Safranal Induces Vasorelaxation by Inhibiting Ca2+ Influx and Na+/Ca2+ Exchanger in Isolated Rat Aortic Rings
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Tissue Preparation
2.3. Role of Endothelium in Safranal-Induced Vasodilation
2.4. Role of Nitric Oxide, Prostaglandins, Guanylate Cyclase, or Phospholipase A2 in Safranal-Induced Vasodilation
2.5. Effect of Safranal on Phenylephrine-Induced Contraction
2.6. Effect of Safranal on Ca2+ Influx and Ca2+-Induced Contraction
2.7. Effect of Safranal on Intracellularly Stored Ca2+
2.8. Role of Na+/Ca2+Exchanger in Safranal-Induced Vasodilation
2.9. Statistical Analysis
3. Results
3.1. Effect of Safranal on Rat Aorta Rings Precontracted with Phenylephrine
3.2. Effects of Indomethacin, L-NAME, Methylene Blue, and Quinacrine on Safranal-Induced Vasodilation
3.3. Effect of Safranal on Phenylephrine-Induced Contraction
3.4. Effect of Safranal on Ca2+-Induced Contraction in Calcium-Free Depolarizing Solution
3.5. Effect of Safranal Pretreatment on the Ca2+ Release from Intracellular Stores
3.6. Safranal Relaxed Ouabain-Induced Contractions
3.7. Safranal Inhibited Ouabain-Induced Contractions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rahmani, A.H.; Ali, A.; Aldebasi, W.H. Saffron (Crocus sativus) and its active ingredients: Role in the prevention and treatment of disease. Pharmacogn. J. 2017, 9, 873–879. [Google Scholar]
- Baba, S.A.; Malik, A.H.; Wani, Z.A.; Mohiuddin, T.; Shah, Z.; Abbas, N.; Ashraf, N. Phytochemical analysis and antioxidant activity of different tissue types of Crocus sativus and oxidative stress alleviating potential of saffron extract in plants, bacteria, and yeast. South Afr. J. Bot. 2015, 99, 80–87. [Google Scholar]
- Alavizadeh, S.H.; Hosseinzadeh, H. Bioactivity assessment and toxicity of crocin: A comprehensive review. Food Chem. Toxicol. 2014, 64, 65–80. [Google Scholar]
- Razavi, B.M.; Seyfabad, M.S.; Hosseinzadeh, H.; Imenshahidi, M. Crocin-induced endothelium-dependent vasodilation in isolated rat aorta. Jundishapur J. Nat. Pharmaceut. Prod. 2017, 12, e32801. [Google Scholar]
- Saadat, S.; Yasavoli, M.; Gholamnezhad, Z.; Aslan, M.R.; Boskabady, M.H. The vasodilatory effect of crocin on rat tracheal smooth muscle and its possible mechanisms. Iran. J. Pharmaceut. Res. 2019, 18, 1358–1370. [Google Scholar]
- Hosseinzadeh, H.; Shamsaie, F.; Mehri, S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents crocin and safranal. Pharmacogn. Mag. 2010, 5, 419–424. [Google Scholar]
- Hosseinzadeh, H.; Younesi, H.M. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002, 2, 7. [Google Scholar] [CrossRef] [Green Version]
- Hosseinzadeh, H.; Khosravan, V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch. Iran Med. 2002, 5, 44–47. [Google Scholar]
- Hosseinzadeh, H.; Ziaee, T.; Sadeghi, A. The effect of saffron, Crocus sativus stigma, extract and its constituents, safranal and crocin on sexual behaviors in normal male rats. Phytomedicine 2008, 15, 491–495. [Google Scholar]
- Hosseinzadeh, H.; Karimi, G.; Niapoor, M. Antidepressant effect of Crocus sativus L. stigma extracts and their constituents, crocin and safranal, in Mice. J. Med. Plants 2004, 3, 48–58. [Google Scholar]
- Abdullaev, F.I. Biological effects of saffron. Biofactors 1993, 4, 83–86. [Google Scholar]
- Nasiri, Z.; Sameni, H.R.; Vakili, A.; Jarrahi, M.; Khorasani, M.Z. Dietary saffron reduced the blood pressure and prevented remodeling of the aorta in L-NAME-induced hypertensive rats. Iran. J. Basic Med. Sci. 2015, 18, 1143–1146. [Google Scholar]
- Imenshahidi, M.; Razavi, B.M.; Faal, A.; Gholampoor, A.; Mousavi, S.M.; Hosseinzadeh, H. The Effect of Chronic Administration of Saffron (Crocus sativus) Stigma Aqueous Extract on Systolic Blood Pressure in Rats. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 175–179. [Google Scholar]
- Delkhosh-Kasmaie, F.; Farshid, A.A.; Tamaddonfard, E.; Imani, M. The effects of safranal, a constitute of saffron, and metformin on spatial learning and memory impairments in type-1 diabetic rats: Behavioral and hippocampal histopathological and biochemical evaluations. Biomed. Pharmacother. 2018, 107, 203–211. [Google Scholar]
- Nassiri-Asl, M.; Hosseinzadeh, H. Neuropharmacology effects of saffron (Crocus sativus) and its active constituents. In Bioactive Nutraceuticals and Dietary Supplements in Neurological and Brain Disease Prevention and Therapy, Role of Complementary and Alternative Supplements; Academic Press: Cambridge, MA, USA, 2015; Chapter 3; pp. 29–39. [Google Scholar] [CrossRef]
- Liu, M.; Amini, A.; Ahmad, Z. Safranal and its analogs inhibit Escherichia coli ATP synthase and cell growth. Int. J. Biol. Macromol. 2016, 95, 145–152. [Google Scholar] [CrossRef] [Green Version]
- Razavi, B.M.; Amanloo, M.A.; Imenshahidi, M.; Hosseinzadeh, H. The vasodilatory activity of safranal in isolated rat aortas is mediated predominantly via an endothelium-independent mechanism- vasodilatory mechanism of safranal. J. Pharmacopunct. 2016, 19, 329–335. [Google Scholar]
- Mehdizadeh, R.; Parizadeh, M.; Khooei, A.-R.; Mehri, S.; Hosseinzadeh, H. Cardioprotective Effect of Saffron Extract and Safranal in Isoproterenol-Induced Myocardial Infarction in Wistar Rats. Iran. J. Basic Med. Sci. 2013, 16, 56–63. [Google Scholar]
- Shafiee, M.; Moghaddam, N.S.A.; Nosrati, M.; Tousi, M.; Avan, A.; Ryzhikov, M.; Parizadeh, M.R.; Fiuji, H.; Rajabian, M.; Bahreyni, A.; et al. Saffron against Components of Metabolic Syndrome: Current Status and Prospective. J. Agric. Food Chem. 2017, 65, 10837–10843. [Google Scholar]
- Godo, S.; Shimokawa, H. Endothelial Functions. Arter. Thromb. Vasc. Biol. 2017, 37, e108–e114. [Google Scholar] [CrossRef] [Green Version]
- Razavi, B.M.; Alyasin, A.; Hosseinzadeh, H.; Imenshahidi, M. Saffron induced vasodilation in isolated rat aorta via endothelium dependent and independent mechanisms. Iran. J. Pharmaceut. Res. 2018, 17, 1018–1025. [Google Scholar]
- Lloréns, S.; Mancini, A.; Serrano-Díaz, J.; D’Alessandro, A.M.; Nava, E.; Alonso, G.L.; Carmona, M. Effects of Crocetin Esters and Crocetin from Crocus sativus L. on Aortic Contractility in Rat Genetic Hypertension. Molecules 2015, 20, 17570–17584. [Google Scholar]
- Pan, Y.; Rong, Y.; You, M.; Ma, Q.; Chen, M.; Hu, F. Royal jelly causes hypotension and vasodilation induced by increasing nitric oxide production. Food Sci. Nutr. 2019, 7, 1361–1370. [Google Scholar] [CrossRef] [Green Version]
- Rees, D.D.; Palmer, R.M.J.; Schulz, R.; Hodson, H.F.; Moncada, S. Characterization of three inhibitors of endothelial nitric oxidesynthase in vitro and in vivo. Br. J. Pharmacol. 1990, 101, 746–752. [Google Scholar]
- Mayer, B.; Brunner, F.; Schmidt, K. Inhibition of nitric oxide synthesis by methylene blue. Biochem. Pharmacol. 1993, 45, 367–374. [Google Scholar]
- Murrant, C.L.; Dodd, J.D.; Foster, A.J.; Inch, K.A.; Muckle, F.R.; Ruiz, D.A.; Simpson, J.A.; Scholl, J.H.P. Prostaglandins induce vasodilatation of the microvasculature during muscle contraction and induce vasodilatation independent of adenosine. J. Physiol. 2014, 592, 1267–1281. [Google Scholar]
- Abdalla, S.S.; Laravuse, R.B.; Will, A. Mechanisms of inhibitory effect of ketamine on Guinea pig isolated main pulmonary artery. Anesth Analg. 1994, 78, 17–22. [Google Scholar]
- Yang, H.; Li, X.; Liu, Y.; Li, X.; Li, X.; Wu, M.; Lv, X.; Chunhua, C.; Ding, X.; Zhang, Y. Crocin improves the endothelial function regulated by KCa3.1 through ERK and Akt signaling pathways. Cell Physiol. Biochem. 2018, 46, 765–780. [Google Scholar]
- Lee, C.-H.; Poburko, D.; Sahouta, P.; Sandhu, J.; Ruehlmann, D.O.; van Breeman, C. The mechanism of phenylephrine-mediated [Ca2+]i oscillations underlying tonic contraction in the rabbit inferior vena cava. J. Physiol. 2001, 534, 641–650. [Google Scholar]
- Barritt, G.J. Receptor operated Ca2+ inflow in animal cells: A variety of pathways tailored to meet different intracellular signaling requirements. Biochem. J. 1999, 337, 153–169. [Google Scholar]
- Jin, J.; Shen, X.; Tai, Y.; Li, S.; Liu, M.; Zhen, C.; Xuan, X.; Zhang, X.; Hu, N.; Zhang, X.; et al. Arterial vasodilation is coupled to inhibition of mitochondrial fission in arterial smooth muscle cells: Comparison of vasodilatory effects of verapamil and phentolamine. Acta Pharm. Sin. B 2017, 7, 319–325. [Google Scholar]
- Leijten, P.A.; van Breeman, C. The relationship between noradrenaline-induced contraction and 45Ca2+ efflux stimulation in rabbit mesenteric artery. Br. J. Pharmacol. 1986, 89, 739–747. [Google Scholar]
- Cifuentes, F.; Palacios, J.; Nwokocha, C.R. Synchronization in the Heart Rate and the Vasomotion in Rat Aorta: Effect of Arsenic Trioxide. Cardiovasc. Toxicol. 2015, 16, 79–88. [Google Scholar] [CrossRef]
- Kreye, V.A.W.; Hofmann, F.; Mühleisen, M. Barium can replace calcium in calmodulin-dependent contractions of skinned renal arteries of the rabbit. Pflugers Arch. 1986, 406, 308–311. [Google Scholar] [CrossRef]
- Juhaszova, M.; Blaustein, M.P. Na+ pump low and high affinity a subunit isoforms are differentlydistributed in cells. Proc. Natl. Acad. Sci. USA 1997, 94, 1800–1805. [Google Scholar]
- Golovina, V.A.; Song, H.; James, P.F.; Lingrel, J.B.; Blaustein, M.P. Na+ pump α2-subunit expression modulates Ca2+ signaling. Am. J. Physiol. Cell Physiol. 2003, 284, C475–C486. [Google Scholar]
- Song, H.; Karashima, E.; Hamlyn, J.M.; Blaustein, M.P. Ouabain-digoxin antagonism in rat arteries and neurones. J. Physiol. 2014, 592, 941–969. [Google Scholar]
- Dias, F.M.V.; Júnior, R.F.R.; Fernandes, A.A.; Fiorim, J.; Travaglia, T.C.F.; Vassallo, D.V.; Stefanon, I. Na+K+-ATPase activity and K+ channels differently contribute to vascular vasodilation in male and female rats. PLoS ONE 2014, 9, e106345. [Google Scholar] [CrossRef]
- Schoner, W.; Scheiner-Bobis, G. Endogenous and exogenous cardiac glycosides: Their roles in hypertension, salt metabolism, and cell growth. Am. J. Physiol. Cell Physiol. 2007, 293, C509–C536. [Google Scholar]
- Hamlyn, J.M.; Manunta, P. Endogenous Cardiotonic Steroids in Kidney Failure: A Review and an Hypothesis. Adv. Chronic Kidney Dis. 2015, 22, 232–244. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.W.; Lingwood, C.A.; Ostrowski, M.A.; Cabral, T.; Cochrane, A. Cardiac glycoside/aglycones inhibit HIV-1 gene expression by a mechanism requiring MEK1/2-ERK1/2 signaling. Sci. Rep. 2018, 8, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Balestrino, M.; Young, J.; Aitken, P. Block of (Na+,K+)ATPase with ouabain induces spreading depression-like depolarization in hippocampal slices. Brain Res. 1999, 838, 37–44. [Google Scholar]
- Kohajda, Z.; Farkas-Morvay, N.; Jost, N.; Nagy, N.; Geramipour, A.; Horváth, A.; Varga, R.S.; Hornyik, T.; Corici, C.; Acsai, K.; et al. The Effect of a Novel Highly Selective Inhibitor of the Sodium/Calcium Exchanger (NCX) on Cardiac Arrhythmias in In Vitro and In Vivo Experiments. PLoS ONE 2016, 11, e0166041. [Google Scholar] [CrossRef]
- Pelat, M.; Barbe, F.; Daveu, C.; Ly-Nguyen, L.; Lartigue, T.; Marque, S.; Tavares, G.; Ballet, V.; Guillon, J.-M.; Steinmeyer, K.; et al. SAR340835, a Novel Selective Na+/Ca2+ Exchanger Inhibitor, Improves Cardiac Function and Restores Sympathovagal Balance in Heart Failure. J. Pharmacol. Exp. Ther. 2021, 377, 293–304. [Google Scholar] [CrossRef]
- Wasserstrom, J.A.; Aistrup, G.L. Digitalis: New actions for an old drug. Am. J. Physiol. Circ. Physiol. 2005, 289, H1781–H1793. [Google Scholar]
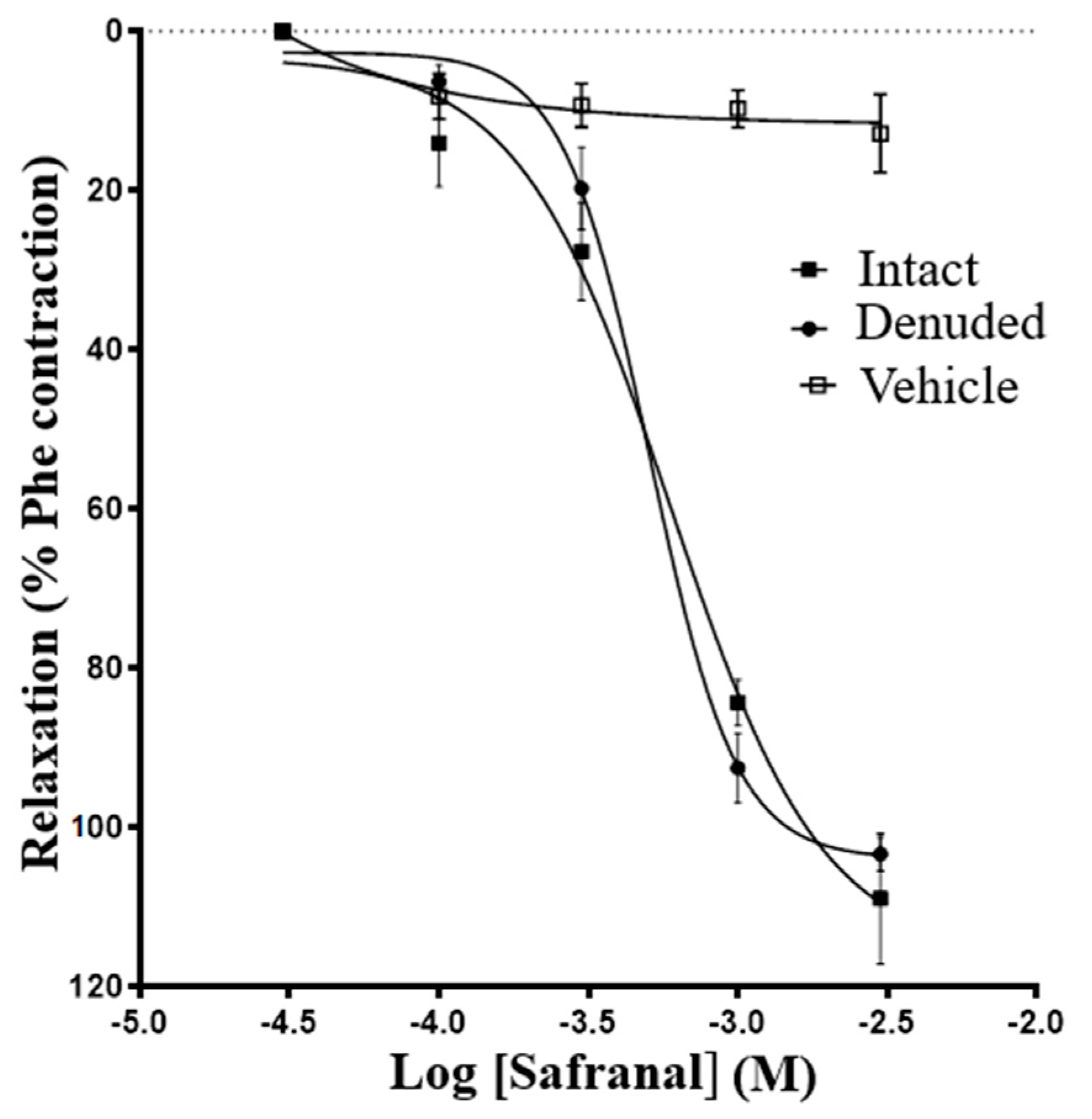
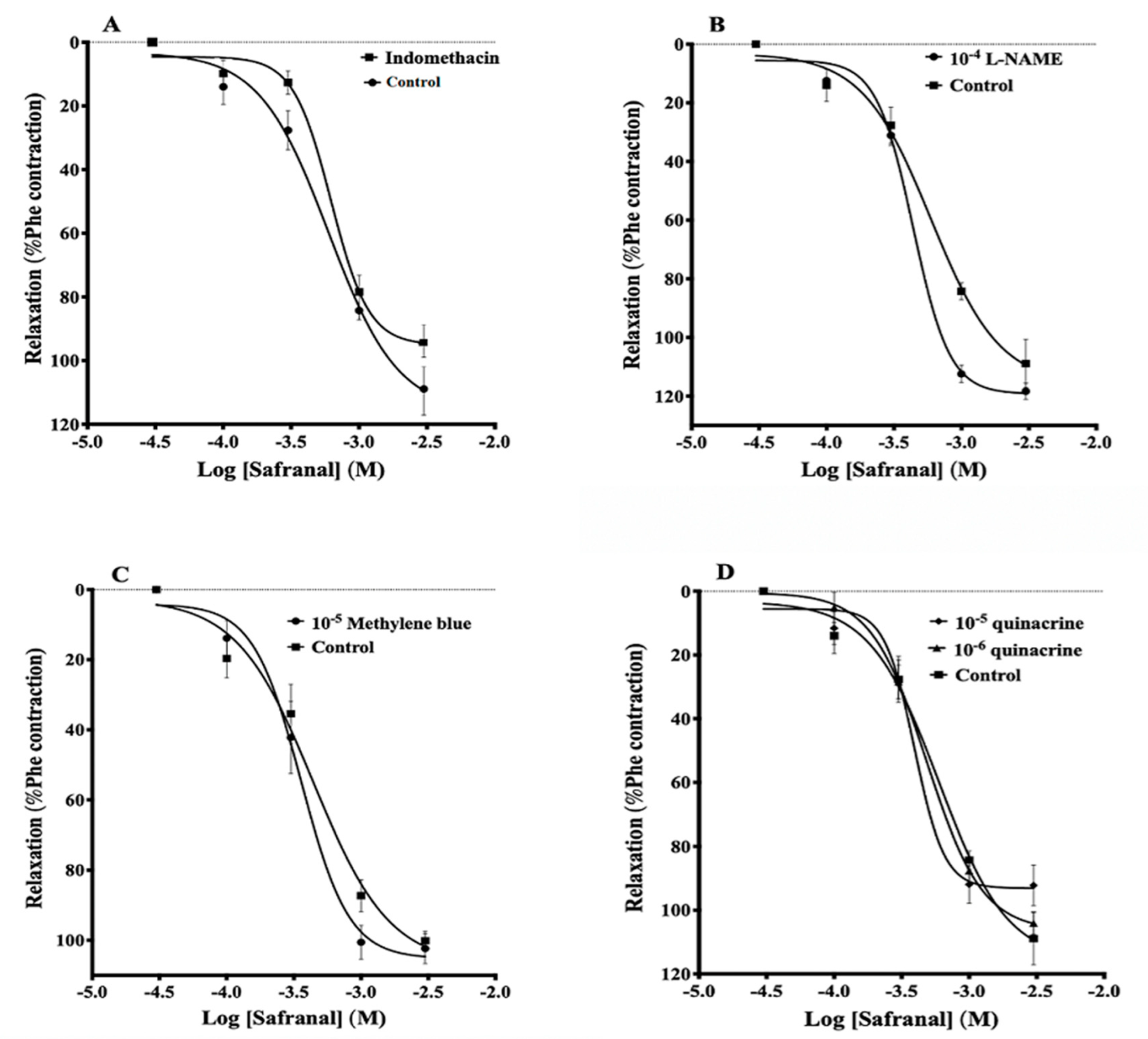

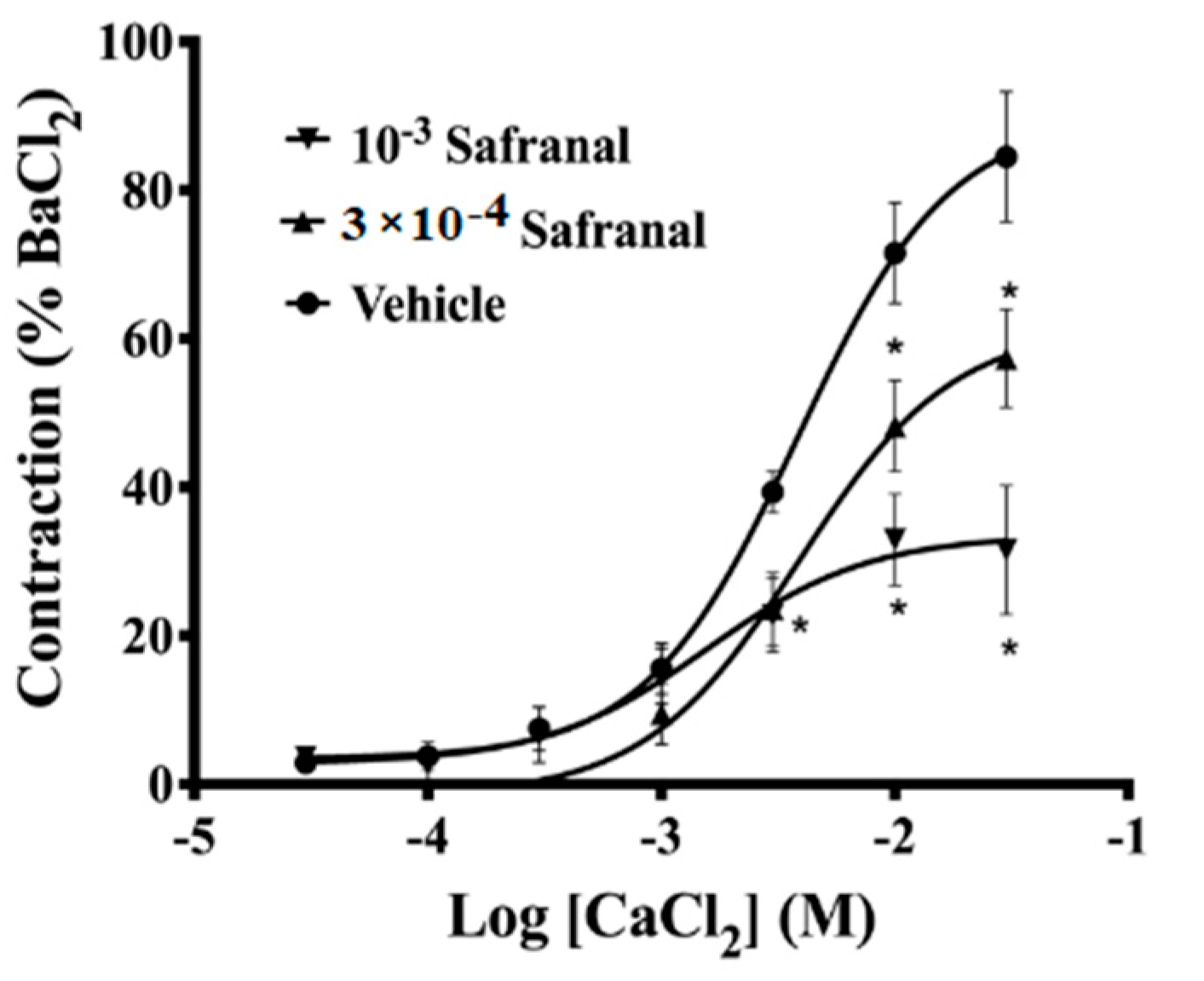
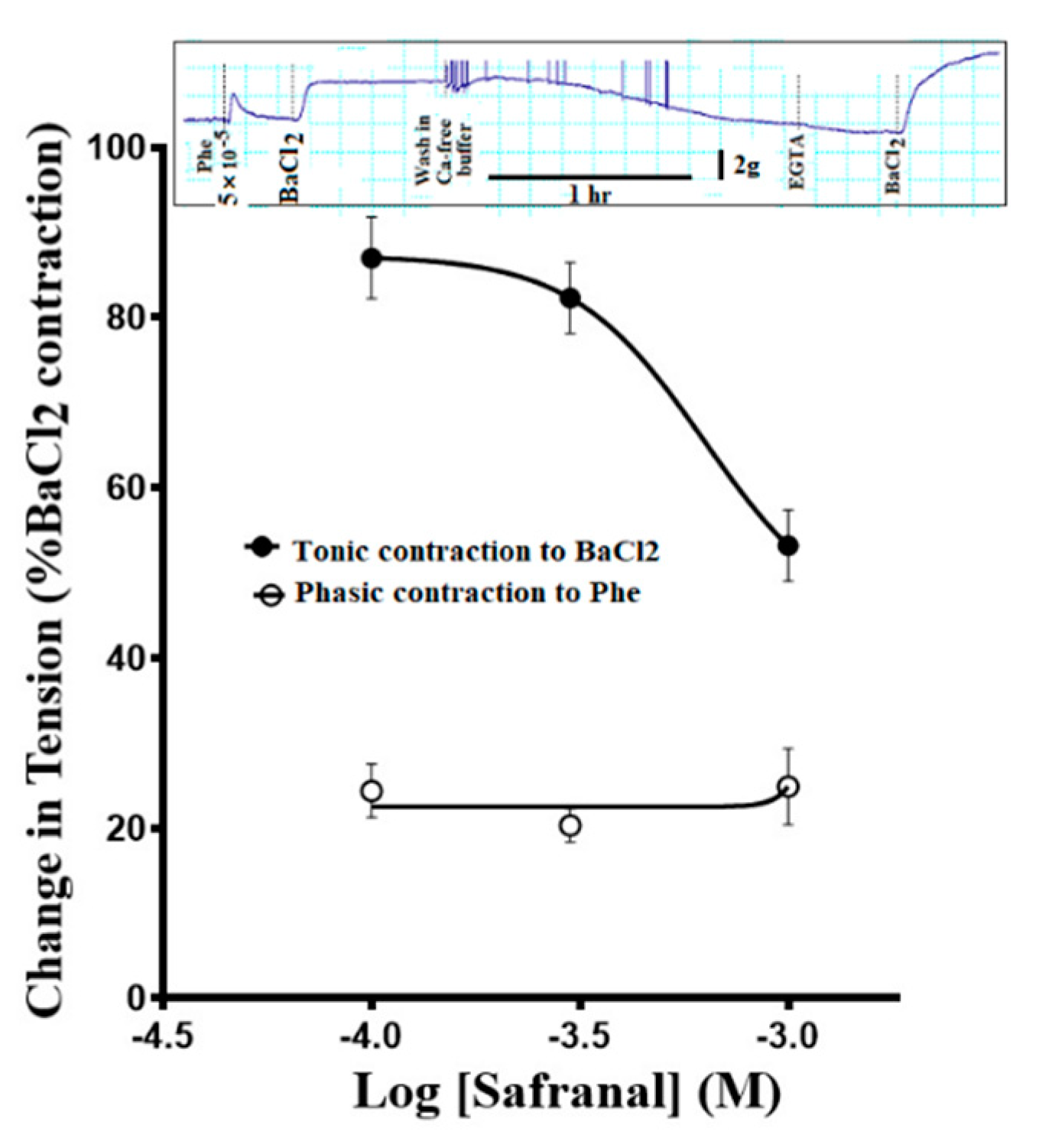


| Relaxant Agent | Treatment | n | IC50 (M) | Max. Relaxation (%Phe) |
|---|---|---|---|---|
| Safranal | Endothelium denuded | 10 | (6 ± 0.6) × 10−4 | 103 ± 2.1 |
| Endothelium intact | 14 | (5 ± 0.6) × 10−4 | 108 ± 8.2 | |
| Acetylcholine | Endothelium denuded | 6 | (3.0 ± 0.3) × 10−4 * | 27.4 ± 5.2 |
| Endothelium intact | 5 | (1.0± 0.7) × 10−4 | 76.2 ± 6.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Saigh, N.N.; Abdalla, S. Safranal Induces Vasorelaxation by Inhibiting Ca2+ Influx and Na+/Ca2+ Exchanger in Isolated Rat Aortic Rings. Molecules 2022, 27, 4228. https://doi.org/10.3390/molecules27134228
Al-Saigh NN, Abdalla S. Safranal Induces Vasorelaxation by Inhibiting Ca2+ Influx and Na+/Ca2+ Exchanger in Isolated Rat Aortic Rings. Molecules. 2022; 27(13):4228. https://doi.org/10.3390/molecules27134228
Chicago/Turabian StyleAl-Saigh, Noor Nadhim, and Shtaywy Abdalla. 2022. "Safranal Induces Vasorelaxation by Inhibiting Ca2+ Influx and Na+/Ca2+ Exchanger in Isolated Rat Aortic Rings" Molecules 27, no. 13: 4228. https://doi.org/10.3390/molecules27134228






