Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure
Abstract
:1. Introduction
2. Results and Discussion
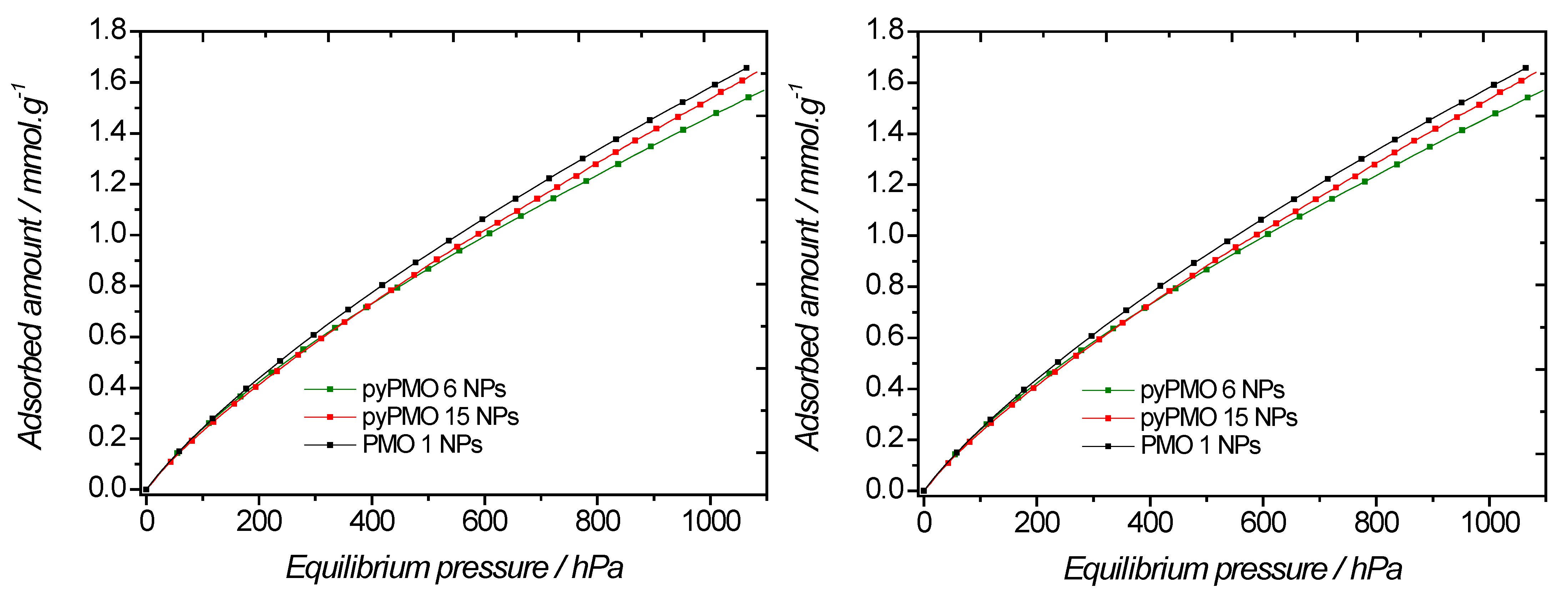
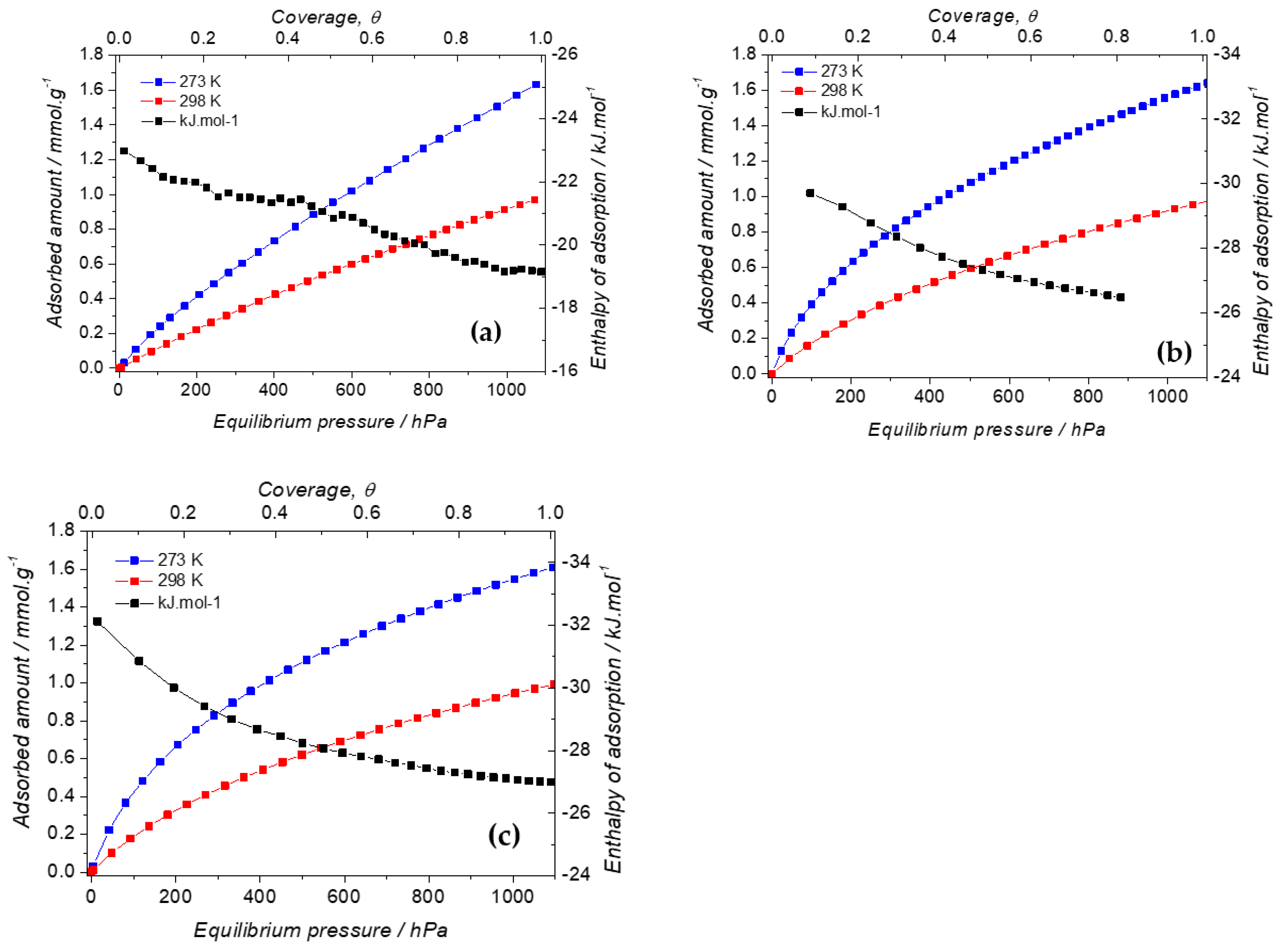
2.1. Effect of the Aminopyridine Moiety on the CO2 Adsorption
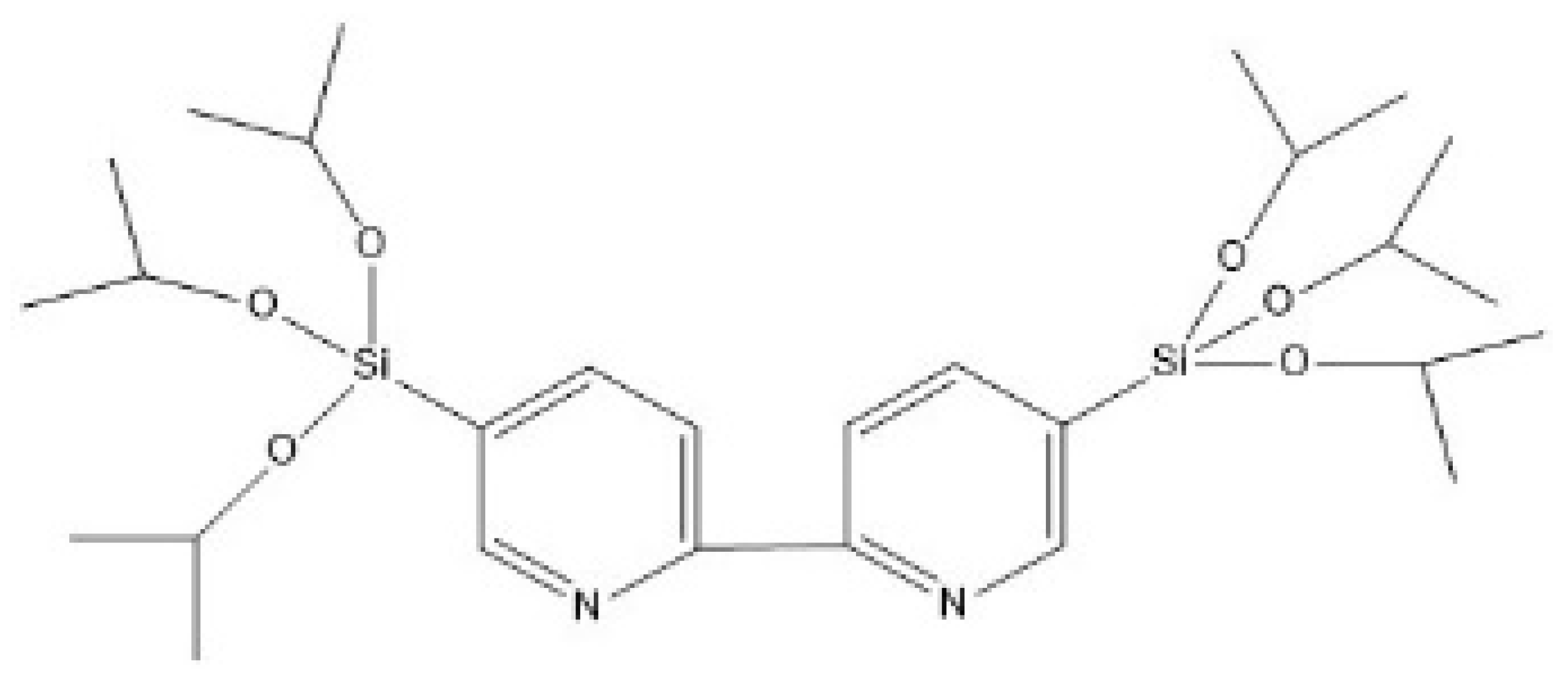
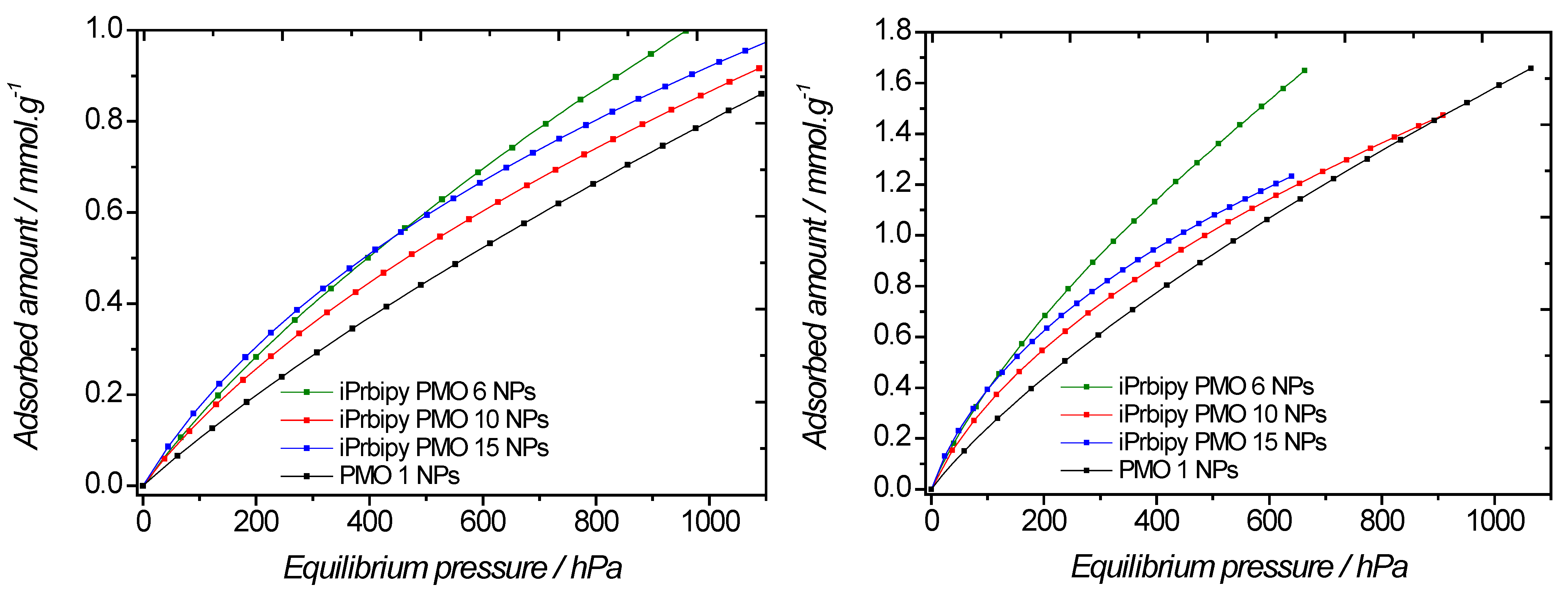
2.2. Effect of the iPrbipyridine Moiety on the CO2 Adsorption
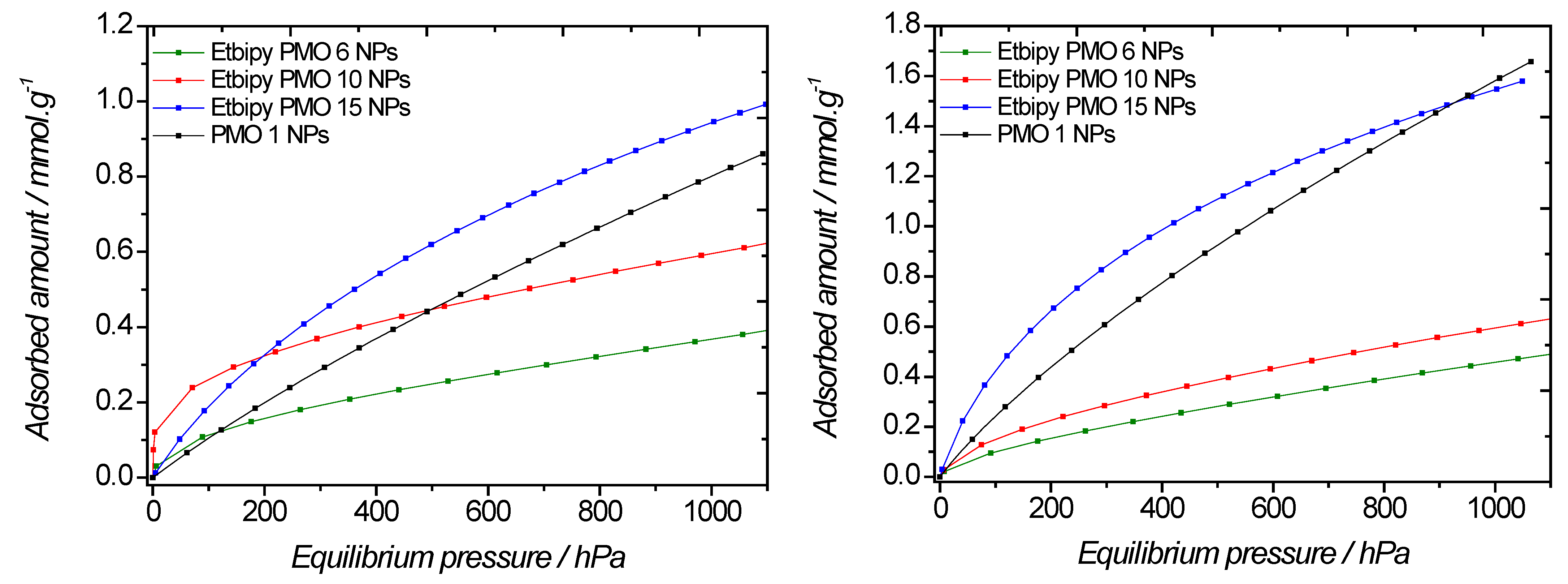
2.3. Effect of the Etbipyridine Moiety on the CO2 Adsorption
2.4. Selection of the Best PMOs for the CO2 Capture
- iPrbipyPMO 6 (94% BTEE/6% iPrbipyridine): 1.04 mmol·g−1;
- EtbipyPMO 15 (85% BTEE/15% Etbipyridine): 0.95 mmol·g−1;
- pyPMO 15 (85% BTEE/15% aminopyridine): 0.92 mmol·g−1.
3. Materials and Methods
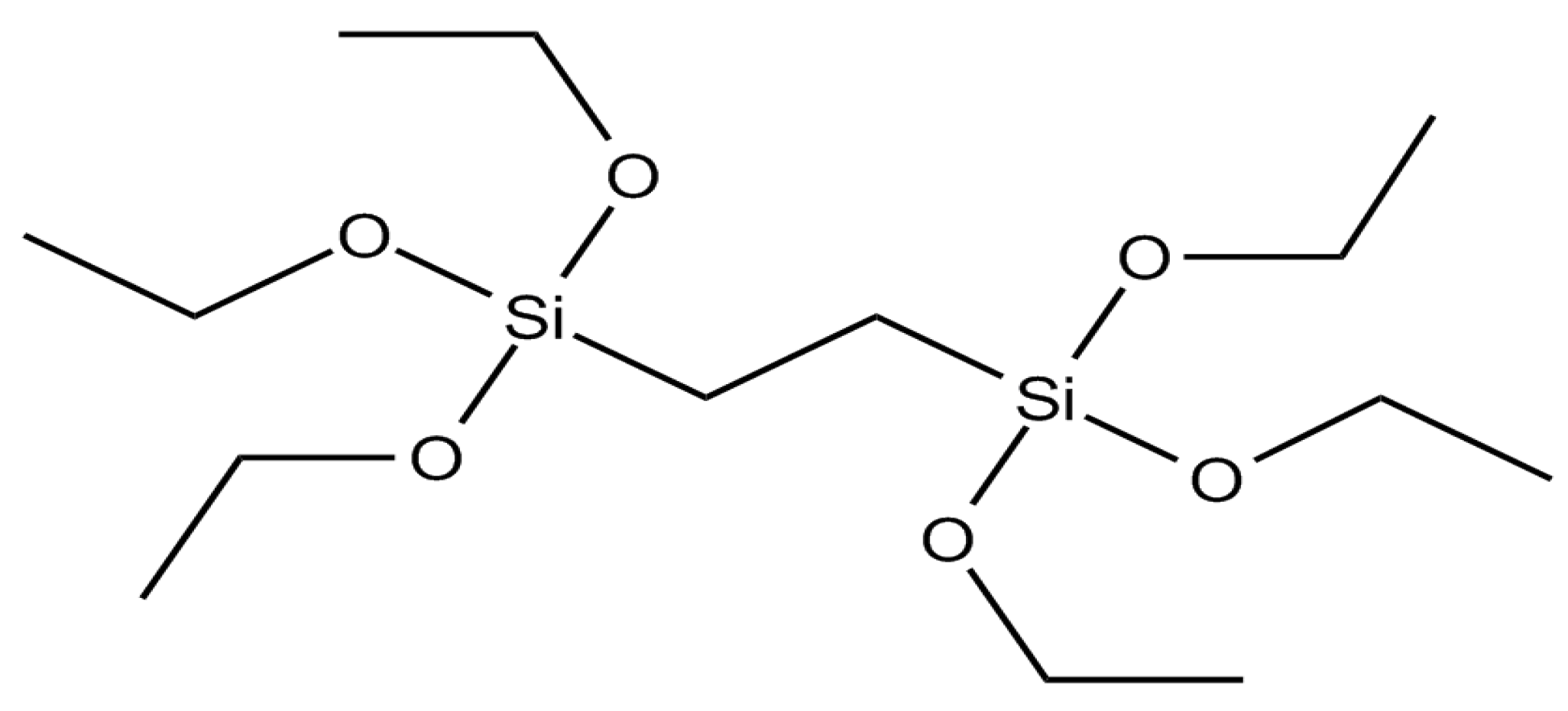
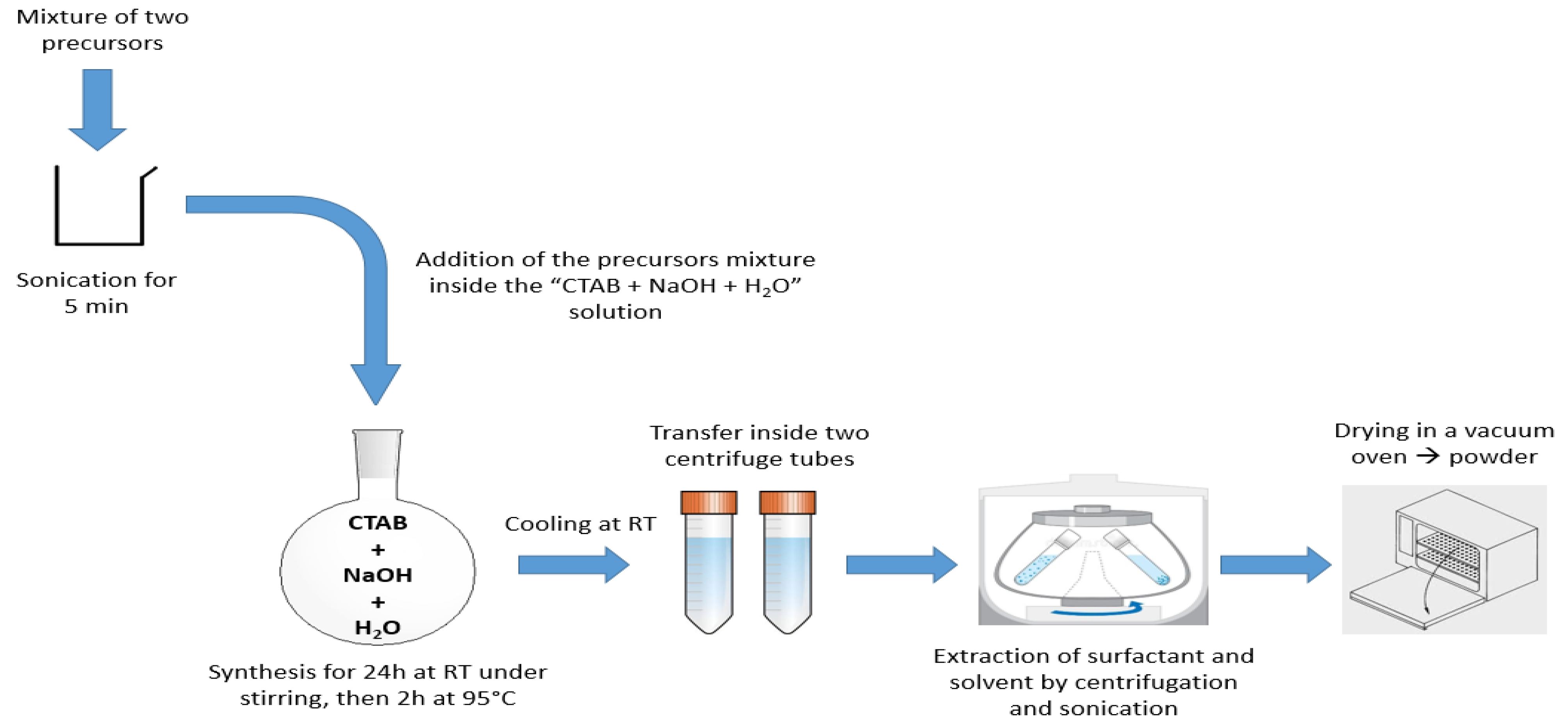
3.1. Synthesis of BTEENPs
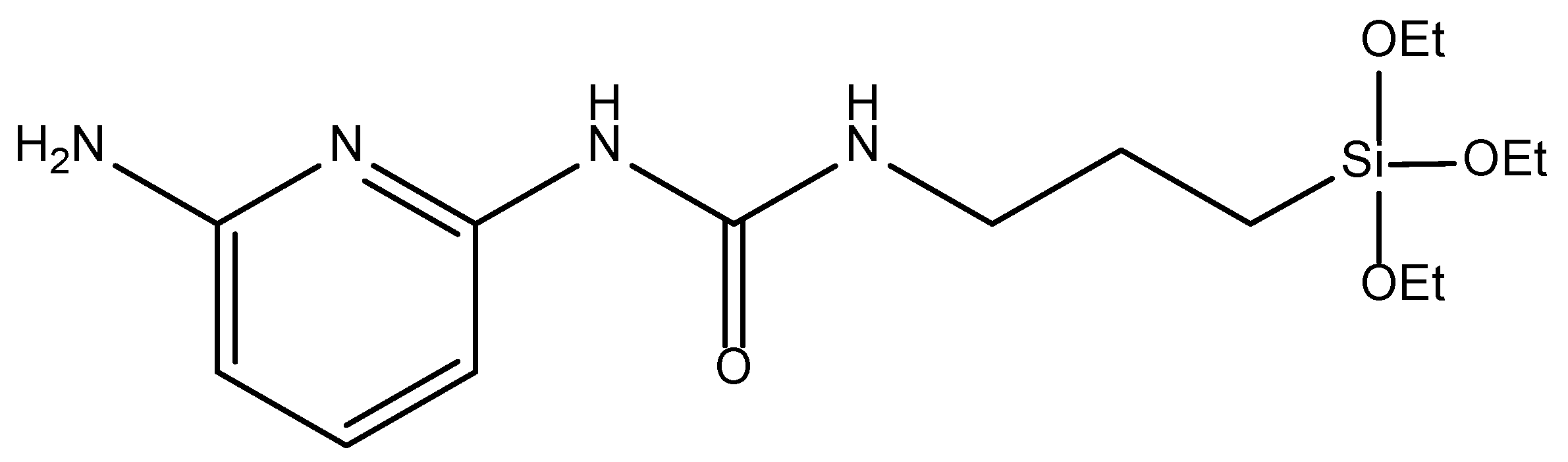
3.2. Synthesis of BTEE-Aminopyridinenps/BTEE-iPrbipyridineNPs/BTEE-EtbipyridineNPs
3.3. DLS Analysis
3.4. FTIR Analysis
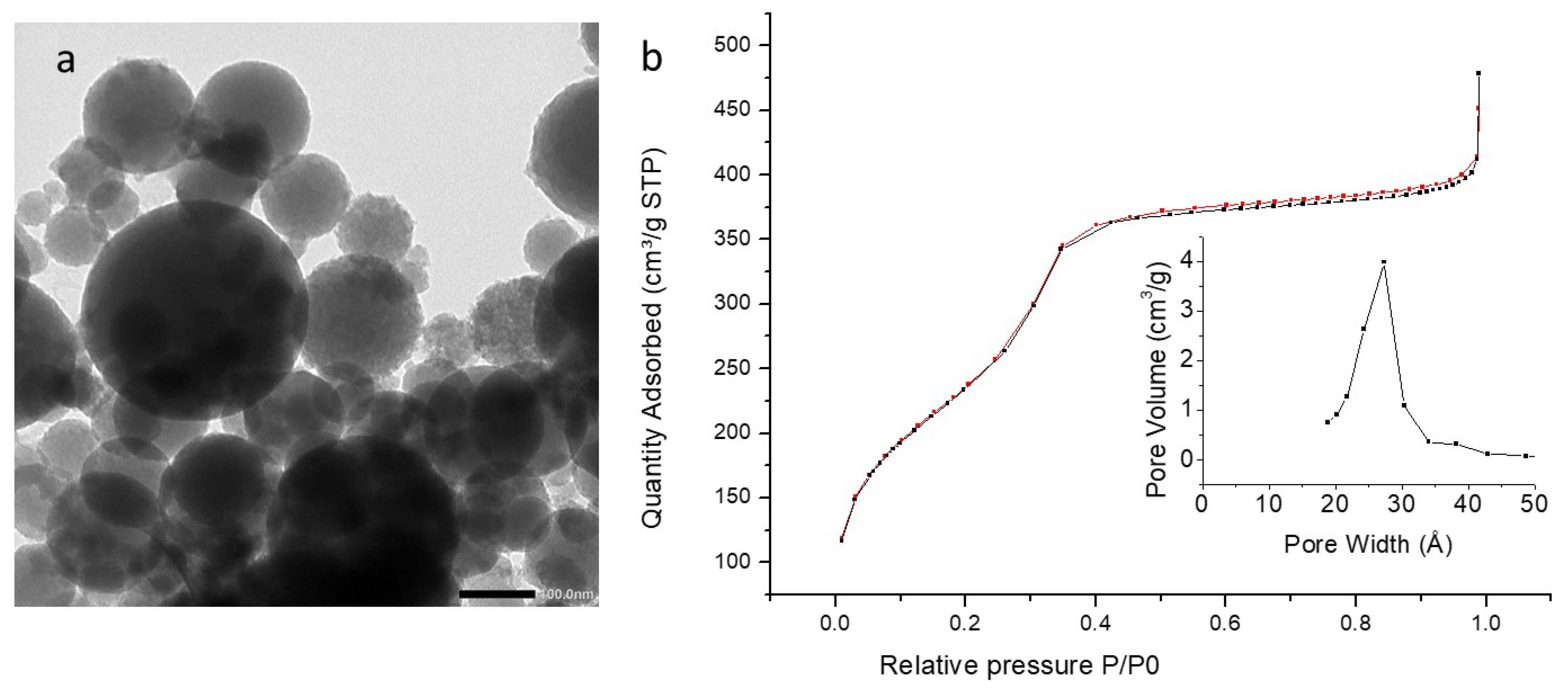
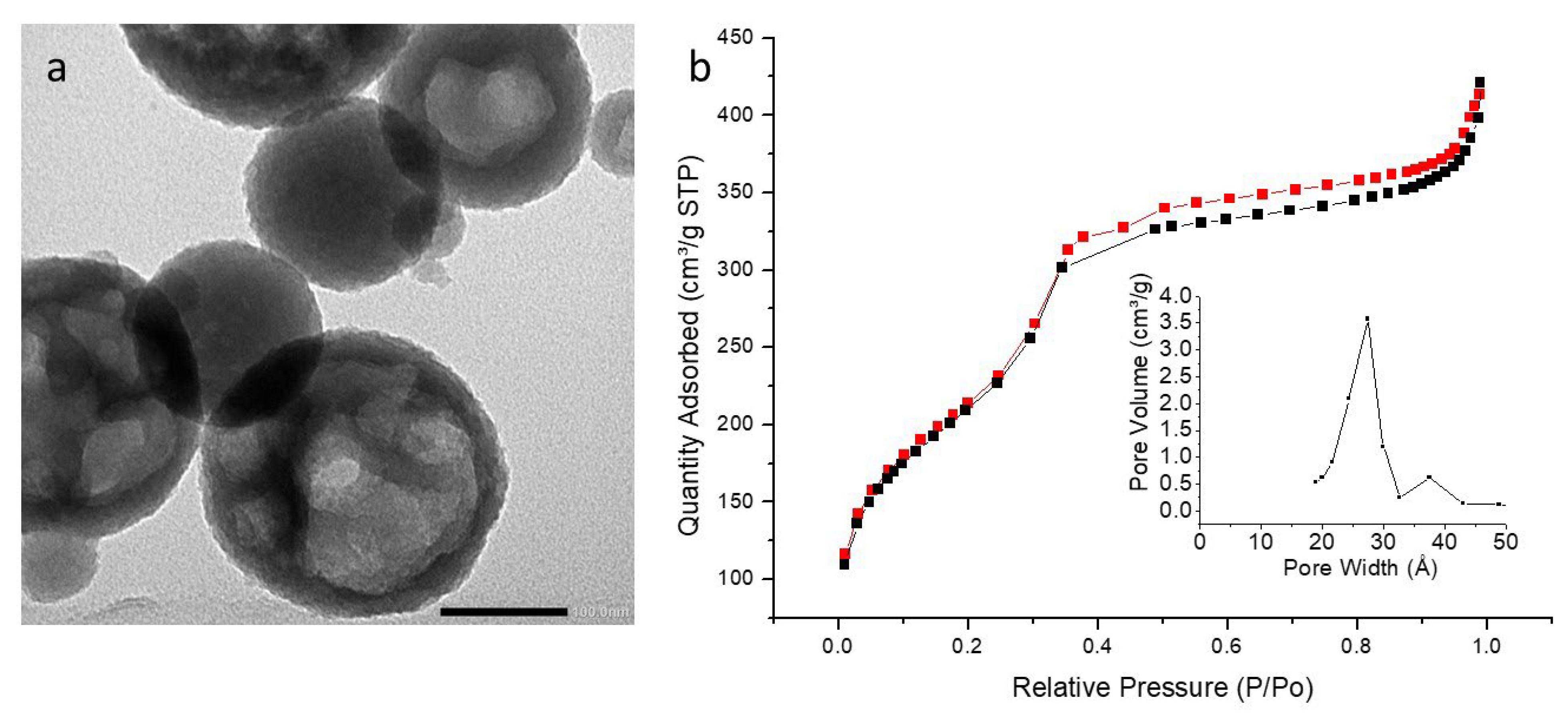
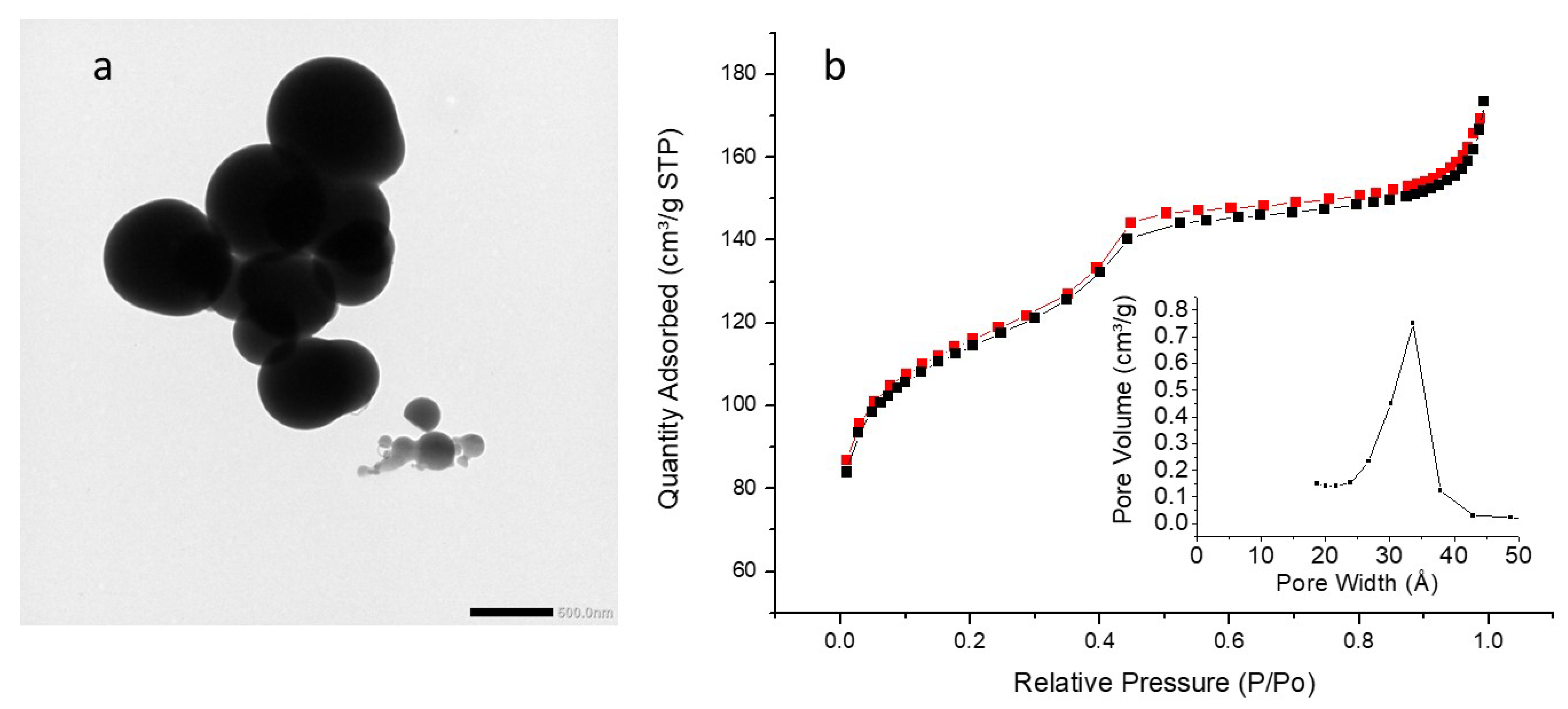
3.5. TEM Analysis
3.6. Textural Analysis
3.7. RMN Analysis
3.8. Elemental Analysis
3.9. CO2 Adsorption Measurement
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Jacobson, M.Z. The health and climate impacts of carbon capture and direct air capture. Energy Environ. Sci. 2019, 12, 3567–3574. [Google Scholar] [CrossRef]
- Rae, J.W.B.; Zhang, Y.G.; Liu, X.; Foster, G.L.; Stoll, H.M.; Whiteford, R.D.M. Atmospheric CO2 over the Past 66 Million Years from Marine Archives. Annu. Rev. Earth Planet. Sci. 2021, 49, 609–641. [Google Scholar] [CrossRef]
- Han, Q.; Bai, X.; Man, Z.; He, H.; Li, L.; Hu, J.; Alsaedi, A.; Hayat, T.; Yu, Z.; Zhang, W.; et al. Convincing Synthesis of Atomically Thin, Single-Crystalline InVO4 Sheets toward Promoting Highly Selective and Efficient Solar Conversion of CO2 into CO. J. Am. Chem. Soc. 2019, 141, 4209–4213. [Google Scholar] [CrossRef] [PubMed]
- Shirley, H.; Su, X.; Sanjanwala, H.; Talukdar, K.; Jurss, J.W.; Delcamp, J.H. Durable Solar-Powered Systems with Ni-Catalysts for Conversion of CO2 or CO to CH4. J. Am. Chem. Soc. 2019, 141, 6617–6622. [Google Scholar] [CrossRef]
- Kar, S.; Goeppert, A.; Prakash, G.K.S. Integrated CO2 Capture and Conversion to Formate and Methanol: Connecting Two Threads. Acc. Chem. Res. 2019, 52, 2892–2903. [Google Scholar] [CrossRef]
- Mou, S.; Wu, T.; Xie, J.; Zhang, Y.; Ji, L.; Huang, H.; Wang, T.; Luo, Y.; Xiong, X.; Tang, B.; et al. Boron Phosphide Nanoparticles: A Nonmetal Catalyst for High-Selectivity Electrochemical Reduction of CO2 to CH3OH. Adv. Mater. 2019, 31, 1903499. [Google Scholar] [CrossRef]
- Falcone, M.; Scopelliti, R.; Mazzanti, M. CO2 and CO/H2 Conversion to Methoxide by a Uranium(IV) Hydride. J. Am. Chem. Soc. 2019, 141, 9570–9577. [Google Scholar] [CrossRef]
- Kamphuis, A.J.; Picchioni, F.; Pescarmona, P.P. CO2-fixation into cyclic and polymeric carbonates: Principles and applications. Green Chem. 2019, 21, 406–448. [Google Scholar] [CrossRef] [Green Version]
- Chiang, C.-L.; Lin, K.-S.; Chuang, H.-W. Direct synthesis of formic acid via CO2 hydrogenation over Cu/ZnO/Al2O3 catalyst. J. Clean. Prod. 2018, 172, 1957–1977. [Google Scholar] [CrossRef]
- Xu, M.; Jupp, A.R.; Ong, M.S.E.; Burton, K.I.; Chitnis, S.S.; Stephan, D.W. Synthesis of Urea Derivatives from CO2 and Silylamines. Angew. Chem. Int. Ed. 2019, 58, 5707–5711. [Google Scholar] [CrossRef]
- Boot-Handford, M.E.; Abanades, J.C.; Anthony, E.J.; Blunt, M.J.; Brandani, S.; Mac Dowell, N.; Fernández, J.R.; Ferrari, M.-C.; Gross, R.; Hallett, J.P.; et al. Carbon capture and storage update. Energy Environ. Sci. 2014, 7, 130–189. [Google Scholar] [CrossRef]
- Haszeldine, R.S. Carbon Capture and Storage: How Green Can Black Be? Science 2009, 325, 1647–1652. [Google Scholar] [CrossRef]
- Cavenati, S.; Grande, C.A.; Rodrigues, A.E. Adsorption Equilibrium of Methane, Carbon Dioxide, and Nitrogen on Zeolite 13X at High Pressures. J. Chem. Eng. Data 2004, 49, 1095–1101. [Google Scholar] [CrossRef]
- Nandi, M.; Okada, K.; Dutta, A.; Bhaumik, A.; Maruyama, J.; Derks, D.; Uyama, H. Unprecedented CO2 uptake over highly porous N-doped activated carbon monoliths prepared by physical activation. Chem. Commun. 2012, 48, 10283–10285. [Google Scholar] [CrossRef] [PubMed]
- Sim, K.; Lee, N.; Kim, J.; Cho, E.-B.; Gunathilake, C.; Jaroniec, M. CO2 Adsorption on Amine-Functionalized Periodic Mesoporous Benzenesilicas. ACS Appl. Mater. Interfaces 2015, 7, 6792–6802. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the Direct Capture of CO2 from Ambient Air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Liu, J.; Thallapally, P.K.; McGrail, B.P.; Brown, D.R.; Liu, J. Progress in adsorption-based CO2 capture by metal–organic frameworks. Chem. Soc. Rev. 2012, 41, 2308–2322. [Google Scholar] [CrossRef]
- He, H.; Sun, Q.; Gao, W.; Perman, J.A.; Sun, F.; Zhu, G.; Aguila, B.; Forrest, K.; Space, B.; Ma, S. A Stable Metal–Organic Framework Featuring a Local Buffer Environment for Carbon Dioxide Fixation. Angew. Chem. Int. Ed. 2018, 57, 4657–4662. [Google Scholar] [CrossRef]
- Zhu, J.; Usov, P.M.; Xu, W.; Celis-Salazar, P.J.; Lin, S.; Kessinger, M.C.; Landaverde-Alvarado, C.; Cai, M.; May, A.M.; Slebodnick, C.; et al. A New Class of Metal-Cyclam-Based Zirconium Metal–Organic Frameworks for CO2 Adsorption and Chemical Fixation. J. Am. Chem. Soc. 2018, 140, 993–1003. [Google Scholar] [CrossRef]
- Rachuri, Y.; Kurisingal, J.F.; Chitumalla, R.K.; Vuppala, S.; Gu, Y.; Jang, J.; Choe, Y.; Suresh, E.; Park, D.-W. Adenine-Based Zn(II)/Cd(II) Metal–Organic Frameworks as Efficient Heterogeneous Catalysts for Facile CO2 Fixation into Cyclic Carbonates: A DFT-Supported Study of the Reaction Mechanism. Inorg. Chem. 2019, 58, 11389–11403. [Google Scholar] [CrossRef]
- Li, X.-Y.; Ma, L.-N.; Liu, Y.; Hou, L.; Wang, Y.-Y.; Zhu, Z. Honeycomb Metal–Organic Framework with Lewis Acidic and Basic Bifunctional Sites: Selective Adsorption and CO2 Catalytic Fixation. ACS Appl. Mater. Interfaces 2018, 10, 10965–10973. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Song, C.; Andresen, J.M.; Miller, B.G.; Scaroni, A.W. Novel Polyethylenimine-Modified Mesoporous Molecular Sieve of MCM-41 Type as High-Capacity Adsorbent for CO2 Capture. Energy Fuels 2002, 16, 1463–1469. [Google Scholar] [CrossRef]
- Wei, Y.; Li, X.; Zhang, R.; Liu, Y.; Wang, W.; Ling, Y.; El-Toni, A.M.; Zhao, D. Periodic Mesoporous Organosilica Nanocubes with Ultrahigh Surface Areas for Efficient CO2 Adsorption. Sci. Rep. 2016, 6, 20769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Canck, E.; Ascoop, I.; Sayari, A.; Van Der Voort, P. Periodic mesoporous organosilicas functionalized with a wide variety of amines for CO2 adsorption. Phys. Chem. Chem. Phys. 2013, 15, 9792–9799. [Google Scholar] [CrossRef] [PubMed]
- Alauzun, J.; Mehdi, A.; Reyé, C.; Corriu, R.J.P. CO2 as a Supramolecular Assembly Agent: A Route for Lamellar Materials with a High Content of Amine Groups. J. Am. Chem. Soc. 2005, 127, 11204–11205. [Google Scholar] [CrossRef] [PubMed]
- Nigar, H.; Garcia-Baños, B.; Peñaranda-Foix, F.L.; Catalá-Civera, J.M.; Mallada, R.; Santamaría, J. Amine-functionalized mesoporous silica: A material capable of CO2 adsorption and fast regeneration by microwave heating. AIChE J. 2016, 62, 547–555. [Google Scholar] [CrossRef]
- Knowles, G.P.; Delaney, S.W.; Chaffee, A.L. Diethylenetriamine[propyl(silyl)]-Functionalized (DT) Mesoporous Silicas as CO2 Adsorbents. Ind. Eng. Chem. Res. 2006, 45, 2626–2633. [Google Scholar] [CrossRef]
- Vilarrasa-García, E.; Cecilia, J.A.; Moya, E.M.O.; Cavalcante, C.L.; Azevedo, D.C.S.; Rodríguez-Castellón, E. “Low Cost” Pore Expanded SBA-15 Functionalized with Amine Groups Applied to CO2 Adsorption. Materials 2015, 8, 2495–2513. [Google Scholar] [CrossRef] [Green Version]
- Yu, L.; Kanezashi, M.; Nagasawa, H.; Tsuru, T. Role of Amine Type in CO2 Separation Performance within Amine Functionalized Silica/Organosilica Membranes: A Review. Appl. Sci. 2018, 8, 1032. [Google Scholar] [CrossRef] [Green Version]
- Croissant, J.G.; Cattoen, X.; Wong Chi Man, M.; Durand, J.-O.; Khashab, N.M. Syntheses and applications of periodic mesoporous organosilica nanoparticles. Nanoscale 2015, 7, 20318–20334. [Google Scholar] [CrossRef]
- Liu, X.; Maegawa, Y.; Goto, Y.; Hara, K.; Inagaki, S. Heterogeneous Catalysis for Water Oxidation by an Iridium Complex Immobilized on Bipyridine-Periodic Mesoporous Organosilica. Angew. Chem. Int. Ed. 2016, 55, 7943–7947. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, Y.; Sekine, M.; Kitamura, K.; Maegawa, Y.; Goto, Y.; Shirai, S.; Inagaki, S.; Ishida, H. Photocatalytic CO2 Reduction by Periodic Mesoporous Organosilica (PMO) Containing Two Different Ruthenium Complexes as Photosensitizing and Catalytic Sites. Chem. A Eur. J. 2017, 23, 10301–10309. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Thiel, I.; Fedorov, A.; Copéret, C.; Mougel, V.; Fontecave, M. Site-isolated manganese carbonyl on bipyridine-functionalities of periodic mesoporous organosilicas: Efficient CO2 photoreduction and detection of key reaction intermediates. Chem. Sci. 2017, 8, 8204–8213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waki, M.; Yamanaka, K.-i.; Shirai, S.; Maegawa, Y.; Goto, Y.; Yamada, Y.; Inagaki, S. Re(bpy)(CO)3Cl Immobilized on Bipyridine-Periodic Mesoporous Organosilica for Photocatalytic CO2 Reduction. Chem. A Eur. J. 2018, 24, 3846–3853. [Google Scholar] [CrossRef]
- Cho, H.S.; Lee, Y.; Wu, J.; Shin, S.R.; Kang, J.K.; Terasaki, O. Understanding Adsorption Behavior of Periodic Mesoporous Organosilica Having a Heterogeneous Chemical Environment: Selective Coverage and Interpenetration of Adsorbates inside the Channel Wall. J. Phys. Chem. C 2019, 123, 24884–24889. [Google Scholar] [CrossRef]
- Waki, M.; Shirai, S.; Yamanaka, K.-I.; Maegawa, Y.; Inagaki, S. Heterogeneous water oxidation photocatalysis based on periodic mesoporous organosilica immobilizing a tris(2,2′-bipyridine)ruthenium sensitizer. RSC Adv. 2020, 10, 13960–13967. [Google Scholar] [CrossRef]
- Yamaguchi, S.; Hashimoto, S. Mechanism of Formic Acid Disproportionation Catalyzed by an Iridium Complex Immobilized on Bipyridine-Periodic Mesoporous Organosilica: A Case Study Based on Kinetics Analysis. Asian J. Org. Chem. 2020, 9, 99–104. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.J.; Kang, J.K.; Jung, J.H. A mesoporous silica functionalized by a covalently bound pyridine derivative for selective optical sensing of thymidine. Mater. Lett. 2007, 61, 5157–5160. [Google Scholar] [CrossRef]
- Zhou, X.; Yi, H.; Tang, X.; Deng, H.; Liu, H. Thermodynamics for the adsorption of SO2, NO and CO2 from flue gas on activated carbon fiber. Chem. Engin. J. 2012, 200–202, 399–404. [Google Scholar] [CrossRef]
- Gelles, T.; Lawson, S.; Rownaghi, A.A.; Rezaei, F. Recent advances in development of amine functionalized adsorbents for CO2 capture. Adsorption 2020, 26, 5–50. [Google Scholar] [CrossRef]
- Chen, C.; Kim, J.; Ahn, W.-S. CO2 capture by amine-functionalized nanoporous materials: A review. Korean J. Chem. Eng. 2014, 31, 1919–1934. [Google Scholar] [CrossRef]
- Mello, M.R.; Phanon, D.; Silveira, G.Q.; Llewellyn, P.L.; Ronconi, C.M. Amine-modified MCM-41 mesoporous silica for carbon dioxide capture. Microporous Mesoporous Mater. 2011, 143, 174–179. [Google Scholar] [CrossRef]
- Kim, S.; Ida, J.; Guliants, V.V.; Lin, Y.S. Tailoring Pore Properties of MCM-48 Silica for Selective Adsorption of CO2. J. Phys. Chem. B 2005, 109, 6287–6293. [Google Scholar] [CrossRef] [PubMed]
- Franchi, R.S.; Harlick, P.J.E.; Sayari, A. Applications of Pore-Expanded Mesoporous Silica. 2. Development of a High-Capacity, Water-Tolerant Adsorbent for CO2. Ind. Eng. Chem. Res. 2005, 44, 8007–8013. [Google Scholar] [CrossRef]
- Schumacher, C.; Gonzalez, J.; Pérez-Mendoza, M.; Wright, P.A.; Seaton, N.A. Design of Hybrid Organic/Inorganic Adsorbents Based on Periodic Mesoporous Silica. Ind. Eng. Chem. Res. 2006, 45, 5586–5597. [Google Scholar] [CrossRef]
- Xu, X.; Song, C.; Andrésen, J.M.; Miller, B.G.; Scaroni, A.W. Preparation and characterization of novel CO2 “molecular basket” adsorbents based on polymer-modified mesoporous molecular sieve MCM-41. Microporous Mesoporous Mater. 2003, 62, 29–45. [Google Scholar] [CrossRef]
- Stephenson, R.M.; Malanowski, S.; Ambrose, D. Handbook of the Thermodynamics of Organic Compounds; Elsevier: New York, NY, USA, 1987. [Google Scholar]
- Sun, L.; Mai, W.; Dang, S.; Qiu, Y.; Deng, W.; Shi, L.; Yan, W.; Zhang, H. Near-infrared luminescence of periodic mesoporous organosilicas grafted with lanthanide complexes based on visible-light sensitization. J. Mater. Chem. 2012, 22, 5121–5127. [Google Scholar] [CrossRef]
- Azzouz, R.; Moreno, V.C.; Herasme-Grullon, C.; Levacher, V.; Estel, L.; Ledoux, A.; Derrouiche, S.; Marsais, F.; Bischoff, L. Efficient Conversion of Epoxides into Carbonates with CO2 and a Single Organocatalyst: Laboratory and Kilogram-Scale Experiments. Synlett 2020, 31, 183–188. [Google Scholar] [CrossRef]
- Umegaki, T.; Enomoto, Y.; Kojima, Y. Metallic ruthenium nanoparticles for hydrogenation of supercritical carbon dioxide. Catal. Sci. Technol. 2016, 6, 409–412. [Google Scholar] [CrossRef]
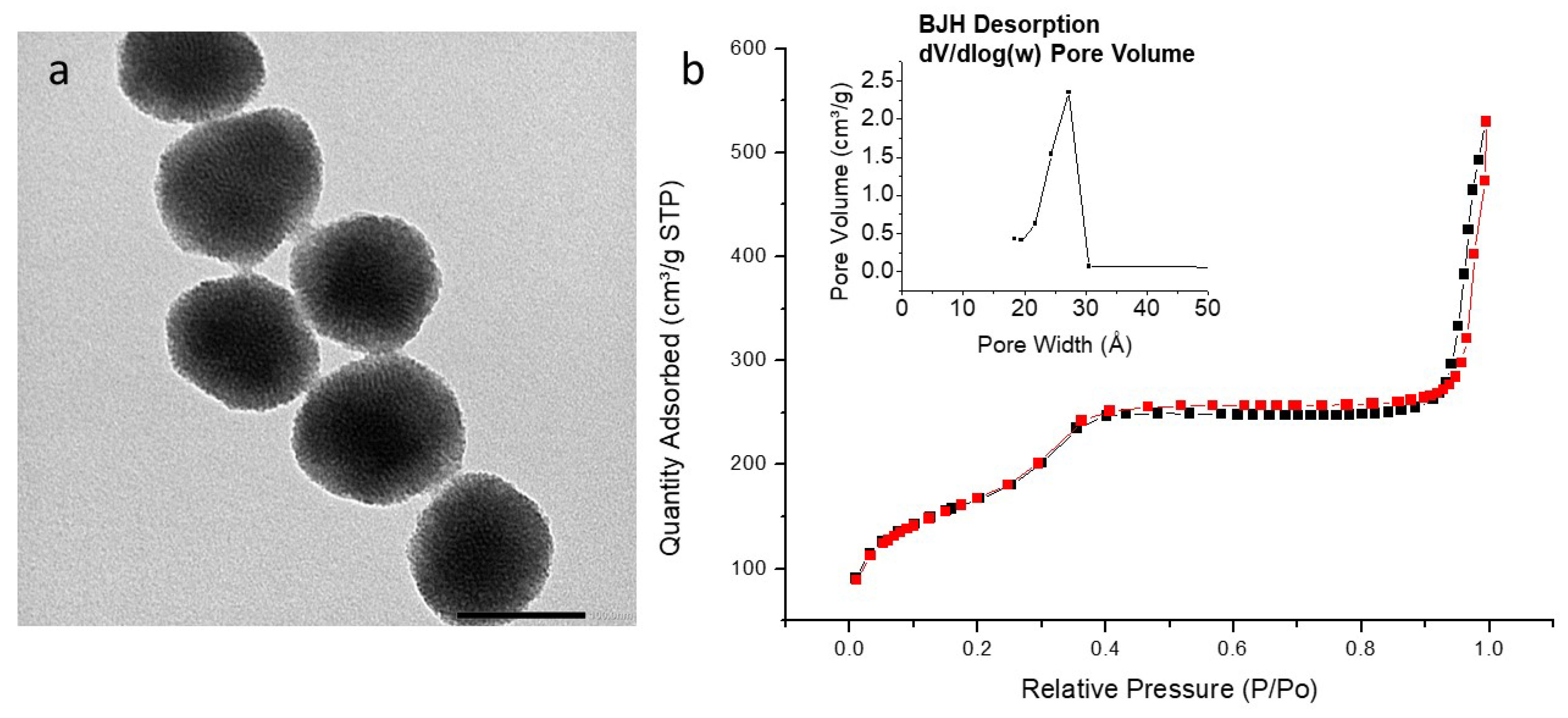





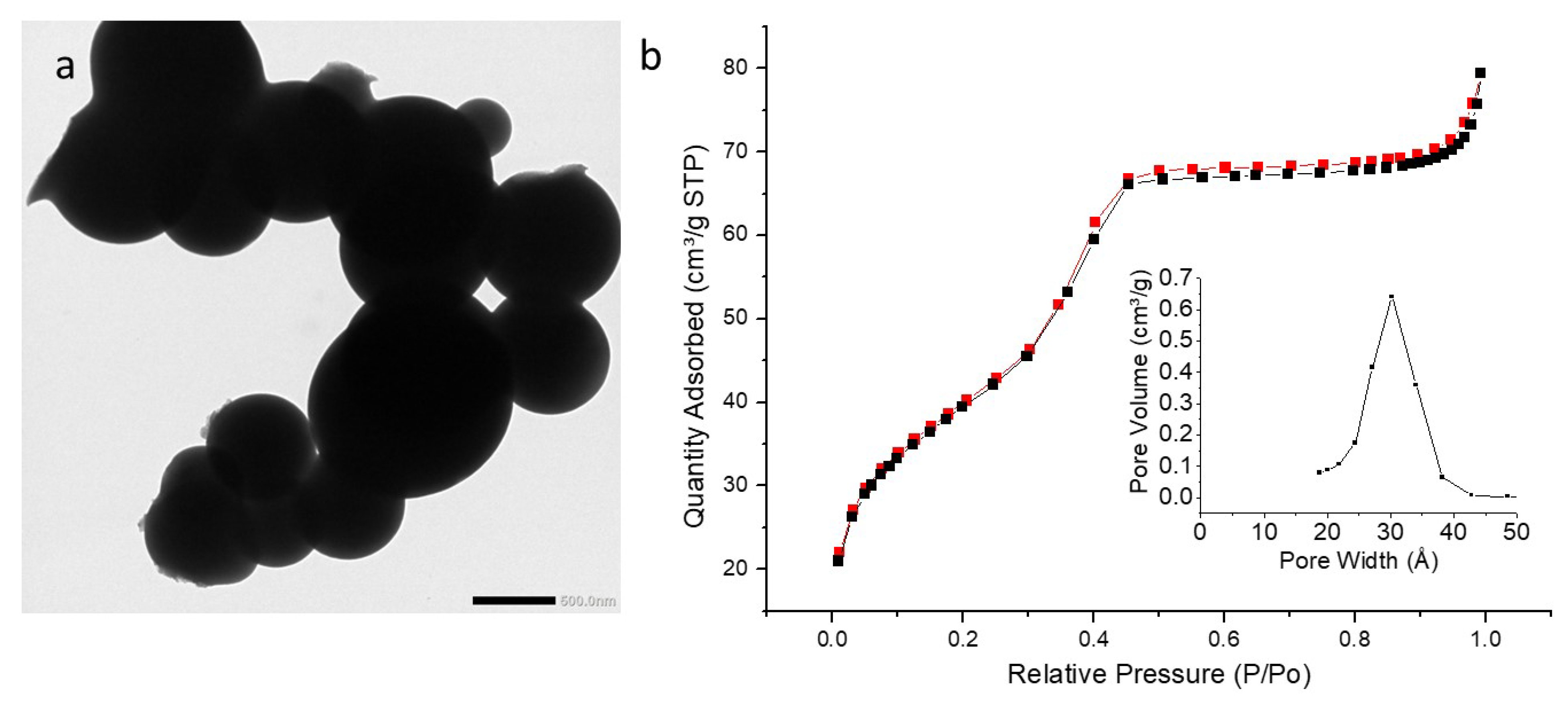
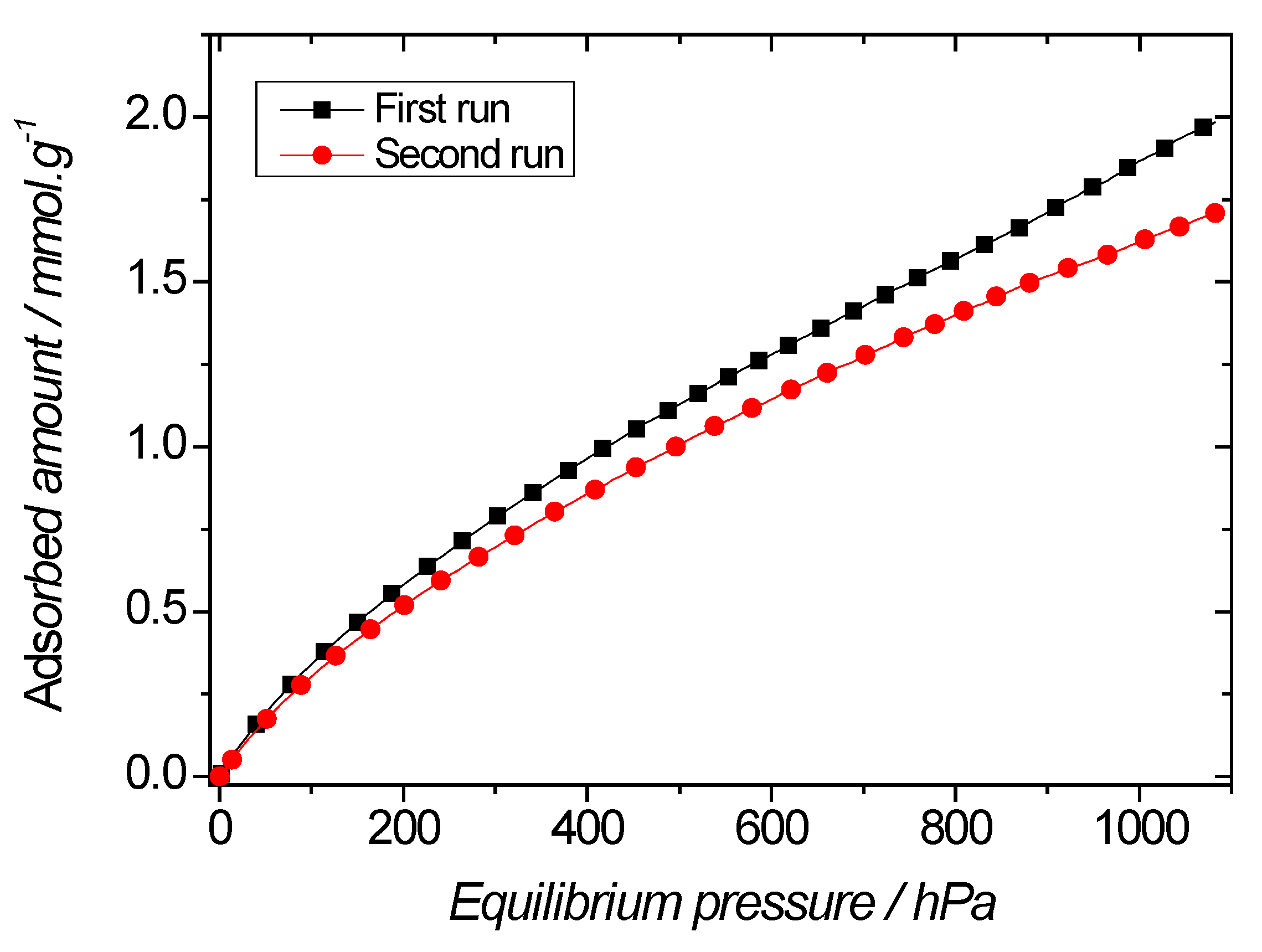
| PMO NPs (Composition) | PMO 1 (100% BTEE) | PyPMO 6 (94% BTEE/ 6% Aminopyridine) | pyPMO 15 (85% BTEE/ 15% Aminopyridine) |
|---|---|---|---|
| Particle size distribution (nm) | 76 | 120 | 99 |
| Standard Deviation (nm) | 12 | 114 | 45 |
| Specific surface area (m2 g−1) | 626 | 918 | 805 |
| Pore size (nm) | 2.7 | 2.7 | 2.5–2.8 |
| PMO | T = 298 K | T = 273 K |
|---|---|---|
| PMO 1 | 0.81 | 1.60 |
| pyPMO 6 | 0.83 | 1.48 |
| pyPMO 15 | 0.92 | 1.55 |
| PMO | T = 298 K | T = 273 K |
|---|---|---|
| iPrbipyPMO 6 | 1.04 | 2.26 |
| iPrbipyPMO 10 | 0.79 | 1.57 |
| iPrbipyPMO 15 | 0.85 | 1.56 |
| PMO (Composition) | iPrbipyPMO 6 (94% BTEE/ 6% Bipyridine) | iPrbipyPMO 10 (90% BTEE/ 10% Bipyridine) | iPrbipyPMO 15 (85% BTEE/ 15% Bipyridine) |
|---|---|---|---|
| Particle size distribution (nm) | 93 | 111 | 102 |
| Standard Deviation (nm) | 46 | 61 | 72 |
| Specific surface area (m2·g−1) | 958 | 796 | 432 |
| Pore size (nm) | 2.5 | 2.5 | 2.5 |
| PMO (Composition) | EtbipyPMO 6 (94% BTEE/6% Etbipyridine) | EtbipyPMO 10 (90% BTEE/10% Etbipyridine) | EtbipyPMO 15 (85% BTEE/15% Etbipyridine) |
|---|---|---|---|
| Particle size distribution (nm) | 301 | 588 | 437 |
| Standard Deviation (nm) | 287 | 300 | 144 |
| Specific surface area (m2·g−1) | 181 | 143 | 372 |
| Pore size (nm) | 2.8 | 3.0 | 3.4 |
| PMO | Adsorption at 105 Pa at 298 K (mmol·g−1) | Adsorption at 105 Pa at 273 K (mmol·g−1) |
|---|---|---|
| EtbipyPMO 6 | 0.37 | 0.46 |
| EtbipyPMO 10 | 0.60 | 0.60 |
| EtbipyPMO 15 | 0.95 | 1.66 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kirren, P.; Barka, L.; Rahmani, S.; Bondon, N.; Donzel, N.; Trens, P.; Bessière, A.; Raehm, L.; Charnay, C.; Durand, J.-O. Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure. Molecules 2022, 27, 4245. https://doi.org/10.3390/molecules27134245
Kirren P, Barka L, Rahmani S, Bondon N, Donzel N, Trens P, Bessière A, Raehm L, Charnay C, Durand J-O. Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure. Molecules. 2022; 27(13):4245. https://doi.org/10.3390/molecules27134245
Chicago/Turabian StyleKirren, Paul, Lucile Barka, Saher Rahmani, Nicolas Bondon, Nicolas Donzel, Philippe Trens, Aurélie Bessière, Laurence Raehm, Clarence Charnay, and Jean-Olivier Durand. 2022. "Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure" Molecules 27, no. 13: 4245. https://doi.org/10.3390/molecules27134245
APA StyleKirren, P., Barka, L., Rahmani, S., Bondon, N., Donzel, N., Trens, P., Bessière, A., Raehm, L., Charnay, C., & Durand, J. -O. (2022). Periodic Mesoporous Organosilica Nanoparticles for CO2 Adsorption at Standard Temperature and Pressure. Molecules, 27(13), 4245. https://doi.org/10.3390/molecules27134245







