The Potential of the Marine Microalga Diacronema lutheri in the Prevention of Obesity and Metabolic Syndrome in High-Fat-Fed Wistar Rats
Abstract
:1. Introduction
2. Results
2.1. Effects of D. lutheri Supplementation on Body Weight and Fat Mass in Wistar Rats Fed a High-Fat Diet
2.1.1. Nutritional Monitoring: Food and Water Intake
2.1.2. Energy Intake
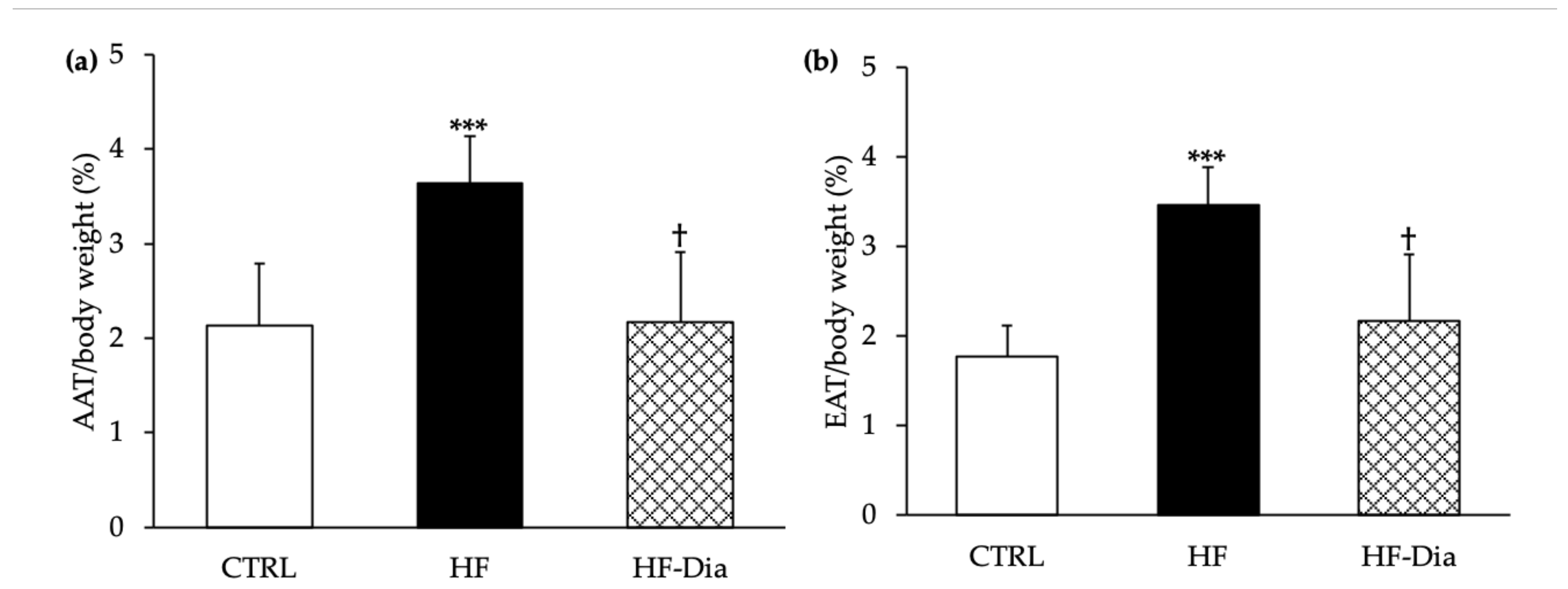
2.1.3. Body Weight and Fat Mass
2.2. Proximate Composition, Fatty Acid, Pigment and Sterol Profile of D. lutheri Biomass
2.2.1. Proximate Composition of D. lutheri Biomass
2.2.2. Fatty Acid Composition of D. lutheri
2.2.3. Pigment, Antioxidant Activity, In Vitro Digestibility and Sterol Composition of D. lutheri Biomass
2.3. Effects of D. lutheri Supplementation on Physiological and Metabolic Disorders in Wistar Rats Fed an HF Diet
2.3.1. Transaminase Levels and Aspartate Amino-Transferase/Alanine Amino-Transferase Ratio
2.3.2. Plasma Lipids, High-Density Lipoprotein Cholesterol/Low-Density Lipoprotein Cholesterol Ratio and Atherogenic Index of Plasma
2.4. Effects of D. lutheri on Glucose Homeostasis and Insulin Sensitivity
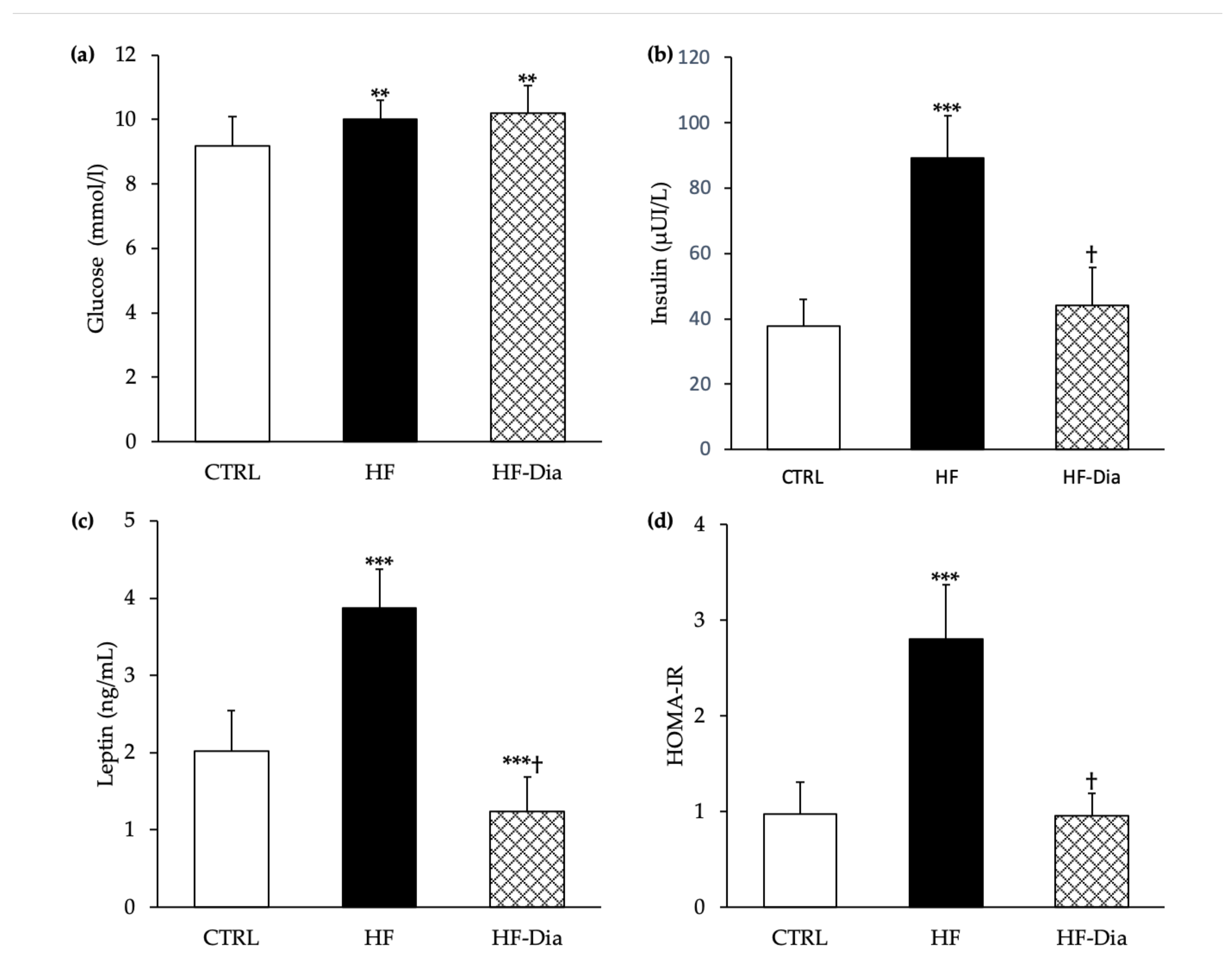
2.4.1. Plasma Glucose, Insulin and Leptin Levels and Homeostasis Model Assessment of Insulin Resistance Index
2.4.2. Glucose and Insulin Tolerance Tests
2.5. Effects of D. lutheri on Inflammatory Status
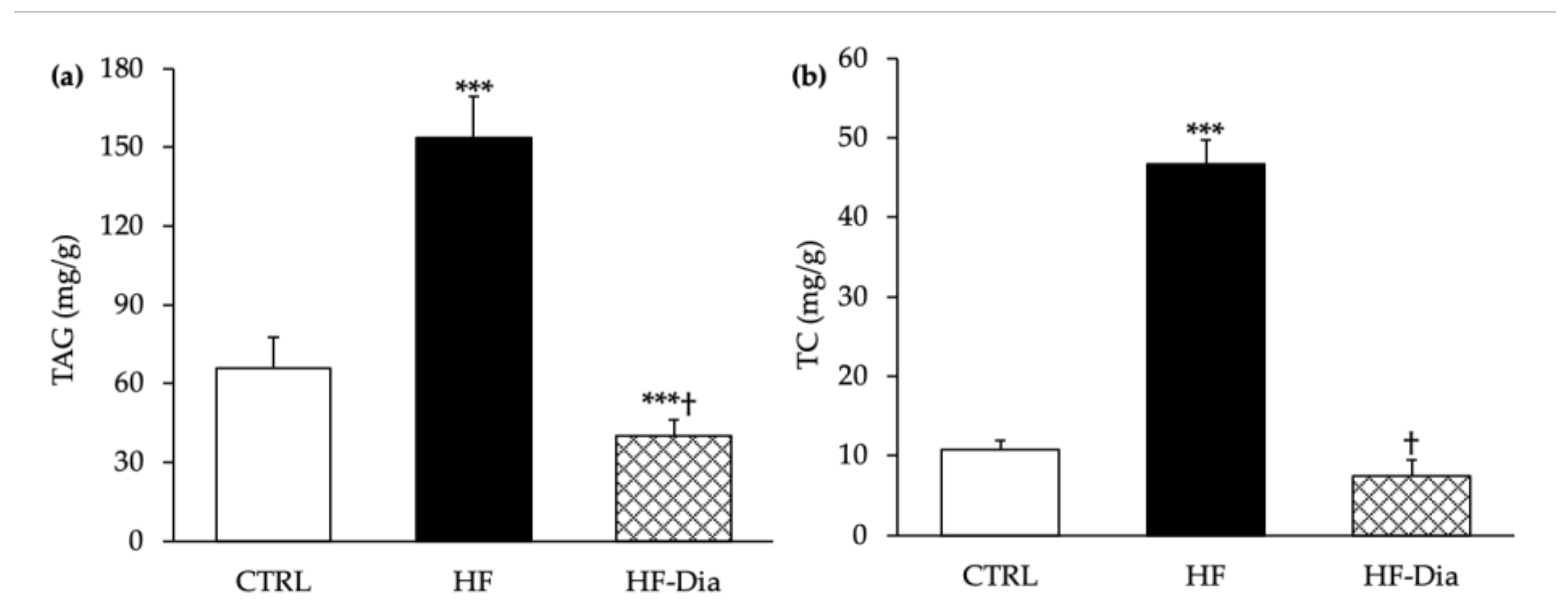
2.6. Effects of D. lutheri on Liver Triacylglycerol and Total Cholesterol Levels
3. Discussion
3.1. Nutritional Profile of D. lutheri Biomass
3.2. D. lutheri Supplementation Reduced Body Weight, Abdominal and Epididymal Adipose Weights in HF-Fed Wistar Rats
3.3. Effects of D. lutheri as a Dietary Supplement in the Prevention of Dyslipidemia and Atherosclerosis
3.4. Effects of D. lutheri Supplementation on Inflammatory Status in HF-Fed Wistar Rats
3.5. Effects of D. lutheri Supplementation on Glycemia and Insulinemia and Its Impact on Glucose Homeostasis and Insulin Sensitivity in HF-Fed Wistar Rats
3.6. Effects of D. lutheri Supplementation on Non-Alcoholic Fatty Liver Disease
4. Materials and Methods
4.1. Animal and Diets
4.2. D. lutheri Biomass Preparation and Characterization
4.3. Gucose and Insulin Tolerance Tests
4.4. Blood and Organ Sampling
4.5. Biochemical Analyses and Hepatic Lipid Measurements
4.6. Stastistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- World Health Organization. Global Health Estimates 2016: Deaths by Cause, Age, Sex, by Country and by Region, 2000–2016; World Health Organization: Genève, Switzerland, 2018. [Google Scholar]
- Chooi, Y.C.; Ding, C.; Magkos, F. The epidemiology of obesity. Metabolism 2018, 92, 6–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grundy, S.M. Metabolic syndrome update. Trends Cardiovasc. Med. 2016, 26, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Saltiel, A.R. Inflammatory links between obesity and metabolic disease. J. Clin. Investig. 2011, 121, 2111–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, J. Mechanisms of insulin resistance in obesity. Front. Med. 2013, 7, 14–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paniagua, J.A. Nutrition, insulin resistance and dysfunctional adipose tissue determine the different components of metabolic syndrome. World J. Diabetes 2016, 7, 483–514. [Google Scholar] [CrossRef]
- Browning, J.D.; Szczepaniak, L.S.; Dobbins, R.; Nuremberg, P.; Horton, J.D.; Cohen, J.C.; Grundy, S.M.; Hobbs, H.H. Prevalence of hepatic steatosis in an urban population in the united states: Impact of ethnicity. Hepatology 2004, 40, 1387–1395. [Google Scholar] [CrossRef] [PubMed]
- Kunesová, M.; Braunerová, R.; Hlavatý, P.; Tvrzická, E.; Stanková, B.; Skrha, J.; Hilgertová, J.; Hill, M.; Kopecký, J.; Wagenknecht, M.; et al. The influence of n-3 polyunsaturated fatty acids and very low calorie diet during a short-term weight reducing regimen on weight loss and serum fatty acid composition in severely obese women. Physiol. Res. 2006, 55, 63–72. [Google Scholar] [CrossRef]
- Bertrand, C.; Pignalosa, A.; Wanecq, E.; Rancoule, C.; Batut, A.; Deleruyelle, S.; Lionetti, L.; Valet, P.; Castan-Laurell, I. Effects of dietary eicosapentaenoic acid (EPA) supplementation in high-fat fed mice on lipid metabolism and apelin/APJ system in skeletal muscle. PLoS ONE 2013, 8, e78874. [Google Scholar] [CrossRef] [Green Version]
- Poudyal, H.; Panchal, S.K.; Ward, L.C.; Brown, L. Effects of ALA, EPA and DHA in high-carbohydrate, high-fat diet-induced metabolic syndrome in rats. J. Nutr. Biochem. 2013, 24, 1041–1052. [Google Scholar] [CrossRef] [PubMed]
- Calder, P.C. Marine omega-3 fatty acids and inflammatory processes: Effects, mechanisms and clinical relevance. BBA—Mol. Cell Biol. Lipids 2015, 1851, 469–484. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Kalupahana, N.S.; Ramalingam, L.; Wang, S.; Rahman, S.M.; Robert-McComb, J.; Moustaid-Moussa, N. Omega-3 fatty acids in obesity and metabolic syndrome: A mechanistic update. J. Nutr. Biochem. 2018, 58, 1–16. [Google Scholar] [CrossRef]
- Albracht-Schulte, K.; Gonzalez, S.; Jackson, A.; Wilson, S.; Ramalingam, L.; Kalupahana, N.; Moustaid-Moussa, N. Eicosapentaenoic acid improves hepatic metabolism and reduces inflammation independent of obesity in high-fat-fed mice and in HepG2 cells. Nutrients 2019, 11, 599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedi, E.; Sahari, M.A. Long-chain polyunsaturated fatty acid sources and evaluation of their nutritional and functional properties. Food Sci. Nutr. 2014, 2, 443–463. [Google Scholar] [CrossRef] [PubMed]
- Lakra, N.; Mahmood, S.; Marwal, A.; Sudheep, N.M.; Anwar, K. Bioengineered plants can be an alternative source of omega-3 fatty acids for human health. In Plant and Human Health; Ozturk, M., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 2, pp. 361–382. [Google Scholar] [CrossRef]
- Chisti, Y. Biodiesel from microalgae. Biotechnol. Adv. 2007, 25, 294–306. [Google Scholar] [CrossRef]
- Bigogno, C. Lipid and fatty acid composition of the green oleaginous alga Parietochloris incisa, the richest plant source of arachidonic acid. Phytochemistry 2002, 60, 497–503. [Google Scholar] [CrossRef]
- Hamed, I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016, 15, 1104–1123. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Wu, Y.; Yang, C.; Liu, B.; Huang, Y. Hypotensive, hypoglycaemic and hypolipidaemic effects of bioactive compounds from microalgae and marine micro-organisms. Int. J. Food Sci. Technol. 2015, 50, 1705–1717. [Google Scholar] [CrossRef]
- Orkesterjournalen, L. Commission Implementing Regulation (EU) 2018/1023 of 23 July 2018 correcting implementing regulation (EU) 2017/2470 establishing the Union list of novel foods. Off. J. Eur. Union 2018, 187, 1–133. [Google Scholar]
- Odjadjare, E.C.; Mutanda, T.; Olaniran, A.O. Potential biotechnological application of microalgae: A critical review. Crit. Rev. Biotechnol. 2017, 37, 37–52. [Google Scholar] [CrossRef]
- Wong, S.K.; Chin, K.-Y.; Suhaimi, F.H.; Fairus, A.; Ima-Nirwana, S. Animal models of metabolic syndrome: A review. Nutr. Metab. 2016, 13, 65. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buettner, R.; Schölmerich, J.; Bollheimer, L.C. High-fat diets: Modeling the metabolic disorders of human obesity in rodents. Obesity 2012, 15, 798–808. [Google Scholar] [CrossRef] [PubMed]
- Kakimoto, P.A.; Kowaltowski, A.J. Effects of high fat diets on rodent liver bioenergetics and oxidative imbalance. Redox Biol. 2016, 8, 216–225. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Meireles, M.; Norberto, S.; Leite, J.; Freitas, J.; Pestana, D.; Faria, A.; Calhau, C. High-fat diet-induced obesity rat model: A comparison between Wistar and Sprague-Dawley Rat. Adipocyte 2016, 5, 11–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lima, M.L.R.P.; Leite, L.H.R.; Gioda, C.R.; Leme, F.O.P.; Couto, C.A.; Coimbra, C.C.; Leite, V.H.R.; Ferrari, T.C.A. A novel Wistar rat model of obesity-related nonalcoholic fatty liver disease induced by sucrose-rich diet. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morançais, M.; Mouget, J.L.; Dumay, J. Proteins and Pigments. In Microalgae in Health and Disease Prevention; Levin, I.A., Fleurence, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 145–175. [Google Scholar] [CrossRef]
- Niccolai, A.; Chini Zittelli, G.; Rodolfi, L.; Biondi, N.; Tredici, M.R. Microalgae of interest as food source: Biochemical composition and digestibility. Algal Res. 2019, 42, 101617. [Google Scholar] [CrossRef]
- Batista, A.P.; Gouveia, L.; Bandarra, N.M.; Franco, J.M.; Raymundo, A. Comparison of microalgal biomass profiles as novel functional ingredient for food products. Algal Res. 2013, 2, 164–173. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, S.; Martinez, E.M.; Espinol, F. Biomass production and biochemical variability of the marine microalga Isochrysis galbana in relation to culture medium. Biochem. Eng. J. 2000, 6, 13–18. [Google Scholar] [CrossRef]
- Vizcaíno, A.J.; Saéz, M.I.; López, G.; Arizcun, M.; Abellán, E.; Martínez, T.F.; Cerón-García, M.C.; Alarcón, F.J. Tetraselmis suecia and Tisochrysis lutea meal as dietary ingredients for gilthead sea bream (Sparus aurata L.) fry. J. Appl. Phycol. 2016, 28, 2843–2855. [Google Scholar] [CrossRef]
- Mayer, C.; Côme, M.; Ulmann, L.; Chini Zittelli, G.; Faraloni, C.; Nazih, H.; Ouguerram, K.; Chénais, B.; Mimouni, V. Preventive effects of the marine microalga Phaeodactylum tricornutum, used as a food supplement, on risk factors associated with metabolic syndrome in Wistar rats. Nutrients 2019, 11, 1069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guiheneuf, F.; Stengel, D.B. Interactive effects of light and temperature on pigments and n-3 LC-PUFA-enriched oil accumulation in batch-cultivated Pavlova lutheri using high-bicarbonate supply. Algal Res. 2017, 23, 113–125. [Google Scholar] [CrossRef]
- Carvalho, A.P.; Monteiro, C.M.; Malcata, F.X. Simultaneous effect of irradiance and temperature on biochemical composition of the microalga Pavlova lutheri. J. Appl. Phycol. 2009, 21, 543–552. [Google Scholar] [CrossRef]
- Khaw, Y.S.; Yusoff, F.M.; Tan, H.T.; Noor Mazli, N.A.I.; Nazarudin, M.F.; Shaharuddin, N.A.; Omar, A.R. The critical studies of fucoxanthin research trends from 1928 to june 2021: A bibliometric review. Mar. Drugs 2021, 19, 606. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, F.; Fanning, K.; Netzel, M.; Turner, W.; Li, Y.; Schenk, P.M. Profiling of carotenoids and antioxidant capacity of microalgae from subtropical coastal and brackish waters. Food Chem. 2014, 165, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Cabral, C.E.; Klein, M.R.S.T. Phytosterols in the Treatment of Hypercholesterolemia and Prevention of Cardiovascular Diseases. Arq. Bras. Cardiol. 2017, 109, 475–482. [Google Scholar] [CrossRef]
- Ahmed, F.; Zhou, W.; Schenk, P.M. Pavlova lutheri is a high-level producer of phytosterols. Algal Res. 2015, 10, 210–217. [Google Scholar] [CrossRef]
- Nagarajan, D.; Varjani, S.; Lee, D.L.; Chang, J.S. Sustainable aquaculture and animal feed from microalgae nutritive value and techno-functional components. Renew. Sustain. Energy Rev. 2021, 150, 111549. [Google Scholar] [CrossRef]
- Machů, L.; Mišurcová, L.; Samek, D.; Hrabě, J.; Fišera, M. In vitro digestibility of different commercial edible algae products. J. Aquat. Food Prod. Technol. 2014, 23, 423–435. [Google Scholar] [CrossRef]
- Ruzickova, J.; Rossmeisl, M.; Prazak, T.; Flachs, P.; Sponarova, J.; Veck, M.; Tvrzicka, E.; Bryhn, M.; Kopecky, J. Omega-3 PUFA of marine origin limit diet-induced obesity in mice by reducing cellularity of adipose tissue. Lipids 2004, 39, 1177–1185. [Google Scholar] [CrossRef]
- Kim, H.-K.; Della-Fera, M.; Lin, J.; Baile, C.A. Docosahexaenoic acid inhibits adipocyte differentiation and induces apoptosis in 3T3-L1 preadipocytes. J. Nutr. 2006, 136, 2965–2969. [Google Scholar] [CrossRef] [Green Version]
- Murali, G.; Desouza, C.V.; Clevenger, M.E.; Ramalingam, R.; Saraswathi, V. Differential effects of eicosapentaenoic acid and docosahexaenoic acid in promoting the differentiation of 3T3-L1 preadipocytes. Prostaglandins Leukot. Essent. Fat. Acids 2014, 90, 13–21. [Google Scholar] [CrossRef]
- Sun, C.; Wei, Z.; Li, Y. DHA regulates lipogenesis and lipolysis genes in mice adipose and liver. Mol. Biol. Rep. 2011, 38, 731–737. [Google Scholar] [CrossRef]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Funayama, K.; Miyashita, K. Fucoxanthin from edible seaweed, Undaria Pinnatifida, shows antiobesity effect through UCP1 expression in white adipose tissues. Biochem. Biophys. Res. Commun. 2005, 332, 392–397. [Google Scholar] [CrossRef]
- Grasa-López, A.; Miliar-García, Á.; Quevedo-Corona, L.; Paniagua-Castro, N.; Escalona-Cardoso, G.; Reyes-Maldonado, E.; Jaramillo-Flores, M.-E. Undaria Pinnatifida and fucoxanthin ameliorate lipogenesis and markers of both inflammation and cardiovascular dysfunction in an animal model of diet-induced obesity. Mar. Drugs 2016, 14, 148. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Cui, Y.; Hu, X.; Liao, X.; Zhang, Y. Chlorophyll supplementation in early life prevents diet-induced obesity and modulates gut microbiota in mice. Mol. Nutr. Food Res. 2019, 63, 1801219. [Google Scholar] [CrossRef]
- Jung, H.A.; Jung, H.J.; Jeong, H.Y.; Kwon, H.J.; Kim, M.-S.; Choi, J.S. Anti-adipogenic activity of the edible brown alga Ecklonia Stolonifera and its constituent fucosterol in 3T3-L1 adipocytes. Arch. Pharm. Res. 2014, 37, 713–720. [Google Scholar] [CrossRef]
- Andersen, R.A. Biology and systematics of heterokont and haptophyte algae. Am. J. Bot. 2004, 91, 1508–1522. [Google Scholar] [CrossRef] [Green Version]
- Ostlund, R.E. Phytosterols in human nutrition. Annu. Rev. Nutr. 2002, 22, 533–549. [Google Scholar] [CrossRef]
- Egert, S.; Kannenberg, F.; Somoza, V.; Erbersdobler, H.F.; Wahrburg, U. Dietary α-linolenic acid, EPA, and DHA have differential effects on LDL fatty acid composition but similar effects on serum lipid profiles in normolipidemic humans. J. Nutr. 2009, 139, 861–868. [Google Scholar] [CrossRef] [Green Version]
- Hoang, M.-H.; Jia, Y.; Jun, H.; Lee, J.H.; Lee, B.Y.; Lee, S.-J. Fucosterol is a selective liver X receptor modulator that regulates the expression of key genes in cholesterol homeostasis in macrophages, hepatocytes, and intestinal cells. J. Agric. Food Chem. 2012, 60, 11567–11575. [Google Scholar] [CrossRef]
- Haimeur, A.; Ulmann, L.; Mimouni, V.; Guéno, F.; Pineau-Vincent, F.; Meskini, N.; Tremblin, G. The role of Odontella aurita, a marine Diatom rich in EPA, as a dietary supplement in dyslipidemia, platelet function and oxidative stress in high-fat fed rats. Lipids Health Dis. 2012, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Beppu, F.; Niwano, Y.; Tsukui, T.; Hosokawa, M.; Miyashita, K. Single and repeated oral dose toxicity study of fucoxanthin (FX), a marine carotenoid, in mice. J. Toxicol. Sci. 2009, 34, 501–510. [Google Scholar] [CrossRef] [Green Version]
- Iio, K.; Okada, Y.; Ishikura, M. Single and 13-week oral toxicity study of fucoxanthin oil from microalgae in rats. Shokuhin Eiseigaku Zasshi 2011, 52, 183–189. [Google Scholar] [CrossRef]
- Adhyaru, B.B.; Jacobson, T.A. New cholesterol guidelines for the management of atherosclerotic cardiovascular disease risk. Endocrinol. Metab. Clin. 2016, 45, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.-W.; Lu, Y.; Li, F.; Yang, C.-J.; Feng, Y.-B.; Li, H.-W.; Yao, W.-F.; Shen, Z.-H. Atherogenic index of plasma is an effective index for estimating abdominal obesity. Lipids Health Dis. 2018, 17, 11. [Google Scholar] [CrossRef] [Green Version]
- de Jesus Raposo, M.; de Morais, A.; de Morais, R. Marine polysaccharides from algae with potential biomedical applications. Mar. Drugs 2015, 13, 2967–3028. [Google Scholar] [CrossRef]
- Luo, X.; Su, P.; Zhang, W. Advances in microalgae-derived phytosterols for functional food and pharmaceutical applications. Mar. Drugs 2015, 13, 4231–4254. [Google Scholar] [CrossRef]
- Lovegrove, A.; Edwards, C.H.; De Noni, I.; Patel, H.; El, S.N.; Grassby, T.; Zielke, C.; Ulmius, M.; Nilsson, L.; Butterworth, P.J.; et al. Role of polysaccharides in food, digestion, and health. Crit. Rev. Food Sci. Nutr. 2017, 57, 237–253. [Google Scholar] [CrossRef] [Green Version]
- Yanai, H.; Masui, Y.; Katsuyama, H.; Adachi, H.; Kawaguchi, A.; Hakoshima, M.; Waragai, Y.; Harigae, T.; Sako, A. An improvement of cardiovascular risk factors by omega-3 polyunsaturated fatty acids. J. Clin. Med. Res. 2018, 10, 281–289. [Google Scholar] [CrossRef] [Green Version]
- Calder, P.C. Dietary modification of inflammation with lipids. Proc. Nutr. Soc. 2002, 61, 345–358. [Google Scholar] [CrossRef]
- Yu, J.; Ma, Y.; Sun, J.; Ran, L.; Li, Y.; Wang, N.; Yu, T.; Gao, W.; Jia, W.; Jiang, R.; et al. Microalgal oil from Schizochytrium sp. prevents HFD-induced abdominal fat accumulation in mice. J. Am. Coll. Nutr. 2017, 36, 347–356. [Google Scholar] [CrossRef]
- Ávila-Román, J.; García-Gil, S.; Rodríguez-Luna, A.; Motilva, V.; Talero, E. Anti-inflammatory and anticancer effects of microalgal carotenoids. Mar. Drugs 2021, 19, 531. [Google Scholar] [CrossRef] [PubMed]
- Guillot, A.; Gao, B. Immunopathogenesis of liver cirrhosis. In Liver Immunology; Gershwin, M.E.M., Vierling, J., Tanaka, A.P., Manns, M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 583–595. [Google Scholar] [CrossRef]
- Koc, F.; Tekeli, M.Y.; Kanbur, M.; Karayigit, M.Ö.; Liman, B.C. The effects of chrysin on lipopolysaccharide-induced sepsis in rats. J. Food Biochem. 2020, 44, e13359. [Google Scholar] [CrossRef] [PubMed]
- Reilly, S.M.; Saltiel, A.R. Adapting to obesity with adipose tissue inflammation. Nat. Rev. Endocrinol. 2017, 13, 633. [Google Scholar] [CrossRef] [PubMed]
- dos Santos Moraes, A.; Pisani, L.P.; Corgosinho, F.C.; Testa Carvalho, L.O.; Masquio, D.C.L.; Jamar, G.; Sanches, R.B.; Oyama, L.M.; Dâmaso, A.R.; Belote, C.; et al. The role of leptinemia state as a mediator of inflammation in obese adults. Horm. Metab. Res. 2013, 45, 605–610. [Google Scholar] [CrossRef]
- Kang, M.-J.; Kim, S.M.; Jeong, S.-M.; Choi, H.-N.; Jang, Y.-H.; Kim, J.-I. Antioxidant effect of Phaeodactylum tricornutum in mice fed high-fat diet. Food Sci. Biotechnol. 2013, 22, 107–113. [Google Scholar] [CrossRef]
- Osegbe, I.; Okpara, H.; Azinge, E. Relationship between serum leptin and insulin resistance among obese nigerian women. Ann. Afr. Med. 2016, 15, 14–19. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, N.; Konishi, F.; Kumamoto, S.; Maruyama, I.; Ando, Y.; Yanagita, T. Beneficial effects of Chlorella on glucose and lipid metabolism in obese rodents on a high-fat diet. Obes. Res. Clin. Pract. 2013, 7, e95–e105. [Google Scholar] [CrossRef]
- Nuño, K.; Villarruel-López, A.; Puebla-Pérez, A.M.; Romero-Velarde, E.; Puebla-Mora, A.G.; Ascencio, F. Effects of the marine microalgae Isochrysis galbana and Nannochloropsis oculata in diabetic rats. J. Funct. Foods 2013, 5, 106–115. [Google Scholar] [CrossRef]
- Vecina, J.F.; Oliveira, A.G.; Araujo, T.G.; Baggio, S.R.; Torello, C.O.; Saad, M.J.A.; de Souza Queiroz, M.L. Chlorella modulates insulin signaling pathway and prevents high-fat diet-induced insulin resistance in mice. Life Sci. 2014, 95, 45–52. [Google Scholar] [CrossRef]
- Nasirian, F.; Sarir, H.; Moradikor, N. Antihyperglycemic and antihyperlipidemic activities of Nannochloropsis oculata microalgae in streptozotocin-induced diabetic rats. Biomol. Concepts 2019, 10, 37–43. [Google Scholar] [CrossRef]
- Yook, J.-S.; Kim, K.-A.; Park, J.E.; Lee, S.-H.; Cha, Y.-S. Microalgal oil supplementation has an anti-obesity effect in C57BL/6J mice fed a high fat diet. Prev. Nutr. Food Sci. 2015, 20, 230–237. [Google Scholar] [CrossRef] [Green Version]
- Maeda, H.; Hosokawa, M.; Sashima, T.; Miyashita, K. Dietary combination of fucoxanthin and fish oil attenuates the weight gain of white adipose tissue and decreases blood glucose in obese/diabetic KK- A y mice. J. Agric. Food Chem. 2007, 55, 7701–7706. [Google Scholar] [CrossRef]
- Peng, J.; Yuan, J.-P.; Wu, C.-F.; Wang, J.-H. Fucoxanthin, a marine carotenoid present in brown seaweeds and diatoms: Metabolism and bioactivities relevant to human health. Mar. Drugs 2011, 9, 1806–1828. [Google Scholar] [CrossRef]
- de Mello-Sampayo, C.; Paterna, A.; Polizzi, A.; Duarte, D.; Batista, I.; Pinto, R.; Gonçalves, P.; Raymundo, A.; Batista, A.P.; Gouveia, L.; et al. Evaluation of marine microalga Diacronema vlkianum biomass fatty acid assimilation in Wistar rats. Molecules 2017, 22, 1097. [Google Scholar] [CrossRef] [Green Version]
- Woo, M.-N.; Jeon, S.-M.; Kim, H.-J.; Lee, M.-K.; Shin, S.-K.; Shin, Y.C.; Park, Y.-B.; Choi, M.-S. Fucoxanthin supplementation improves plasma and hepatic lipid metabolism and blood glucose concentration in high-fat fed C57BL/6N mice. Chem. Biol. Interact. 2010, 186, 316–322. [Google Scholar] [CrossRef]
- Liland, N.S.; Pittman, K.; Whatmore, P.; Torstensen, B.E.; Sissener, N.H. Fucosterol causes small changes in lipid storage and brassicasterol affects some markers of lipid metabolism in atlantic salmon hepatocytes. Lipids 2018, 53, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Abdul, Q.A.; Choi, R.J.; Jung, H.A.; Choi, J.S. Health benefit of fucosterol from marine algae: A review. J. Sci. Food Agric. 2015, 96, 1856–1866. [Google Scholar] [CrossRef]
- Topuz, O.K. Algal oil: A novel source of omega-3 fatty acids for human nutrition. Series F. Biotech. 2016, 20, 178–183. [Google Scholar]
- Mayer, C.; Richard, L.; Côme, M.; Ulmann, L.; Nazih, H.; Chénais, B.; Ouguerram, K.; Mimouni, V. The marine microalga, Tisochrysis lutea, protects against metabolic disorders associated with metabolic syndrome and obesity. Nutrients 2021, 13, 430. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Marsh, J.B.; Weinstein, D.B. Simple charring method for determination of lipids. J. Lipid Res. 1966, 7, 574–576. [Google Scholar] [CrossRef]
- Vohra, D.F. Determination of photosynthetic pigments in sea-water. In Monographs Onocéanographie Methodology; UNESCO, Ed.; UNESCO: Paris, France, 1966; p. 66. [Google Scholar]
- Van Heukelem, L.; Thomas, C.S. Computer-assisted high-performance liquid chromatography method development with applications to the isolation and analysis of phytoplankton pigments. J. Chromatogr. A 2001, 910, 31–49. [Google Scholar] [CrossRef]
- Boisen, S.; Fernández, J.A. Prediction of the total tract digestibility of energy in feedstuffs and pig diets by in vitro analyses. Anim. Feed Sci. Technol. 1997, 68, 277–286. [Google Scholar] [CrossRef]
- Batista, A.P.; Niccolai, A.; Fradinho, P.; Fragoso, S.; Bursic, I.; Rodolfi, L.; Biondi, N.; Tredici, M.R.; Sousa, I.; Raymundo, A. Microalgae biomass as an alternative ingredient in cookies: Sensory, physical and chemical properties, antioxidant activity and in vitro digestibility. Algal Res. 2017, 26, 161–171. [Google Scholar] [CrossRef]
- Bondet, V.; Brand-Williams, W.; Berset, C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT—Food Sci. Technol. 1997, 30, 609–615. [Google Scholar] [CrossRef]
- Frohlich, J. Fractional esterification rate of cholesterol and ratio of triglycerides to HDL-cholesterol are powerful predictors of positive findings on coronary angiography. Clin. Chem. 2003, 49, 1873–1880. [Google Scholar] [CrossRef]
- Matthews, D.R.; Hosker, J.P.; Rudenski, A.S.; Naylor, B.A.; Treacher, D.F.; Turner, R.C. Homeostasis model assessment: Insulin resistance and beta-cell function from fasting plasma glucose andinsulin concentrations in man. Diabetologia 1985, 28, 412–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
 ), control group; HF (
), control group; HF (  ), high-fat group; HF-Dia (
), high-fat group; HF-Dia (  ), HF supplemented with D. lutheri group. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference in the HF-Dia group compared with the HF group (p < 0.05).
), HF supplemented with D. lutheri group. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference in the HF-Dia group compared with the HF group (p < 0.05).
 ), control group; HF (
), control group; HF (  ), high-fat group; HF-Dia (
), high-fat group; HF-Dia (  ), HF supplemented with D. lutheri group. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference in the HF-Dia group compared with the HF group (p < 0.05).
), HF supplemented with D. lutheri group. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference in the HF-Dia group compared with the HF group (p < 0.05).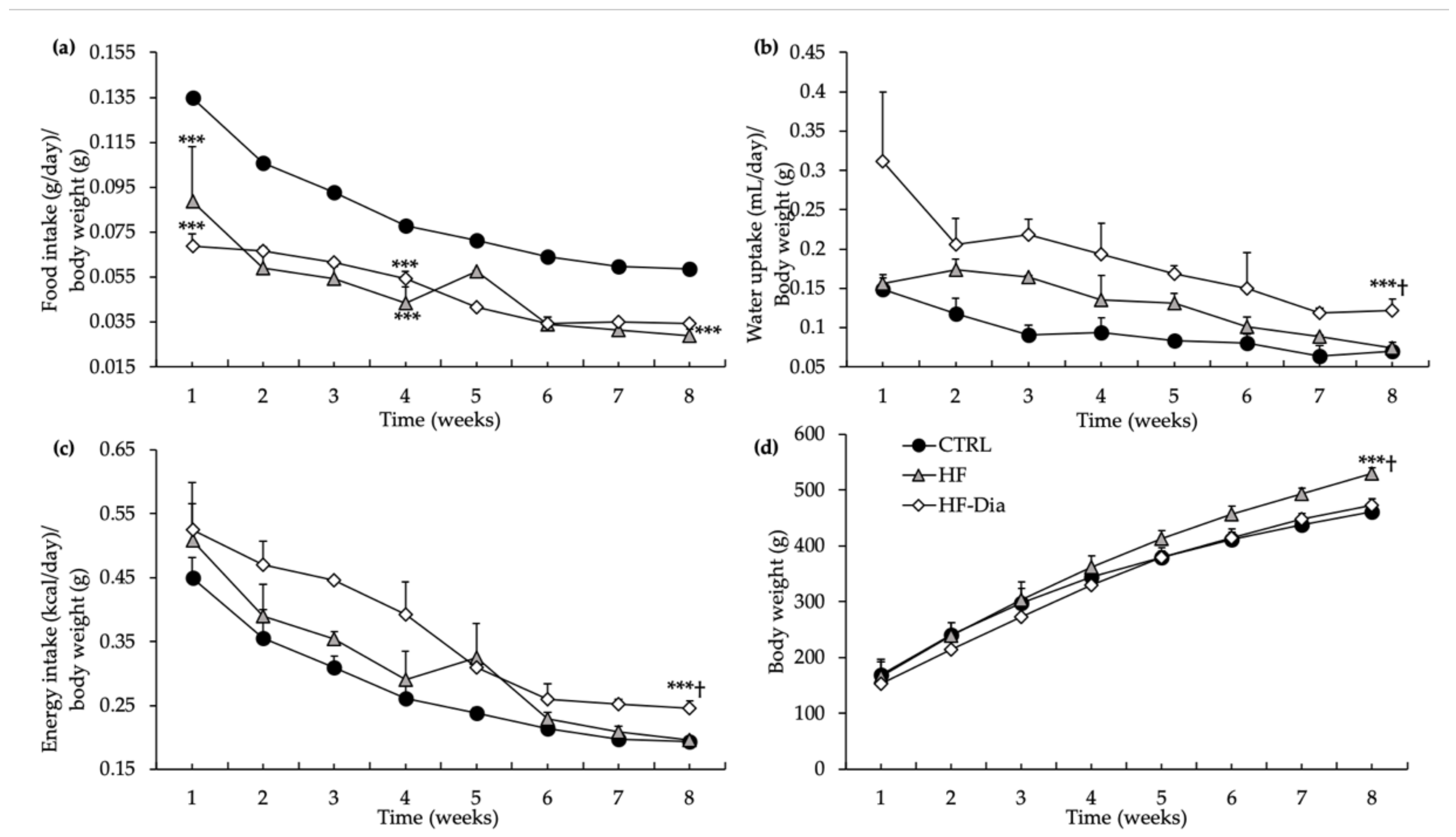
 ), control group; GTT, glucose tolerance test; HF (
), control group; GTT, glucose tolerance test; HF (  ), high-fat group; HF-Dia (
), high-fat group; HF-Dia (  ), high-fat group supplemented with D. lutheri; ITT, insulin tolerance test. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference compared with the HF group (p < 0.05).
), high-fat group supplemented with D. lutheri; ITT, insulin tolerance test. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference compared with the HF group (p < 0.05).
 ), control group; GTT, glucose tolerance test; HF (
), control group; GTT, glucose tolerance test; HF (  ), high-fat group; HF-Dia (
), high-fat group; HF-Dia (  ), high-fat group supplemented with D. lutheri; ITT, insulin tolerance test. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference compared with the HF group (p < 0.05).
), high-fat group supplemented with D. lutheri; ITT, insulin tolerance test. Values are means (n = 6), with standard deviations represented by vertical bars. Statistical significance was determined using ANOVA with post hoc Fisher’s test and mean values were significantly different from those of the CTRL group with *** p < 0.001. † Significant difference compared with the HF group (p < 0.05).
| Plasma Biochemical Parameters | CTRL | HF | HF-Dia |
|---|---|---|---|
| ASAT (UI/L) | 58.72 ± 6.22 | 51.80 *** ± 7.83 | 52.53 *** ± 5.06 |
| ALAT (UI/L) | 44.46 ± 4.57 | 52.10 *** ± 4.10 | 56.29 ***† ± 4.78 |
| ASAT/ALAT | 1.36 ± 0.11 | 1.24 ** ± 0.17 | 1.03 **† ± 0.11 |
| TAG (mmol/L) | 3.07 ± 0.84 | 6.85 *** ± 1.16 | 4.02 ***† ± 0.55 |
| TC (mmol/L) | 2.10± 0.36 | 2.72 *** ± 0.45 | 3.68 ***† ± 0.31 |
| HDL-C(mmol/L) | 2.03 ± 0.27 | 1.72 *** ± 0.20 | 2.60 ***† ± 0.22 |
| LDL-C (mmol/L) | 0.44 ± 0.12 | 0.96 *** ± 0.21 | 0.98 *** ± 0.20 |
| HDL-C/LDL-C | 2.65 ± 0.31 | 1.62 *** ± 0.39 | 2.08 ***† ± 0.41 |
| AIP | 0.32 ± 0.08 | 0.51 *** ± 0.13 | 0.18 ***† ± 0.05 |
| Parameters | CTRL | HF | HF-Dia |
|---|---|---|---|
| Plasma parameters | |||
| IL-6 (pg/mL) | 26.83 ± 2.49 | 51.80 *** ± 7.83 | 56.87 ***† ± 8.27 |
| TNF-α (pg/mL) | 22.13 ± 2.96 | 41.08 *** ± 3.30 | 42.11 *** ± 5.54 |
| IL-4 (pg/mL) | 41.11 ± 6.37 | 25.87 ***± 6.54 | 44.70 ***† ± 6.32 |
| Adipocyte parameter | |||
| IL-10 (ng/mL) | 0.73 ± 0.11 | 0.46 *** ± 0.08 | 0.73 ***† ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mayer, C.; Côme, M.; Ulmann, L.; Martin, I.; Zittelli, G.C.; Faraloni, C.; Ouguerram, K.; Chénais, B.; Mimouni, V. The Potential of the Marine Microalga Diacronema lutheri in the Prevention of Obesity and Metabolic Syndrome in High-Fat-Fed Wistar Rats. Molecules 2022, 27, 4246. https://doi.org/10.3390/molecules27134246
Mayer C, Côme M, Ulmann L, Martin I, Zittelli GC, Faraloni C, Ouguerram K, Chénais B, Mimouni V. The Potential of the Marine Microalga Diacronema lutheri in the Prevention of Obesity and Metabolic Syndrome in High-Fat-Fed Wistar Rats. Molecules. 2022; 27(13):4246. https://doi.org/10.3390/molecules27134246
Chicago/Turabian StyleMayer, Claire, Martine Côme, Lionel Ulmann, Isabelle Martin, Graziella Chini Zittelli, Cecilia Faraloni, Khadija Ouguerram, Benoît Chénais, and Virginie Mimouni. 2022. "The Potential of the Marine Microalga Diacronema lutheri in the Prevention of Obesity and Metabolic Syndrome in High-Fat-Fed Wistar Rats" Molecules 27, no. 13: 4246. https://doi.org/10.3390/molecules27134246
APA StyleMayer, C., Côme, M., Ulmann, L., Martin, I., Zittelli, G. C., Faraloni, C., Ouguerram, K., Chénais, B., & Mimouni, V. (2022). The Potential of the Marine Microalga Diacronema lutheri in the Prevention of Obesity and Metabolic Syndrome in High-Fat-Fed Wistar Rats. Molecules, 27(13), 4246. https://doi.org/10.3390/molecules27134246








