Molecular Modeling Approaches Can Reveal the Molecular Interactions Established between a Biofilm and the Bioactive Compounds of the Essential Oil of Piper divaricatum
Abstract
:1. Introduction
2. Results and Discussions
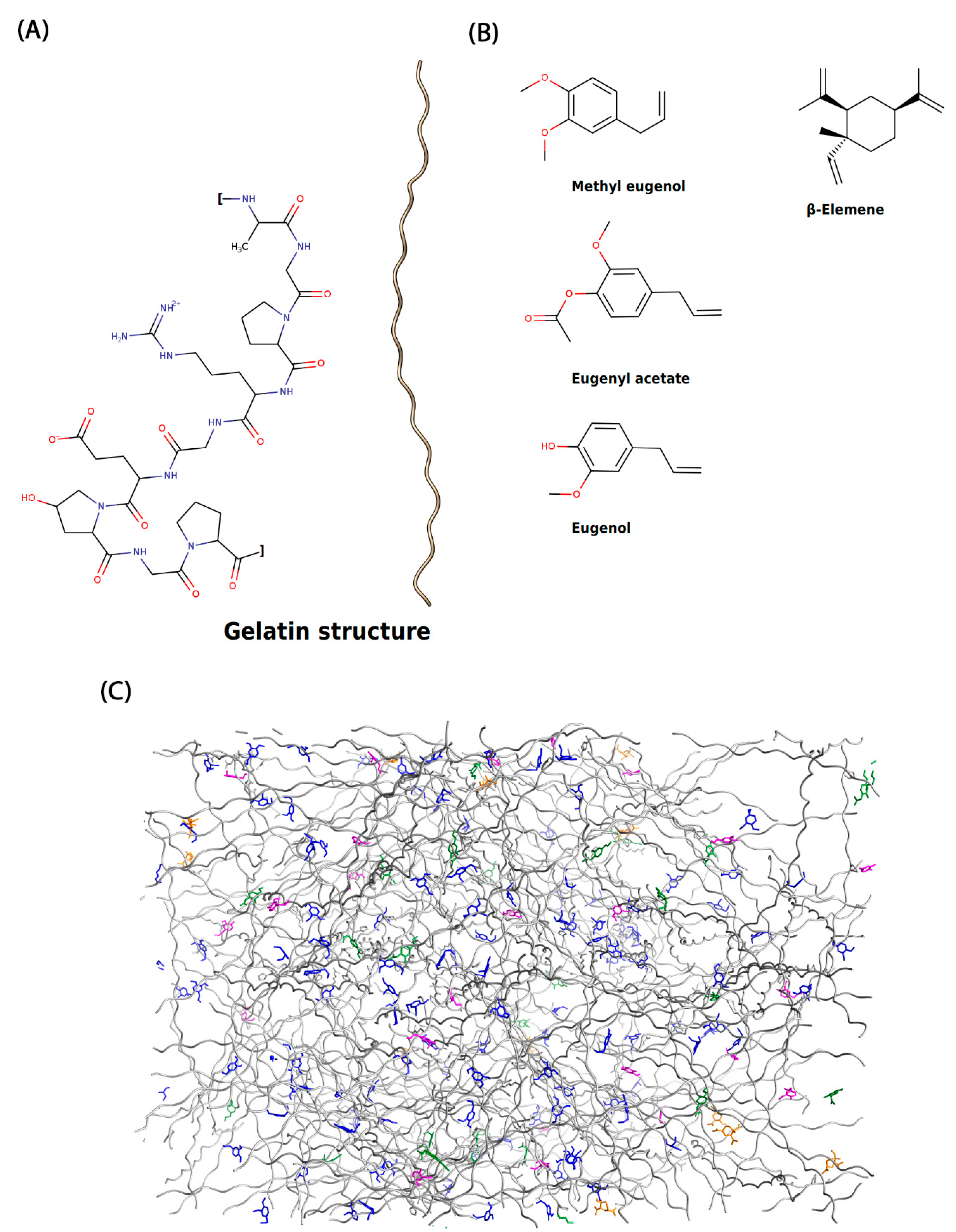
Molecular Analysis of the Biofilm Matrix
3. Materials and Methods
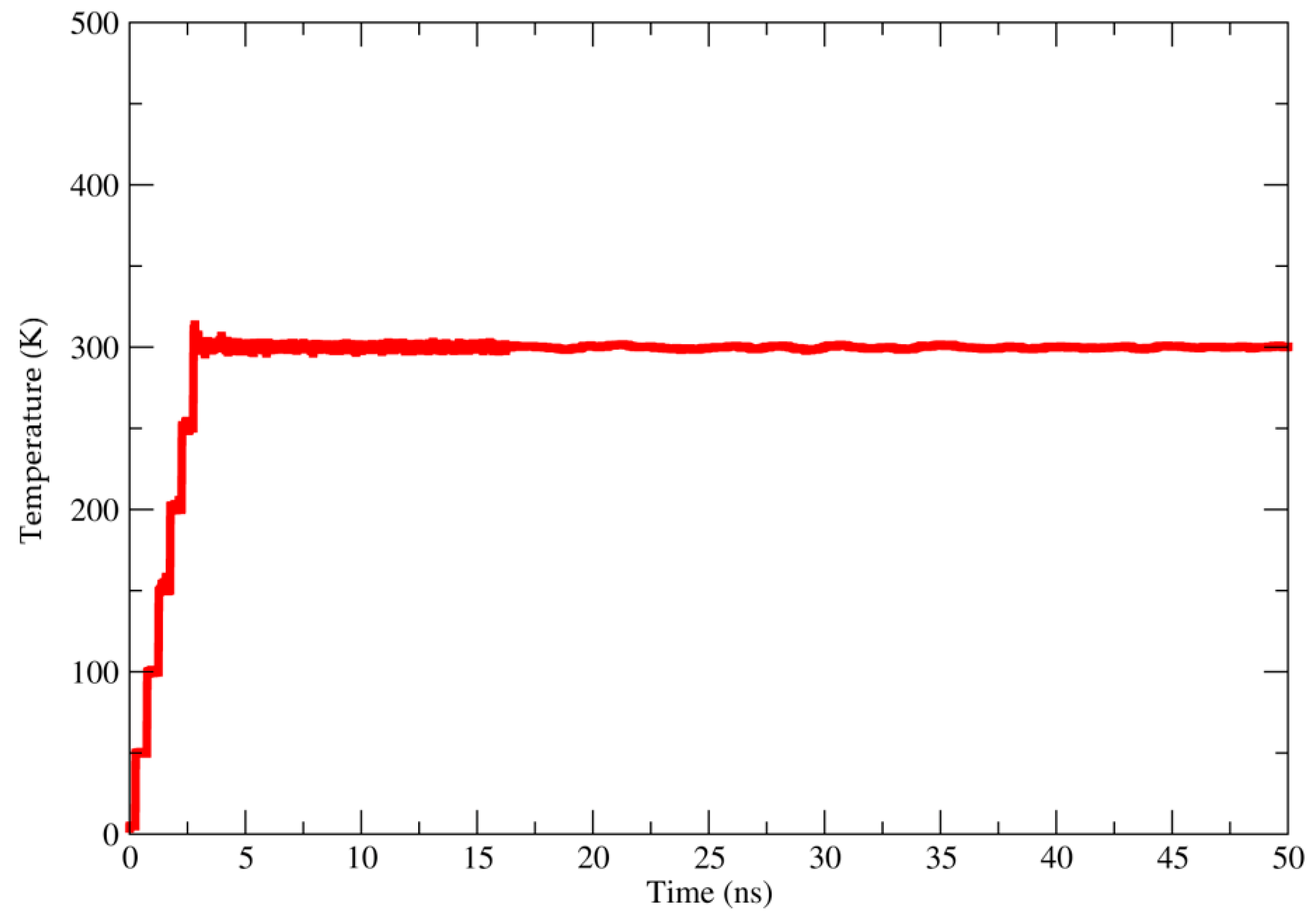
Molecular Modeling Approaches
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Eriksen, M.; Lebreton, L.C.M.; Carson, H.S.; Thiel, M.; Moore, C.J.; Borerro, J.C.; Galgani, F.; Ryan, P.G.; Reisser, J. Plastic Pollution in the World’s Oceans: More than 5 Trillion Plastic Pieces Weighing over 250,000 Tons Afloat at Sea. PLoS ONE 2014, 9, e111913. [Google Scholar] [CrossRef] [Green Version]
- Rhodes, C.J. Plastic Pollution and Potential Solutions. Sci. Prog. 2018, 101, 207–260. [Google Scholar] [CrossRef] [PubMed]
- Windsor, F.M.; Durance, I.; Horton, A.A.; Thompson, R.C.; Tyler, C.R.; Ormerod, S.J. A Catchment-Scale Perspective of Plastic Pollution. Glob. Chang. Biol. 2019, 25, 1207–1221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kedzierski, M.; Lechat, B.; Sire, O.; Le Maguer, G.; Le Tilly, V.; Bruzaud, S. Microplastic Contamination of Packaged Meat: Occurrence and Associated Risks. Food Packag. Shelf Life 2020, 24, 100489. [Google Scholar] [CrossRef]
- Hwang, J.; Choi, D.; Han, S.; Jung, S.Y.; Choi, J.; Hong, J. Potential Toxicity of Polystyrene Microplastic Particles. Sci. Rep. 2020, 10, 7391. [Google Scholar] [CrossRef] [PubMed]
- Avella, M.; De Vlieger, J.J.; Errico, M.E.; Fischer, S.; Vacca, P.; Volpe, M.G. Biodegradable Starch/Clay Nanocomposite Films for Food Packaging Applications. Food Chem. 2005, 93, 467–474. [Google Scholar] [CrossRef]
- Du, F.; Cai, H.; Zhang, Q.; Chen, Q.; Shi, H. Microplastics in Take-out Food Containers. J. Hazard. Mater. 2020, 399, 122969. [Google Scholar] [CrossRef]
- Syahida, N.; Fitry, I.; Zuriyati, A.; Hanani, N. Effects of Palm Wax on the Physical, Mechanical and Water Barrier Properties of Fish Gelatin Films for Food Packaging Application. Food Packag. Shelf Life 2020, 23, 100437. [Google Scholar] [CrossRef]
- Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Development of Active Gelatin Films by Means of Valorisation of Food Processing Waste: A Review. Food Hydrocoll. 2017, 68, 192–198. [Google Scholar] [CrossRef]
- Mehta, M.J.; Kumar, A. Ionic Liquid Assisted Gelatin Films: Green, UV Shielding, Antioxidant, and Antibacterial Food Packaging Materials. ACS Sustain. Chem. Eng. 2019, 7, 8631–8636. [Google Scholar] [CrossRef]
- Yadav, S.; Mehrotra, G.K.; Bhartiya, P.; Singh, A.; Dutta, P.K. Preparation, Physicochemical and Biological Evaluation of Quercetin Based Chitosan-Gelatin Film for Food Packaging. Carbohydr. Polym. 2020, 227, 115348. [Google Scholar] [CrossRef]
- Shahbazi, Y. The Properties of Chitosan and Gelatin Films Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora Clinopodioides Essential Oil as Biodegradable Materials for Active Food Packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive Characterization of Active Chitosan-Gelatin Blend Films Enriched with Different Essential Oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Ahmad, M.; Benjakul, S.; Prodpran, T.; Agustini, T.W. Physico-Mechanical and Antimicrobial Properties of Gelatin Film from the Skin of Unicorn Leatherjacket Incorporated with Essential Oils. Food Hydrocoll. 2012, 28, 189–199. [Google Scholar] [CrossRef]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef]
- Bezerra, F.W.F.W.F.; De Oliveira, M.S.S.; Bezerra, P.N.N.; Cunha, V.M.B.M.B.; Silva, M.P.P.; da Costa, W.A.A.; Pinto, R.H.H.; Cordeiro, R.M.; Da Cruz, J.N.; Chaves Neto, A.M.J.; et al. Extraction of Bioactive Compounds. In Green Sustainable Process for Chemical and Environmental Engineering and Science; Elsevier: Amsterdam, The Netherlands, 2020; pp. 149–167. ISBN 9780128173886. [Google Scholar]
- Do Nascimento, L.D.; de Moraes, A.A.B.; da Costa, K.S.; Galúcio, J.M.P.; Taube, P.S.; Costa, C.M.L.; Cruz, J.N.; Andrade, E.H.d.A.; de Faria, L.J.G. Bioactive Natural Compounds and Antioxidant Activity of Essential Oils from Spice Plants: New Findings and Potential Applications. Biomolecules 2020, 10, 988. [Google Scholar] [CrossRef]
- Cascaes, M.M.; Carneiro, S.O.d.; Do Nascimento, L.D.; De Moraes, Â.A.B.; De Oliveira, M.S.; Cruz, J.N.; Skelding, G.M.; Guilhon, P.; Andrade, E.H.D.A. Essential Oils from Annonaceae Species from Brazil: A Systematic Review of Their Phytochemistry, and Biological Activities. Int. J. Mol. Sci. 2021, 22, 12140. [Google Scholar] [CrossRef]
- Ramos Da Silva, L.R.; Ferreira, O.O.; Cruz, J.N.; De Jesus Pereira Franco, C.; Dos Anjos, T.O.; Cascaes, M.M.; Da Costa, W.A.; Andrade, E.H.D.A.; De Oliveira, M.S. Lamiaceae Essential Oils, Phytochemical Profile, Antioxidant, and Biological Activities. Evid.-Based Complement. Altern. Med. 2021, 2021, 6748052. [Google Scholar] [CrossRef]
- da Rocha Galucio, N.C.; de Araújo Moysés, D.; Pina, J.R.S.; Marinho, P.S.B.; Gomes Júnior, P.C.; Cruz, J.N.; Vale, V.V.; Khayat, A.S.; do Rosario Marinho, A.M. Antiproliferative, Genotoxic Activities and Quantification of Extracts and Cucurbitacin B Obtained from Luffa operculata (L.) Cogn. Arab. J. Chem. 2022, 15, 103589. [Google Scholar] [CrossRef]
- Silva, S.G.; de Oliveira, M.S.; Cruz, J.N.; da Costa, W.A.; da Silva, S.H.M.; Barreto Maia, A.A.; de Sousa, R.L.; Carvalho Junior, R.N.; de Aguiar Andrade, E.H. Supercritical CO2 Extraction to Obtain Lippia thymoides Mart. & Schauer (Verbenaceae) Essential Oil Rich in Thymol and Evaluation of Its Antimicrobial Activity. J. Supercrit. Fluids 2021, 168, 105064. [Google Scholar] [CrossRef]
- Lee, K.Y.; Lee, J.H.; Yang, H.J.; Song, K. Bin Production and Characterisation of Skate Skin Gelatin Films Incorporated with Thyme Essential Oil and Their Application in Chicken Tenderloin Packaging. Int. J. Food Sci. Technol. 2016, 51, 1465–1472. [Google Scholar] [CrossRef]
- Ramos, M.; Valdés, A.; Beltrán, A.; Garrigós, M. Gelatin-Based Films and Coatings for Food Packaging Applications. Coatings 2016, 6, 41. [Google Scholar] [CrossRef] [Green Version]
- da Silva Júnior, O.S.; Franco, C.d.J.P.; de Moraes, A.A.B.; Cruz, J.N.; da Costa, K.S.; do Nascimento, L.D.; de Aguiar Andrade, E.H. In Silico Analyses of Toxicity of the Major Constituents of Essential Oils from Two Ipomoea L. Species. Toxicon 2021, 195, 111–118. [Google Scholar] [CrossRef]
- Rego, C.M.A.; Francisco, A.F.; Boeno, C.N.; Paloschi, M.V.; Lopes, J.A.; Silva, M.D.S.; Santana, H.M.; Serrath, S.N.; Rodrigues, J.E.; Lemos, C.T.L.; et al. Inflammasome NLRP3 Activation Induced by Convulxin, a C-Type Lectin-like Isolated from Crotalus durissus terrificus Snake Venom. Sci. Rep. 2022, 12, 4706. [Google Scholar] [CrossRef]
- Mesquita, K.D.S.M.; Feitosa, B.D.S.; Cruz, J.N.; Ferreira, O.O.; Franco, C.D.J.P.; Cascaes, M.M.; Oliveira, M.S.D.; Andrade, E.H.D.A. Chemical Composition and Preliminary Toxicity Evaluation of the Essential Oil from Peperomia circinnata Link Var. circinnata. (Piperaceae) in Artemia salina Leach. Molecules 2021, 26, 7359. [Google Scholar] [CrossRef]
- Santana de Oliveira, M.; da Silva, V.M.P.; Cantão Freitas, L.; Silva, S.G.; Cruz, J.N.; de Aguiar Andrade, E.H. Extraction Yield, Chemical Composition, Preliminary Toxicity of Bignonia nocturna (Bignoniaceae) Essential Oil and in Silico Evaluation of the Interaction. Chem. Biodivers. 2021, 18, e2000982. [Google Scholar] [CrossRef]
- Costa, E.B.B.; Silva, R.C.C.; Espejo-Román, J.M.M.; Neto, M.F.D.A.F.D.A.; Cruz, J.N.N.; Leite, F.H.A.H.A.; Silva, C.H.T.P.H.T.P.; Pinheiro, J.C.C.; Macêdo, W.J.C.J.C.; Santos, C.B.R.B.R. Chemometric Methods in Antimalarial Drug Design from 1,2,4,5-Tetraoxanes Analogues. SAR QSAR Environ. Res. 2020, 31, 1–19. [Google Scholar] [CrossRef]
- Neto, R.d.A.M.M.; Santos, C.B.R.R.; Henriques, S.V.C.C.; Machado, L.D.O.; Cruz, J.N.; da Silva, C.H.D.P.; Federico, L.B.; Oliveira, E.H.D.; de Souza, M.P.C.C.; da Silva, P.N.B.B.; et al. Novel Chalcones Derivatives with Potential Antineoplastic Activity Investigated by Docking and Molecular Dynamics Simulations. J. Biomol. Struct. Dyn. 2020, 40, 2204–2216. [Google Scholar] [CrossRef]
- Albuquerque, G.A.; Bezerra, F.W.F.; de Oliveira, M.S.; da Costa, W.A.; de Carvalho Junior, R.N.; Joele, M.R.S.P. Supercritical CO2 Impregnation of Piper divaricatum Essential Oil in Fish (Cynoscion acoupa) Skin Gelatin Films. Food Bioprocess Technol. 2020, 13, 1765–1777. [Google Scholar] [CrossRef]
- Hanani, Z.A.N.; Roos, Y.H.; Kerry, J.P. Use and Application of Gelatin as Potential Biodegradable Packaging Materials for Food Products. Int. J. Biol. Macromol. 2014, 71, 94–102. [Google Scholar] [CrossRef]
- Dennington, R.; Keith, T.; Millam, J. GaussView; Version 5; Semichem Inc.: Shawnee Mission, KS, USA, 2009. [Google Scholar]
- Case, D.A.; Cheatham, T.E.; Darden, T.; Gohlke, H.; Luo, R.; Merz, K.M.; Onufriev, A.; Simmerling, C.; Wang, B.; Woods, R.J. The Amber Biomolecular Simulation Programs. J. Comput. Chem. 2005, 26, 1668–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castro, A.L.G.; Cruz, J.N.; Sodré, D.F.; Correa-Barbosa, J.; Azonsivo, R.; de Oliveira, M.S.; de Sousa Siqueira, J.E.; da Rocha Galucio, N.C.; de Oliveira Bahia, M.; Burbano, R.M.R.; et al. Evaluation of the Genotoxicity and Mutagenicity of Isoeleutherin and Eleutherin Isolated from Eleutherine plicata Herb. Using Bioassays and in Silico Approaches. Arab. J. Chem. 2021, 14, 103084. [Google Scholar] [CrossRef]
- Lima, A.R.J.A.d.M.; Siqueira, A.S.; Möller, M.L.S.; de Souza, R.C.; Cruz, J.N.; Lima, A.R.J.; da Silva, R.C.; Aguiar, D.C.F.; Junior, J.L.; Gonçalves, E.C. In Silico Improvement of the Cyanobacterial Lectin Microvirin and Mannose Interaction. J. Biomol. Struct. Dyn. 2020, 40, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Becke, A.D. Density-Functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, K.L.B.; Cruz, J.N.; Silva, L.B.; Ramos, R.S.; Neto, M.F.A.; Lobato, C.C.; Ota, S.S.B.; Leite, F.H.A.; Borges, R.S.; da Silva, C.H.T.P.; et al. Identification of Novel Chemical Entities for Adenosine Receptor Type 2a Using Molecular Modeling Approaches. Molecules 2020, 25, 1245. [Google Scholar] [CrossRef] [Green Version]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Barone, V.; Mennucci, B.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 09 Citation; Gaussian Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
- Martinez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A Package for Building Initial Configurations for Molecular Dynamics Simulations. J. Comput. Chem. 2009, 30, 2157–2164. [Google Scholar] [CrossRef]
- Santos, C.B.R.; Santos, K.L.B.; Cruz, J.N.; Leite, F.H.A.; Borges, R.S.; Taft, C.A.; Campos, J.M.; Silva, C.H.T.P. Molecular Modeling Approaches of Selective Adenosine Receptor Type 2A Agonists as Potential Anti-Inflammatory Drugs. J. Biomol. Struct. Dyn. 2021, 39, 3115–3127. [Google Scholar] [CrossRef]
- Darden, T.; York, D.; Pedersen, L. Particle Mesh Ewald: An N·log(N) Method for Ewald Sums in Large Systems. J. Chem. Phys. 1993, 98, 10089–10092. [Google Scholar] [CrossRef] [Green Version]
- Ryckaert, J.P.; Ciccotti, G.; Berendsen, H.J.C. Numerical Integration of the Cartesian Equations of Motion of a System with Constraints: Molecular Dynamics of n-Alkanes. J. Comput. Phys. 1977, 23, 327–341. [Google Scholar] [CrossRef] [Green Version]
- Lzaguirre, J.A.; Catarello, D.P.; Wozniak, J.M.; Skeel, R.D. Langevin Stabilization of Molecular Dynamics. J. Chem. Phys. 2001, 114, 2090–2098. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cruz, J.N.; de Oliveira, M.S.; Andrade, E.H.d.A.; Rodrigues Lima, R. Molecular Modeling Approaches Can Reveal the Molecular Interactions Established between a Biofilm and the Bioactive Compounds of the Essential Oil of Piper divaricatum. Molecules 2022, 27, 4199. https://doi.org/10.3390/molecules27134199
Cruz JN, de Oliveira MS, Andrade EHdA, Rodrigues Lima R. Molecular Modeling Approaches Can Reveal the Molecular Interactions Established between a Biofilm and the Bioactive Compounds of the Essential Oil of Piper divaricatum. Molecules. 2022; 27(13):4199. https://doi.org/10.3390/molecules27134199
Chicago/Turabian StyleCruz, Jorddy Neves, Mozaniel Santana de Oliveira, Eloisa Helena de Aguiar Andrade, and Rafael Rodrigues Lima. 2022. "Molecular Modeling Approaches Can Reveal the Molecular Interactions Established between a Biofilm and the Bioactive Compounds of the Essential Oil of Piper divaricatum" Molecules 27, no. 13: 4199. https://doi.org/10.3390/molecules27134199







