Evaluation of Acidic Ionic Liquids as Catalysts for Furfural Production from Eucalyptus nitens Wood
Abstract
:1. Introduction
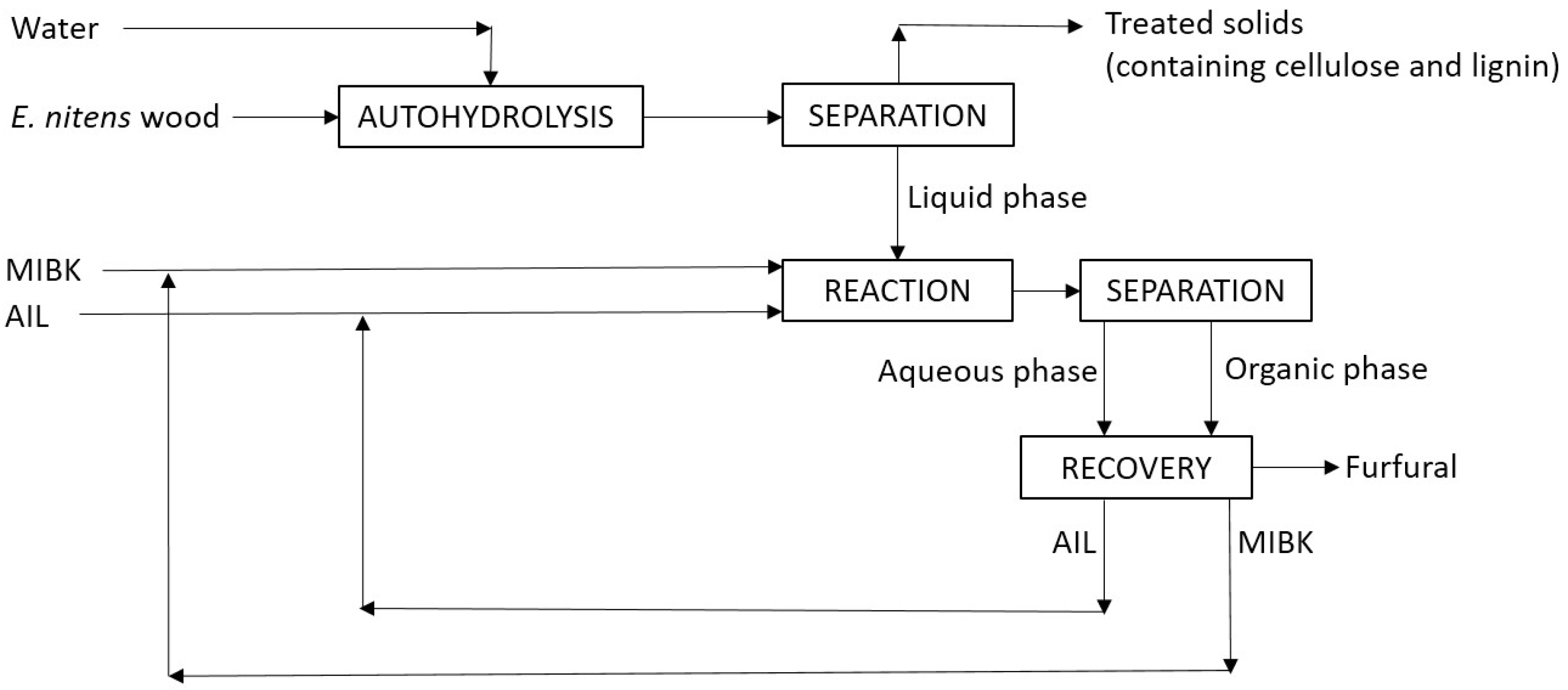
- (a)
- Partial hydrolysis of pentosans (xylan or arabinan, with formula (C5H8O4)n) via autohydrolysis, involving the uptake of 1 mol water/mol anhydrosugar, to yield soluble low-molecular-weight polysaccharides or oligosaccharides made up of anhydropentoses (with formula (C5H8O4)k, where n and k are the polymerization degrees of pentosans and soluble higher saccharides, respectively;
- (b)
- Hydrolysis of the above low-molecular-weight polysaccharides or oligosaccharides made up of anhydropentoses into pentoses (xylose or arabinose, with formula C5H10O5, consuming 1 mol water/mol anhydrosugar);
- (c)
- Dehydration of the above pentoses (xylose or arabinose) into furfural (C5H4O2), releasing 3 mol water/mol pentose.
2. Results and Discussion
2.1. Wood Processing
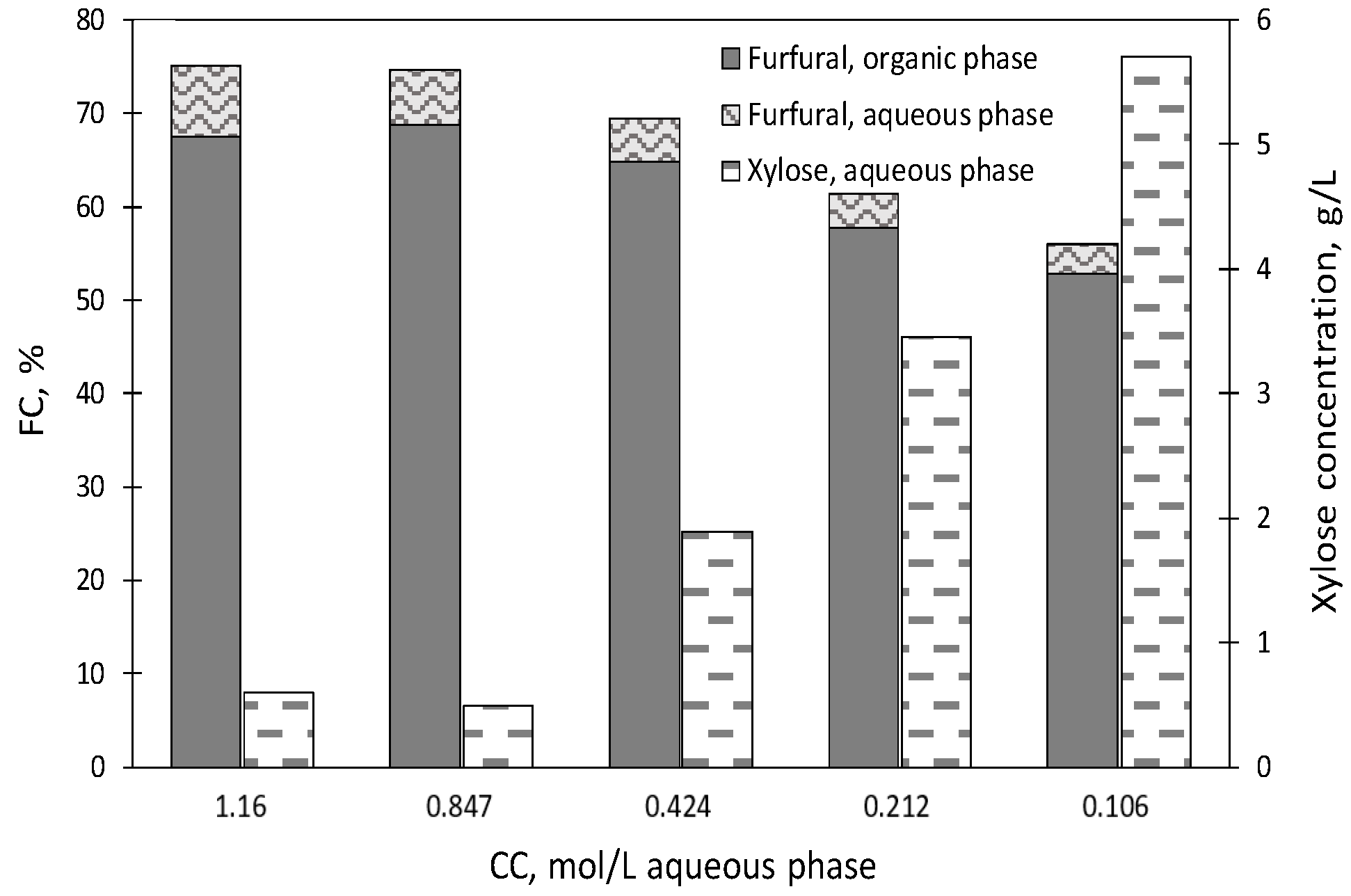
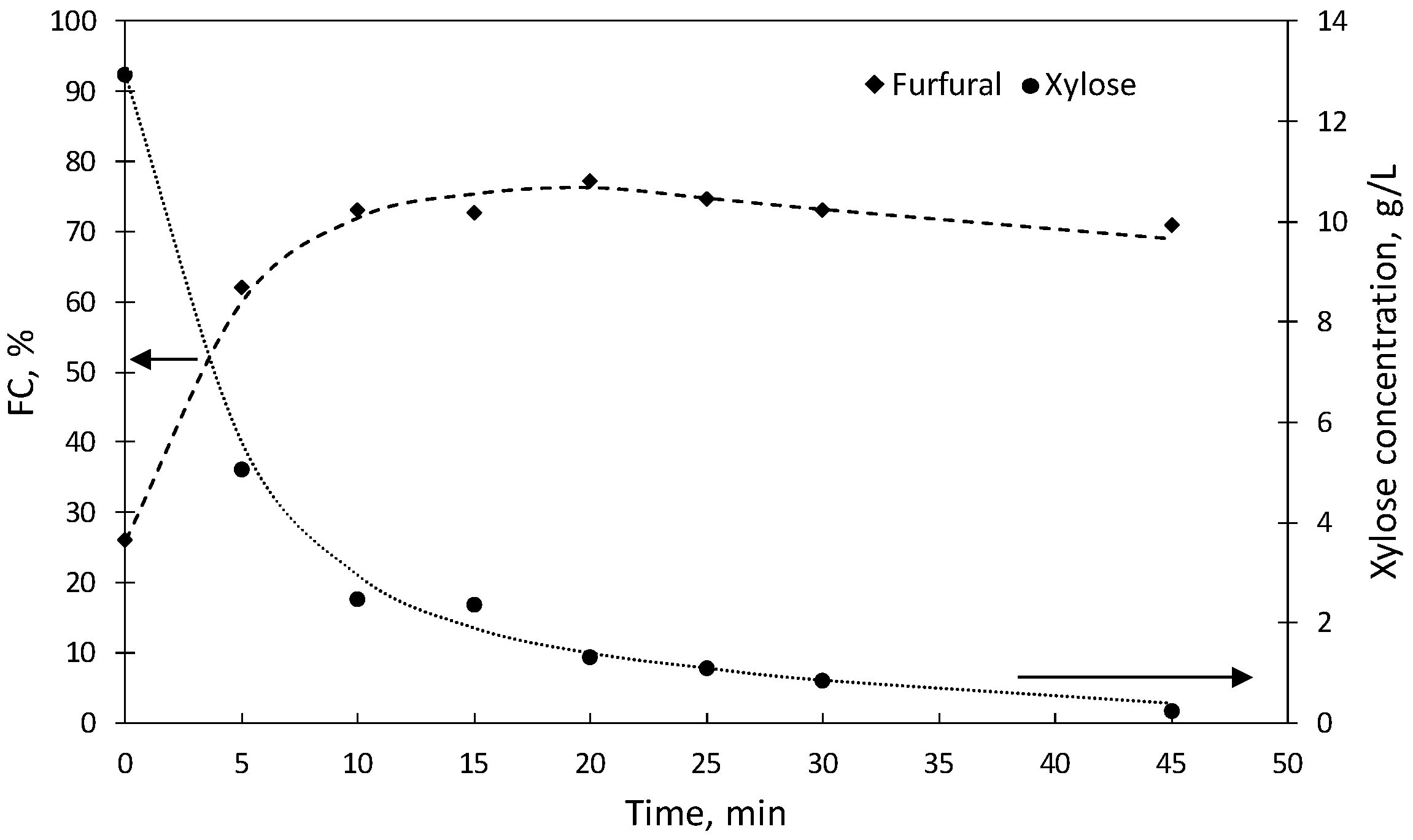
2.2. Experiments Using [C4mim]HSO4 as a Catalyst
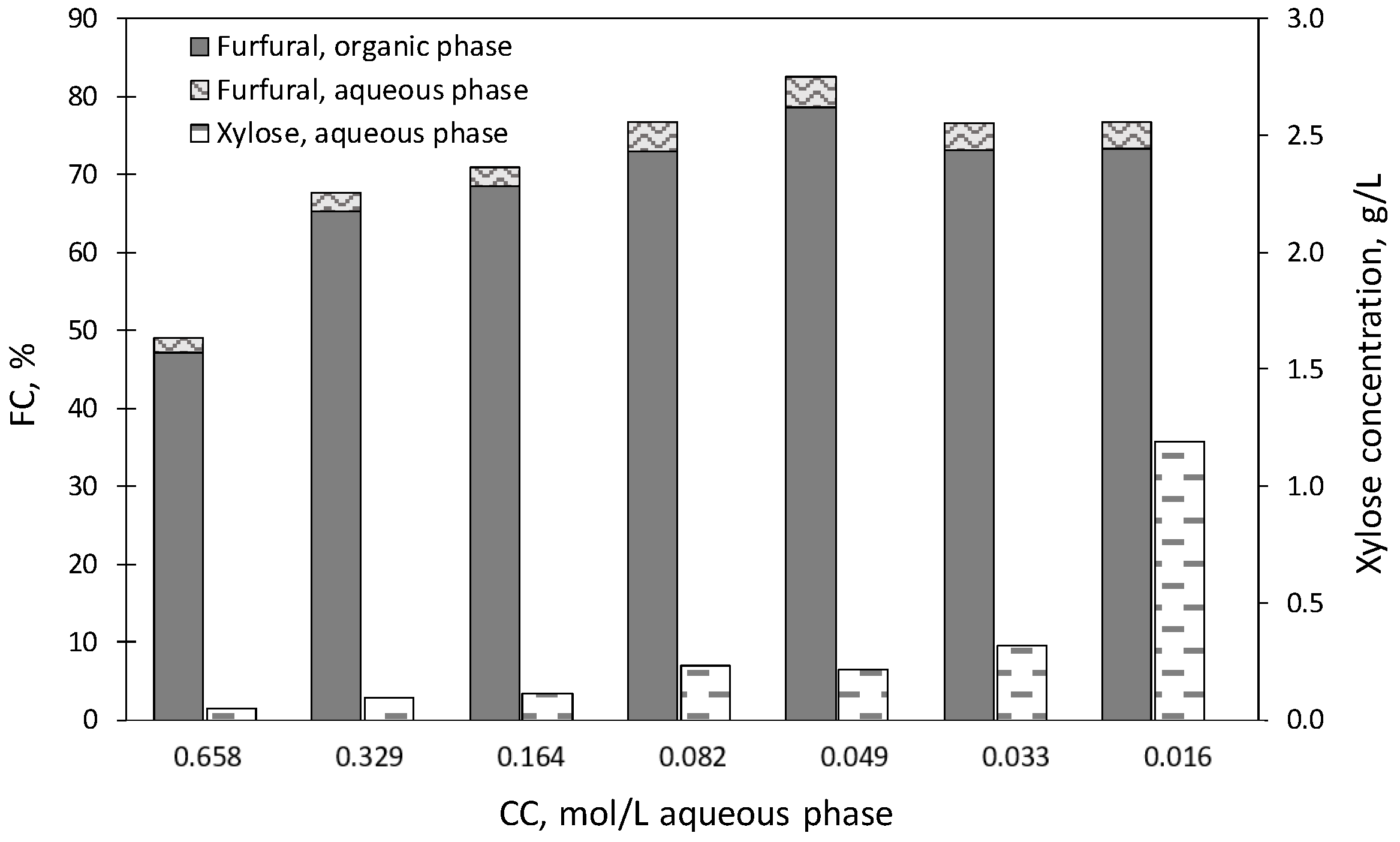
2.3. Experiments Using [C3SO3Hmim]HSO4 as a Catalyst
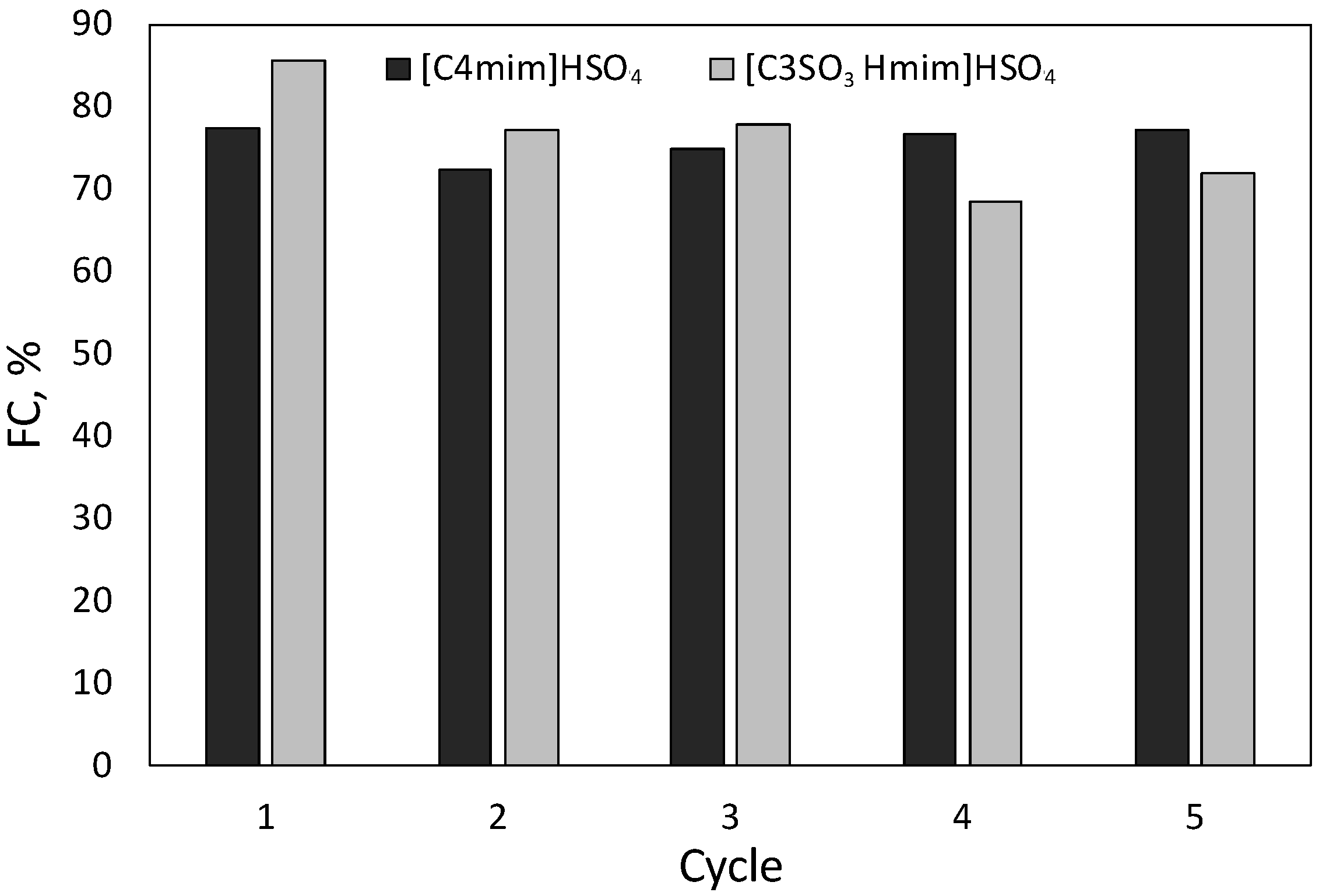
2.4. Catalyst Recycling
3. Materials and Methods
3.1. Materials and Reaction in Biphasic Media
3.2. Analytical Methods
3.3. AIL Reutilization
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- European Commission. The European Green Deal. Communication from the Commission to the European Parliament, the European Council, the Council, the European Economic and Social Committee and the Committee of the Regions. Brussels. 2019. Available online: https://eur-lex.europa.eu/resource.html?uri=cellar:b828d165-1c22-11ea-8c1f-01aa75ed71a1.0002.02/DOC_1&format=PDF (accessed on 22 April 2022).
- International Energy Agency. IEA Bioenergy Task 42 Biorefining. Sustainable and Synergetic Processing of Biomass into Marketable Food & Feed Ingredients, Chemicals, Materials and Energy (Fuels, Power, Heat). 2014. Available online: https://www.ieabioenergy.com/wp-content/uploads/2014/09/IEA-Bioenergy-Task42-Biorefining-Brochure-SEP2014_LR.pdf (accessed on 22 April 2022).
- Liguori, R.; Faraco, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Velvizhi, G.; Balakumar, K.; Shetti, N.P.; Ahmad, E.; Pant, K.K.; Aminabhavi, T.M. Integrated biorefinery processes for conversion of lignocellulosic biomass to value added materials: Paving a path towards circular economy. Bioresour. Technol. 2022, 343, 126151. [Google Scholar] [CrossRef] [PubMed]
- Cherubini, F.; Strømman, A.H. Life cycle assessment of bioenergy systems: State of the art and future challenges. Bioresour. Technol. 2011, 102, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Magaton, A.D.A.S.; Colodette, J.L.; De Gouvêa, A.F.G.; Gomide, J.L.; Muguet, M.C.D.S.; Pedrazzi, C. Eucalyptus wood quality and its impact on kraft pulp production and use. TAPPI J. 2009, 8, 32–39. [Google Scholar]
- Pérez, S.; Renedo, C.J.; Ortiz, A.; Mañana, M.; Silió, D. Energy evaluation of the Eucalyptus globulus and the Eucalyptus nitens in the north of Spain (Cantabria). Thermochim. Acta 2006, 451, 57–64. [Google Scholar] [CrossRef]
- Valente, C.; Gonçalves, C.I.; Monteiro, F.; Gaspar, J.; Silva, M.; Sottomayor, M.; Paiva, M.R.; Branco, M. Economic outcome of classical biological control: A case study on the Eucalyptus snout bettle, Gonipterus platensis, and the parasitoid Anaphes nitens. Ecol. Econ. 2018, 149, 40–47. [Google Scholar] [CrossRef]
- Pérez-Cruzado, C.; Muñoz-Sáez, F.; Basurco, F.; Riesco, G.; Rodríguez-Soalleiro, R. Combining empirical models and the process-based model 3-PG to predict Eucalyptus nitens plantations growth in Spain. Forest Ecol. Manag. 2011, 262, 1067–1077. [Google Scholar] [CrossRef]
- Gullón, B.; Dávila, I.; García-Torreiro, M.; Yáñez, R.; Labidi, J.; Gullón, P. Production and emerging applications of bioactive oligosaccharides from biomass hemicelluloses by hydrothermal processing. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass; Ruiz, H.A., Thomsen, H.T., Trajano, H.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 253–283. [Google Scholar] [CrossRef]
- Michelin, M.; Romaní, A.; Salgado, J.M.; Domingues, L.; Teixeira, J.A. Production of hemicellulases, xylitol, and furan from hemicellulosic hydrolysates using hydrothermal pretreatment. In Hydrothermal Processing in Biorefineries: Production of Bioethanol and High Added-Value Compounds of Second and Third Generation Biomass; Ruiz, H.A., Thomsen, H.T., Trajano, H.L., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 285–315. [Google Scholar] [CrossRef]
- Ruiz, H.A.; Rodríguez-Jasso, M.A.; Fernandes, B.D.; Vicente, A.A.; Teixeira, J.A. Hydrothermal processing as an alternative for upgrading agriculture residues and marine biomass according to the biorefinery concept: A review. Renew. Sustain. Energy Rev. 2013, 21, 35–51. [Google Scholar] [CrossRef] [Green Version]
- González-Muñoz, M.J.; Rivas, S.; Santos, V.; Parajó, J.C. Aqueous processing of Pinus pinaster wood: Kinetics of polysaccharide breakdown. Chem. Eng. J. 2013, 231, 380–387. [Google Scholar] [CrossRef]
- Rivas, S.; González-Muñoz, M.J.; Santos, V.; Parajó, J.C. Production of furans from hemicellulosic saccharides in biphasic reaction systems. Holzforschung 2013, 67, 923–929. [Google Scholar] [CrossRef]
- Vila, C.; Romero, J.; Francisco, J.L.; Garrote, G.; Parajó, J.C. Extracting value from Eucalyptus wood before kraft pulping: Effects of hemicelluloses solubilization on pulp properties. Bioresour. Technol. 2011, 102, 5251–5254. [Google Scholar] [CrossRef]
- Vila, C.; Romero, J.; Francisco, J.L.; Santos, V.; Parajó, J.C. On the recovery of hemicellulose before kraft pulping. BioResources 2012, 7, 4179–4189. [Google Scholar]
- Werpy, T.; Petersen, G. Top Value Added Chemicals from Biomass: Volume I—Results of Screening for Potential Candidates from Sugars and Synthesis Gas; NREL/TP-510-35523; National Renewable Energy Laboratory: Golden, CO, USA, 2004. Available online: http://www.nrel.gov/docs/fy04osti/35523.pdf (accessed on 22 April 2022).
- Bozell, J.J.; Petersen, G.R. Technology development for the production of biobased products from biorefinery carbohydrates—the US Department of Energy’s “Top 10” revisited. Green Chem. 2010, 12, 539–555. [Google Scholar] [CrossRef]
- Peleteiro, S.; Rivas, S.; Alonso, J.L.; Santos, V.; Parajó, J.C. Furfural production using ionic liquids: A review. Bioresour. Technol. 2016, 202, 181–191. [Google Scholar] [CrossRef]
- Zeitsch, K.J. The chemistry and technology of furfural and its many byproducts. In Sugar Series; Elsevier Science: Amsterdam, The Netherlands, 2000; Volume 13, pp. 98–104. ISBN 9780080528991. [Google Scholar]
- Zhang, L.; Xi, G.; Yu, K.; Yu, H.; Wang, X. Furfural Production from biomass-derived carbohydrates and lignocellulosic residues via heterogeneous acid catalysts. Ind. Crop Prod. 2017, 98, 68–75. [Google Scholar] [CrossRef]
- Peleteiro, S.; Santos, V.; Garrote, G.; Parajó, J.C. Furfural production from Eucalyptus wood using an acidic ionic liquid. Carbohydr. Polym. 2016, 146, 20–25. [Google Scholar] [CrossRef]
- Hoydonckx, H.E.; Van Rhijn, W.M.; Van Rhijn, W.; De Vos, D.E.; Jacobs, P.A. Furfural and derivatives. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co.: Weinheim, Germany, 2007. [Google Scholar] [CrossRef]
- Peleteiro, S.; Raspolli-Galetti, A.; Antoneti, C.; Santos, V.; Parajó, J.C. Manufacture of furfural from xylan-containing biomass by acidic processing of hemicellulose-derived saccharides in biphasic media using microwave heating. J. Wood Chem. Technol. 2018, 38, 198–213. [Google Scholar] [CrossRef]
- Kim, E.S.; Liu, S.; Abu-Omar, M.M.; Mosier, N.S. Selective conversion of biomass hemicellulose to furfural using maleic acid with microwave eating. Energy Fuels 2012, 26, 1298–1304. [Google Scholar] [CrossRef]
- Earle, M.J.; Seddon, K.R. Ionic liquids: Green solvents for the future. ACS Symp. Ser. 2002, 819, 10–25. [Google Scholar] [CrossRef] [Green Version]
- Huddleston, J.G.; Visser, A.E.; Reichert, W.M.; Willauer, H.D.; Broker, G.A.; Rogers, R.D. Characterization and comparison of hydrophilic and hydrophobic room temperature ionic liquids incorporating the imidazolium cation. Green Chem. 2001, 3, 156–164. [Google Scholar] [CrossRef]
- Stark, A. Ionic liquids in the biorefinery: A critical assessment of their potential. Energy Environ. Sci. 2011, 4, 19–32. [Google Scholar] [CrossRef]
- Guenic, S.L.; Delbecq, F.; Ceballos, C.; Len, C. Microwave-assisted dehydration of D-xylose into furfural by diluted inexpensive inorganic salts solution in a biphasic system. J. Mol. Catal. A Chem. 2015, 410, 1–7. [Google Scholar] [CrossRef]
- Yemis, O.; Mazza, G. Acid-catalyzed conversion of xylose, xylan and xtraw into furfural. Bioresour. Technol. 2011, 102, 7371–7378. [Google Scholar] [CrossRef]
- Prat, D.; Wells, A.; Hayler, J.; Sneddon, H.; McElroy, C.R.; Abou-Shehada, S.; Dunn, P.J. CHEM21 selection guide of classical- and less classical-solvents. Green Chem. 2015, 18, 288–296. [Google Scholar] [CrossRef] [Green Version]
- Tao, F.; Song, H.; Chou, L. Efficient process for the conversion of xylose to furfural with acidic ionic liquid. Can. J. Chem. 2011, 89, 83–87. [Google Scholar] [CrossRef]
- Peleteiro, S.; da Costa Lopes, A.M.; Garrote, G.; Parajó, J.C.; Bogel-Łukasik, R. Simple and efficient furfural production from xylose in media containing 1-butyl- 3-methylimidazolium hydrogen sulfate. Ind. Eng. Chem. Res. 2015, 54, 8368–8373. [Google Scholar] [CrossRef] [Green Version]
- Penín, L.; Santos, V.; del Río, J.C.; Parajó, J.C. Assesment on the chemical fractionation of Eucalyptus nitens wood: Characterization of the products derived from the structural components. Bioresour. Technol. 2019, 281, 269–276. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous petreatments. Philos. Trans. R. Soc. A 1987, 321, 523–536. [Google Scholar]
- Peleteiro, S.; Santos, V.; Parajó, J.C. Furfural production in biphasic media using an acidic ionic liquid as a catalyst. Carbohydr. Polym. 2016, 153, 421–428. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, Y.; Lin, H.; Chen, J.; Zhu, L.; Luo, Z. Conversion of C5 carbohydrates into furfural catalyzed by a Lewis acidic ionic liquid in renewable γ-valerolactone. Green Chem. 2017, 19, 3869–3879. [Google Scholar] [CrossRef]
- Xia, Q.; Peng, H.; Zhang, Y.; Fu, G.; Liu, Y.; Xiao, Z.; Huang, L.; Bi, H. Microwave-assisted furfural production from xylose and bamboo hemicellulose in a biphasic medium. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Matsagar, B.M.; Munshi, M.K.; Kelkar, A.A.; Dhepe, P.L. Conversion of concentrated sugar solutions into 5-hydroxymethyl furfural and furfural using Brönsted acidic ionic liquids. Catal. Sci. Technol. 2015, 5, 5086–5090. [Google Scholar] [CrossRef]
- Lin, H.; Chen, J.; Zhao, Y.; Wang, S. Conversion of C5 Carbohydrates into furfural catalyzed by SO3H-functionalized ionic liquid in renewable γ-valerolactone. Energy Fuels 2017, 31, 3929–3934. [Google Scholar] [CrossRef]
- López, M.; Rivas, S.; Vila, C.; Santos, V.; Parajó, J.C. Performance of 1-(3-sulfopropyl)-3-methylimidazolium hydrogen sulfate as a catalyst for hardwood upgrading into bio-based platform chemicals. Catalysts 2020, 10, 937. [Google Scholar] [CrossRef]

| Experiment | OAR (g/g) | FC (%) | ||
|---|---|---|---|---|
| Aqueous Phase | Organic Phase | Total | ||
| 1 | 1 | 12.4 ± 0.11 | 60.1 ± 0.50 | 72.5 ± 0.39 |
| 2 | 2 | 7.5 ± 0.004 | 67.5 ± 0.71 | 75.0 ± 0.71 |
| 3 | 3 | 4.9 ± 0.06 | 70.4 ± 0.57 | 75.3 ± 0.51 |
| 4 | 4 | 3.4 ± 0.01 | 71.2 ± 0.26 | 74.6 ± 0.27 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Penín, L.; López, M.; Santos, V.; Parajó, J.C. Evaluation of Acidic Ionic Liquids as Catalysts for Furfural Production from Eucalyptus nitens Wood. Molecules 2022, 27, 4258. https://doi.org/10.3390/molecules27134258
Penín L, López M, Santos V, Parajó JC. Evaluation of Acidic Ionic Liquids as Catalysts for Furfural Production from Eucalyptus nitens Wood. Molecules. 2022; 27(13):4258. https://doi.org/10.3390/molecules27134258
Chicago/Turabian StylePenín, Lucía, Mar López, Valentín Santos, and Juan Carlos Parajó. 2022. "Evaluation of Acidic Ionic Liquids as Catalysts for Furfural Production from Eucalyptus nitens Wood" Molecules 27, no. 13: 4258. https://doi.org/10.3390/molecules27134258
APA StylePenín, L., López, M., Santos, V., & Parajó, J. C. (2022). Evaluation of Acidic Ionic Liquids as Catalysts for Furfural Production from Eucalyptus nitens Wood. Molecules, 27(13), 4258. https://doi.org/10.3390/molecules27134258






