QTOF-ESI MS Characterization and Antioxidant Activity of Physalis peruviana L. (Cape Gooseberry) Husks and Fruits from Costa Rica
Abstract
:1. Introduction
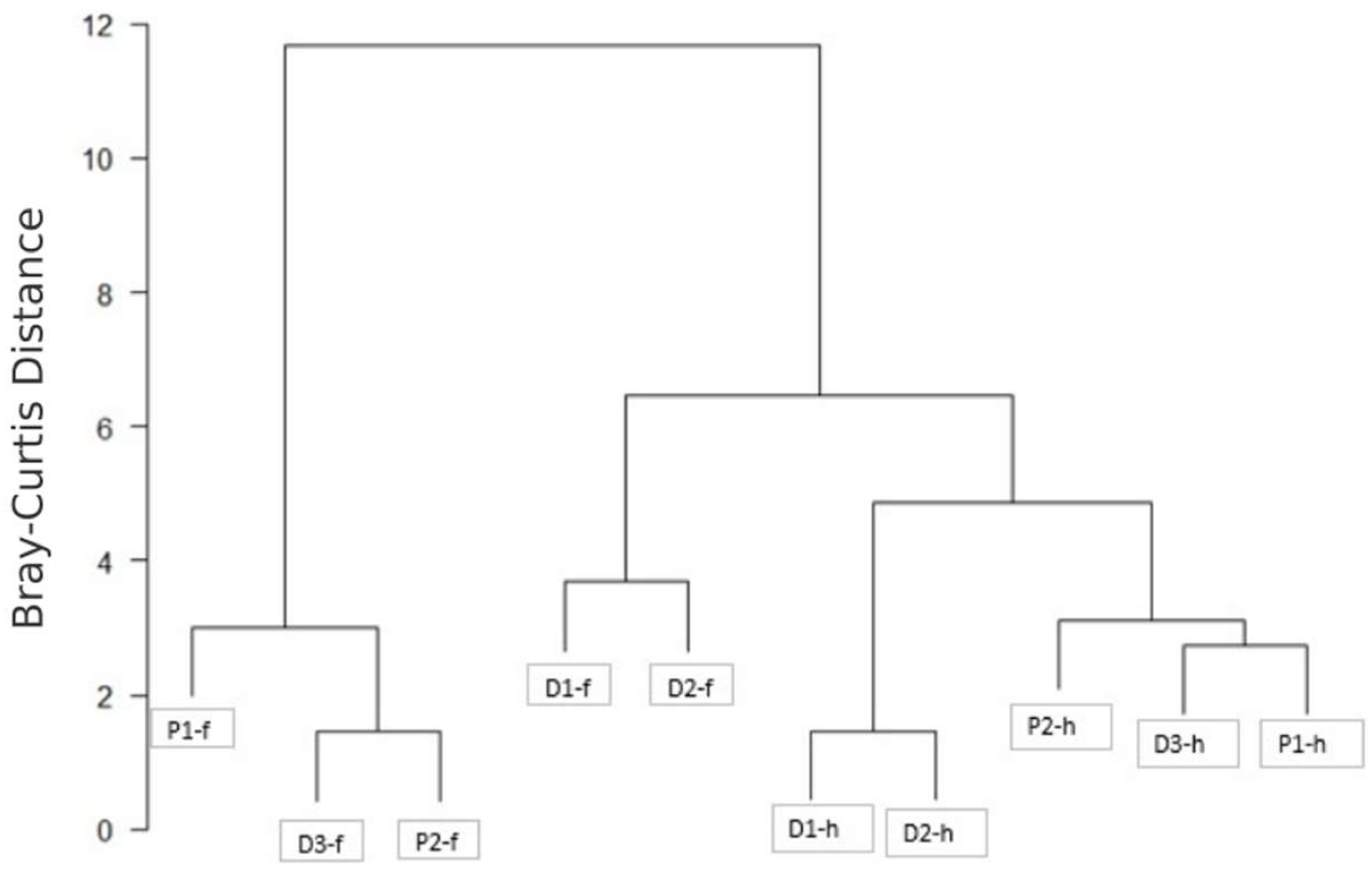
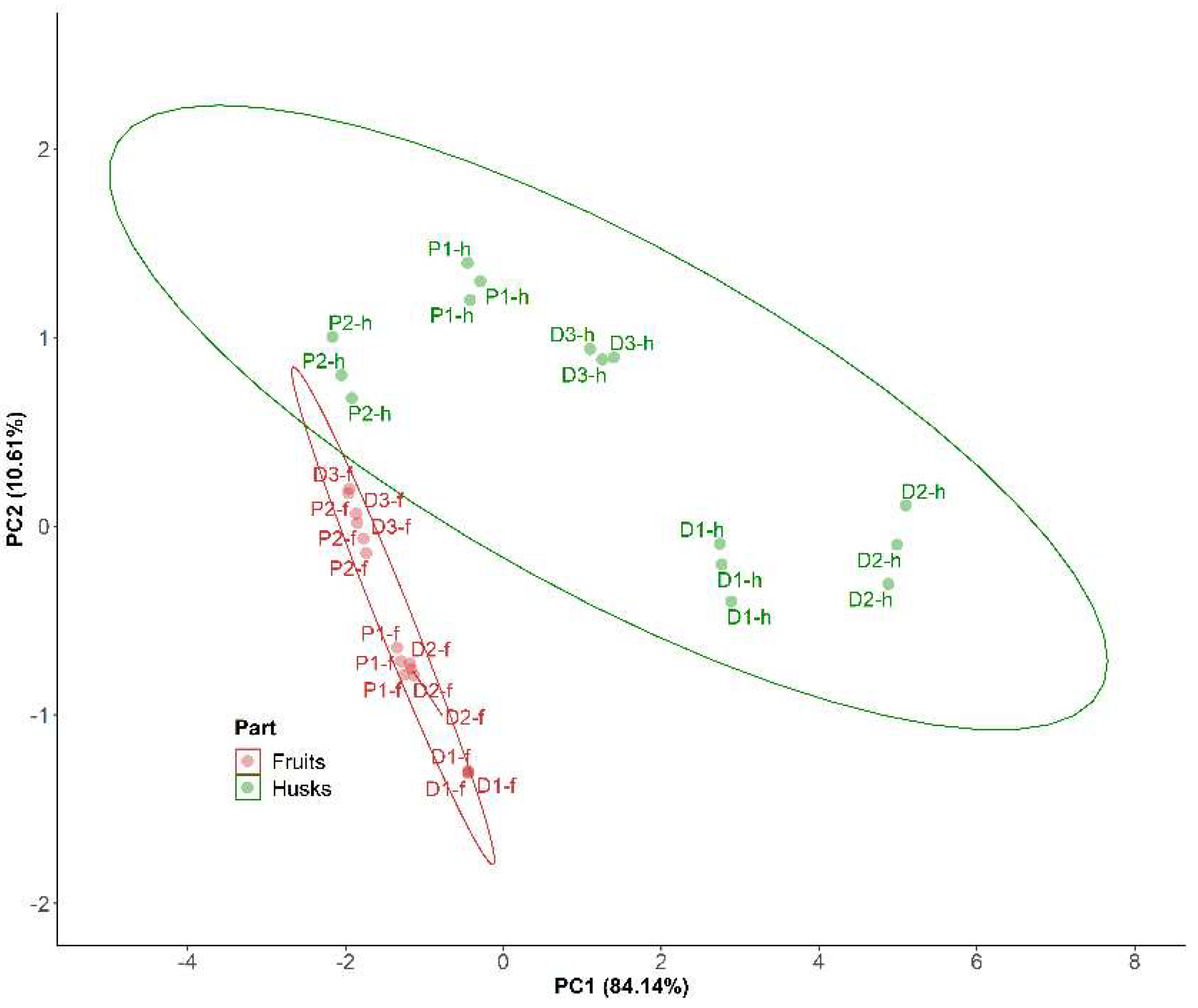
2. Results and Discussion
2.1. P. peruviana Husk and Fruit Secondary Metabolite Profile by UPLC-ESI-MS Analysis
2.1.1. Withanolides
2.1.2. Sucrose Ester Derivatives
2.1.3. Flavonoids
2.2. UPLC-DAD Analysis of Physalis peruviana Extracts
2.2.1. Determination of β-Carotene in P. peruviana Fruits
2.2.2. Determination of Flavonoids in P. peruviana Fruits and Husks
2.3. Folin–Ciocalteau Evaluation of P. peruviana Extracts
2.4. Evaluation of Antioxidant Activity of Physalis peruviana Extracts
3. Materials and Methods
3.1. Physalis peruviana Samples, Chemicals and Reagents
3.2. Extraction of P. Peruviana Samples
3.3. Analysis of P. Peruviana Extracts by UPLC-ESI-MS and by UPLC-DAD
3.4. Determination of Folin–Ciocalteu Total Polyphenolic Contents
3.5. DPPH Radical Scavenging Activity
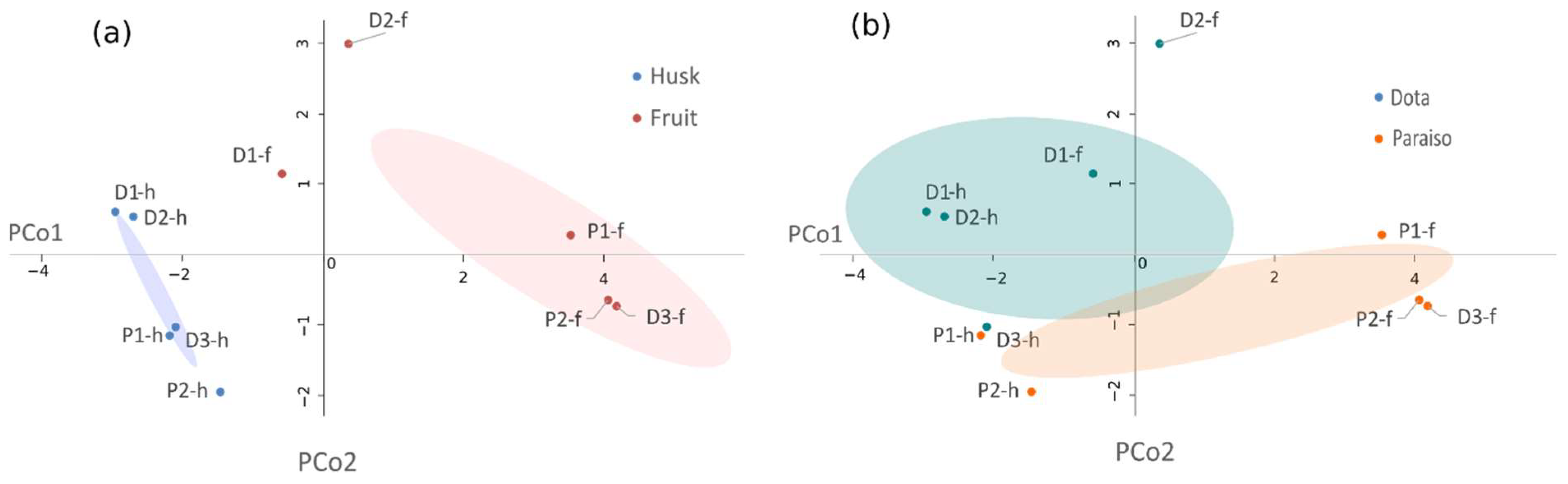
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Singh, N.; Singh, S.; Maurya, P.; Arya, M.; Khan, F.; Dwivedi, D.H.; Saraf, S.A. An Updated Review on Physalis peruviana Fruit: Cultivational, Nutraceutical and Pharmaceutical Aspects. Indian J. Nat. Prod. Resour. 2019, 14, 1. [Google Scholar]
- Ahmad, S.; Malik, A.; Yasmin, R.; Ullah, N.; Gul, W.; Khan, P.M.; Nawaz, H.R.; Afza, N. Withanolides from Physalis peruviana. Phytochemistry 1999, 50, 647–651. [Google Scholar] [CrossRef]
- Puente, L.A.; Pinto-Muñoz, C.A.; Castro, E.S.; Cortés, M. Physalis peruviana Linnaeus, the Multiple Properties of a Highly Functional Fruit: A Review. Food Res. Int. 2011, 44, 1733–1740. [Google Scholar] [CrossRef]
- Zavala, D.; Quispe, A.; Posso, M.; Rojas, J.; Vaisberg, A. Efecto citotóxico de Physalis peruviana (capulí) en cáncer de colon y leucemia mieloide crónica. An. Fac. Med. 2006, 67, 283. [Google Scholar] [CrossRef] [Green Version]
- Toro, R.M.; Aragón, D.M.; Ospina, L.F.; Ramos, F.A.; Castellanos, L. Phytochemical Analysis, Antioxidant and Anti-Inflammatory Activity of Calyces from Physalis peruviana. Nat. Prod. Commun. 2014, 9, 1934578X1400901. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.-C.; Choi, B.Y. Withaferin-A—A Natural Anticancer Agent with Pleitropic Mechanisms of Action. Int. J. Mol. Sci. 2016, 17, 290. [Google Scholar] [CrossRef] [Green Version]
- Kathare, J.M.; Mbaria, J.M.; Nguta, J.M.; Moriasi, G.A. Antimicrobial, Cytotoxicity, Acute Oral Toxicity and Qualitative Phytochemical Screening of the Aqueous and Methanolic Extracts of Physalis peruviana L (Solanaceae). Appl. Microbiol. Open Access 2021, 7, 189. [Google Scholar]
- Franco, L.; Ocampo, Y.; Gómez, H.; De la Puerta, R.; Espartero, J.; Ospina, L. Sucrose Esters from Physalis peruviana Calyces with Anti-Inflammatory Activity. Planta Med. 2014, 80, 1605–1614. [Google Scholar] [CrossRef]
- Tuğçe, D. Antioxidant and Cytotoxic Activity of Physalis peruviana. Med. Plant Res. 2014, 4, 30–34. [Google Scholar] [CrossRef]
- Arun, M.; Asha, V.V. Preliminary Studies on Antihepatotoxic Effect of Physalis peruviana Linn. (Solanaceae) against Carbon Tetrachloride Induced Acute Liver Injury in Rats. J. Ethnopharmacol. 2007, 111, 110–114. [Google Scholar] [CrossRef]
- Ballesteros-Vivas, D.; Alvarez-Rivera, G.; León, C.; Morantes, S.J.; Ibánez, E.; Parada-Alfonso, F.; Cifuentes, A.; Valdés, A. Anti-Proliferative Bioactivity against HT-29 Colon Cancer Cells of a Withanolides-Rich Extract from Golden Berry (Physalis peruviana L.) Calyx Investigated by Foodomics. J. Funct. Foods 2019, 63, 103567. [Google Scholar] [CrossRef]
- Mier-Giraldo, H.; Díaz-Barrera, L.E.; Delgado-Murcia, L.G.; Valero-Valdivieso, M.F.; Cáez-Ramírez, G. Cytotoxic and Immunomodulatory Potential Activity of Physalis Peruviana Fruit Extracts on Cervical Cancer (HeLa) and Fibroblast (L929) Cells. J. Evid. Based Complementary Altern. Med. 2017, 22, 777–787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodríguez Ulloa, S.; Rodríguez Ulloa, E.M. Efecto de la ingesta de Physalis peruviana (aguaymanto) sobre la glicemia postprandial en adultos jóvenes. Rev. Médica Val. 2019, 4, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Cseke, L.J.; Kaufman, P.B.; Kirakosyan, A.; Brielmann, H.L.; Warber, S.; Duke, J.A. Natural Products from Plants, 2nd ed.; Taylor & Francis, CRC Press: Boca Raton, FL, USA, 2006. [Google Scholar] [CrossRef]
- Dhar, N.; Razdan, S.; Rana, S.; Bhat, W.W.; Vishwakarma, R.; Lattoo, S.K. A Decade of Molecular Understanding of Withanolide Biosynthesis and In Vitro Studies in Withania somnifera (L.) Dunal: Prospects and Perspectives for Pathway Engineering. Front. Plant Sci. 2015, 6, 1031. [Google Scholar] [CrossRef] [Green Version]
- Sharada, A.C.; Solomon, F.E.; Devi, P.U.; Udupa, N.; Srinivasan, K.K. Antitumor and Radiosensitizing Effects of Withaferin a on Mouse Ehrlich Ascites Carcinoma in Vivo. Acta Oncol. 1996, 35, 95–100. [Google Scholar] [CrossRef] [Green Version]
- Sultana, F.; Neog, M.K.; Rasool, M. Withaferin A, a steroidal lactone encapsulated mannose decorated liposomes ameliorates rheumatoid arthritis by intriguing the macrophage repolarization in adjuvant-inducedarthritic rats. Colloids Surf. B Biointerfaces 2017, 155, 349–365. [Google Scholar] [CrossRef]
- Akhoon, B.A.; Rathor, L.; Pandey, R. Withanolide A Extends the Lifespan in Human EGFR-Driven Cancerous Caenorhabditis Elegans. Exp. Gerontol. 2018, 104, 113–117. [Google Scholar] [CrossRef]
- Mukherjee, S.; Kumar, G.; Patnaik, R. Withanolide a Penetrates Brain via Intra-Nasal Administration and Exerts Neuroprotection in Cerebral Ischemia Reperfusion Injury in Mice. Xenobiotica 2020, 50, 957–966. [Google Scholar] [CrossRef]
- Ramadan, M.F. Physalis Peruviana Pomace Suppresses Highcholesterol Diet-Induced Hypercholesterolemia in Rats. Grasas Y Aceites 2012, 63, 411–422. [Google Scholar] [CrossRef]
- Kour, K.; Pandey, A.; Suri, K.A.; Satti, N.K.; Gupta, K.K.; Bani, S. Restoration of stress-induced altered T cell function and corresponding cytokines patterns by Withanolide A. Int. Immunopharmacol. 2009, 9, 1137–1144. [Google Scholar] [CrossRef]
- Rodriguez-Amaya, D.B.; Kimura, M.; Godoy, H.T.; Amaya-Farfan, J. Updated Brazilian Database on Food Carotenoids: Factors Affecting Carotenoid Composition. J. Food Compos. Anal. 2008, 21, 445–463. [Google Scholar] [CrossRef]
- Mueller, L.; Boehm, V. Antioxidant Activity of β-Carotene Compounds in Different in Vitro Assays. Molecules 2011, 16, 1055–1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gurmu, F.; Hussein, S.; Laing, M. The Potential of Orange-Fleshed Sweet Potato to Prevent Vitamin A Deficiency in Africa. Int. J. Vitam. Nutr. Res. 2014, 84, 65–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, T.; Shnimizu, M.; Moriwaki, H. Cancer Chemoprevention by Carotenoids. Molecules 2012, 17, 3202–3242. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorization. Part 2: Characterization of bioactive compounds from goldenberry (Physalis peruviana L.) calyx extracts using hyphenated techniques. J. Chromatogr. A 2019, 11, 144–154. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and Anti-Inflammatory Activities of Quercetin and Its Derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Magar, R.T.; Sohng, J.K. A Review on Structure, Modifications and Structure-Activity Relation of Quercetin and Its Derivatives. J. Microbiol. Biotechnol. 2020, 30, 11–20. [Google Scholar] [CrossRef]
- Ganeshpurkar, A.; Saluja, A.K. The Pharmacological Potential of Rutin. Saudi Pharm. J. 2017, 25, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.-R.; Khan, W.; Bakht, J.; Nair, M.G. New Antiinflammatory Sucrose Esters in the Natural Sticky Coating of Tomatillo (Physalis Philadelphica), an Important Culinary Fruit. Food Chem. 2016, 196, 726–732. [Google Scholar] [CrossRef] [Green Version]
- Bernal, C.-A.; Castellanos, L.; Aragón, D.M.; Martínez-Matamoros, D.; Jiménez, C.; Baena, Y.; Ramos, F.A. Peruvioses A to F, Sucrose Esters from the Exudate of Physalis peruviana Fruit as α-Amylase Inhibitors. Carbohydr. Res. 2018, 461, 4–10. [Google Scholar] [CrossRef]
- Huang, M.; He, J.X.; Hu, H.X.; Zhang, K.; Wang, X.N.; Zhao, B.B.; Lou, H.X.; Ren, D.M.; Shen, T. Withanolides from the genus Physalis: A review on their phytochemical and pharmacological aspects. J. Pharm. Pharmacol. 2019, 72, 649–669. [Google Scholar] [CrossRef] [PubMed]
- Wijeratne, E.M.; Xu, Y.M.; Scherz-Shouval, R.; Marron, M.T.; Rocha, D.D.; Liu, M.X.; Costa-Lotufo, L.V.; Santagata, S.; Lindquist, S.; Whitesell, L.; et al. Structure-activity relationships for withanolides as inducers of the cellular heat-shock response. J. Med. Chem. 2014, 10, 2851–2863. [Google Scholar] [CrossRef]
- Chen, L.X.; He, H.; Qiu, F. Natural withanolides: An overview. Nat. Prod. Rep. 2011, 28, 705–740. [Google Scholar] [CrossRef] [PubMed]
- Ghulam, S.M.; Ali, A.; Ali, R.A.; Yousuf, S.; Rahman, A.U.; Choudhary, M.I. Analysis and development of structure-fragmentation relationships in withanolides using an electrospray ionization quadropole time-of-flight tandem mass spectrometry hybrid instrument. Rapid Commun. Mass Spectrom. 2011, 25, 104–114. [Google Scholar] [CrossRef]
- Dong, B.; An, L.; Yang, X.; Zhang, X.; Zhang, J.; Tuerhong, M.; Jin, D.Q.; Ohizumi, Y.; Lee, D.; Xu, J.; et al. Withanolides from Physalis peruviana showing nitric oxide inhibitory effects and affinities with iNOS. Bioorg. Chem. 2019, 87, 585–593. [Google Scholar] [CrossRef]
- Maldonado, E.; Amador, S.; Martínez, M.; Pérez-Castorena, A.L. Virginols A–C, three new withanolides from Physalis virginiana. Steroids 2010, 75, 346–349. [Google Scholar] [CrossRef]
- Chang, L.C.; Sang-Ngern, M.; Pezzuto, J.M.; Ma, C. The Daniel K. Inouye College of Pharmacy Scripts: Poha Berry (Physalis peruviana) with Potential Anti-inflammatory and Cancer Prevention Activities. Hawaii J. Med. Public Health 2016, 75, 353–359. [Google Scholar]
- Ahmad, S.; Malik, A.; Afza, N.; Yasmin, R. A new withanolide glycoside from Physalis peruviana. J. Nat. Prod. 1999, 62, 493–494. [Google Scholar] [CrossRef]
- Yang, S.H.; Liu, Y.; Wang, Q.; Sun, Y.P.; Guan, W.; Liu, Y.; Yang, B.Y.; Kuang, H.X. UPLC-MS/MS Identification and Quantification of Withanolides from Six Parts of the Medicinal Plant Datura metel L. Molecules 2020, 25, 1260. [Google Scholar] [CrossRef] [Green Version]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of Flavone, Flavonol, and Flavanone Aglycones by Negative Ion Liquid Chromatography Electrospray Ion Trap Mass Spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef] [Green Version]
- Fathoni, A.; Saepudin, E.; Cahyana, A.H.; Rahayu, D.U.C.; Haib, J. Identification of nonvolatile compounds in clove (Syzygium aromaticum) from Manado. AIP Conf. Proc. 2017, 1862, 030079. [Google Scholar] [CrossRef] [Green Version]
- Justesen, U. Collision-induced fragmentation of deprotonated methoxylated flavonoids, obtained by electrospray ionization mass spectrometry. J. Mass Spectrom. 2001, 36, 169–178. [Google Scholar] [CrossRef] [PubMed]
- Chagas, V.T.; de Coelho, R.M.R.S.; Gaspar, R.S.; da Silva, S.A.; Mastrogiovanni, M.; de Mendonça, C.J.; de Ribeiro, M.N.S.; de Paes, A.M.A.; Trostchansky, A. Protective Effects of a Polyphenol-Rich Extract from Syzygium cumini (L.) Skeels Leaf on Oxidative Stress-Induced Diabetic Rats. Oxid. Med. Cell. Longev. 2018, 2018, 5386079:1–5386079:13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- March, R.E.; Miao, X.S. A fragmentation study of a kaempferol using electrospray quadrupole time-of-flight mass spectrometry at high mass resolution. Int. J. Mass Spectrom. 2004, 231, 157–167. [Google Scholar] [CrossRef]
- Olivares-Tenorio, M.L.; Dekker, M.; Verkerk, R.; van Boekel, M.A.J.S. Health-promoting compounds in cape gooseberry (Physalis peruviana L.): Review from a supply chain perspective. Trends Food Sci. Technol. 2016, 57, 83–92. [Google Scholar] [CrossRef]
- Rene, C.; Zelada, E.; Ureña, M.O.; Ritva, P.; Carrasco, R. Determinacion de compuestos bioactivos del Aguaymato (Physalis peruviana, Linnaeus, 1753) y de su conserva en almíbar maximizando la retención de ácido ascórbico. Rev. ECIPeru 2019, 4, 6–10. [Google Scholar] [CrossRef]
- Carrasco, R.R. De Determinación De La Capacidad Antioxidante y Compuestos Bioactivos De Frutas Nativas Peruanas. Rev. Soc. Química Perú 2008, 74, 108–124. [Google Scholar]
- Mier, H.; Cáez, G. Contenido de polifenoles, carotenos y capacidad antioxidante en frutos de uchuva (Physalis peruviana) en relación a su estado de maduración. ReCiTelA 2011, 11, 103–115. [Google Scholar]
- Bazalar Pereda, M.S.; Nazareno, M.A.; Viturro, C.I. Nutritional and Antioxidant Properties of Physalis peruviana L. Fruits from the Argentinean Northern Andean Region. Plant Foods Hum. Nutr. 2019, 74, 68–75. [Google Scholar] [CrossRef]
- Xu, Y.; Gong, F.; Dixon, S.J.; Brereton, R.G.; Soini, H.A.; Novotny, M.V.; Penn, D.J. Application of Dissimilarity Indices, Principal Coordinates Analysis, and Rank Tests to Peak Tables in Metabolomics of the Gas Chromatography/Mass Spectrometry of Human Sweat. Anal. Chem. 2007, 79, 5633–5641. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I.; Safwat, G.; Gamal, M.; Megahed, B.M.H. Chemical Composition and Biological Activity of Physalis peruviana L. Gesunde Pflanz. 2019, 71, 113–122. [Google Scholar] [CrossRef]
- Licodiedidoff, S.; Koslowski, L.A.D.; Ribani, R.H. Flavonóis e atividade antioxidante do fruto Physalis peruviana L. em dois estádios de maturação. Acta Sci. Technol. 2013, 35, 393–399. [Google Scholar] [CrossRef] [Green Version]
- Cardona, M.; Toro, M.; Costa, G.; Ospina, L.; Castellanos, L.; Ramos, F.; Aragón, D.M. Influence of extraction process on antioxidant activity and rutin content in Physalis peruviana calyces extract. J. Appl. Pharm. Sci. 2017, 7, 164–168. [Google Scholar] [CrossRef] [Green Version]
- Medina, S.; Collado-González, J.; Ferreres, F.; Londoño-Londoño, J.; Jiménez-Cartagena, C.; Guy, A.; Durand, T.; Galano, J.M.; Gil-Izquierdo, Á. Potential of Physalis peruviana calyces as a low-cost valuable resource of phytoprostanes and phenolic compounds. J. Sci. Food Agric. 2019, 99, 2194–2204. [Google Scholar] [CrossRef]
- Gironés-Vilaplana, A.; Baenas, N.; Villaño, D.; Speisky, H.; García-Viguera, C.; Moreno, D.A. Evaluation of Latin-American fruits rich in phytochemicals with biological effects. J. Funct. Foods 2014, 7, 599–608. [Google Scholar] [CrossRef]
- Platzer, M.; Kiese, S.; Herfellner, T.; Schweiggert-Weisz, U.; Eisner, P. How Does the Phenol Structure Influence the Results of the Folin-Ciocalteu Assay? Antioxidants 2021, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Luaces, P.; Pascual, M.; Pérez, A.G.; Sanz, C. An Easy-to-Use Procedure for the Measurement of Total Phenolic Compounds in Olive Fruit. Antioxidants 2021, 10, 1656. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu regent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Vega-Gálvez, A.; López, J.; Torres-Ossandón, M.J.; Galotto, M.J.; Puente-Díaz, L.; Quispe-Fuentes, I.; Di Scala, K. High hydrostatic pressure effect on chemical composition, color, phenolic acids and antioxidant capacity of Cape gooseberry pulp (Physalis peruviana L.). LWT Food Sci. Technol. 2014, 58, 519–526. [Google Scholar] [CrossRef]
- Valdenegro, M.; Fuentes, L.; Herrera, R.; Moya-León, M.A. Changes in antioxidant capacity during development and ripening of goldenberry (Physalis peruviana L.) fruit and in response to 1-methylcyclopropene treatment. Postharvest Biol. Technol. 2012, 67, 110–117. [Google Scholar] [CrossRef]
- Muñoz, P.; Parra, F.; Simirgiotis, M.J.; Sepúlveda Chavera, G.F.; Parra, C. Chemical Characterization, Nutritional and Bioactive Properties of Physalis peruviana Fruit from High Areas of the Atacama Desert. Foods 2021, 10, 2699. [Google Scholar] [CrossRef] [PubMed]
- Rockenbach, I.; Rogrigues, E.; Ciriele, C.; Gonzaga, L.; Lima, A.; Mancini, J.; Fett, R. Acidos fenólicos e atividade antioxidante em fruto de Physalis peruviana L. Alim. Nutr. 2009, 19, 271–276. Available online: https://www.researchgate.net/publication/49599931 (accessed on 31 January 2022).
- Bravo, K.; Sepulveda-Ortega, S.; Lara-Guzman, O.; Navas-Arboleda, A.A.; Osorio, E. Influence of Cultivar and Ripening Time on Bioactive Compounds and Antioxidant Properties in Cape Gooseberry (Physalis peruviana L.). J. Sci. Food Agric. 2014, 95, 1562–1569. [Google Scholar] [CrossRef] [PubMed]
- Ballesteros-Vivas, D.; Álvarez-Rivera, G.; Sanchez-Camargo, A.; Ibáñez, E.; Parada-Alfonso, F.; Cifuentes, A. A multi-analytical platform based on pressurized-liquid extraction, in vitro assays and liquid chromatography/gas chromatography coupled to high resolution mass spectrometry for food by-products valorisation. Part 1: Withanolides-rich fractions from goldenberry (Physalis peruviana L.) calyces obtained after extraction optimization as case study. J. Chromatogr. A 2019, 1584, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Lizcano, L.J.; Bakkali, F.; Ruiz-Larrea, B.; Ruiz-Sanz, J.I. Antioxidant activity and polyphenol content of aqueous extracts from Colombia Amazonian plants with medicinal use. Food Chem. 2010, 119, 1566–1570. [Google Scholar] [CrossRef]
- Aryal, S.; Baniya, M.K.; Danekhu, K.; Kunwar, P.; Gurung, R.; Koirala, N. Total Phenolic content, Flavonoid content and antioxidant potential of wild vegetables from western Nepal. Plants 2019, 8, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niki, E. Antioxidant Capacity: Which Capacity and How to Assess It? J. Berry Res. 2011, 1, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Foti, M.; Daquino, C.; Geraci, C. Electron-Transfer Reaction of Cinnamic Acids and Their Methyl Esters with the DPPH• Radical in Alcoholic Solutions. J. Org. Chem. 2004, 69, 2309–2314. [Google Scholar] [CrossRef]
- Gomes, S.M.C.; Ghica, M.E.; Rodrigues, I.A.; De Souza Gil, E.; Oliveira-Brett, A.M. Flavonoids electrochemical detection in fruit extracts and total antioxidant capacity evaluation. Talanta 2016, 154, 284–291. [Google Scholar] [CrossRef] [PubMed]
- Izli, N.; Yildiz, G.; Ünal, H.; Işik, E.; Uylaşer, V. Effect of different drying methods on drying characteristics, colour, total phenolic content and antioxidant capacity of Goldenberry (Physalis peruviana L.). Int. J. Food Sci. Technol. 2014, 49, 9–17. [Google Scholar] [CrossRef]
- Namiesnik, J.; Vearasilp, K.; Leontowicz, H.; Leontowicz, M.; Ham, K.-S.; Kang, S.-G.; Park, Y.-K.; Arancibia-Avila, P.; Toledo, F.; Gorinstein, S. Comparative assessment of two extraction procedures for determination of bioactive compounds in some berries used for daily food consumption. Int. J. Food Sci. Technol. 2014, 49, 337–346. [Google Scholar] [CrossRef]
- Castro, J.; Ocampo, Y.; Franco, L. Cape gooseberry [Physalis peruviana L.] calyces ameliorate TNBS acid-induced colitis in rats. J. Crohn’s Colitis 2015, 9, 1004–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutière, P.; Woillez, M.; Mérillon, J.M. Comparative Study of Antioxidant Properties and Total Phenolic Content of 30 Plant Extracts of Industrial Interest Using DPPH, ABTS, FRAP, SOD, and ORAC Assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef]
- Liu, D.; Shi, J.; Colina Ibarra, A.; Kakuda, Y.; Jun Xue, S. The scavenging capacity and synergistic effects of lycopene, vitamin E, vitamin C, and β-carotene mixtures on the DPPH free radical. LWT Food Sci. Technol. 2008, 41, 1344–1349. [Google Scholar] [CrossRef]
- Quirós-Fallas, M.I.; Vargas-Huertas, F.; Quesada-Mora, S.; Azofeifa-Cordero, G.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A.; Navarro-Hoyos, M. Polyphenolic HRMS Characterization, Contents and Antioxidant Activity of Curcuma longa Rhizomes from Costa Rica. Antioxidants 2022, 11, 620. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Hoyos, M.; Acuña-Quirós, M.; Quirós-Fallas, M.I.; Vargas-Huertas, F.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A. UPLC-HRMS Polyphenolic Characterization, Contents and Antioxidant Activity of Zingiber officinale Roscoe rhizomes from Costa Rica. Processes 2022, 10, 691. [Google Scholar] [CrossRef]
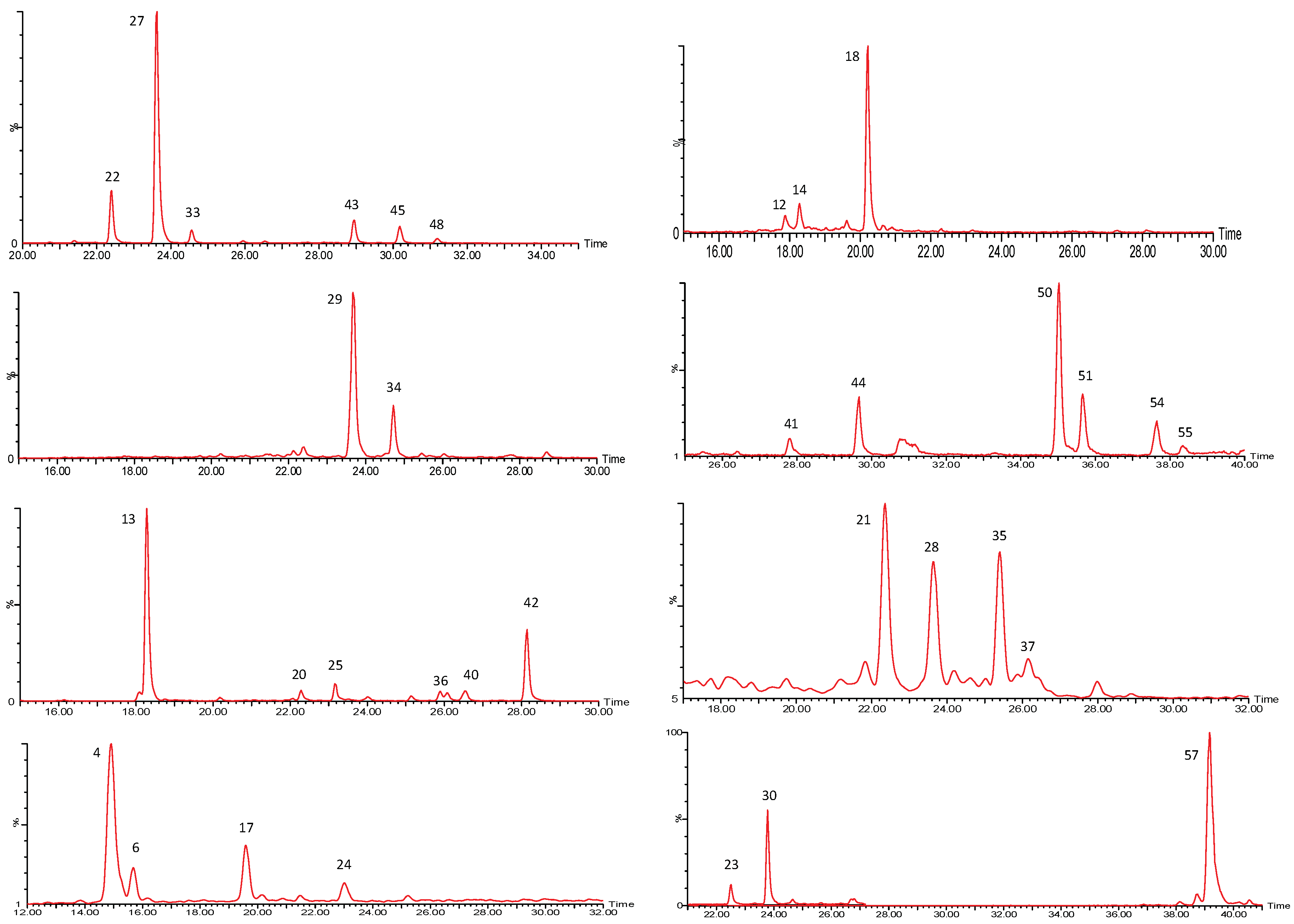

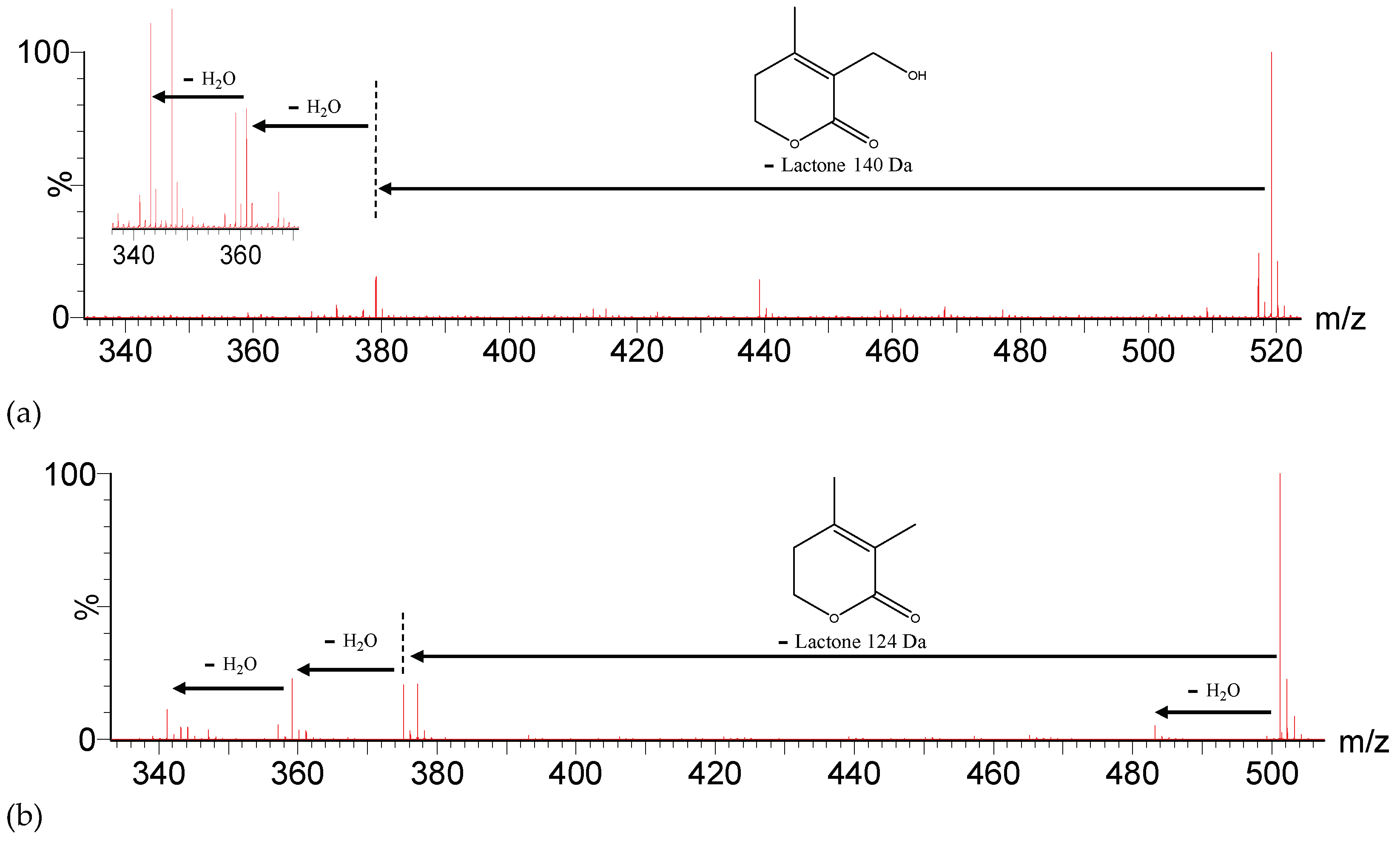


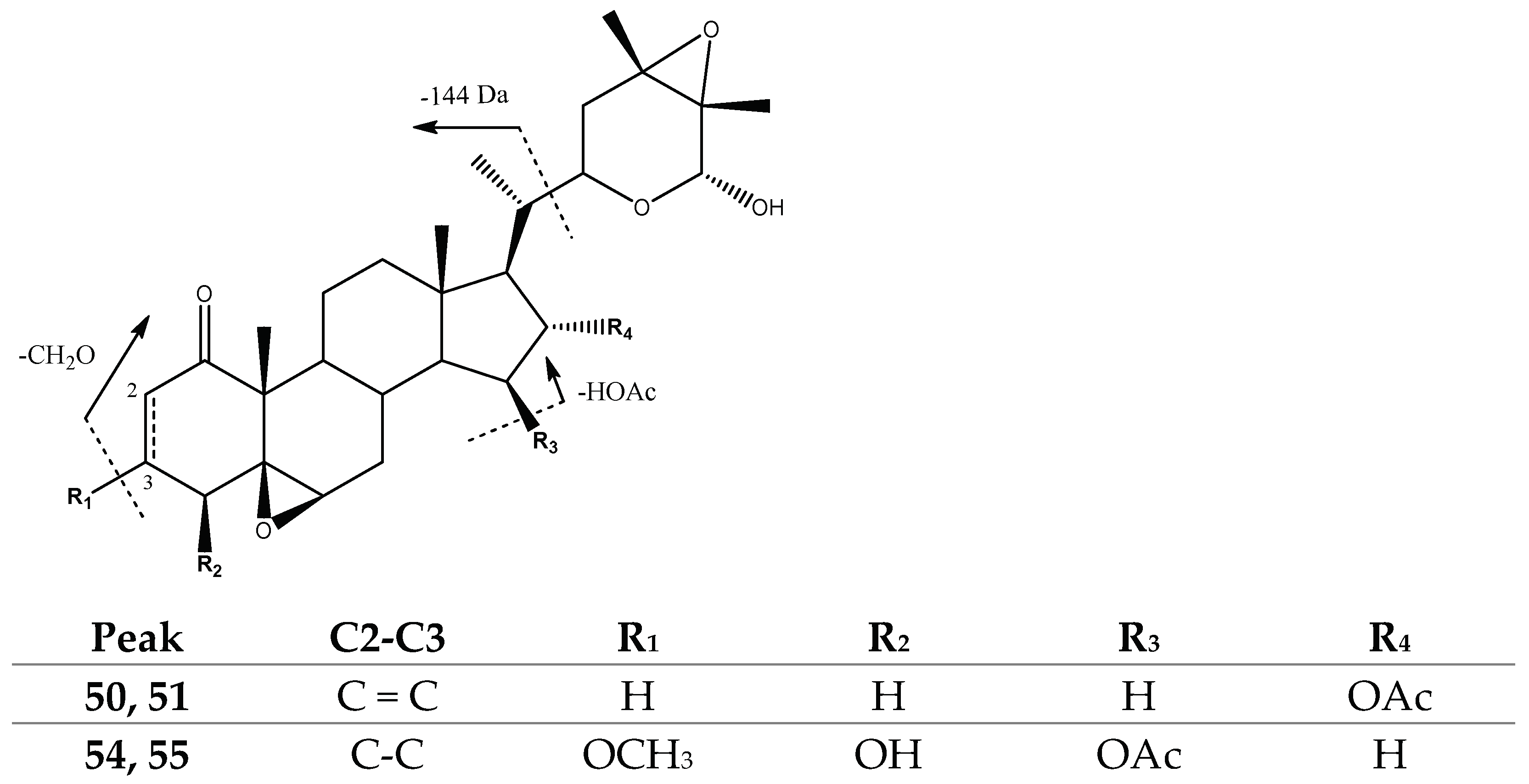
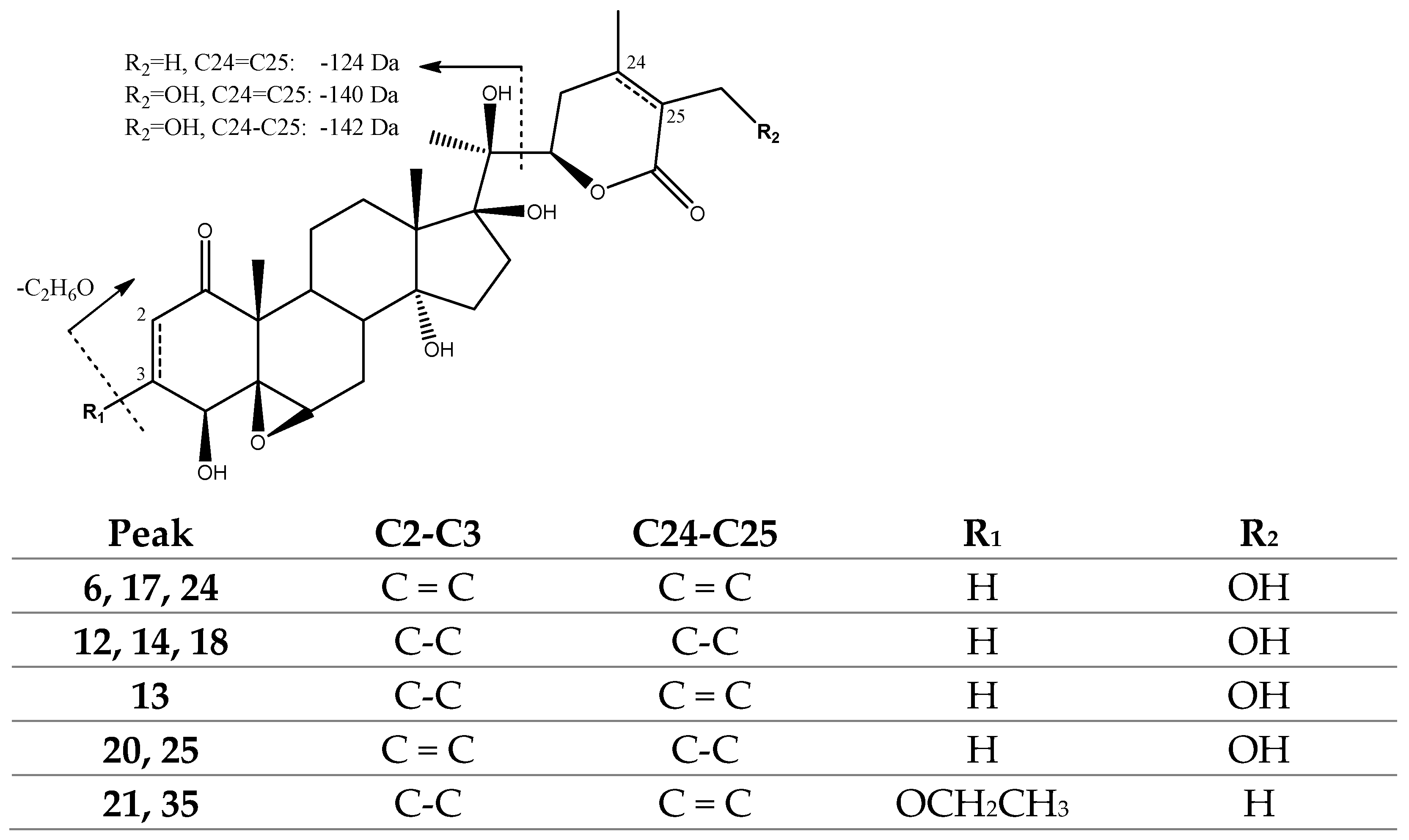

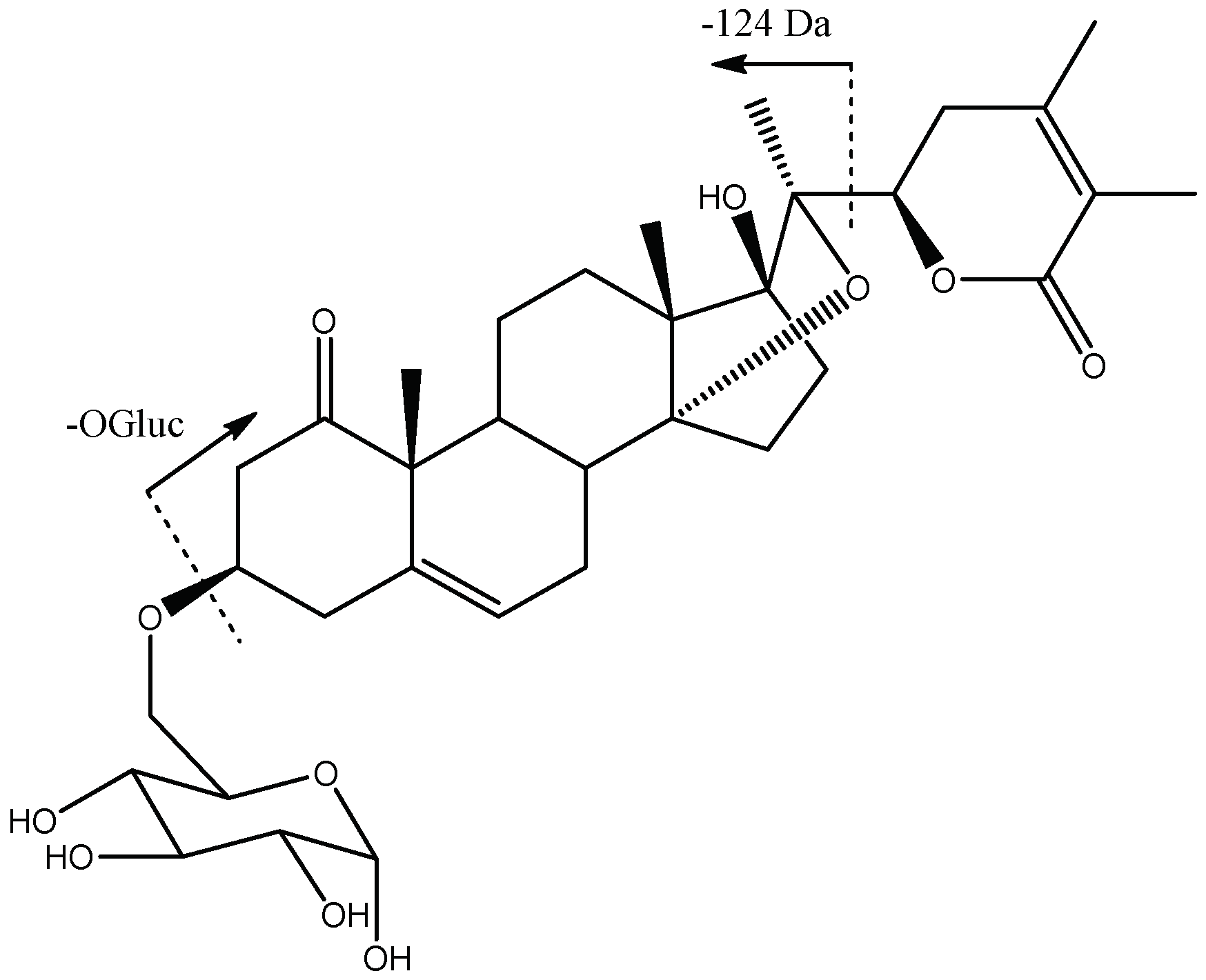
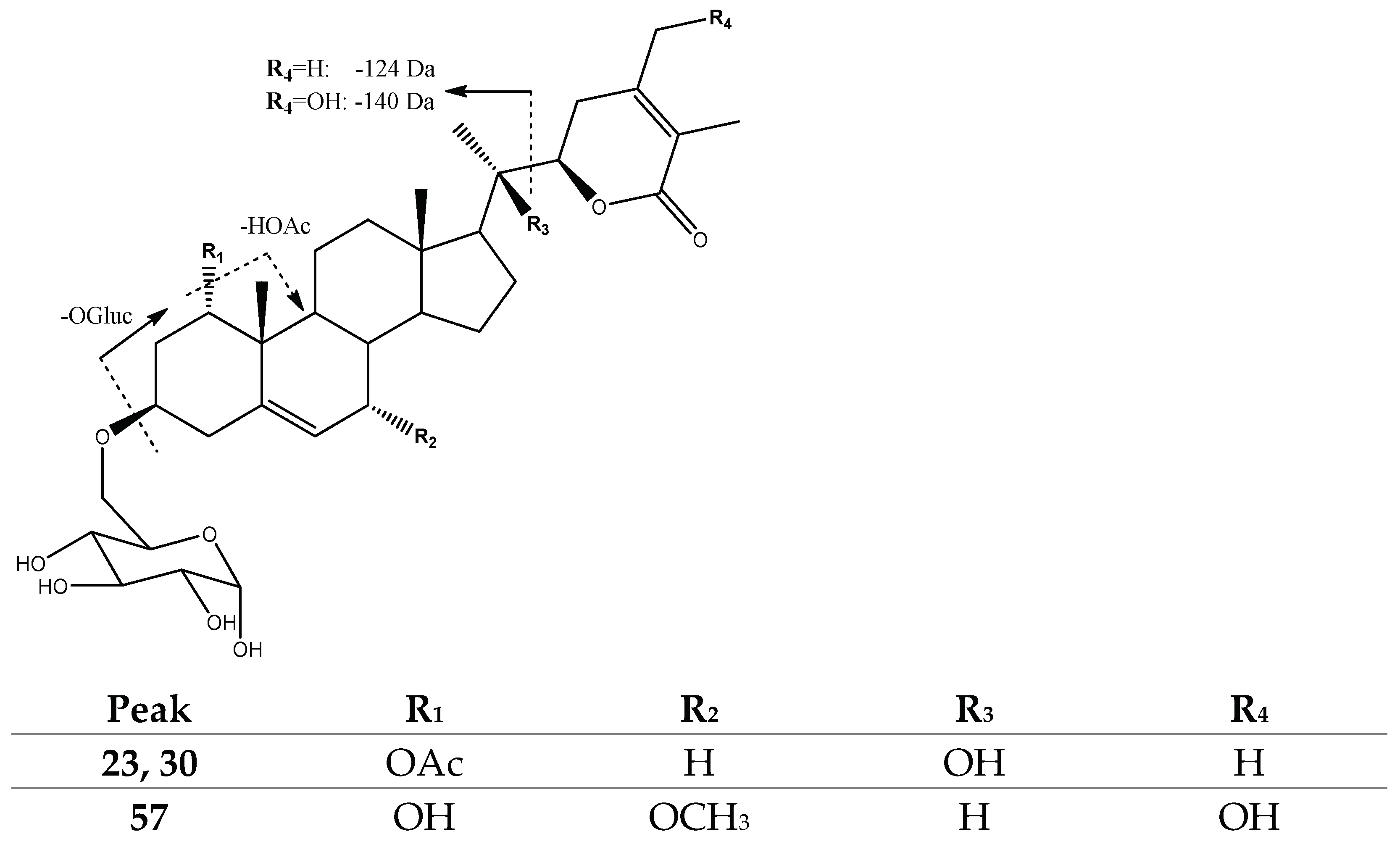
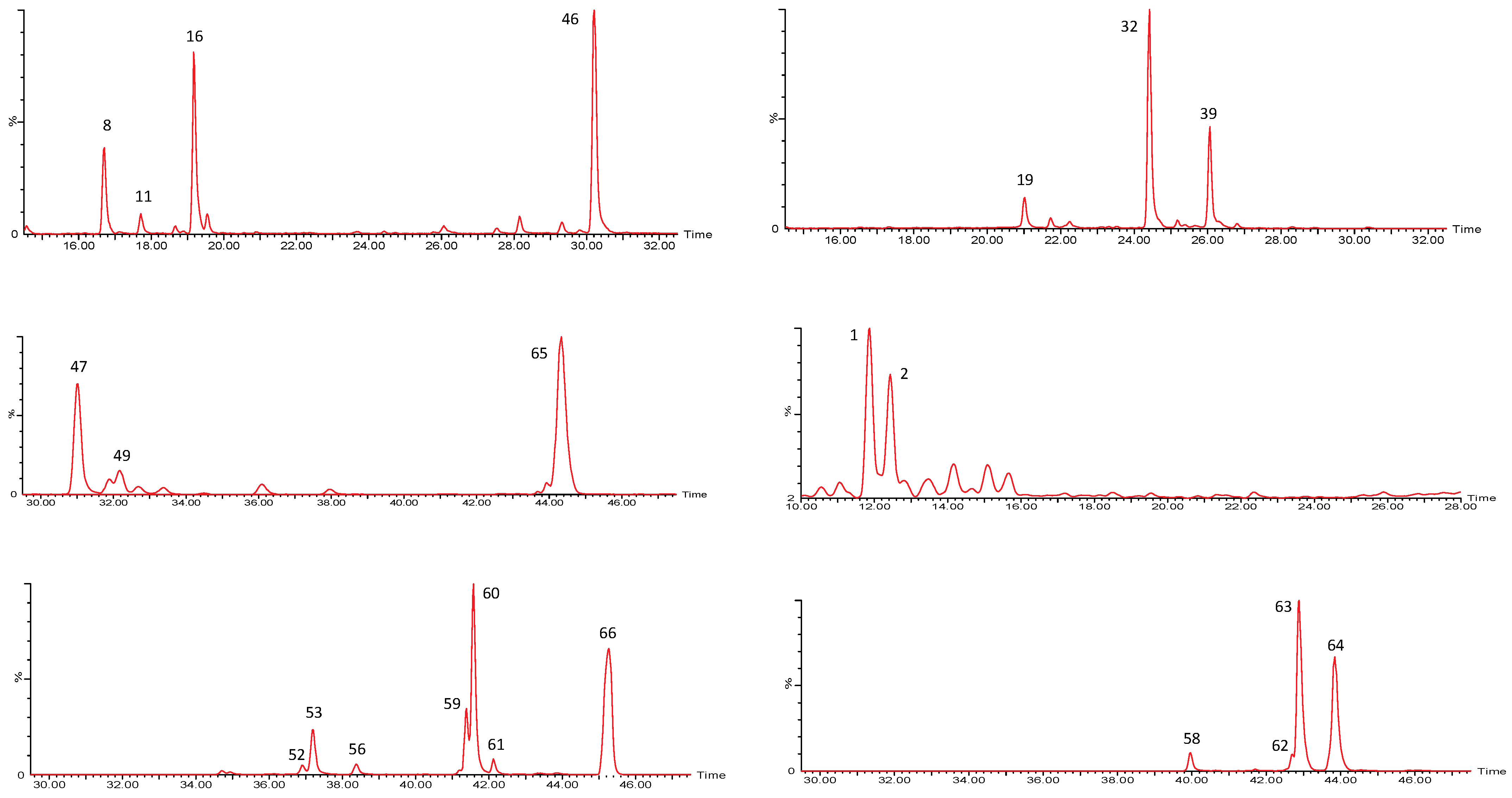
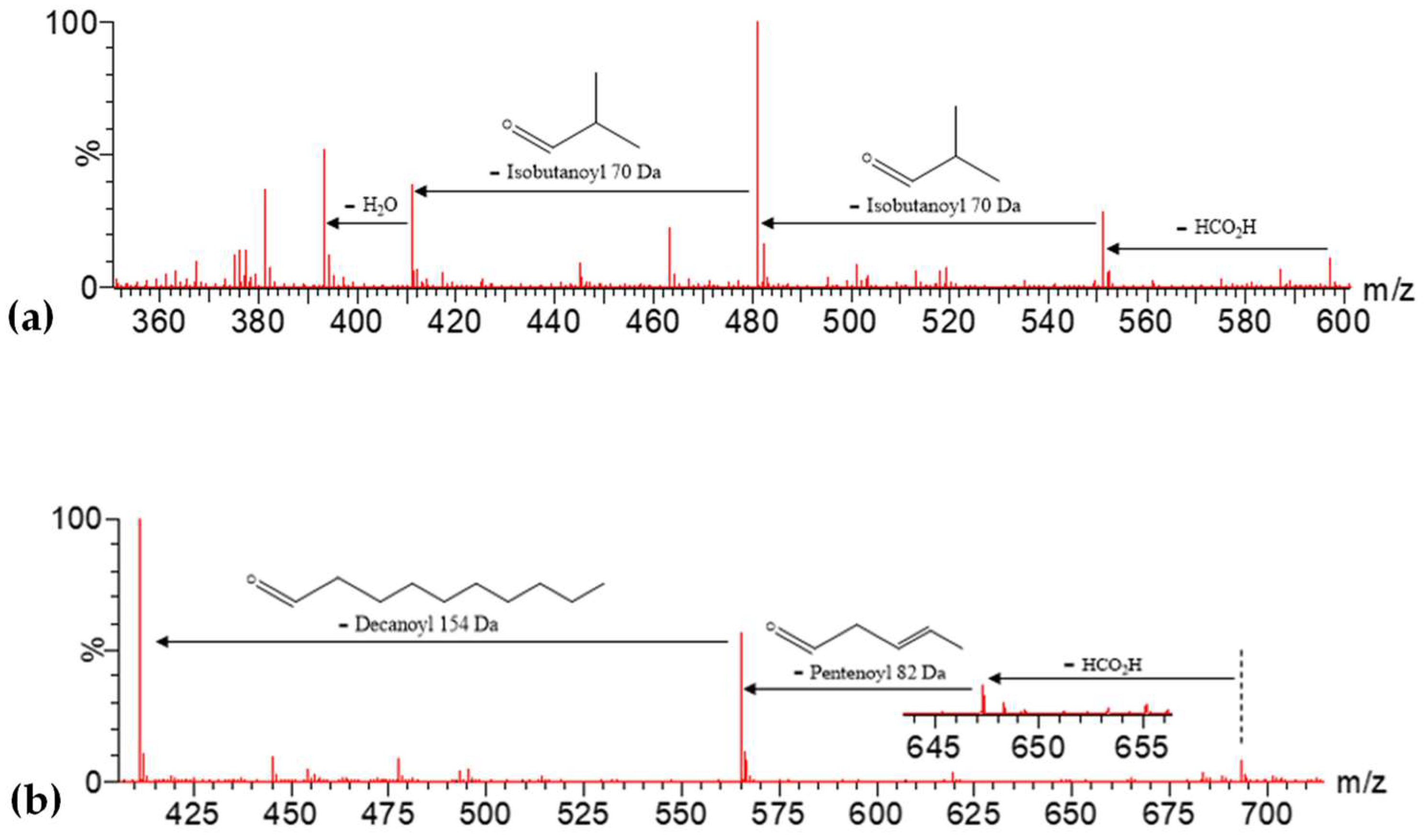


| Peak No. | [M-H]− | Lactone Moiety (Da) | [Precursor] -Lac (m/z) | Other Fragments |
|---|---|---|---|---|
| 4 | 543.2612 | 124 | [543]: 419 | 483[543-HOAc]−,359[483-Lac]−, 341[359-H2O]−, 323 [341-H2O]− |
| 6, 17, 24 | 517.2471 | 140 | [517]: 377 | 499[517-H2O]−, 359[377-H2O]−, 341[359-H2O]− |
| 12, 14, 18 | 521.2756 | 142 | [521]: 379 | 503[521-H2O]−, 361[379-H2O]−, 343[361-H2O]− |
| 13 | 519.2601 | 140 | [519]: 379 | 501[519-H2O]−, 361[379-H2O]−, 343[361-H2O]− |
| 20, 25 | 519.2601 | 142 | [519]: 377 | 501[519-H2O]−, 359[377-H2O]−, 341[359-H2O]− |
| 21, 35 | 547.2926 | 124 | [465]: 341 | 501[547-EtOH]−, 483[501-H2O]−, 465[483-H2O]− |
| 22 | 501.2494 | 140 | [501]: 361 | 483[501-H2O]−, 343[361-H2O]−, 325[341-H2O]− |
| 23, 30 | 661.3590 | 124 | [481]: 357 | 601[647-HOAc]−, 481[661-OGluc]−, 339[357-H2O]− |
| 27,33 | 501.2494 | 124 | [501]: 377 | 483[501-H2O]−, 359[377-H2O]−, 341[359-H2O]− |
| 28 | 631.3137 | 124 | [433]: 309 | 631[613-H2O]−, 451[631-OGluc]−, 433[451-H2O]− |
| 29, 34 | 503.2650 | 142 | [503]: 361 | 485[503-H2O]−, 343[361-H2O]−, 325[343-H2O]− |
| 36, 40, 42 | 485.2550 | 124 | [485]: 361 | 467[485-H2O]−, 343[361-H2O]− |
| 37, 43, 45 | 487.2701 | 142 | [487]: 345 | 469[487-H2O]−, 327[345-H2O]− |
| 41, 44 | 471.2725 | 158 | [471]: 313 | 453[471-H2O]−, 435[453-H2O]− |
| 48 | 469.2607 | 124 | [469]: 345 | 451[469-H2O]−, 327 [345-H2O]− |
| 50, 51 | 513.2831 | 144 | [453]: 309 | 495[513-H2O]−, 453[513-HOAc]− |
| 54, 55 | 561.3042 | 144 | [471]: 327 | 531[561-CH2O]−, 471[531-HOAc]−, 453[471-H2O]− |
| 57 | 649.3606 | 140 | [469]: 329 | 631[649-H2O]−, 469[649-OGluc]−, |
| Peak No. | [M-H +FA]− | [M-H]− (m/z) | R group (Da) | [M-H-R]− (m/z) | Other Fragments (m/z) |
|---|---|---|---|---|---|
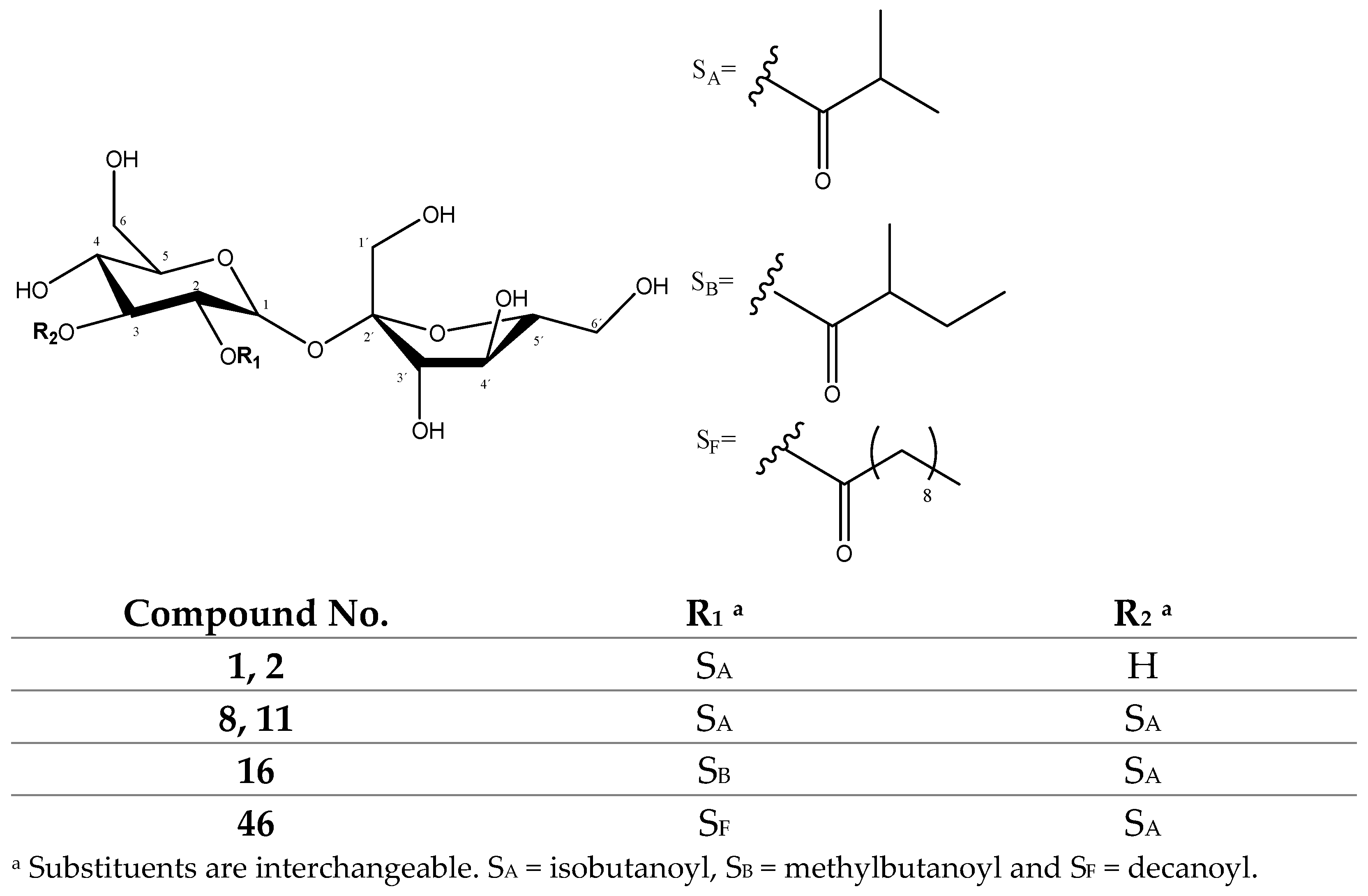
| 1, 2 | 457.1558 | 411 | 70 | 341 | 323[Suc-H-H2O]−, 181[Suc-H-Gluc]− |
| 8, 11 | 527.1980 | 481 | 70 | 411 | 393[411-H2O]−, 323[Suc-H-H2O]− |
| 16 | 541.2151 | 495 | 84 | 411 | 393[411-H2O]−, 323[Suc-H-H2O]−, 161[Suc-H-OGluc]− |
| 19 | 609.2408 | 563 | 82 | 481 | 411[481-70]−, 393[411-H2O]− |
| 32 | 597.2380 | 551 | 70 | 481 | 411[481-70]−, 393[411-H2O]− |
| 39 | 623.2604 | 577 | 84 | 493 | 411[493-82]−, 323[Suc-H-H2O]− |
| 46 | 611.2938 | 565 | 154 | 411 | 495[565-70]−, 323[Suc-H-H2O]− |
| 47 | 653.3061 | 607 | 126 | 481 | 537[607-70]−, 393[481-70-H2O]− |
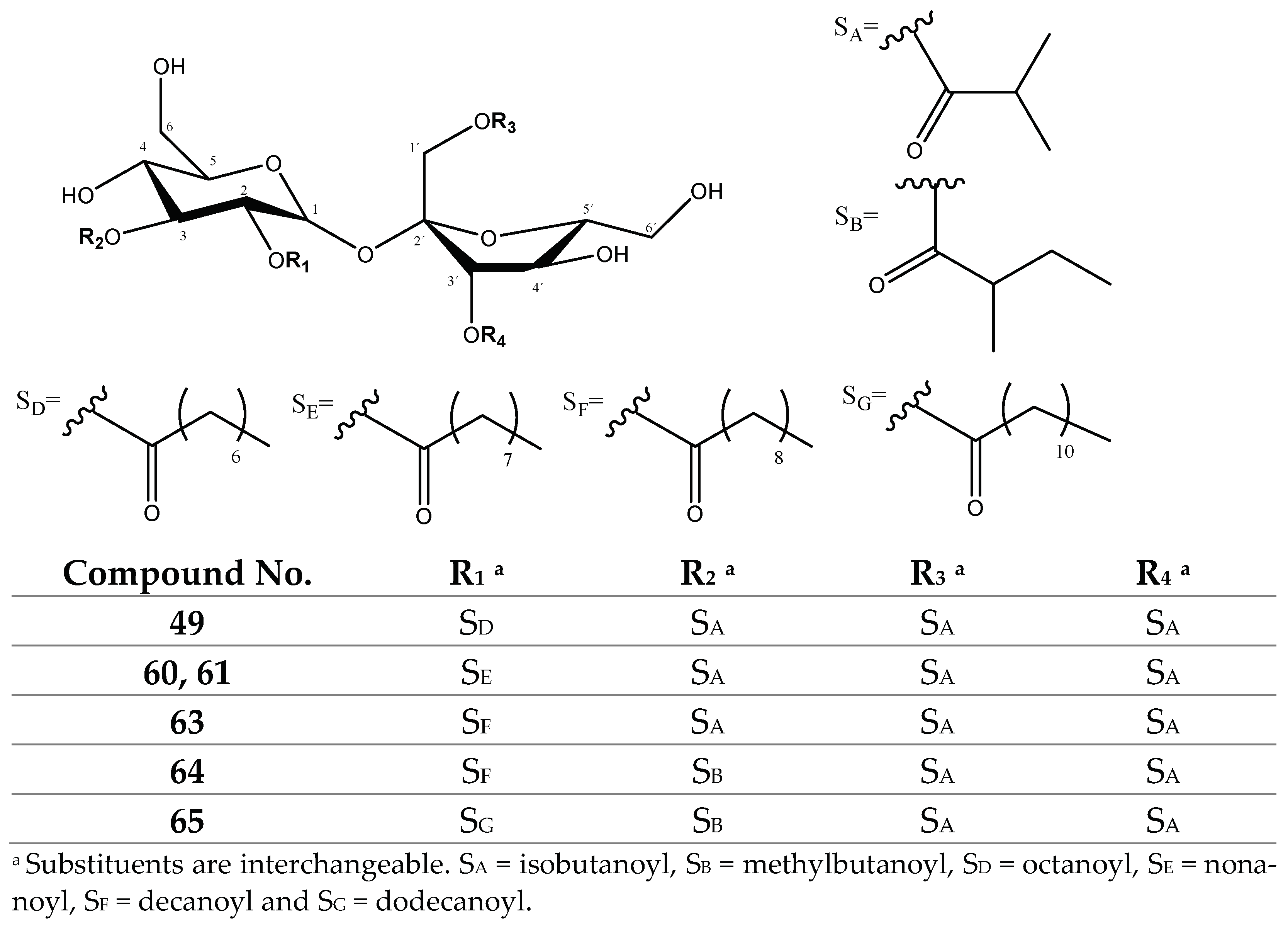
| 49 | 723.3472 | 677 | 126 | 551 | 607[677-70]−, 481[551-70]− |
| 52, 58, 62 | 693.3303 | 647 | 82 | 565 | 493 [647-154]−, 411[493-82]− |
| 53 | 667.3208 | 621 | 140 | 481 | 551[621-70]−, 411 [481-70]− |
| 56, 59 | 681.3366 | 635 | 154 | 481 | 411 [481-70]−, 393[411-H2O]− |
| 60, 61 | 737.3571 | 691 | 140 | 551 | 621[691-70]−, 481 [551-70]−, 393[481-70-H2O]− |
| 63 | 751.3787 | 705 | 154 | 551 | 635[705-70]−, 481 [551-70]−, 393[481-70-H2O]− |
| 64 | 765.3963 | 719 | 154 | 565 | 635[719-84]−, 481 [565-84]−, 393[481-70-H2O]− |
| 65 | 793.4285 | 747 | 182 | 565 | 663[747-84]−, 481 [565-84]−, 393[481-70-H2O]− |
| 66 | 779.4506 | 733 | 182 | 551 | 593[733-140]−, 411 [481-70]− |
| Peak No. | [M-H]− | Glycoside group (Da) | Aglycone (m/z) | Other Fragments (m/z) |
|---|---|---|---|---|
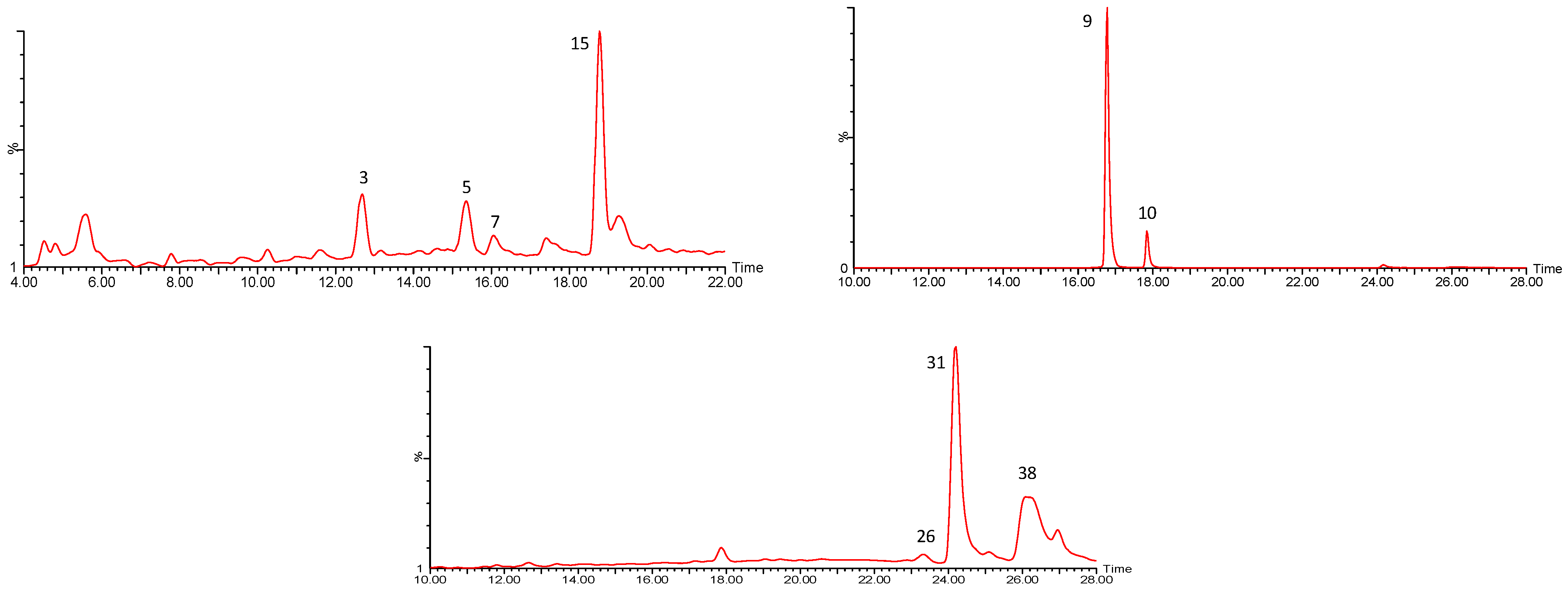
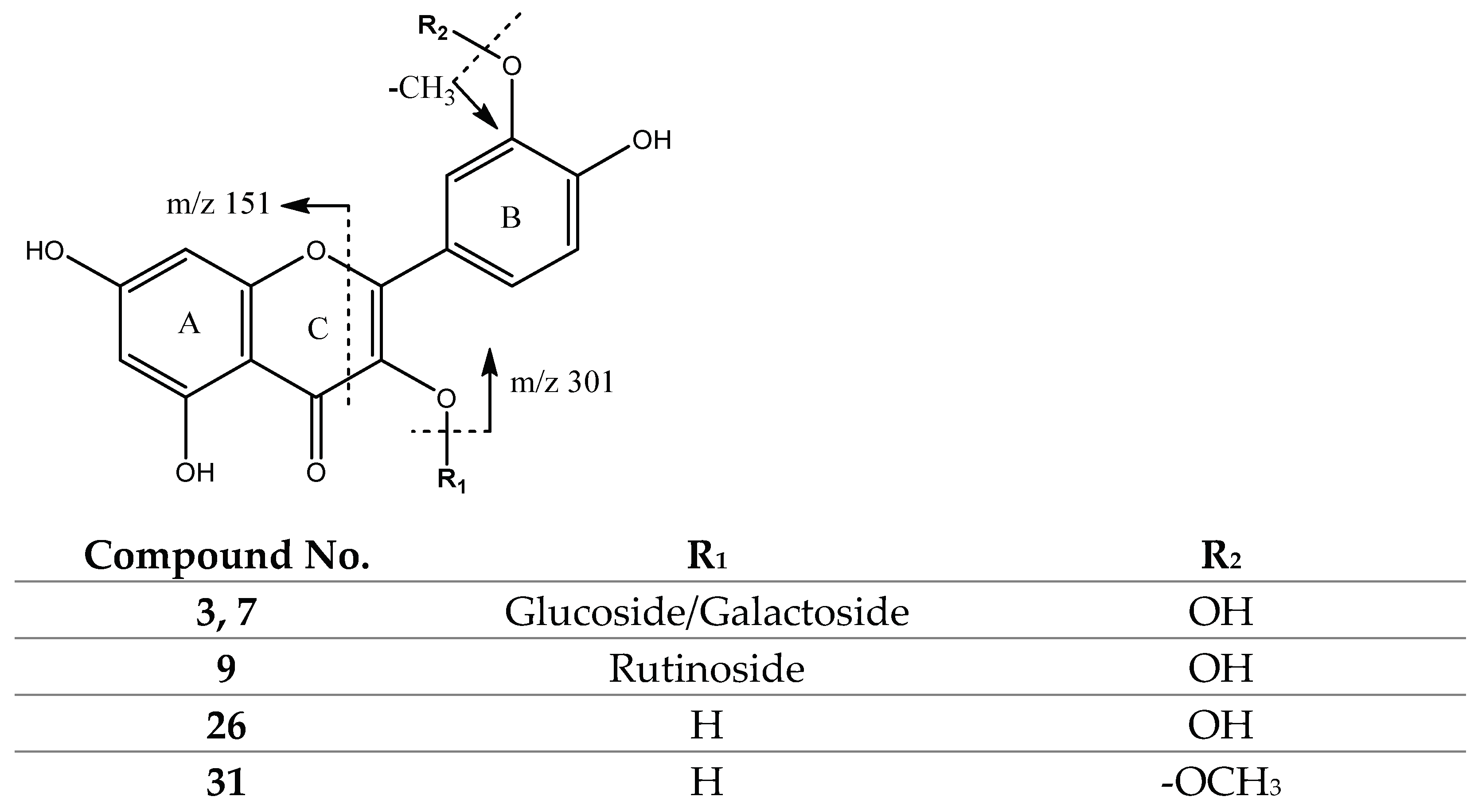
| 3,7 | 463.0894 | Glucose/Galactose (162) | 301 | |
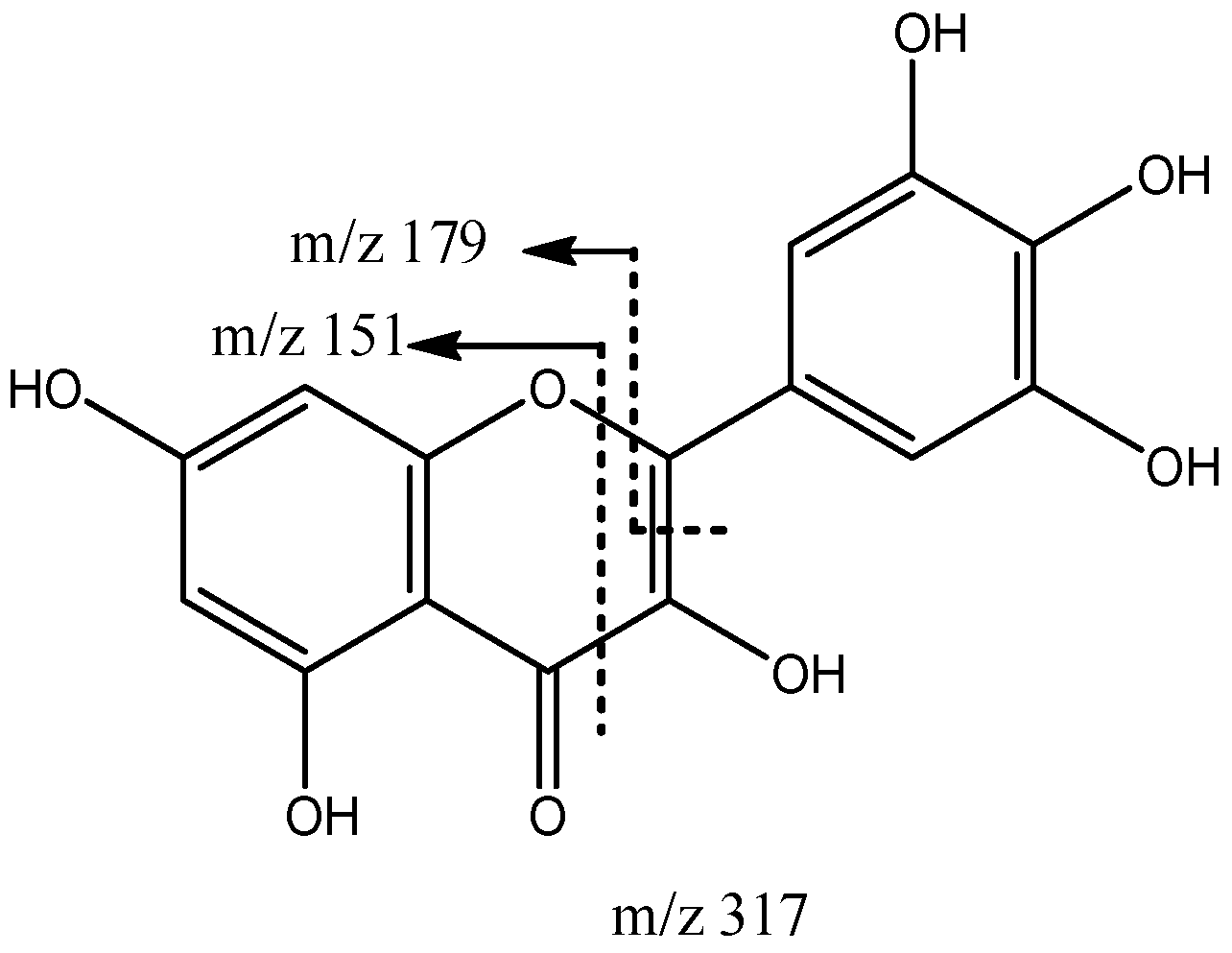
| 5 | 317.0305 | 317 | 151, 179 | |
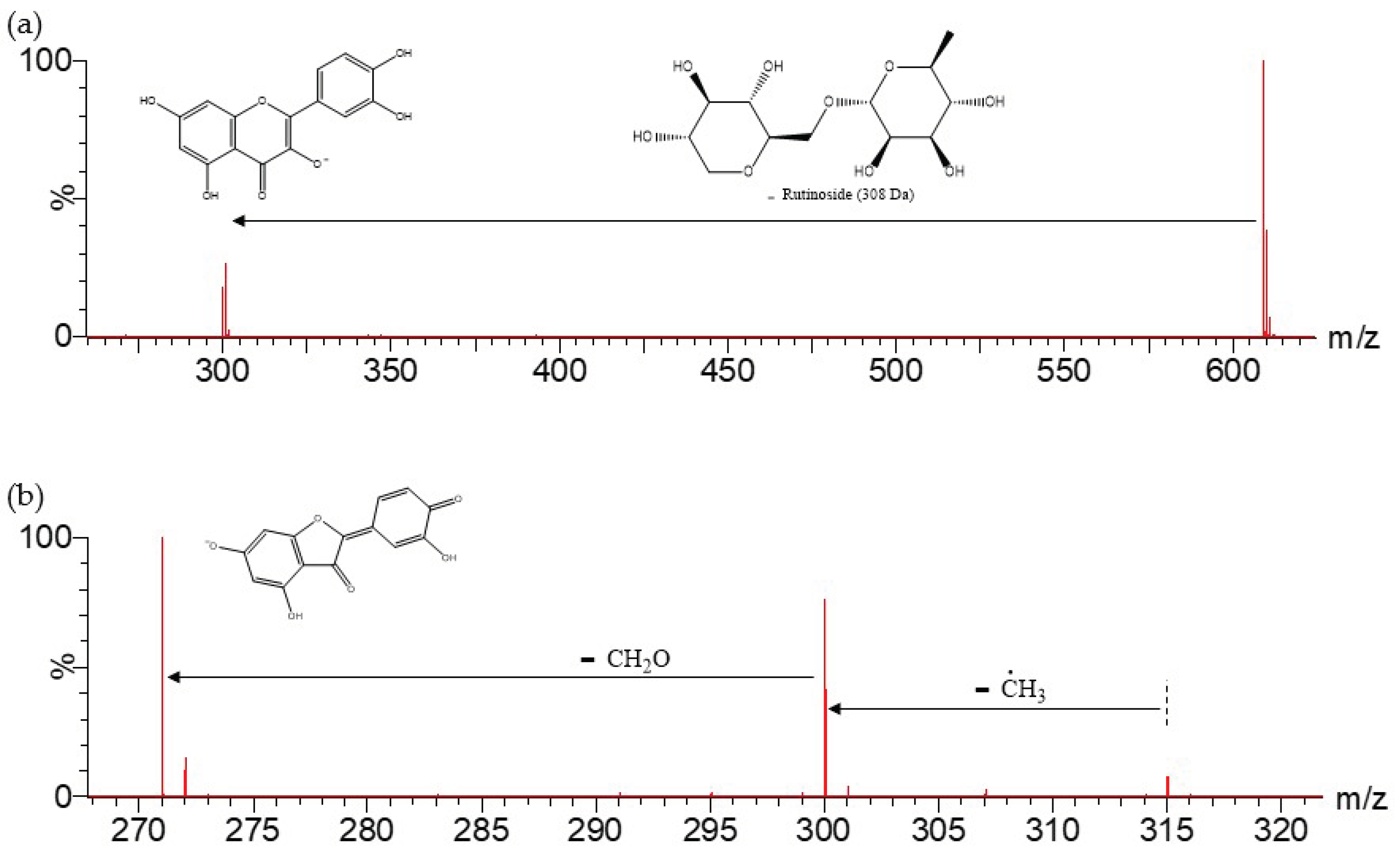
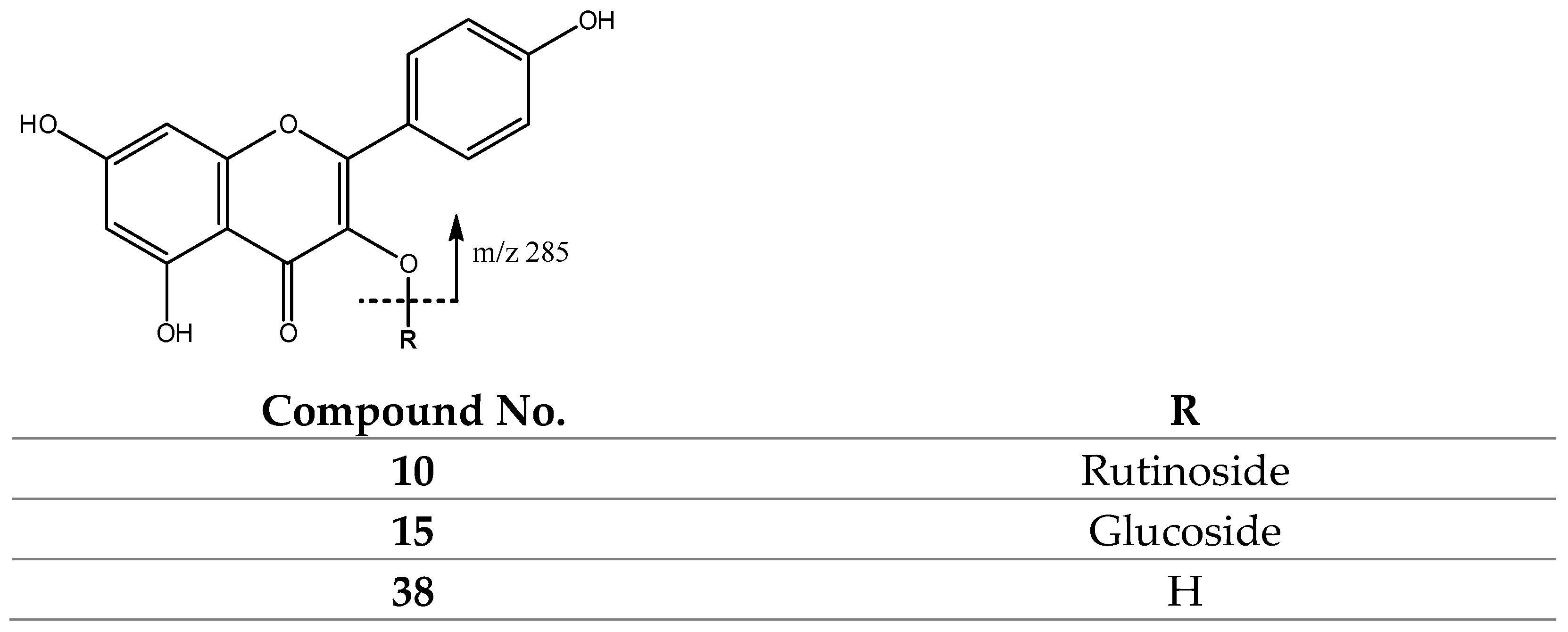
| 9 | 609.1442 | Rutinoside (308) | 301 | |
| 10 | 593.1504 | Rutinoside (308) | 285 | |
| 15 | 447.0947 | Glucose/Galactose (162) | 285 | |
| 26 | 301.0362 | 301 | 243, 163, 151 | |
| 31 | 315.0504 | 315 | 300, 271 | |
| 38 | 285.0410 | 285 | 257, 255, 243 |
| Product | β-Carotene (μg/g) 1,2,3 |
|---|---|
| D1-f | 60.10 a ± 0.08 |
| D2-f | 53.67 d ± 0.09 |
| D3-f | 58.60 b ± 0.38 |
| P1-f | 55.90 c ± 1.01 |
| P2-f | 43.00 e ± 0.21 |
| Product 1 | Quercetin Glucoside/Galactoside (QG) (mg/g) 2,3,4 | Product 1 | Quercetin Glucoside/Galactoside (QG) (mg/g) 2,3,4 | Quercetin (QC) (mg/g) 2,3,4 | Rutin (RU) (mg/g) 2,3,4 |
|---|---|---|---|---|---|
| D1-f | 0.25 a ± 0.02 | D1-h | 0.58 a ± 0.05 | 0.21 a ± 0.02 | 0.75 b ± 0.01 |
| D2-f | 0.15 b ± 0.01 | D2-h | 0.64 a ± 0.03 | 0.23 a ± 0.02 | 1.96 a ± 0.04 |
| D3-f | 0.09 d ± 0.00 | D3-h | 0.33 b ± 0.01 | 0.22 a ± 0.01 | 0.74 b ± 0.04 |
| P1-f | 0.11 c ± 0.01 | P1-h | 0.19 c ± 0.01 | 0.22 a ± 0.01 | 0.15 c ± 0.01 |
| P2-f | 0.10 cd ± 0.00 | P2-h | 0.04 d ± 0.00 | 0.05 b ± 0.00 | 0.14 c ± 0.01 |
| Product | FC (mg GAE/g) 1,2,3 | Product | FC (mg GAE/g) 1,2,3 |
|---|---|---|---|
| D1-f | 2.60 a ± 0.01 | D1-h | 4.28 b ± 0.08 |
| D2-f | 1.89 b ± 0.03 | D2-h | 5.12 a ± 0.12 |
| D3-f | 1.74 b ± 0.03 | D3-h | 2.59 c ± 0.07 |
| P1-f | 1.88 b ± 0.08 | P1-h | 1.14 d ± 0.01 |
| P2-f | 1.51 c ± 0.10 | P2-h | 0.84 e ± 0.01 |
| Product | IC50 (mg/mL) 1,2,3 | Product | IC50 (mg/mL) 1,2,3 |
|---|---|---|---|
| D1-f | 2.60 e ± 0.01 | D1-h | 2.25 d ± 0.02 |
| D2-f | 3.20 d ± 0.04 | D2-h | 1.62 e ± 0.09 |
| D3-f | 4.49 a ± 0.21 | D3-h | 3.19 c ± 0.14 |
| P1-f | 3.24 c ± 0.10 | P1-h | 3.98 b ± 0.16 |
| P2-f | 4.33 b ± 0.23 | P2-h | 4.72 a ± 0.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Navarro-Hoyos, M.; Arnáez-Serrano, E.; Quirós-Fallas, M.I.; Vargas-Huertas, F.; Wilhelm-Romero, K.; Vásquez-Castro, F.; Alvarado-Corella, D.; Sánchez-Kopper, A. QTOF-ESI MS Characterization and Antioxidant Activity of Physalis peruviana L. (Cape Gooseberry) Husks and Fruits from Costa Rica. Molecules 2022, 27, 4238. https://doi.org/10.3390/molecules27134238
Navarro-Hoyos M, Arnáez-Serrano E, Quirós-Fallas MI, Vargas-Huertas F, Wilhelm-Romero K, Vásquez-Castro F, Alvarado-Corella D, Sánchez-Kopper A. QTOF-ESI MS Characterization and Antioxidant Activity of Physalis peruviana L. (Cape Gooseberry) Husks and Fruits from Costa Rica. Molecules. 2022; 27(13):4238. https://doi.org/10.3390/molecules27134238
Chicago/Turabian StyleNavarro-Hoyos, Mirtha, Elizabeth Arnáez-Serrano, María Isabel Quirós-Fallas, Felipe Vargas-Huertas, Krissia Wilhelm-Romero, Felipe Vásquez-Castro, Diego Alvarado-Corella, and Andrés Sánchez-Kopper. 2022. "QTOF-ESI MS Characterization and Antioxidant Activity of Physalis peruviana L. (Cape Gooseberry) Husks and Fruits from Costa Rica" Molecules 27, no. 13: 4238. https://doi.org/10.3390/molecules27134238







