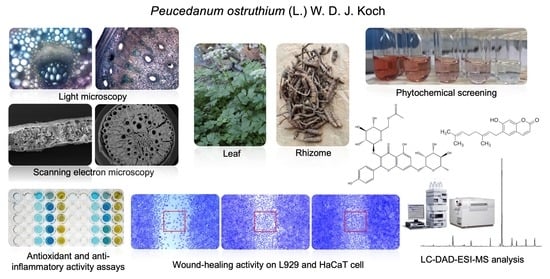
Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch
Abstract
:1. Introduction
2. Results
2.1. Micromorphological Analysis
2.2. Phytochemical Analyses
2.3. Antioxidant and Anti-Inflammatory Activities
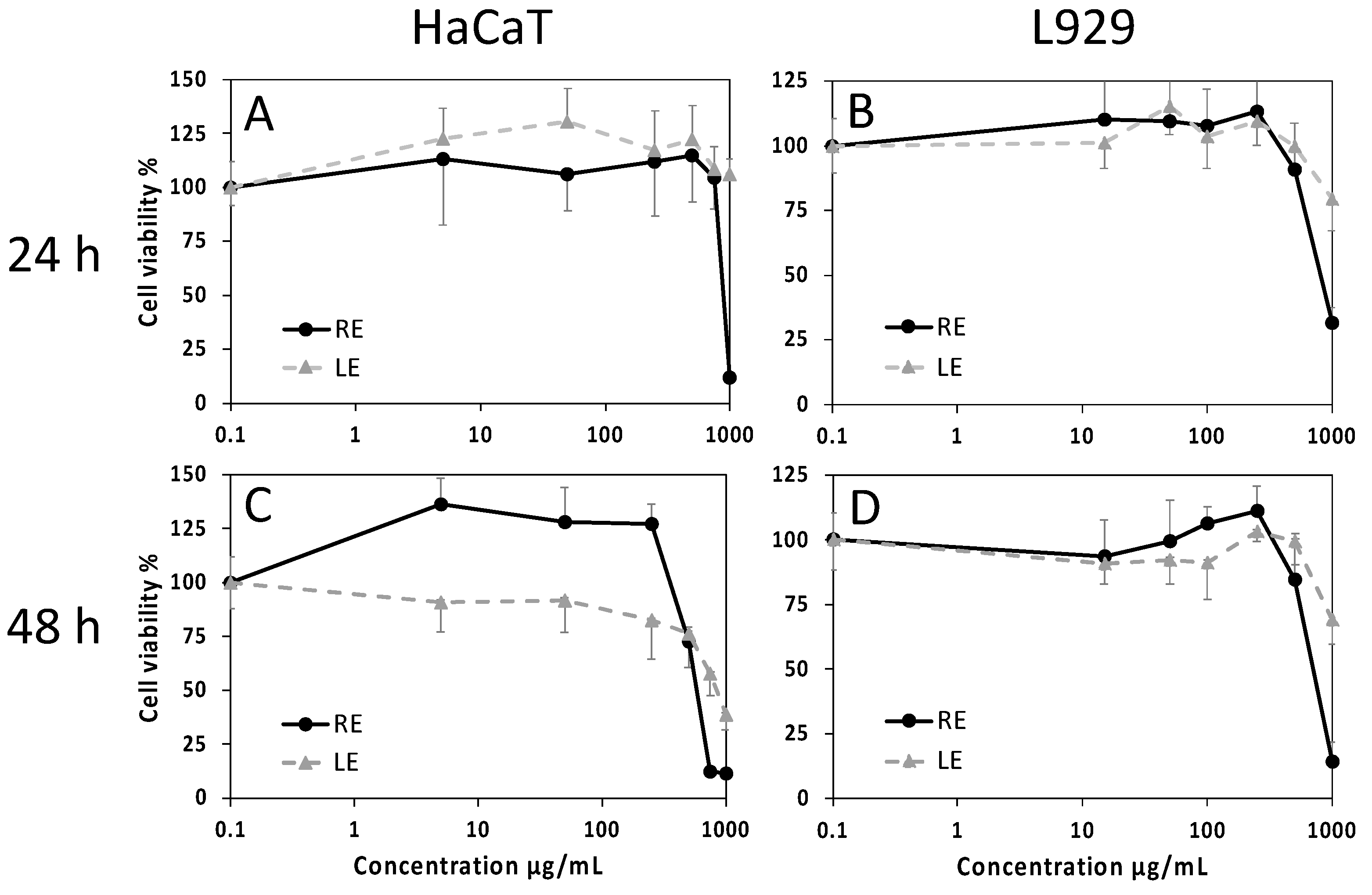
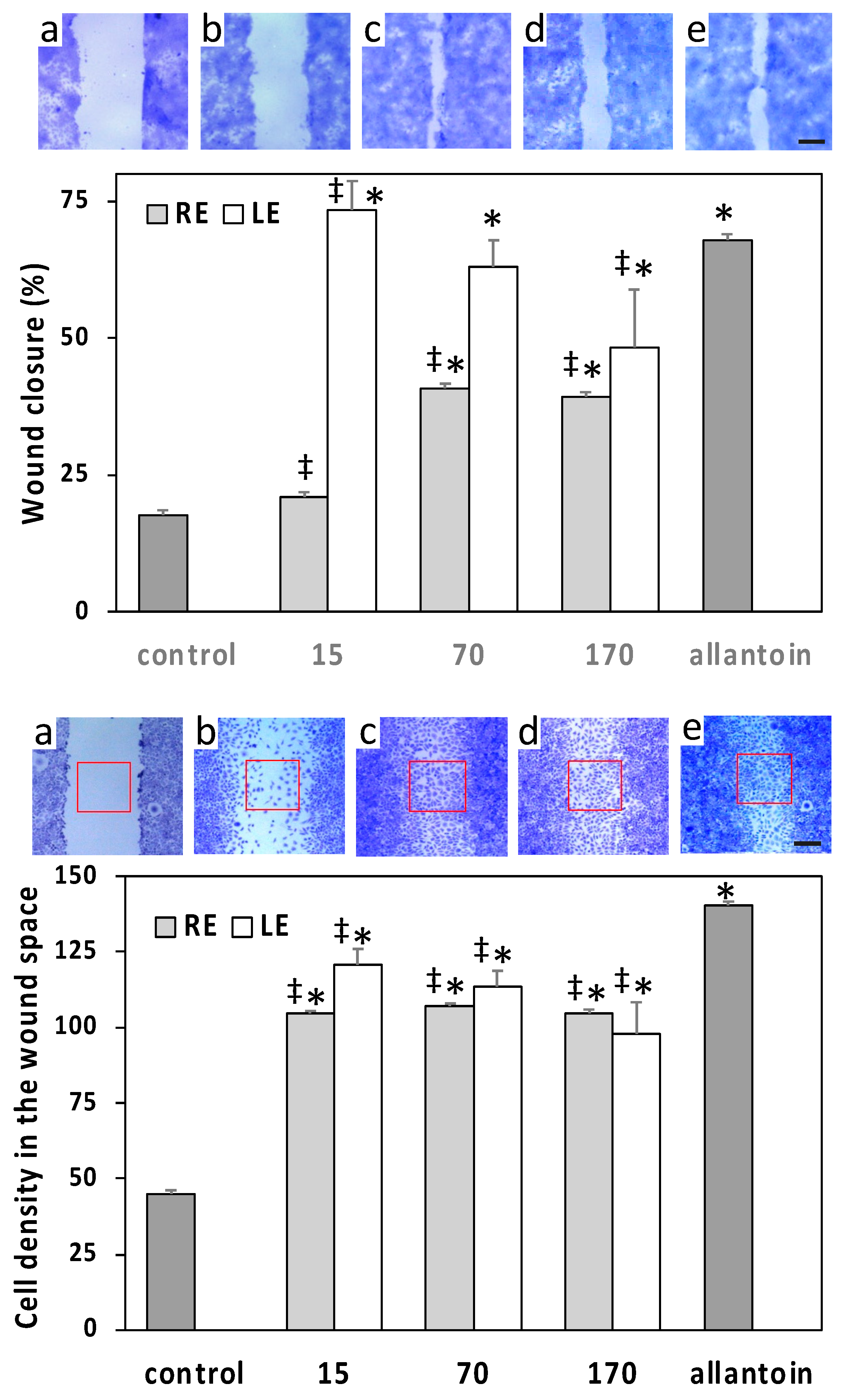
2.4. Cell Viability and Wound-Healing Activity
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Chemicals and Cells
4.3. Micromorphological Analyses
4.4. Sample Preparation
4.5. Phytochemical Screening
4.5.1. Total Phenols
4.5.2. Flavonoids
4.5.3. Vanillin Index
4.5.4. Proanthocyanidins
4.6. Polyphenol Profile by RP-LC-DAD-ESI-MS Analysis
4.7. Antioxidant Activity
4.7.1. DPPH Assay
4.7.2. TEAC Assay
4.7.3. FRAP Assay
4.7.4. ORAC Assay
4.8. Anti-Inflammatory Activity
4.8.1. Bovine Serum Albumin (BSA) Denaturation Assay
4.8.2. Protease Inhibition Assay
4.8.3. Lipoxygenase (LOX) and Cyclooxygenase (COX-2) Inhibition Assays
4.9. Cell Viability and Wound Healing Assays
4.9.1. Cell Viability Assay
4.9.2. Wound Healing Test
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Coulter, J.M.; Rose, J.N. Monograph of the North American Umbelliferae; Contributions from the United States National Herbarium: Washington, DC, USA, 1900; Volume 7, pp. 9–256. [Google Scholar]
- Danna, C.; Poggio, L.; Smeriglio, A.; Mariotti, M.; Cornara, L. Ethnomedicinal and Ethnobotanical Survey in the Aosta Valley Side of the Gran Paradiso National Park (Western Alps, Italy). Plants 2022, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Petelka, J.; Plagg, B.; Säumel, I.; Zerbe, S. Traditional medicinal plants in South Tyrol (northern Italy, southern Alps): Biodiversity and use. J. Ethnobiol. Ethnomed. 2020, 16, 74. [Google Scholar] [CrossRef] [PubMed]
- Abbet, C.; Mayor, R.; Roguet, D.; Spichiger, R.; Hamburger, M.; Potterat, O. Ethnobotanical survey on wild alpine food plants in Lower and Central Valais (Switzerland). J. Ethnopharmacol. 2014, 151, 624–634. [Google Scholar] [CrossRef]
- Gokay, O.; Kuhner, D.; Los, M.; Gotz, F.; Bertsche, U.; Albert, K. An efficient approach for the isolation, identification and evaluation of antimicrobial plant components on an analytical scale, demonstrated by the example of Radix imperatoriae. Anal. Bioanal. Chem. 2010, 398, 2039–2047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adams, M.; Berset, C.; Kessler, M.; Hamburger, M. Medicinal herbs for the treatment of rheumatic disorders—A survey of European herbals from the 16th and 17th century. J. Ethnopharmacol. 2009, 121, 343–359. [Google Scholar] [CrossRef]
- Cisowski, W.; Sawicka, U.; Mardarowicz, M.; Asztemborska, M.; Luczkiewicz, M. Essential oil from herb and rhizome of Peucedanum ostruthium (L. Koch.) ex DC. Z. Nat. C J. Biosci. 2001, 56, 930–932. [Google Scholar] [CrossRef]
- Hiermann, A.; Schantl, D. Antiphlogistic and antipyretic activity of Peucedanum ostruthium. Planta Med. 1998, 64, 400–403. [Google Scholar] [CrossRef]
- Sarkhail, P. Traditional uses, phytochemistry and pharmacological properties of the genus Peucedanum: A review. J. Ethnopharmacol. 2014, 156, 235–270. [Google Scholar] [CrossRef]
- Lammel, C.; Zwirchmayr, J.; Seigner, J.; Rollinger, J.M.; de Martin, R. Peucedanum ostruthium Inhibits E-Selectin and VCAM-1 Expression in Endothelial Cells through Interference with NF-kappaB Signaling. Biomolecules 2020, 10, 1215. [Google Scholar] [CrossRef]
- Urbain, A.; Marston, A.; Hostettmann, K. Coumarins from Peucedanum ostruthium as inhibitors of acetylcholinesterase. Pharm. Biol. 2005, 43, 647–650. [Google Scholar] [CrossRef] [Green Version]
- Joa, H.; Vogl, S.; Atanasov, A.G.; Zehl, M.; Nakel, T.; Fakhrudin, N.; Heiss, E.H.; Picker, P.; Urban, E.; Wawrosch, C.; et al. Identification of ostruthin from Peucedanum ostruthium rhizomes as an inhibitor of vascular smooth muscle cell proliferation. J. Nat. Prod. 2011, 74, 1513–1516. [Google Scholar] [CrossRef]
- Schinkovitz, A.; Gibbons, S.; Stavri, M.; Cocksedge, M.J.; Bucar, F. Ostruthin: An antimycobacterial coumarin from the roots of Peucedanum ostruthium. Planta Med. 2003, 69, 369–371. [Google Scholar] [CrossRef] [Green Version]
- Cousyn, G.; Dalfrà, S.; Scarpa, B.; Geelen, J.; Anton, R.; Serafini, M.; Delmulle, L. Project BELFRIT: Harmonizing the Use of Plants in Food Supplements in the European Union: Belgium, France and Italy—A first Step. Eur. Food Feed. Law Rev. 2013, 8, 187–196. [Google Scholar]
- Palmioli, A.; Bertuzzi, S.; de Luigi, A.; Colombo, L.; la Ferla, B.; Salmona, M.; de Noni, I.; Airoldi, C. bioNMR-based identification of natural anti-Abeta compounds in Peucedanum ostruthium. Bioorg. Chem. 2019, 83, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Vitalini, S.; Palmioli, A.; Orlando, F.; Scari, G.; Airoldi, C.; de Noni, I.; Bocchi, S.; Iriti, M. Phytotoxicity, nematicidal activity and chemical constituents of Peucedanum ostruthium (L.) WDJKoch (Apiaceae). Ind. Crop. Prod. 2021, 166, 113499. [Google Scholar] [CrossRef]
- Vogl, S.; Zehl, M.; Picker, P.; Urban, E.; Wawrosch, C.; Reznicek, G.; Saukel, J.; Kopp, B. Identification and quantification of coumarins in Peucedanum ostruthium (L.) Koch by HPLC-DAD and HPLC-DAD-MS. J. Agric. Food Chem. 2011, 59, 4371–4377. [Google Scholar] [CrossRef] [PubMed]
- McCardell, J.H.; Heritier, J.; Simonnet, X.; Carlen, C. P 10: Peucedanum ostruthium (L.) Koch: Morphological and phytochemical variability of twelve accessions from the Swiss alpine region. Julius-Kuhn-Arch 2016, 453, 121–123. [Google Scholar]
- Nani, M.; Leone, A.; Bom, V.P.; Buszinski, A.F.; de Souza, R.O.; Pinheiro, V.A.; Danapoulos, P.; Swikidisa, R.; Marquele-Oliveira, F.; Frade, M.A.C.; et al. Evaluation and Comparison of Wound Healing Properties of an Ointment (AlpaWash) Containing Brazilian Micronized Propolis and Peucedanum ostruthium Leaf Extract in Skin Ulcer in Rats. Int. J. Pharm. Compd. 2018, 22, 154–163. [Google Scholar]
- Nguyen, V.T.; Sakoff, J.A.; Scarlett, C.J. Physicochemical Properties, Antioxidant and Anti-proliferative Capacities of Dried Leaf and Its Extract from Xao tam phan (Paramignya trimera). Chem. Biodivers 2017, 14, e1600498. [Google Scholar] [CrossRef]
- Yang, H.B.; Gao, H.R.; Ren, Y.J.; Fang, F.X.; Tian, H.T.; Gao, Z.J.; Song, W.; Huang, S.M.; Zhao, A.F. Effects of isoimperatorin on proliferation and apoptosis of human gastric carcinoma cells. Oncol. Lett. 2018, 15, 7993–7998. [Google Scholar] [CrossRef]
- Liu, W.; Li, J.; Zhang, X.; Zu, Y.; Yang, Y.; Liu, W.; Xu, Z.; Gao, H.; Sun, X.; Jiang, X.; et al. Current Advances in Naturally Occurring Caffeoylquinic Acids: Structure, Bioactivity, and Synthesis. J. Agric. Food Chem. 2020, 68, 10489–10516. [Google Scholar] [CrossRef] [PubMed]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin Improves Inflammation, Oxidative Stress, and Impaired Wound Healing in Atopic Dermatitis Model of Human Keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.P.; Mani, I.; Iversen, L.; Ziboh, V.A. Effects of naturally-occurring flavonoids and biflavonoids on epidermal cyclooxygenase and lipoxygenase from guinea-pigs. Prostaglandins Leukot Essent Fat. Acids 1998, 58, 17–24. [Google Scholar] [CrossRef]
- Boukamp, P.; Petrussevska, R.T.; Breitkreutz, D.; Hornung, J.; Markham, A.; Fusenig, N.E. Normal keratinization in a spontaneously immortalized aneuploid human keratinocyte cell line. J. Cell Biol. 1988, 106, 761–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gahan, P.B. Plant Histochemistry and Cytochemistry. An Introduction; Academic Press: London, UK, 1984. [Google Scholar]
- Chieco, C.; Rotondi, A.; Morrone, L.; Rapparini, F.; Baraldi, R. An ethanol-based fixation method for anatomical and micro-morphological characterization of leaves of various tree species. Biotech. Histochem. 2013, 88, 109–119. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Trombetta, D.; Ragusa, S.; Circosta, C. New Insights on Euphorbia dendroides L. (Euphorbiaceae): Polyphenol Profile and Biological Properties of Hydroalcoholic Extracts from Aerial Parts. Plants 2021, 10, 1621. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Ragusa, S.; Monforte, M.T.; D’Angelo, V.; Circosta, C. Phytochemical analysis and evaluation of antioxidant and anti-acetylcholinesterase activities of Euphorbia dendroides L. (Euphorbiaceae) latex. Plant Biosystems 2019, 153, 498–505. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Barreca, D.; D’Angelo, V.; Germano, M.P.; Trombetta, D. Polyphenolic profile and biological activities of black carrot crude extract (Daucus carota L. ssp. sativus var. atrorubens Alef.). Fitoterapia 2018, 124, 49–57. [Google Scholar] [CrossRef]
- Barreca, D.; Lagana, G.; Leuzzi, U.; Smeriglio, A.; Trombetta, D.; Bellocco, E. Evaluation of the nutraceutical, antioxidant and cytoprotective properties of ripe pistachio (Pistacia vera L., variety Bronte) hulls. Food Chem. 2016, 196, 493–502. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; D’Angelo, V.; Germano, M.P.; Trombetta, D. Antioxidant, Anti-Inflammatory and Anti-Angiogenic Properties of Citrus lumia Juice. Front. Pharmacol. 2020, 11, 593506. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; Barreca, D.; Calderaro, A.; Bisignano, C.; Ginestra, G.; Bellocco, E.; Trombetta, D. In Vitro Evaluation of the Antioxidant, Cytoprotective, and Antimicrobial Properties of Essential Oil from Pistacia vera L. Variety Bronte Hull. Int. J. Mol. Sci. 2017, 18, 1212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monforte, M.T.; Smeriglio, A.; Germano, M.P.; Pergolizzi, S.; Circosta, C.; Galati, E.M. Evaluation of antioxidant, antiinflammatory, and gastroprotective properties of Rubus fruticosus L. fruit juice. Phytother. Res. 2018, 32, 1404–1414. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Denaro, M.; de Francesco, C.; Cornara, L.; Barreca, D.; Bellocco, E.; Ginestra, G.; Mandalari, G.; Trombetta, D. Feijoa Fruit Peel: Micro-morphological Features, Evaluation of Phytochemical Profile, and Biological Properties of Its Essential Oil. Antioxidants 2019, 8, 320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smeriglio, A.; Bonasera, S.; Germano, M.P.; D’Angelo, V.; Barreca, D.; Denaro, M.; Monforte, M.T.; Galati, E.M.; Trombetta, D. Opuntia ficus-indica (L.) Mill. fruit as source of betalains with antioxidant, cytoprotective, and anti-angiogenic properties. Phytother. Res. 2019, 33, 1526–1537. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; de Francesco, C.; Denaro, M.; Trombetta, D. Prickly Pear Betalain-Rich Extracts as New Promising Strategy for Intestinal Inflammation: Plant Complex vs. Main Isolated Bioactive Compounds. Front. Pharmacol. 2021, 12, 722398. [Google Scholar] [CrossRef]
- Smeriglio, A.; Denaro, M.; di Gristina, E.; Mastracci, L.; Grillo, F.; Cornara, L.; Trombetta, D. Pharmacognostic approach to evaluate the micromorphological, phytochemical and biological features of Citrus lumia seeds. Food Chem. 2022, 375, 131855. [Google Scholar] [CrossRef]
- Bazzicalupo, M.; Cornara, L.; Burlando, B.; Cascini, A.; Denaro, M.; Smeriglio, A.; Trombetta, D. Carpobrotus edulis (L.) N.E.Br. extract as a skin preserving agent: From traditional medicine to scientific validation. J. Integr. Med. 2021, 19, 526–536. [Google Scholar] [CrossRef]
- Muniandy, K.; Gothai, S.; Tan, W.S.; Kumar, S.S.; Esa, N.M.; Chandramohan, G.; Al-Numair, K.S.; Arulselvan, P. In Vitro Wound Healing Potential of Stem Extract of Alternanthera sessilis. Evid. Based Complement. Altern. Med. 2018, 2018, 3142073. [Google Scholar] [CrossRef] [Green Version]



| Assay | LE | RE |
|---|---|---|
| Total phenols (mg GAE a/100 g DE b) | 10,668.30 ± 581.55 | 9538.00 ± 622.24 |
| Flavonoids (mg RE c/100 g DE) | 52,914.94 ± 384.84 * | 13,694.83 ± 561.33 |
| Flavan-3-ols (mg CE d/100 g DE) | 200.19 ± 1.58 * | 334.89 ± 12.66 |
| Proanthocyanidins (mg CyE e/100 g DE) | 0.078 ± 0.00 * | 0.003 ± 0.00 |
| Polimerization index f | 2575.46 * | 111.630 |
| Compound | [M-H]− | [M-H]+ | λmax | LE | RE | |
|---|---|---|---|---|---|---|
| (m/z) | (m/z) | (nm) | mg/100 g DE | |||
| 1 | 3-O-Caffeoylquinic acid | 353 | 355 | 296, 326 | 4.05 ± 0.14 * | 195.55 ± 1.67 |
| 2 | 5-O-Caffeoylquinic acid | 353 | 353 | 296, 326 | 5.14 ± 0.05 a,* | 114.36 a ± 1.44 |
| 3 | 4-O-Caffeoylquinic acid | 353 | 355 | 296, 326 | 135.0 ± 0.57 a,* | 16.28 a ± 0.08 |
| 4 | 5-O-p-Coumaroylquinic acid | 337 | 339 | 296, 324 | 3.70 ± 0.02 b | - |
| 5 | 5-O-Feruloylquinic acid | 367 | 369 | 296, 324 | 5.05 ± 0.08 c,* | 59.80 c ± 0.05 |
| 6 | 4-O-Feruloylquinic acid | 367 | 369 | 296, 324 | - | 1.93 c ± 0.01 |
| 7 | p-Coumaroyl glucose | 325 | 327 | 226, 315 | 0.13 ± 0.00 b | - |
| 8 | Quercetin-3-O-rutinoside | 609 | 611 | 257, 354 | 95.28 ± 0.84 | - |
| 9 | 3,4-di-O-Caffeoylquinic acid | 515 | 517 | 296, 324 | 11.08 ± 0.05 a,* | 2.69 a ± 0.02 |
| 10 | Hesperidin | 609 | 611 | 284, 332 | - | 9.57 ± 0.06 |
| 11 | Quercetin-3-O-(6″acetyl-glucoside) | 505 | 507 | 256, 356 | 138.52 ± 1.88 d | - |
| 12 | 3,7-Dimethylquercetin | 329 | 331 | 257, 358 | 2.28 ± 0.03 e | - |
| 13 | Oxypeucedanin-hexoside | 465 | 467 | 313 | 46.98 ± 0.42 f,* | 0.43 f ± 0.01 |
| 14 | Kaempferol 3-O-acetyl-glucoside | 489 | 491 | 265, 328 | 501.24 ± 0.66 g | - |
| 15 | Osthenol-7-O-glucoside | - | 393 | 270, 320 | - | 0.73 h ± 0.01 |
| 16 | Oxypeucedanin-malonyl-hexoside | - | 553 | 270, 315 | - | 0.14 f ± 0.00 |
| 17 | Oxypeucedanin hydrate | - | 305 | 311 | 1.14 ± 0.01 f,* | 5.67 f ± 0.04 |
| 18 | Oxypeaucedanin 2′-acetate-3′glucoside | - | 509 | 311 | 3.25 ± 0.02 f,* | 2.13 f ± 0.01 |
| 19 | Oxypeucedanin | - | 287 | 309 | 5.81 ± 0.02 f,* | 3.05 f ± 0.03 |
| 20 | Oxypeucedanin ethanolate | - | 333 | 311 | 8.62 ± 0.04 f | - |
| 21 | Ostruthol | - | 387 | 309 | - | 1.45 ± 0.02 |
| 22 | Isoimperatorin | - | 271 | 300 | - | 29.55 ± 0.08 |
| 23 | Imperatorin | - | 271 | 310 | - | 7.31 ± 0.05 |
| 24 | Ostruthin | - | 299 | 330 | - | 281.88 ± 2.24 |
| Percentage distribution (%) of phytochemical classes | ||||||
| Phenolic acids | 16.97 | 53.32 | ||||
| Flavonoids | 76.23 | 1.31 | ||||
| Coumarins | 6.80 | 45.37 | ||||
| Assay | LE | RE | Reference Standard b |
|---|---|---|---|
| Antioxidant activities | |||
| 2,2-Diphenyl-1-picrylhydrazyl (DPPH) | 24.11 (20.14–28.87) * | 152.73 (61.54–379.06) | 8.57 (4.88–10.22) § |
| Trolox equivalent antioxidant capacity (TEAC) | 12.14 (10.34–14.24) * | 51.07 (35.56–73.35) | 4.89 (2.24–6.95) § |
| Ferric reducing antioxidant power (FRAP) | 19.37 (15.59–24.07) * | 47.18 (39.75–56.01) | 5.38 (3.86–8.01) § |
| Oxygen radical absorbance capacity (ORAC) | 1.03 (0.76–1.40) | 1.35 (1.09–1.69) | 0.72 (0.38–0.92) § |
| Anti-inflammatory activities | |||
| BSA a denaturation assay | 15.16 (12.97–17.72) * | 57.06 (47.72–69.70) | 17.58 (15.05–19.68) ° |
| Protease inhibitory activity | 24.78 (19.75–31.09) | 30.04 (23.42–38.51) | 6.88 (3.26–9.44) § |
| Enzyme | LE | RE | Standard | ||
|---|---|---|---|---|---|
| 150 µg/mL | 300 µg/mL | 150 µg/mL | 300 µg/mL | ||
| COX-2 | 67.3 ± 11.5 | 43.8 ± 4.4 | n.d. | n.d. | 87.9 ± 0.1 * |
| LOX | 52.0 ± 27.3 ‡ | 78.7 ± 8.8 # | 11.3 ± 11.3 | 65.4 ± 13.5 # | 96 ± 3.5 * |
| Leaf Extract | Rhizome Extract | |||
|---|---|---|---|---|
| IC50 | IC05 | IC50 | IC05 | |
| HaCaT | 897 (760–1059) | 252 (113–559) | 439 (416–463) | 364 (293–451) |
| L929 | 1094 (607–1970) | 801 (189–3394) | 681 (603–768) | 385 (320–462) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Danna, C.; Bazzicalupo, M.; Ingegneri, M.; Smeriglio, A.; Trombetta, D.; Burlando, B.; Cornara, L. Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules 2022, 27, 4271. https://doi.org/10.3390/molecules27134271
Danna C, Bazzicalupo M, Ingegneri M, Smeriglio A, Trombetta D, Burlando B, Cornara L. Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules. 2022; 27(13):4271. https://doi.org/10.3390/molecules27134271
Chicago/Turabian StyleDanna, Cristina, Miriam Bazzicalupo, Mariarosaria Ingegneri, Antonella Smeriglio, Domenico Trombetta, Bruno Burlando, and Laura Cornara. 2022. "Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch" Molecules 27, no. 13: 4271. https://doi.org/10.3390/molecules27134271
APA StyleDanna, C., Bazzicalupo, M., Ingegneri, M., Smeriglio, A., Trombetta, D., Burlando, B., & Cornara, L. (2022). Anti-Inflammatory and Wound Healing Properties of Leaf and Rhizome Extracts from the Medicinal Plant Peucedanum ostruthium (L.) W. D. J. Koch. Molecules, 27(13), 4271. https://doi.org/10.3390/molecules27134271