 76, 1415–1419. [Google Scholar] [CrossRef]
- Esteban, B.; Riba, J.R.; Baquero, G.; Rius, A.; Puig, R. Temperature Dependence of Density and Viscosity of Vegetable Oils. Biomass Bioenergy 2012, 42, 164–171. [Google Scholar] [CrossRef] [Green Version]
- Teixeira, R.S.; Rocha, P.R.; Polonini, H.C.; Brandão, M.A.F.; Chaves, M.G.A.M.; Raposo, N.R.B. Mushroom Tyrosinase Inhibitory Activity and Major Fatty Acid Constituents of Amazonian Native Flora Oils. Brazilian J. Pharm. Sci. 2012, 48, 399–404. [Google Scholar] [CrossRef] [Green Version]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Cossetin, L.F.; Sagrillo, M.R.; Nascimento, K.; Silva, A.S.; Machado, A.K.; Cruz, I.B.M.; Stefani, L.M.; et al. Antihyperglycemic, Antioxidant Activities of Tucumã Oil (Astrocaryum vulgare) in Alloxan-Induced Diabetic Mice, and Identification of Fatty Acid Profile by Gas Chromatograph: New Natural Source to Treat Hyperglycemia. Chem. Biol. Interact. 2017, 270, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Silva, R.B.; Silva-Júnior, E.V.; Rodrigues, L.C.; Andrade, L.H.C.; Silva, S.I.; Harand, W.; Oliveira, A.F.M. A Comparative Study of Nutritional Composition and Potential Use of Some Underutilized Tropical Fruits of Arecaceae. An. Acad. Bras. Cienc. 2015, 87, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, K.T.; Tavares, T.S.; Nunes, C.A. The Chemical, Thermal and Textural Characterization of Fractions from Macauba Kernel Oil. Food Res. Int. 2020, 130, 108925. [Google Scholar] [CrossRef] [PubMed]
- Souza, T.G.S.; Silva, M.M.; Feitoza, G.S.; Alcântara, L.F.M.; Silva, M.A.; Oliveira, A.M.; Aguiar, J.C.R.O.F.; Navarro, D.M.A.F.; Aguiar Júnior, F.C.A.; Silva, M.V.; et al. Biological Safety of Syagrus coronata (Mart.) Becc. Fixed Oil: Cytotoxicity, Acute Oral Toxicity, and Genotoxicity Studies. J. Ethnopharmacol. 2021, 272, 113941. [Google Scholar] [CrossRef]
- Bony, E.; Boudard, F.; Brat, P.; Dussossoy, E.; Portet, K.; Poucheret, P.; Giaimis, J.; Michel, A. Awara (Astrocaryum vulgare M.) Pulp Oil: Chemical Characterization, and Anti-Inflammatory Properties in a Mice Model of Endotoxic Shock and a Rat Model of Pulmonary Inflammation. Fitoterapia 2012, 83, 33–43. [Google Scholar] [CrossRef]
- Santos, M.M.R.; Fernandes, D.S.; Cândido, C.J.; Cavalheiro, L.F.; Silva, A.F.; Nascimento, V.A.; Ramos Filho, M.M.; Santos, E.F.; Hiane, P.A. Physical-Chemical, Nutritional and Antioxidant Properties of Tucumã (Astrocaryum huaimi Mart.) Fruits. Semin. Ciências Agrárias 2018, 39, 1517. [Google Scholar] [CrossRef]
- Santos, M.F.G.; Mamede, R.V.S.; Rufino, M.S.M.; Brito, E.S.; Alves, R.E. Amazonian Native Palm Fruits as Sources of Antioxidant Bioactive Compounds. Antioxidants 2015, 4, 591–602. [Google Scholar] [CrossRef] [Green Version]
- Bony, E.; Boudard, F.; Dussossoy, E.; Portet, K.; Brat, P.; Giaimis, J.; Michel, A. Chemical Composition and Anti-Inflammatory Properties of the Unsaponifiable Fraction from Awara (Astrocaryum vulgare M.) Pulp Oil in Activated J774 Macrophages and in a Mice Model of Endotoxic Shock. Plant Foods Hum. Nutr. 2012, 67, 384–392. [Google Scholar] [CrossRef]
- Lima, J.R.O.; Silva, R.B.; Silva, C.C.M.; Santos, L.S.S.; Santos, J.R.; Moura, E.M.; Moura, C.V.R. Biodiesel de Babaçu (Orbignya sp.) Obtido Por via Etanólica. Quim. Nova 2007, 30, 600–603. [Google Scholar] [CrossRef]
- Shibahara, A.; Yamamoto, K.; Nakayama, T.; Kajimoto, G. Cis-Vaccenic Acid in Pulp Lipids of Commonly Available Fruits. J. Am. Oil Chem. Soc. 1987, 64, 397–401. [Google Scholar] [CrossRef]
- Shibahara, A.; Yamamoto, K.; Nakayama, T.; Kajimoto, G. Cis-Vaccenic Acid in Mango Pulp Lipids. Lipids 1986, 21, 388–394. [Google Scholar] [CrossRef]
- Alves, A.Q.; Silva, V.A.; Goés, A.J.S.; Silva, M.S.; Oliveira, G.G.; Bastos, I.V.G.A.; Castro Neto, A.G.; Alves, A.J. The Fatty Acid Composition of Vegetable Oils and Their Potential Use in Wound Care. Adv. Ski. Wound Care 2019, 32, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Speranza, P.; Falcão, A.O.; Macedo, J.A.; Silva, L.H.M.; Rodrigues, A.M.C.; Macedo, G.A. Amazonian Buriti Oil: Chemical Characterization and Antioxidant Potential. Grasas Aceites 2016, 67, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Barbi, R.C.T.; Hornung, P.S.; Ávila, S.; Alves, F.E.S.B.; Beta, T.; Ribani, R.H. Ripe and Unripe Inajá (Maximilia maripa) Fruit: A New High Source of Added Value Bioactive Compounds. Food Chem. 2020, 331, 127333. [Google Scholar] [CrossRef] [PubMed]
- Santos, M.F.G.; Alves, R.E.; Ruíz-Méndez, M.V. Minor Components in Oils Obtained from Amazonian Palm Fruits. Grasas Aceites 2013, 64, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Oboh, F.O.J.; Oderinde, R.A. Analysis of the Pulp and Pulp Oil of the Tucum (Astrocaryum vulgare Mart) Fruit. Food Chem. 1988, 30, 277–287. [Google Scholar] [CrossRef]
- Matos, K.A.N.; Lima, D.P.; Barbosa, A.P.P.; Mercadante, A.Z.; Chisté, R.C. Peels of Tucumã (Astrocaryum vulgare) and Peach Palm (Bactris gasipaes) Are by-Products Classified as Very High Carotenoid Sources. Food Chem. 2019, 272, 216–221. [Google Scholar] [CrossRef]
- Costa, P.A.; Ballus, C.A.; Teixeira-Filho, J.; Godoy, H.T. Phytosterols and Tocopherols Content of Pulps and Nuts of Brazilian Fruits. Food Res. Int. 2010, 43, 1603–1606. [Google Scholar] [CrossRef]
- Sagrillo, M.R.; Garcia, L.F.M.; Souza Filho, O.C.; Duarte, M.M.M.F.; Ribeiro, E.E.; Cadoná, F.C.; Cruz, I.B.M. Tucumã Fruit Extracts (Astrocaryum aculeatum Meyer) Decrease Cytotoxic Effects of Hydrogen Peroxide on Human Lymphocytes. Food Chem. 2015, 173, 741–748. [Google Scholar] [CrossRef] [Green Version]
- Bobbio, F.O.; Druzian, J.I.; Abrão, P.A.; Bobbio, P.A.; Fadelli, S. Identificação e Quantificação Das Antocianinas Do Fruto Do Açaizeiro (Euterpe oleracea) Mart. Ciência e Tecnol. Aliment. 2000, 20, 388–390. [Google Scholar] [CrossRef]
- Machado, A.K.; Andreazza, A.C.; Silva, T.M.; Boligon, A.A.; Nascimento, V.; Scola, G.; Duong, A.; Cadoná, F.C.; Ribeiro, E.E.; da Cruz, I.B.M. Neuroprotective effects of açaí (Euterpe oleracea Mart.) against rotenone in vitro exposure. Oxid. Med. Cell. Longev. 2016, 2016, 8940850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.; Talcott, S.T. Chemical Composition, Antioxidant Properties, and Thermal Stability of a Phytochemical Enriched Oil from Açai (Euterpe oleracea Mart.). J. Agric. Food Chem. 2008, 56, 4631–4636. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Palencia, L.A.; Talcott, S.T.; Safe, S.; Mertens-Talcott, S. Absorption and Biological Activity of Phytochemical-Rich Extracts from Açai (Euterpe oleracea Mart.) Pulp and Oil in Vitro. J. Agric. Food Chem. 2008, 56, 3593–3600. [Google Scholar] [CrossRef] [PubMed]
- Marques, E.D.S.; Froder, J.G.; de Oliveira, P.R.; Perazzo, F.F.; Rosa, P.C.P.; Gaivão, I.O.D.M.; Mathias, M.I.C.; Maistro, E.L. Cytotoxic Effects of Euterpe oleraceae Fruit Oil (Açaí) in Rat Liver and Thyroid Tissues. Rev. Bras. Farmacogn. 2019, 29, 54–61. [Google Scholar] [CrossRef]
- Marques, E.S.; Froder, J.G.; Carvalho, J.C.T.; Rosa, P.C.P.; Perazzo, F.F.; Maistro, E.L. Evaluation of the Genotoxicity of Euterpe oleraceae Mart. (Arecaceae) Fruit Oil (Açaí), in Mammalian Cells in Vivo. Food Chem. Toxicol. 2016, 93, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Neves, L.C.; Tosin, J.M.; Benedette, R.M.; Cisneros-Zevallos, L. Post-Harvest Nutraceutical Behaviour during Ripening and Senescence of 8 Highly Perishable Fruit Species from the Northern Brazilian Amazon Region. Food Chem. 2015, 174, 188–196. [Google Scholar] [CrossRef]
- Bessa, C.M.A.S.; Nascimento, R.S.; Alves, R.C.C.; Anselmo, J.M.; Silva, A.P.S.; Silva, A.G.; Lima, V.L.M.; Tavares, J.F.; Silva, L.C.N.; Silva, M.V.; et al. Syagrus coronata Seed Oils Have Antimicrobial Action against Multidrug-Resistant Staphylococcus aureus. J. Med. Plants Res. 2016, 10, 310–317. [Google Scholar] [CrossRef]
- Santos, L.M.M.; Nascimento, J.S.; Santos, M.A.G.; Marriel, N.B.; Bezerra-Silva, P.C.; Rocha, S.K.L.; Silva, A.G.; Correia, M.T.S.; Paiva, P.M.G.; Martins, G.F.; et al. Fatty Acid-Rich Volatile Oil from Syagrus coronata Seeds Has Larvicidal and Oviposition-Deterrent Activities against Aedes aegypti. Physiol. Mol. Plant Pathol. 2017, 100, 35–40. [Google Scholar] [CrossRef]
- Kershaw, J.; Kim, K.H. The Therapeutic Potential of Piceatannol, a Natural Stilbene, in Metabolic Diseases: A Review. J. Med. Food 2017, 20, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Gao, L.; Chen, C. Role of Medium-Chain Fatty Acids in Healthy Metabolism: A Clinical Perspective. Trends Endocrinol. Metab. 2021, 32, 351–366. [Google Scholar] [CrossRef]
- Nunes, Â.A.; Buccini, D.F.; Jaques, J.A.S.; Portugal, L.C.; Guimarães, R.C.A.; Favaro, S.P.; Caldas, R.A.; Carvalho, C.M.E. Effect of Dietary Acrocomia aculeata Kernel Oil Rich in Medium Chain Fatty Acids on Type 2 Diabetic Rats. J. Funct. Foods 2020, 75, 104295. [Google Scholar] [CrossRef]
- Santos, A.C.V.; Fernandes, C.C.; Lopes, L.M.; Sousa, A.H. Use of Plant Oils from the Southwestern Amazon for the Control of Maize Weevil. J. Stored Prod. Res. 2015, 63, 67–70. [Google Scholar] [CrossRef]
- Silva, C.S.M.; Araújo, J.A.; Silveira, T.S.; Castro, K.C.F.; Baratto, L.C.; Kaminski, R.C.K.; Santos, G.B.; Nunes, K.M. Wound Healing Activity of Topical Formulations Containing Mauritia flexuosa Oil. Rev. Bras. Farmacogn. 2021, 31, 225–231. [Google Scholar] [CrossRef]
- Monge-Fuentes, V.; Muehlmann, L.A.; Longo, J.P.F.; Silva, J.R.; Fascineli, M.L.; Azevedo, R.B.; Souza, P.; Faria, F.; Degterev, I.A.; Rodriguez, A.; et al. Photodynamic Therapy Mediated by Acai Oil (Euterpe oleracea Martius) in Nanoemulsion: A Potential Treatment for Melanoma. J. Photochem. Photobiol. B Biol. 2017, 166, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Falcão, A.D.O.; Speranza, P.; Ueta, T.; Martins, I.M.; Macedo, G.A.; Macedo, J.A. Antioxidant Potential and Modulatory Effects of Restructured Lipids from the Amazonian Palms on Liver Cells. Food Technol. Biotechnol. 2017, 55, 553–561. [Google Scholar] [CrossRef]
- Nascimento, K.; Baldissera, M.D.; Souza, C.D.F.; Brum, G.F.; Ramos, A.P.; Riéffel, R.C.; Pappis, L.; Filho, W.P.D.S.; Gundel, A.; Machado, A.K.; et al. Evaluation of the in Vivo Safety of Tucumã Oil Nanocapsules in an Experimental Model of Silver Catfish Rhamdia Quelen. Nat. Prod. Res. 2020, 36, 649–653. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Doleski, P.H.; Grando, T.H.; Sagrillo, M.R.; Silva, A.S.; Leal, D.B.R.; Monteiro, S.G. Treatment with Tucumã Oil (Astrocaryum vulgare) for Diabetic Mice Prevents Changes in Seric Enzymes of the Purinergic System: Improvement of Immune System. Biomed. Pharmacother. 2017, 94, 374–379. [Google Scholar] [CrossRef]
- Baldissera, M.D.; Souza, C.F.; Grando, T.H.; Sagrillo, M.R.; Cossetin, L.F.; da Silva, A.S.; Stefani, L.M.; Monteiro, S.G. Tucumã Oil (Astrocaryum vulgare) Ameliorates Hepatic Antioxidant Defense System in Alloxan-Induced Diabetic Mice. J. Food Biochem. 2018, 42, e12468. [Google Scholar] [CrossRef]
- Leite, T.C.; Picoli, F.; Lopes, D.D.A.; Baldissera, M.D.; Souza, C.F.; Baldisserotto, B.; Costa, A.P.O.; Nora, L.; Marcelino, A.H.; Da Silva, A.S. The Effects of Açaí Oil Addition in Tilapia Diets on Performance, Hepatic Energy Metabolism Enzymes and Antioxidant Responses. Aquac. Res. 2021, 52, 395–402. [Google Scholar] [CrossRef]
- Nobre, C.B.; Sousa, E.O.; Silva, J.M.F.L.; Coutinho, H.D.M.; Costa, J.G.M. Chemical Composition and Antibacterial Activity of Fixed Oils of Mauritia flexuosa and Orbignya speciosa Associated with Aminoglycosides. Eur. J. Integr. Med. 2018, 23, 84–89. [Google Scholar] [CrossRef]
- Pérez, M.M.; Gonçalves, E.C.S.; Salgado, J.C.S.; Rocha, M.S.; Almeida, P.Z.; Vici, A.C.; Infante, J.C.; Guisán, J.M.; Rocha-Martin, J.; Costa Pessela, B.; et al. Production of Omegas-6 and 9 from the Hydrolysis of Açaí and Buriti Oils by Lipase Immobilized on a Hydrophobic Support. Molecules 2018, 23, 3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pereira, Y.F.; Costa, M.D.S.; Tintino, S.R.; Rocha, J.E.; Rodrigues, F.F.G.; Feitosa, M.K.D.S.B.; de Menezes, I.R.A.; Coutinho, H.D.M.; Costa, J.G.M.; Sousa, E.O. Modulation of the Antibiotic Activity by the Mauritia flexuosa (Buriti) Fixed Oil against Methicillin-Resistant Staphylococcus aureus (MRSA) and Other Multidrug-Resistant (MDR) Bacterial Strains. Pathogens 2018, 7, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parente, M.O.M.; Rocha, K.S.; Bessa, R.J.B.; Parente, H.N.; Zanine, A.D.M.; Machado, N.A.F.; Lourenço Júnior, J.D.B.; Bezerra, L.R.; Landim, A.V.; Alves, S.P. Effects of the Dietary Inclusion of Babassu Oil or Buriti Oil on Lamb Performance, Meat Quality and Fatty Acid Composition. Meat Sci. 2020, 160, 107971. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.M.D. Use of Syagrus coronata Oil to Aid in the Treatment of Acne. Master’s Thesis, Federal University of Pernambuco, Recife, Brazil, 2020. (In Portuguese). [Google Scholar]
- Silva, T.M.; Oliveira, R.L.; Barbosa, L.P.; Garcez Neto, A.F.; Bagaldo, A.R.; Jesus, I.B.; Macome, F.M.; Ribeiro, C.V.D.M. Componentes Corporais de Caprinos Jovens 3/4 Boer Submetidos a Dietas Com Óleo de Licuri (Syagrus coronata). Arq. Bras. Med. Vet. e Zootec. 2010, 62, 1448–1454. [Google Scholar] [CrossRef] [Green Version]
- Pereira, R.A.G.; Oliveira, C.J.B.; Medeiros, A.N.; Costa, R.G.; Bomfim, M.A.D.; Queiroga, R.C.R.E. Physicochemical and Sensory Characteristics of Milk from Goats Supplemented with Castor or Licuri Oil. J. Dairy Sci. 2010, 93, 456–462. [Google Scholar] [CrossRef] [Green Version]
- Barbosa, L.P.; Oliveira, R.L.; Silva, T.M.; Jesus, I.B.; Garcez Neto, A.F.; Bagaldo, A.R. Morfometria Testicular de Cabritos Alimentados Com Óleo de Licuri (Syagrus coronata). Arq. Bras. Med. Vet. Zootec. 2012, 64, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.S.; Oliveira, R.L.; Bagaldo, A.R.; Neto, A.F.G.; Ribeiro, C.V.D.M.; Lanna, D.P.D. Composition and Fatty Acid Profile of Milk from Cows on Pasture Subjected to Licuri Oil Supplement. Rev. Bras. Zootec. 2011, 40, 2858–2865. [Google Scholar] [CrossRef] [Green Version]
- Lima, L.S.; Oliveira, R.L.; Garcez Neto, A.F.; Bagaldo, A.R.; Abreu, C.L.; Silva, T.M.; Carvalho, S.T.; Bezerra, L.R. Licuri Oil Supplements for Lactating Cows on Pasture. Can. J. Anim. Sci. 2015, 95, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Gomes, G.V.L.; Sola, M.R.; Rochetti, A.L.; Fukumasu, H.; Vicente, A.A.; Pinho, S.C. β-Carotene and α-Tocopherol Coencapsulated in Nanostructured Lipid Carriers of Murumuru (Astrocaryum murumuru) Butter Produced by Phase Inversion Temperature Method: Characterisation, Dynamic in Vitro Digestion and Cell Viability Study. J. Microencapsul. 2019, 36, 43–52. [Google Scholar] [CrossRef] [Green Version]
- Neves, M.C.T.; Lopes, A.; Oliveira, M.C.J.; Iamaguti, P.S.; Lira, T.A.M.; Moreti, T.C.F.; Lima, L.P.; Koike, G.H.A. Effects of Murumuru (Astrocaryum murumuru Mart.) and Soybean Biodiesel Blends on Tractor Performance and Smoke Density. Aust. J. Crop Sci. 2018, 12, 878–885. [Google Scholar] [CrossRef]
- Dourado, D.; Lôbo, M.; Pereira, N.D.P. Cosmetic Performance of Emulgels Containing Fatty Raw Material from Brazilian Organic Agriculture. In Agricultural Research Updates; Gorawala, P., Mandhatri, S., Eds.; Nova Science Publishers: Hauppauge, NY, USA, 2018; Volume 24, pp. 141–168. ISBN 9781536141382. [Google Scholar]
- Barbosa, B.S.; Koolen, H.H.F.; Barreto, A.C.; Silva, J.D.; Figliuolo, R.; Nunomura, S.M. The Use of Tucumã of Amazonas Kernel Oil in the Biodiesel Production. Acta Amaz. 2009, 39, 371–376. [Google Scholar] [CrossRef] [Green Version]
- Pantoja, S.S.; Mescouto, V.A.; Costa, C.E.F.; Zamian, J.R.; Rocha Filho, G.N.; Nascimento, L.A.S. High-Quality Biodiesel Production from Buriti (Mauritia flexuosa) Oil Soapstock. Molecules 2019, 24, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, J.L.F.; Batista, L.M.B.; Santos, N.A.; Araújo, A.M.M.; Fernandes, V.J., Jr.; Araujo, A.S.; Alves, A.P.M.; Gondim, A.D. Clay-Supported Zinc Oxide as Catalyst in Pyrolysis and Deoxygenation of Licuri (Syagrus coronata) Oil. Renew. Energy 2021, 168, 1377–1387. [Google Scholar] [CrossRef]
- Lima, C.G.; Pianovski, A.R.; Vilela, A.F.G.; Silva, K.K.; Carvalho, V.F.M.; Musis, C.R.; Machado, S.R.P.; Ferrari, M. O/W/O Multiple Emulsions Containing Amazon Oil: Babassu Oil (Orbignya oleifera). J. Dispers. Sci. Technol. 2010, 31, 622–626. [Google Scholar] [CrossRef]
- Gumiero, V.C.; Rocha Filho, P.A. Babassu Nanoemulsions Have Physical and Chemical Stability. J. Dispers. Sci. Technol. 2012, 33, 1569–1573. [Google Scholar] [CrossRef]
- Wasule, D.D.; Nawandar, S.Y.; Kaur, H. Evaluation of Babassu Oil as Skin Moisturizer. World J. Pharm. Sci. 2014, 2, 1–6. [Google Scholar]
- Vinhal, J.O.; Lima, C.F.; Barbosa, L.C.A. Analytical Pyrolysis of the Kernel and Oil of Babassu Palm (Orbignya phalerata). J. Anal. Appl. Pyrolysis 2014, 107, 73–81. [Google Scholar] [CrossRef]
- Valério, A.; Araújo, P.H.H.; Sayer, C. Preparation of Poly(urethane-urea) Nanoparticles Containing Açaí Oil by Miniemulsion Polymerization. Polimeros 2013, 23, 451–455. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, M.; Rocha-Filho, P.A. Multiple Emulsions Containing Amazon Oil:Açaí Oil (Euterpe oleracea). Rev. Bras. Farmacogn. 2011, 21, 737–743. [Google Scholar] [CrossRef] [Green Version]
- Leal, L.B.; Sousa, G.D.; Seixas, K.B.; Souza, P.H.N.; Santana, D.P. Determination of the Critical Hydrophile-Lipophile Balance of Licuri Oil from Syagrus coronata: Application for Topical Emulsions and Evaluation of Its Hydrating Function. Brazilian J. Pharm. Sci. 2013, 49, 167–173. [Google Scholar] [CrossRef]
- Rocha, A.A.; Almeida, M.F.; Coelho, B.S.; Soares, L.S.; Veloso, C.M. Effect of the Addition of Licuri Oil (Syagrus coronata) and Tween 80 in a Film of Araruta Starch (Maranta arundinacea L.). Hig. Aliment. 2019, 33, 1400–1404. (In Portuguese) [Google Scholar]
- Silva, S.H.T.; Bader-Mittermaier, S.; Silva, L.B.; Doer, G.; Eisner, P. Electrophoretic Characterization, Amino Acid Composition and Solubility Properties of Macauba (Acrocomia aculeata L.) Kernel Globulins. Food Biosci. 2021, 40, 100908. [Google Scholar] [CrossRef]
- Vieira, W.T.; Bispo, M.D.; Farias, S.M.; Almeida, A.S.V.; Silva, T.L.; Vieira, M.G.A.; Soletti, J.I.; Balliano, T.L. Activated Carbon from Macauba Endocarp (Acrocomia aculeate) for Removal of Atrazine: Experimental and Theoretical Investigation Using Descriptors Based on DFT. J. Environ. Chem. Eng. 2021, 9, 105155. [Google Scholar] [CrossRef]
- Umpierres, C.S.; Thue, P.S.; Lima, E.C.; Reis, G.S.; Brum, I.A.S.; Alencar, W.S.; Dias, S.L.P.; Dotto, G.L. Microwave-Activated Carbons from Tucumã (Astrocaryum aculeatum) Seed for Efficient Removal of 2-Nitrophenol from Aqueous Solutions. Environ. Technol. 2018, 39, 1173–1187. [Google Scholar] [CrossRef]
- Thue, P.S.; Umpierres, C.S.; Lima, E.C.; Lima, D.R.; Machado, F.M.; dos Reis, G.S.; da Silva, R.S.; Pavan, F.A.; Tran, H.N. Single-Step Pyrolysis for Producing Magnetic Activated Carbon from Tucumã (Astrocaryum aculeatum) Seed and Nickel(II) Chloride and Zinc(II) Chloride. Application for Removal of Nicotinamide and Propanolol. J. Hazard. Mater. 2020, 398, 122903. [Google Scholar] [CrossRef]
- Mendonça, I.M.; Paes, O.A.R.L.; Maia, P.J.S.; Souza, M.P.; Almeida, R.A.; Silva, C.C.; Duvoisin, S., Jr; de Freitas, F.A. New Heterogeneous Catalyst for Biodiesel Production from Waste Tucumã Peels (Astrocaryum aculeatum Meyer): Parameters Optimization Study. Renew. Energy 2019, 130, 103–110. [Google Scholar] [CrossRef]
- Jobim, M.L.; Santos, R.C.V.; Alves, C.F.S.; Oliveira, R.M.; Mostardeiro, C.P.; Sagrillo, M.R.; Souza Filho, O.C.; Garcia, L.F.M.; Manica-Cattani, M.F.; Ribeiro, E.E.; et al. Antimicrobial Activity of Amazon Astrocaryum aculeatum Extracts and Its Association to Oxidative Metabolism. Microbiol. Res. 2014, 169, 314–323. [Google Scholar] [CrossRef]
- Jantsch, M.H.; Bernardes, V.M.; Oliveira, J.S.; Passos, D.F.; Dornelles, G.L.; Manzoni, A.G.; Cabral, F.L.; Silva, J.L.G.; Schetinger, M.R.C.; Leal, D.B.R. Tucumã (Astrocaryum aculeatum) Prevents Memory Loss and Oxidative Imbalance in the Brain of Rats with Hyperlipidemia. J. Food Biochem. 2021, 45, e13636. [Google Scholar] [CrossRef]
- Guex, C.G.; Cassanego, G.B.; Dornelles, R.C.; Casoti, R.; Engelmann, A.M.; Somacal, S.; Maciel, R.M.; Duarte, T.; Borges, W.D.S.; Andrade, C.M.; et al. Tucumã (Astrocaryum aculeatum) Extract: Phytochemical Characterization, Acute and Subacute Oral Toxicity Studies in Wistar Rats. Drug Chem. Toxicol. 2020, 45, 810–821. [Google Scholar] [CrossRef]
- Menezes, B.P.; Rodrigues, L.S.; Lourenço, J.D.B., Jr.; Silva, A.G.M.; Andrade, S.J.T.; Silva, J.A.R.; Faturi, C.; Garcia, A.R.; Nahúm, B.D.S.; Barbosa, A.V.C.; et al. Intake, Digestibility, and Nitrogen Balance of Rations Containing Different Levels of Murumuru Meal in Sheep Diets. Semin. Agrar. 2016, 37, 415–428. [Google Scholar] [CrossRef] [Green Version]
- Silva, A.P.D.S.; Rosalen, P.L.; Camargo, A.C.; Lazarini, J.G.; Rocha, G.; Shahidi, F.; Franchin, M.; Alencar, S.M.D. Inajá Oil Processing By-Product: A Novel Source of Bioactive Catechins and Procyanidins from a Brazilian Native Fruit. Food Res. Int. 2021, 144, 110353. [Google Scholar] [CrossRef] [PubMed]
- Taniguchi, K.; Karin, M. NF-B, Inflammation, Immunity and Cancer: Coming of Age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef] [PubMed]
- Souza, M.H.S.L.; Monteiro, C.A.; Figueredo, P.M.S.; Nascimento, F.R.F.; Guerra, R.N.M. Ethnopharmacological Use of Babassu (Orbignya phalerata Mart) in Communities of Babassu Nut Breakers in Maranhão, Brazil. J. Ethnopharmacol. 2011, 133, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Gombert, A.K.; Pinto, A.L.; Castilho, L.R.; Freire, D.M.G. Lipase Production by Penicillium restrictum in Solid-State Fermentation Using Babassu Oil Cake as Substrate. Process Biochem. 1999, 35, 85–90. [Google Scholar] [CrossRef]
- Palma, M.B.; Pinto, A.L.; Gombert, A.K.; Seitz, K.H.; Kivatinitz, S.C.; Castilho, L.R.; Freire, D.M.G. Lipase Production by Penicillium restrictum Using Solid Waste of Industrial Babassu Oil Production as Substrate. Appl. Biochem. Biotechnol.—Part A Enzym. Eng. Biotechnol. 2000, 84–86, 1137–1145. [Google Scholar] [CrossRef]
- Cardoso, Z.S.; Rodrigues, I.A.; Mendonça, C.J.S.; Rodrigues, J.R.P.; Ribeiro, W.R.A.; Silva, W.O.; Maciel, A.P. Evaluating the Electrochemical Characteristics of Babassu Coconut Mesocarp Ethanol Produced to Be Used in Fuel Cells. J. Braz. Chem. Soc. 2018, 29, 1732–1741. [Google Scholar] [CrossRef]
- Martins, G.R.; Amaral, F.R.L.; Brum, F.L.; Mohana-Borges, R.; Moura, S.S.T.; Ferreira, F.A.; Sangenito, L.S.; Santos, A.L.S.; Figueiredo, N.G.; Silva, A.S.A.D. Chemical Characterization, Antioxidant and Antimicrobial Activities of Açaí Seed (Euterpe oleracea Mart.) Extracts Containing A- and B-Type Procyanidins. LWT 2020, 132, 109830. [Google Scholar] [CrossRef]
- Bagaldo, A.R.; Miranda, G.S.; Soares, M.S.F., Jr.; Araújo, F.L.; Matoso, R.V.M.; Chizzotti, M.L.; Bezerra, L.R.; Oliveira, R.L. Effect of Licuri Cake Supplementation on Performance, Digestibility, Ingestive Behavior, Carcass Traits and Meat Quality of Grazing Lambs. Small Rumin. Res. 2019, 177, 18–24. [Google Scholar] [CrossRef]
- Daza, A.; Souza, J.G.; Monnerat, J.P.I.S.; Ribeiro, C.V.D.M. Performance of Growing Lambs Supplemented with Ground Licuri (Syagrus coronata). Anim. Biosci. 2021, 34, 1014–1021. [Google Scholar] [CrossRef]
- Santos, F.M.; Santos, J.D.R.; Carvalho, F.A.L.; Queiroz, M.A.Á.; Yamamoto, S.M.; Guimarães, O.D. Licury Cake in Lamb Feed: Characteristics of Carcass and Non-Carcass Components. Cienc. Agrotecnol. 2015, 39, 260–268. [Google Scholar] [CrossRef] [Green Version]
- Costa, J.B.; Oliveira, R.L.; Silva, T.M.; Ribeiro, R.D.X.; Silva, A.M.; Leão, A.G.; Bezerra, L.R.; Rocha, T.C. Intake, Digestibility, Nitrogen Balance, Performance, and Carcass Yield of Lambs Fed Licuri Cake. J. Anim. Sci. 2016, 94, 2973–2980. [Google Scholar] [CrossRef] [PubMed]
- Borja, M.S.; Oliveira, R.L.; Ribeiro, C.V.D.M.; Bagaldo, A.R.; Carvalho, G.G.P.; Silva, T.M.; Lima, L.S.; Barbosa, L.P. Effects of Feeding Licury (Syagrus coronate) Cake to Growing Goats. Asian-Australasian J. Anim. Sci. 2010, 23, 1436–1444. [Google Scholar] [CrossRef]
- Costa, J.B.; Oliveira, R.L.; Silva, T.M.; Barbosa, A.M.; Borja, M.S.; Pellegrini, C.B.; Oliveira, V.S.; Ribeiro, R.D.X.; Bezerra, L.R. Fatty Acid, Physicochemical Composition and Sensory Attributes of Meat from Lambs Fed Diets Containing Licuri Cake. PLoS ONE 2018, 13, e0206863. [Google Scholar] [CrossRef]
- Scalet, V.; Róz, A.L.; Santos, L.R.O.; Hansted, A.L.S.; Pires, A.A.F.; Nakashima, G.T.; Tomeleri, J.O.P.; Yamaju, F.M. Waste of the Licuri (Syagrus coronata) Nut Shells: An Alternative Energy Source. Rev. Bras. Energias Renov. 2019, 8. [Google Scholar] [CrossRef] [Green Version]








| Scientific Name | Common Name | Oil Content (%) | Raw Material Code | References | |
|---|---|---|---|---|---|
| Acrocomia aculeata | Macaúba | Pulp | 9.77–28.94 | MAP | [28,29] |
| Kernel | 3.43–47.50 | MAK | [29,30,31] | ||
| Astrocaryum aculeatum | Tucumã-do-Amazonas | Pulp | 21.25–61.60 | TAP | [32,33,34] |
| Kernel | 28.40–45.50 | TAK | [35] | ||
| Astrocaryum murumuru | Murumuru | Pulp | 2.60 | MUP | [36] |
| Kernel | 27.70–44.00 | MUK | [37,38] | ||
| Astrocaryum vulgare | Tucumã-do-Pará | Pulp | 1.90–40.49 | TPP | [12,37,39,40] |
| Kernel | 9.60–29.59 | TPK | [37,39] | ||
| Attalea maripa | Inajá | Pulp | 35.52–56.20 | INP | [12,41] |
| Kernel | 31.30–67.69 | INK | [39,42,43] | ||
| Attalea speciosa | Babassu | Kernel | 50.31–66.00 | BBK | [44,45] |
| Bactris gasipaes | Pupunha | Pulp | 7.70–61.70 | PUP | [46,47,48] |
| Kernel | 11.50–23.50 | PUK | [39,48,49] | ||
| Butia spp. | Butiá | Pulp | 2.60–2.73 | BUP | [50,51] |
| Kernel | 31.96–53.60 | BUK | [52,53] | ||
| Euterpe oleracea | Açaí | Pulp | 7.00–48.00 | ACP | [54] |
| Kernel | 0.22–2.84 | ACK | [55,56] | ||
| Mauritia flexuosa | Buriti | Pulp | 19.00–51.67 | BRP | [12,57,58] |
| Kernel | 7.01–9.20 | BRK | [59] | ||
| Oenocarpus bataua | Patauá | Pulp | 14.40–51.60 | PTP | [12,57,60] |
| Kernel | 0.06–1.30 | PTK | [37,61] | ||
| Oenocarpus bacaba | Bacaba | Pulp | 7.40–60.39 | BAP | [62,63,64] |
| Kernel | 0.83–4.10 | BAK | [37,61] | ||
| Syagrus coronata | Licuri | Pulp | 4.11–4.50 | LIP | [65,66,67] |
| Kernel | 39.00–50.00 | LIK | [65,66,68,69] | ||
| Oil | FFA (% Oleic Acid) | AV (mg KOH g−1) | PV (mEq O2 kg−1) | IV (g I2 100 g−1) | SV (mg KOH g−1) | RI, 40 °C | UM (%) | OSI, 110 °C (h) | MP (°C) | KV, 40 °C (mm2 s−1) | ρ, 40 °C (kg m−3) | References |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MAP | 1.72–5.63 | - | 3.16–5.0 | - | - | - | - | 16 | - | - | - | [31,72,146] |
| MAK | 0.94–4.9 | 112 | 1.9–6.9 | 25.5–30.9 | 230.0–235.4 | - | - | 0.07–26 | - | 41.9 | 919 e | [30,68] |
| TAP | - | 19.10–20.63 | 11.8 | 10.88 | 196.1–298.39 | - | - | - | 19.1–20.3 | 41.8–42.2 | - | [32,134] |
| TAK | 0.1–0.17 a | 0.26–0.48 | - | 08-11 | 208.60–246.00 | - | - | 20–44 | 19.5–33.0 | 44.2–46.1 | - | [35,134] |
| MUK | 0.36 | 0.8 b–5.16 | 5.0 b–22.85 | 11.0 b–13.12 | 228.3 b–258.24 | 1.45 b–1.46 | 0.11 b–0.16 | 18 d–40.0 b | 25–37 | 29 | 904.17–907.8 e | [10,11,19,147] |
| TPP | 0.7–2.75 | 3.80–15.25 | 0.5–10.52 | 67.77–73.6 | 180.40–202.71 | 1.43–1.47 | 1.8–2.2 | 10.1 c | 13.8 | - | 910 c–975 | [37,40,135,148,149] |
| TPK | 0.1 | 0.45–10.91 | 1.19–9.0 | 12.05–18.07 | 236.40–246.43 | 1.43–1.45 | 0.47–1.1 | 3.87 d | 30.3 | 15.40–30.5 | 860–904.15 | [10,37,39,135] |
| INP | 1 | - | 6.5–176 | 74.15–74.86 | 210–214 | - | 0.8 | 6.3 c | - | - | [42,148] | |
| INK | 4.85–5.06 | 2.6 | 4.0–25 | 16.48–16.79 | 262–263 | 1.45 | 0.6 | - | - | - | 930 | [39,42] |
| BBK | 0.05–0.08 a | 1.06 | 0.22–0.91 | 10.36 | 248.73–249.50 | 1.45–1.47 | - | 2–17 d | 23.9–26.52 | - | 918.63–919.10 e | [11,94,138] |
| PUP | 2 | 2.45 | 5.47–6.4 | - | - | - | 1.3 | 34.2 c | - | - | - | [47,148] |
| PUK | - | 1.70–12.20 | 68.6 | - | - | 1.45 | 0.8–3.0 | - | - | - | 900 | [39,49] |
| ACP | - | 3.66 b | - | 71 b | 199 b | 1.46 b | - | - | - | - | 952 e | [150] |
| ACK | - | 93.1 | 38.46–62.05 | 91.3 | 186.1 | 1.45 | - | - | - | - | 924.3 e | [55] |
| BRP | 1.14–6.22 | 2.13–5.76 | 7.4–14.20 | 70.17–77.40 | 183.91–192.88 | 1.46–1.47 | 0.5–1.3 | 16.9 c-18.3 | 37.2 b-25.1 | 40.8 | 921.2 e | [11,19,59,148,151] |
| BRK | - | - | - | - | - | - | - | 3.55–6.91 | - | - | - | [59] |
| PTP | 0.4 | - | 18.3 | 76.3 | - | 1.46 | 0.8 | - | <8.0 | - | - | [37] |
| PTK | - | 14.52 | 15.94 | 75.85 | 174.12 | - | 0.81 | 4.97 d | - | 38.96 | 900.03 | [10,37] |
| BAP | 2.4–63.0 | 1.65 | 11.9–76.1 | 51.35–75.8 | 225 | 1.46 | 1.0–2.6 | 5.65–11.9 c | 32.6 | - | - | [37,64,148,152] |
| BAK | 35.6 | - | - | 80.9 | - | 1.45 | - | - | <10.0 | - | - | [37] |
| LIK | 1.4 | 0.72–1.5 | - | 13.9–18.5 | - | - | - | 10.7–69.6 | - | 23.4–27.6 | 920 e–924 e | [68,153,154] |
| Pulp Oils | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| FAs | MAP | TAP | TPP | INP | PUP | BUP | ACP | BRP | PTP | BAP | LIP | |
| C6:0 | 0.1 | - | - | - | - | 6.73–6.88 | - | - | - | - | - | |
| C7:0 | 0.1 | - | - | - | - | - | - | - | - | - | - | |
| C8:0 | 0.1–0.3 | - | - | - | - | 1.28–1.42 | - | - | - | - | - | |
| C9:0 | 0.23 | - | - | - | - | - | - | - | - | - | - | |
| C10:0 | 0.07–0.10 | - | 0.80 | - | - | 0.29–0.40 | - | - | - | - | - | |
| C11:0 | 0.04 | - | - | - | - | - | - | - | - | - | - | |
| C12:0 | 0.08–0.26 | 0.20 | - | 0.68–4.60 | - | 2.71–3.64 | 0.04–0.08 | 0.02–0.10 | - | - | - | |
| C13:0 | 0.03 | - | - | - | - | - | - | - | - | - | - | |
| C14:0 | 0.02–0.64 | 0.50 | 0.10 | 1.57–10.70 | 0.10 | 3.05–3.55 | 0.11–0.14 | 0.10–0.12 | - | 0.15–0.29 | - | |
| C15:0 | 0.08 | - | - | - | - | - | - | 0.04 | - | - | - | |
| C16:0 | 23.37–29.75 | 7.89–10.40 | 21.20–24.60 | 19.84–25.10 | 24.10–50.57 | 18.81–19.35 | 22.80–26.78 | 17.59–20.80 | 11.3–12.2 | 17.19–25.90 | 42.31 | |
| C16:1n7 | 5.21 | - | 0.10 | 0.10–0.30 | 3.39–7.40 | 0.48–0.49 | 4.20–5.40 | 0.25–0.26 | 0.6–0.7 | 0.68–1.10 | - | |
| C17:0 | 0.29 | 1.70 | 0.10 | - | 0.11 | - | - | 0.05–0.08 | - | - | - | |
| C17:1 | - | - | - | - | - | - | - | - | - | - | - | |
| C18:0 | 1.31–4.23 | 4.90–6.72 | 2.60–5.10 | 1.60–3.50 | 0.80–3.50 | 1.82–2.00 | 1.40–1.90 | 1.35–1.60 | 3.1–3.9 | 2.19–4.70 | 32.35 | |
| C18:1n9 | 54.79–63.21 | 64.20–73.81 | 64.7–67.62 | 39.20–60.82 | 36.27–60.80 | 47.33–48.07 | 51.46–62.30 | 71.60–78.55 | 77.7–79.1 | 46.20–66.24 | 25.34 | |
| C18:1n11 | - | - | - | - | - | - | 3.39–4.80 | - | - | - | ||
| C18:2n6 | 5.42–10.26 | 11.00–12.14 | 1.15–5.30 | 6.47–12.90 | 2.50–5.40 | 11.00–12.17 | 7.28–10.60 | 1.13–2.50 | 3.6–4.9 | 12.12–20.00 | - | |
| C18:3n3 | 1.32–3.19 | 1.00 | 0.20–3.10 | 0.20–6.60 | 1.20–1.40 | 3.13–3.66 | 0.30–0.81 | 0.09–1.40 | 0.5–0.6 | 0.58–0.72 | - | |
| C20:0 | 0.17 | 0.40–1.89 | 4.10 | 3.20 | - | 0.16–0.21 | 0.10–0.12 | 0.66–1.65 | 0.1 | 0.06–0.19 | - | |
| C20:1n9 | - | - | - | 1.30 | - | 0.10–0.13 | - | 0.70 | - | - | - | |
| C22:0 | - | 0.10 | 0.10 | - | - | 0.07–0.09 | - | - | - | 0.01–0.07 | - | |
| Σ SFA | 24.97–36.29 | 13.19–21.51 | 23.80–34.90 | 23.69–47.10 | 24.90–50.57 | 34.92–37.54 | 26.70–28.84 | 19.77–24.39 | 14.40–16.20 | 19.60–31.15 | 74.66 | |
| Σ MUFA | 54.79–68.42 | 64.20–73.81 | 64.70–67.62 | 39.30–62.42 | 46.70–68.20 | 47.91–48.69 | 59.73–62.30 | 71.85–79.51 | 78.30–79.80 | 44.88–67.34 | 25.34 | |
| Σ PUFA | 6.74–13.45 | 11.00–13.14 | 1.35–8.40 | 6.67–19.50 | 3.70–6.80 | 14.13–15.83 | 7.83–11.10 | 1.22–3.90 | 4.10–5.50 | 12.70–20.72 | 0.00 | |
| References | [28,31] | [34,134] | [12,148,159,160] | [12,41,136,148] | [46,47,148] | [51] | [139,141,159] | [11,12,148] | [10,11,12,57,159] | [63,148] | [161] | |
| Kernel oils | ||||||||||||
| FAs | MAK | TAK | MUK | TPK | INK | BBK | PUK | BUK | ACK | BRK | PTK | LIK |
| C6:0 | 0.03–0.80 | - | - | - | - | 0.54–0.55 | - | 0.4 | - | - | - | - |
| C7:0 | 0.01 | - | - | - | - | - | - | - | - | - | - | - |
| C8:0 | 1.06–6.20 | 1.80–2.20 | 1.19–2.70 | 1.94 | 3.80 | 7.50–8.57 | - | 7.8 | - | - | 0.02 | 9.00–13.00 |
| C9:0 | 0.32 | - | - | - | - | - | - | - | - | - | - | - |
| C10:0 | 3.94–4.20 | 1.80–2.30 | 1.31–2.00 | 1.95 | 4.00 | 6.55–7.42 | - | 8.0 | - | - | 0.03 | 5.80–8.00 |
| C11:0 | 0.12 | - | - | - | - | 2.10–2.22 | - | - | - | - | - | - |
| C12:0 | 31.96–45.70 | 52.30–57.50 | 47.15–51.60 | 43.50–50.89 | 10.06–40.50 | 47.62–49.97 | 33.30–60.60 | 42.1 | 2.90–8.74 | - | 0.20–1.37 | 36.10–48.00 |
| C13:0 | 0.14 | - | 0.08 | - | - | 0.03–1.51 | - | - | - | - | 0.10–0.19 | - |
| C14:0 | 8.30–12.14 | 23.40–27.10 | 25.80–28.75 | 25.20–28.60 | 13.59–25.50 | 13.85–14.22 | 18.90–27.80 | 10.5 | 4.60–22.86 | - | 0.09–0.94 | 14.00–16.40 |
| C15:0 | 0.47 | - | - | - | - | - | - | - | - | - | 0.30–0.50 | - |
| C16:0 | 6.90–11.11 | 4.70–5.90 | 6.00–7.09 | 6.23–7.50 | 9.00–20.55 | 6.59–7.30 | 6.00–9.60 | 6.0 | 16.10–16.27 | 18.07–24.28 | 9.68–25.96 | 5.00–8.80 |
| C16:1n7 | 0.29 | - | - | - | 0.16–0.29 | 0.21–0.23 | - | - | 0.61 | - | 0.31–1.61 | - |
| C17:0 | 0.35 | - | - | - | - | 0.01–0.06 | - | - | - | - | 0.06–0.10 | - |
| C17:1 | - | - | - | - | - | - | - | - | - | - | 0.05–0.11 | |
| C18:0 | 2.40–6.63 | 1.90–2.70 | 2.57–2.92 | 2.74 | 1.85–2.40 | 2.69–3.31 | 5.10 | 4.0 | 1.37–3.20 | 3.83–5.50 | 0.87–5.09 | 2.00–4.00 |
| C18:1n9 | 24.10–29.10 | 5.0–7.0 | 5.70–7.97 | 8.10–13.60 | 10.80–43.39 | 8.60–9.57 | 12.90–24.30 | 16.9 | 26.79–51.30 | 27.59–52.96 | 64.78–81.91 | 7.00–12.00 |
| C18:1n11 | - | - | - | - | - | - | - | - | - | - | 0.97–3.41 | - |
| C18:2n6 | 2.85–3.40 | 2.1–3.1 | 3.00–3.62 | 2.94–3.30 | 2.40–6.96 | 0.27–1.52 | - | 4.2 | 14.60–25.52 | 16.24–36.04 | 1.18–5.97 | 3.00–3.10 |
| C18:3n3 | - | - | 0.12 | - | 7.62–7.81 | 1.36–1.64 | - | - | 0.84 | 1.74–13.33 | 0.08–1.21 | - |
| C20:0 | 0.33 | 0.08–0.11 | 0.10 | - | - | - | - | 0.1 | 2.30 | - | 0.03–0.60 | - |
| C20:1n9 | - | 0.05 | - | - | - | - | 0.04 | 1.50 | - | 0.07–0.13 | - | |
| C21:0 | - | - | - | - | - | 0.04 | - | - | - | - | - | - |
| C22:0 | 0.12 | 0.06 | 0.03 | - | - | - | - | - | - | - | - | - |
| C24:0 | 0.19 | 0.04–0.07 | - | - | - | - | - | - | - | - | - | - |
| Σ SFA | 54.59–88.83 | 85.98–97.87 | 84.02–95.27 | 74.93–93.62 | 34.50–96.75 | 84.48–95.17 | 75.70–85.50 | 78.90 | 29.10 | 21.90–29.78 | 11.33–34.80 | 71.90–98.20 |
| Σ MUFA | 24.10–29.39 | 5.00–7.05 | 6.85–7.97 | 8.10–13.60 | 10.96–43.68 | 8.81–9.79 | 12.90 | 16.94 | 52.80 | 27.59–52.96 | 66.18–87.17 | 7.00–12.00 |
| Σ PUFA | 2.85–3.40 | 2.10–3.10 | 3.26–3.62 | 2.94–3.30 | 10.02–14-77 | 1.52–1.95 | 0.00 | 4.20 | 14.60 | 17.98–49.37 | 1.26–7.18 | 3.00–3.10 |
| References | [30,31,162] | [35] | [10,11,37] | [10,39] | [39,42] | [11,138] | [39,49] | [52] | [55,56] | [59] | [10,11,12,57,159] | [68,153,154,163] |
| TAG | MUK | TPP | TPK | INP | BBK | PUP | BRP | PTP | BAP | LIK |
|---|---|---|---|---|---|---|---|---|---|---|
| CCC | - | - | - | - | - | - | - | - | - | 3.12 |
| CLM | - | - | - | - | - | - | - | - | - | 14.47 |
| CLL | - | - | - | - | - | - | - | - | - | 21.03 |
| COL | - | - | - | - | 4.54–4.95 | - | - | - | - | - |
| CpCpC | - | - | - | - | 0.41–0.44 | - | - | - | - | - |
| CpCpL | - | - | - | - | 1.57–1.69 | - | - | - | - | - |
| CpLC | - | - | - | - | 2.80–3.01 | - | - | - | - | - |
| CpLM | - | - | - | - | - | - | - | - | - | 4.54 |
| CpLL | - | - | - | - | - | - | - | - | - | 25.76 |
| CpLP | - | - | - | - | - | - | - | - | - | 4.55 |
| CpLO | - | - | - | - | - | - | - | - | - | 2.76 |
| CpOL | - | - | - | - | 4.48–4.91 | - | - | - | - | - |
| CpOC | - | - | - | - | 1.10–1.19 | - | - | - | - | - |
| CpOCp | - | - | - | - | 0.49–0.53 | - | - | - | - | - |
| CpLnL | - | - | - | - | 0.82 | - | - | - | - | - |
| LLL | 13.73 | - | 19.52 | - | 14.46–15.13 | - | - | - | - | - |
| LLM | 14.69 | - | 22.87 | - | 10.33–10.72 | - | - | - | - | 11.53 |
| LLC | - | - | - | - | 8.45–8.95 | - | - | - | - | - |
| LLCp | - | - | - | - | 7.67–8.11 | - | - | - | - | - |
| LLO | - | - | 5.91 | - | - | - | - | - | - | 1.86 |
| LLP | 7.15 | - | - | - | 6.54–7.04 | - | - | - | - | - |
| LLS | - | - | - | - | 3.63–4.07 | - | - | - | - | - |
| LMM | - | - | - | - | - | - | - | - | 4.53 | |
| LOL | - | - | - | - | 11.30–12.55 | - | - | - | - | - |
| LOM | - | - | - | - | 5.80–6.54 | - | - | - | - | - |
| LOP | - | - | - | - | 3.32–3.54 | - | - | - | - | - |
| LOS | - | - | - | - | 1.66–1.68 | - | - | - | - | - |
| LLnL | - | - | - | - | 2.10–2.13 | - | - | - | - | - |
| LLnM | - | - | - | - | 1.07–1.11 | - | - | - | - | - |
| LLnP | - | - | - | - | 0.60–0.62 | - | - | - | - | - |
| LLiL | - | - | - | - | 0.40 | - | - | - | - | - |
| LaLaP | - | - | - | 0.4 | - | - | - | - | - | - |
| LaMP | - | - | - | 0.9 | - | - | - | - | - | - |
| LaOL | - | - | - | 1.8 | - | - | - | - | - | - |
| LaOLa | - | - | - | 0.8 | - | - | - | - | - | - |
| LaPP | - | - | - | 1.2 | - | - | - | - | - | - |
| LaOM | - | - | - | 3.2 | - | - | - | - | - | - |
| LaLM | - | - | - | 1.5 | - | - | - | - | - | - |
| MML | 7.85 | - | 14.62 | - | - | - | - | - | - | - |
| MOO | - | - | - | 3.9 | - | - | 1.2 | - | - | - |
| MOL | - | - | - | 3.5 | - | - | - | - | - | - |
| MOS | - | - | - | - | 0.41–0.42 | - | - | - | - | - |
| MOP | - | - | - | 10.4 | - | - | - | - | - | - |
| MPP | - | - | - | 1.9 | - | - | - | - | - | - |
| MOM+LaOP | - | - | - | 7.9 | - | - | - | - | - | - |
| MLM | - | - | - | 3.3 | - | - | - | - | - | - |
| MLP+LaOO | - | - | - | 6.7 | - | - | - | - | - | - |
| PPL | - | - | - | - | 1.43–1.65 | - | - | - | - | - |
| PPLn | - | - | - | - | - | - | 1.0 | - | - | - |
| PPO | - | - | - | - | - | 7.4 | - | |||
| PPoP | - | - | - | - | - | 3.7 | - | - | - | - |
| PPP | - | 1.8 | - | 1.6 | - | 3.1 | - | - | 0.9 | - |
| PLM | 7.28 | - | 7.50 | - | - | - | - | - | - | - |
| PLS | - | 1.2 | - | 0.9 | - | 1.6 | - | 4.0 | - | |
| PLL | - | 3.3 | - | 1.6 | - | - | 0.6 | - | 5.9 | - |
| PLP | - | 1.1 | - | 5.7 | - | - | - | - | 8.3 | - |
| POL | - | - | - | 6.2 | - | 6.6 | 2.5–3.9 | - | 17.7 | - |
| POLn | - | - | - | - | - | - | 3.9 | - | - | - |
| POP | - | 22.5 | - | 11.5 | - | 32.4 | 9.4 | - | 10.3 | - |
| POPo | - | - | - | - | - | 12.2 | - | - | - | - |
| POO | - | 38.8 | - | 12.5 | - | 28.5 | 25.1–38.8 | - | 19.0 | - |
| POS | - | 3.4 | - | - | - | 2.1 | 1.3 | - | 3.1 | - |
| OLL | - | - | - | - | - | - | - | - | 2.8 | - |
| OLLn | - | - | - | - | - | - | 1.6 | - | - | - |
| OLnL | - | - | - | - | 0.80 | - | - | - | - | - |
| OLnLn | - | - | - | - | - | - | 1.4 | - | - | - |
| OOP | - | - | - | - | - | - | - | 19.27 | - | - |
| OOL | - | - | - | 2.1 | - | 0.8 | 1.7–6.9 | - | 8.0 | - |
| OOLn | - | - | - | - | - | - | 11.1 | - | - | - |
| OOLi | - | - | - | - | - | - | - | 9.53 | - | - |
| OOO | - | 19.4 | - | 3.9 | - | 6.1 | 28.8–35.6 | 38.83 | 9.3 | - |
| OOS | - | - | - | - | - | - | - | 7.95 | - | - |
| SLL | - | 2.4 | - | - | - | - | - | - | 2.1 | - |
| SLP | - | - | - | - | 0.53–0.64 | - | - | - | - | - |
| SOO | - | 3.3 | - | 0.8 | - | 1.1 | 1.6–3.5 | - | 2.4 | - |
| SOL | - | 1.1 | - | - | - | - | 2.3 | - | 4.0 | - |
| References | [10] | [148] | [10] | [148] | [138] | [148] | [148,172] | [10] | [148] | [69] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teixeira, G.L.; Ibañez, E.; Block, J.M. Emerging Lipids from Arecaceae Palm Fruits in Brazil. Molecules 2022, 27, 4188. https://doi.org/10.3390/molecules27134188
Teixeira GL, Ibañez E, Block JM. Emerging Lipids from Arecaceae Palm Fruits in Brazil. Molecules. 2022; 27(13):4188. https://doi.org/10.3390/molecules27134188
Chicago/Turabian StyleTeixeira, Gerson Lopes, Elena Ibañez, and Jane Mara Block. 2022. "Emerging Lipids from Arecaceae Palm Fruits in Brazil" Molecules 27, no. 13: 4188. https://doi.org/10.3390/molecules27134188