Chemometric Analysis of Urinary Volatile Organic Compounds to Monitor the Efficacy of Pitavastatin Treatments on Mammary Tumor Progression over Time
Abstract
:1. Introduction
2. Results
2.1. Urine Collection and Data Screening
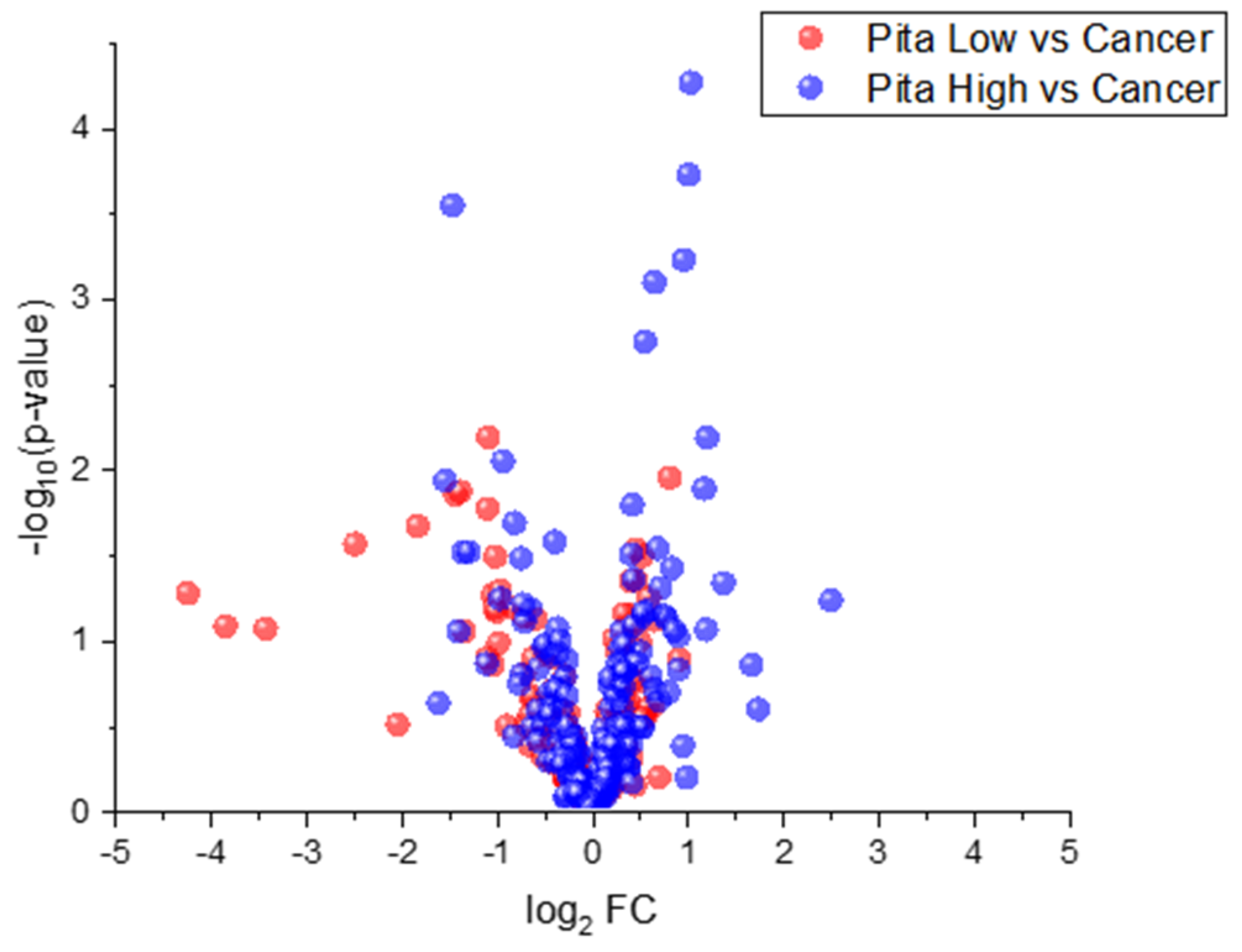
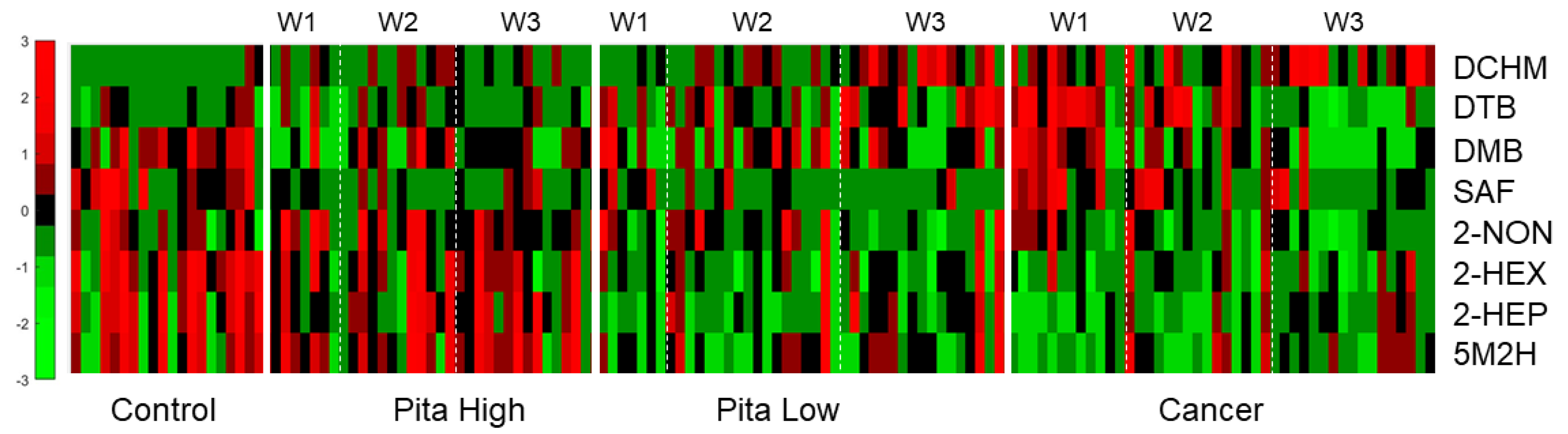
2.2. Univariate Statistical Analyses
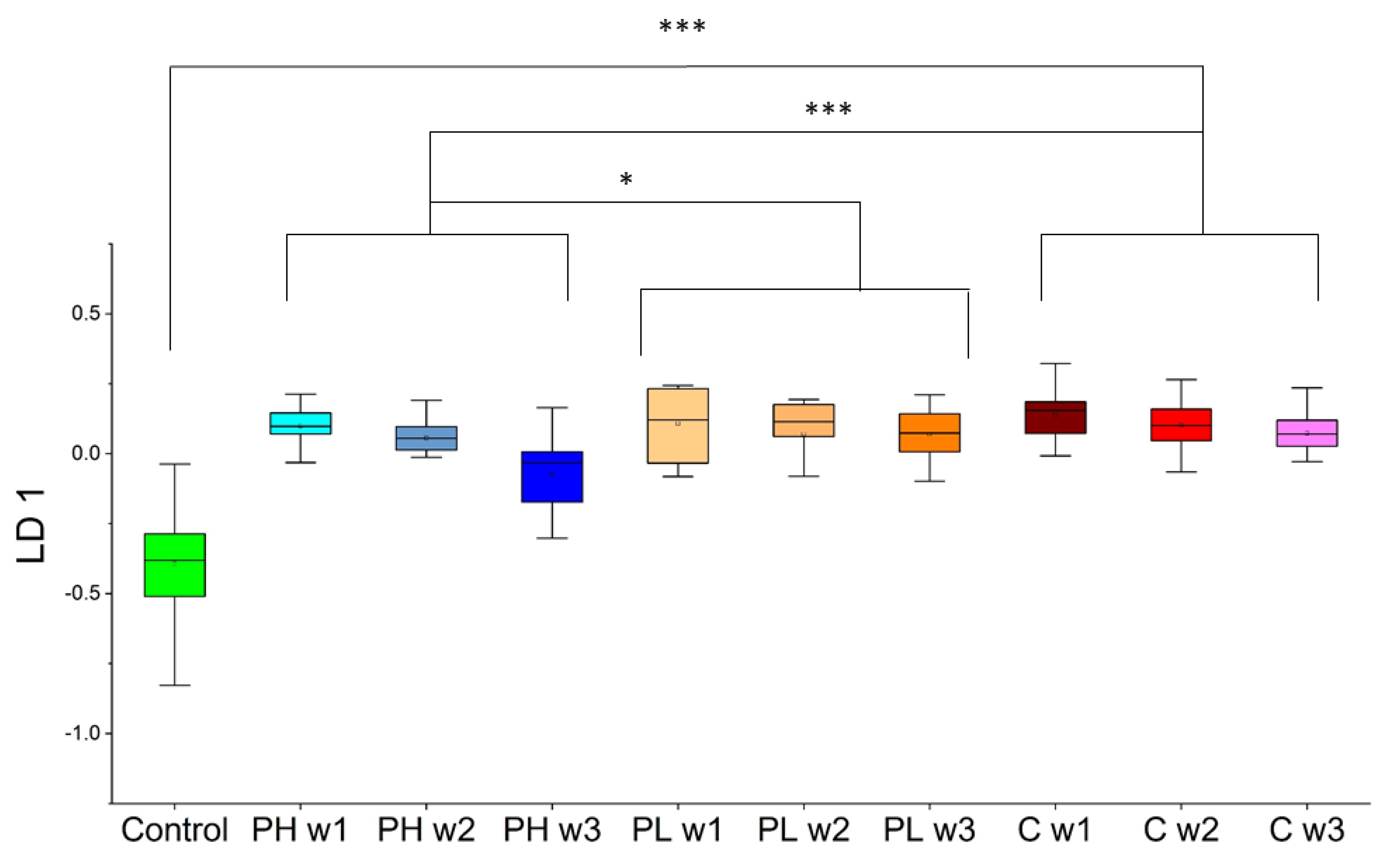
2.3. Multivariate Chemometric Analyses
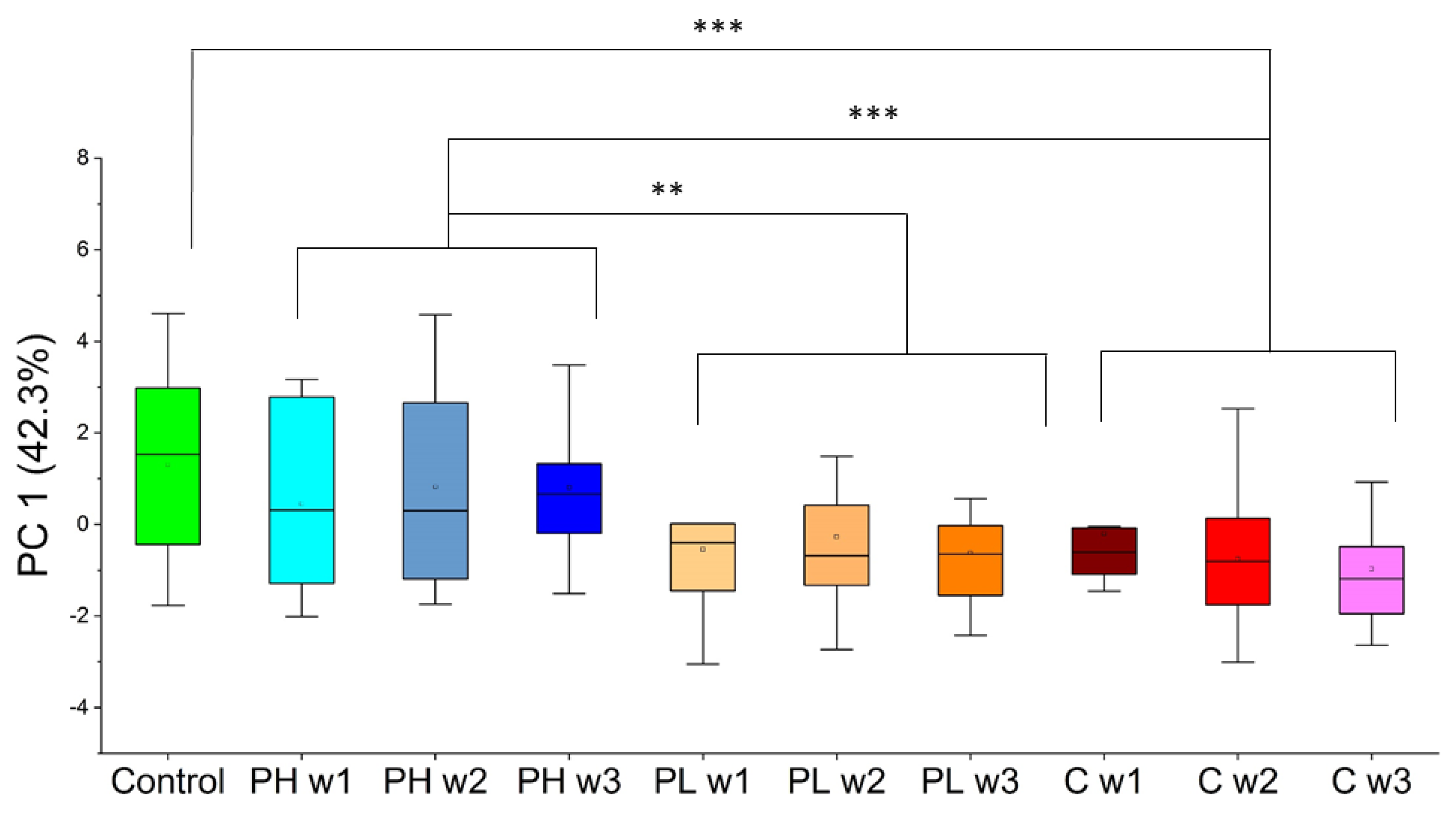
2.3.1. Approach 1
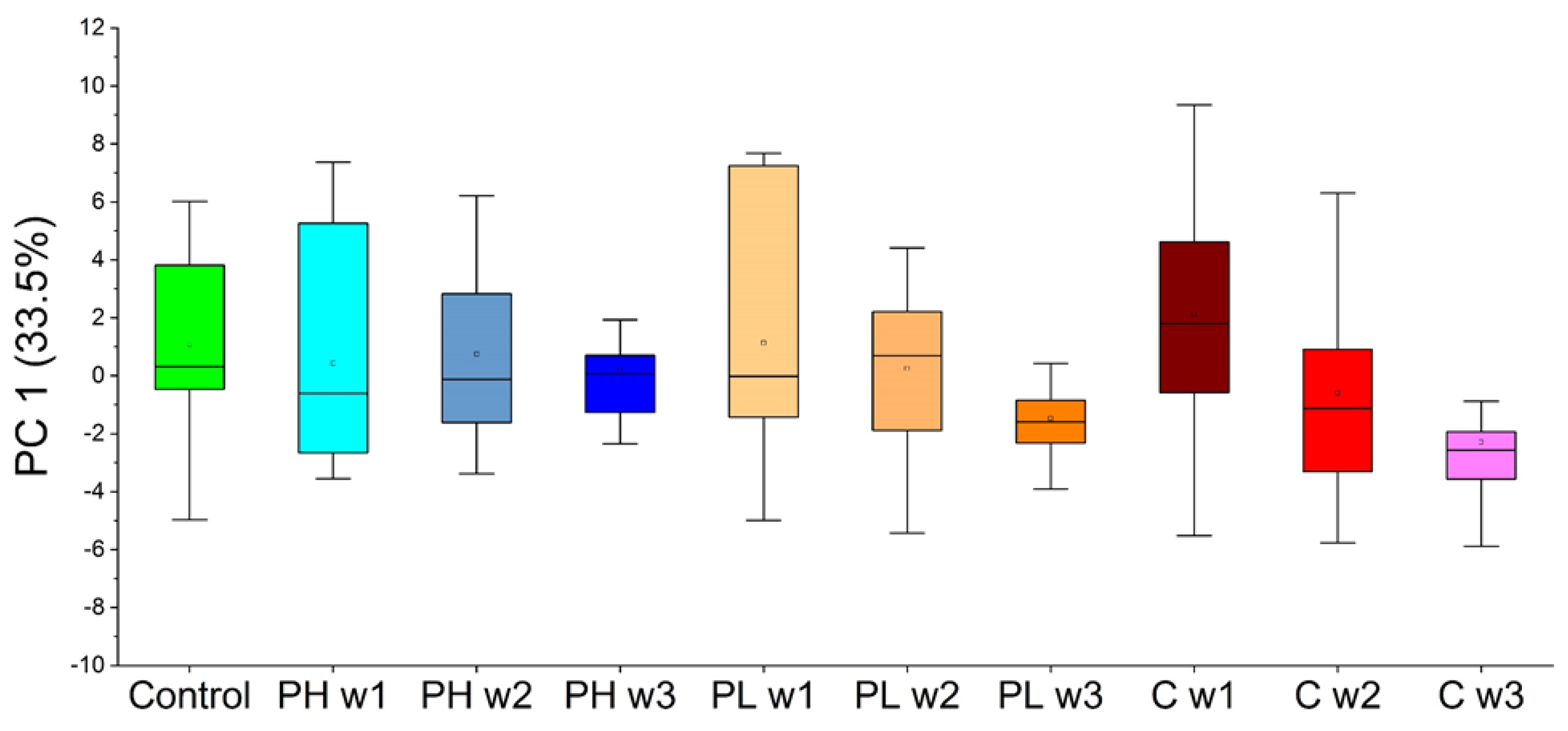
2.3.2. Approach 2
3. Discussion
4. Materials and Methods
4.1. Instrumentation and Materials
4.2. Tumor Injection, Drug Administration, and Urine Collection
4.3. SPME GC-MS QTOF Analysis
4.4. Data Processing and VOC Identification
4.5. Chemometric Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Siegel, R.L.; Miller, K.D.; Fuchs, H.E.; Jemal, A. Cancer Statistics, 2021. CA. Cancer J. Clin. 2021, 71, 7–33. [Google Scholar] [CrossRef] [PubMed]
- Ginsburg, O.; Yip, C.; Brooks, A.; Cabanes, A.; Caleffi, M.; Dunstan Yataco, J.A.; Gyawali, B.; McCormack, V.; McLaughlin de Anderson, M.; Mehrotra, R.; et al. Breast Cancer Early Detection: A Phased Approach to Implementation. Cancer 2020, 126, 2379–2393. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishna, S.; Agarwal, S.; Parikh, P.M.; Kaur, K.; Panwar, S.; Sharma, S.; Dey, A.; Saxena, K.K.; Chandra, M.; Sud, S. Role of Magnetic Resonance Imaging in Breast Cancer Management. South Asian J. Cancer 2018, 7, 69–71. [Google Scholar] [CrossRef] [PubMed]
- Lavra, L.; Catini, A.; Ulivieri, A.; Capuano, R.; Baghernajad Salehi, L.; Sciacchitano, S.; Bartolazzi, A.; Nardis, S.; Paolesse, R.; Martinelli, E.; et al. Investigation of VOCs Associated with Different Characteristics of Breast Cancer Cells. Sci. Rep. 2015, 5, 13246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rakha, E.A.; Ellis, I.O. An Overview of Assessment of Prognostic and Predictive Factors in Breast Cancer Needle Core Biopsy Specimens. J. Clin. Pathol. 2006, 60, 1300–1306. [Google Scholar] [CrossRef]
- Van Seijen, M.; Lips, E.H.; Thompson, A.M.; Nik-Zainal, S.; Futreal, A.; Hwang, E.S.; Verschuur, E.; Lane, J.; Jonkers, J.; Rea, D.W.; et al. Ductal Carcinoma in Situ: To Treat or Not to Treat, That Is the Question. Br. J. Cancer 2019, 121, 285–292. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Treatment of Breast Cancer by Stage. Available online: https://www.cancer.org/cancer/breast-cancer/treatment/treatment-of-breast-cancer-by-stage.html#written_by (accessed on 23 February 2022).
- Ma, Y.; Wang, Q.; Dong, Q.; Zhan, L.; Zhang, J. How to Differentiate Pseudoprogression from True Progression in Cancer Patients Treated with Immunotherapy. Am. J. Cancer Res. 2019, 9, 1546–1553. [Google Scholar]
- McCulloch, M.; Jezierski, T.; Broffman, M.; Hubbard, A.; Turner, K.; Janecki, T. Diagnostic Accuracy of Canine Scent Detection in Early- and Late-Stage Lung and Breast Cancers. Integr. Cancer Ther. 2006, 5, 30–39. [Google Scholar] [CrossRef]
- Khalid, T.; Aggio, R.; White, P.; De Lacy Costello, B.; Persad, R.; Al-Kateb, H.; Jones, P.; Probert, C.S.; Ratcliffe, N. Urinary Volatile Organic Compounds for the Detection of Prostate Cancer. PLoS ONE 2015, 10, e0143283. [Google Scholar] [CrossRef]
- Saalberg, Y.; Wolff, M. VOC Breath Biomarkers in Lung Cancer. Clin. Chim. Acta 2016, 459, 5–9. [Google Scholar] [CrossRef]
- Lima, A.R.; Pinto, J.; Carvalho-Maia, C.; Jerónimo, C.; Henrique, R.; Bastos, M.D.L.; Carvalho, M.; Guedes de Pinho, P. A Panel of Urinary Volatile Biomarkers for Differential Diagnosis of Prostate Cancer from Other Urological Cancers. Cancers 2020, 12, 2017. [Google Scholar] [CrossRef] [PubMed]
- Siegel, A.P.; Daneshkhah, A.; Hardin, D.S.; Shrestha, S.; Varahramyan, K.; Agarwal, M. Analyzing Breath Samples of Hypoglycemic Events in Type 1 Diabetes Patients: Towards Developing an Alternative to Diabetes Alert Dogs. J. Breath Res. 2017, 11, 026007. [Google Scholar] [CrossRef] [PubMed]
- Woollam, M.; Siegel, A.P.; Grocki, P.; Saunders, J.L.; Sanders, D.B.; Agarwal, M.; Davis, M.D. Preliminary Method for Profiling Volatile Organic Compounds in Breath That Correlate with Pulmonary Function and Other Clinical Traits of Subjects Diagnosed with Cystic Fibrosis: A Pilot Study. J. Breath Res. 2022, 16, 027103. [Google Scholar] [CrossRef] [PubMed]
- Janfaza, S.; Khorsand, B.; Nikkhah, M.; Zahiri, J. Digging Deeper into Volatile Organic Compounds Associated with Cancer. Biol. Methods Protoc. 2019, 4, bpz014. [Google Scholar] [CrossRef]
- Woollam, M.; Teli, M.; Liu, S.; Daneshkhah, A.; Siegel, A.P.; Yokota, H.; Agarwal, M. Urinary Volatile Terpenes Analyzed by Gas Chromatography–Mass Spectrometry to Monitor Breast Cancer Treatment Efficacy in Mice. J. Proteome Res. 2020, 19, 1913–1922. [Google Scholar] [CrossRef]
- Woollam, M.; Teli, M.; Angarita-Rivera, P.; Liu, S.; Siegel, A.P.; Yokota, H.; Agarwal, M. Detection of Volatile Organic Compounds (VOCs) in Urine via Gas Chromatography-Mass Spectrometry QTOF to Differentiate Between Localized and Metastatic Models of Breast Cancer. Sci. Rep. 2019, 9, 2526. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Cataneo, R.N.; Saunders, C.; Hope, P.; Schmitt, P.; Wai, J. Volatile Biomarkers in the Breath of Women with Breast Cancer. J. Breath Res. 2010, 4, 026003. [Google Scholar] [CrossRef]
- Phillips, M.; Cataneo, R.N.; Ditkoff, B.A.; Fisher, P.; Greenberg, J.; Gunawardena, R.; Kwon, C.S.; Rahbari-Oskoui, F.; Wong, C. Volatile Markers of Breast Cancer in the Breath. Breast J. 2003, 9, 184–191. [Google Scholar] [CrossRef] [Green Version]
- Phillips, M.; Cataneo, R.N.; Cruz-Ramos, J.A.; Huston, J.; Ornelas, O.; Pappas, N.; Pathak, S. Prediction of Breast Cancer Risk with Volatile Biomarkers in Breath. Breast Cancer Res. Treat. 2018, 170, 343–350. [Google Scholar] [CrossRef]
- Silva, C.L.; Passos, M.; Câmara, J.S. Solid Phase Microextraction, Mass Spectrometry and Metabolomic Approaches for Detection of Potential Urinary Cancer Biomarkers—A Powerful Strategy for Breast Cancer Diagnosis. Talanta 2012, 89, 360–368. [Google Scholar] [CrossRef]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Implementing a Central Composite Design for the Optimization of Solid Phase Microextraction to Establish the Urinary Volatomic Expression: A First Approach for Breast Cancer. Metabolomics 2019, 15, 64. [Google Scholar] [CrossRef] [PubMed]
- Woollam, M.; Wang, L.; Grocki, P.; Liu, S.; Siegel, A.P.; Kalra, M.; Goodpaster, J.V.; Yokota, H.; Agarwal, M. Tracking the Progression of Triple Negative Mammary Tumors over Time by Chemometric Analysis of Urinary Volatile Organic Compounds. Cancers 2021, 13, 1462. [Google Scholar] [CrossRef] [PubMed]
- Corsini, A.; Bellosta, S.; Baetta, R.; Fumagalli, R.; Paoletti, R.; Bernini, F. New Insights into the Pharmacodynamic and Pharmacokinetic Properties of Statins. Pharmacol. Ther. 1999, 84, 413–428. [Google Scholar] [CrossRef]
- Chou, R.; Dana, T.; Blazina, I.; Daeges, M.; Bougatsos, C.; Grusing, S.; Jeanne, T.L. Statin Use for the Prevention of Cardiovascular Disease in Adults: A Systematic Review for the U.S. Preventive Services Task Force; U.S. Preventive Services Task Force Evidence Syntheses, Formerly Systematic Evidence Reviews; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2016. [Google Scholar]
- Iannelli, F.; Lombardi, R.; Milone, M.R.; Pucci, B.; De Rienzo, S.; Budillon, A.; Bruzzese, F. Targeting Mevalonate Pathway in Cancer Treatment: Repurposing of Statins. Recent Pat. Anticancer Drug Discov. 2018, 13, 184–200. [Google Scholar] [CrossRef]
- Sopková, J.; Vidomanová, E.; Strnádel, J.; Škovierová, H.; Halašová, E. The Role of Statins as Therapeutic Agents in Cancer. Gen. Physiol. Biophys. 2017, 36, 501–511. [Google Scholar] [CrossRef]
- Iarrobino, N.A.; Gill, B.; Bernard, M.E.; Mishra, M.V.; Champ, C.E. Targeting Tumor Metabolism with Statins During Treatment for Advanced-Stage Pancreatic Cancer. Am. J. Clin. Oncol. 2018, 41, 1125–1131. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Y.; Chen, A.; Teli, M.; Kondo, R.; Jalali, A.; Fan, Y.; Liu, S.; Zhao, X.; Siegel, A.; et al. Pitavastatin Slows Tumor Progression and Alters Urine-Derived Volatile Organic Compounds through the Mevalonate Pathway. FASEB J. 2019, 33, 13710–13721. [Google Scholar] [CrossRef] [Green Version]
- Silva, C.L.; Perestrelo, R.; Silva, P.; Tomás, H.; Câmara, J.S. Volatile Metabolomic Signature of Human Breast Cancer Cell Lines. Sci. Rep. 2017, 7, 43969. [Google Scholar] [CrossRef] [Green Version]
- Lima, A.R.; Pinto, J.; Azevedo, A.I.; Barros-Silva, D.; Jerónimo, C.; Henrique, R.; de Lourdes Bastos, M.; Guedes de Pinho, P.; Carvalho, M. Identification of a Biomarker Panel for Improvement of Prostate Cancer Diagnosis by Volatile Metabolic Profiling of Urine. Br. J. Cancer 2019, 121, 857–868. [Google Scholar] [CrossRef]
- Santos, P.M.; del Nogal Sánchez, M.; Pozas, Á.P.C.; Pavón, J.L.P.; Cordero, B.M. Determination of Ketones and Ethyl Acetate—A Preliminary Study for the Discrimination of Patients with Lung Cancer. Anal. Bioanal. Chem. 2017, 409, 5689–5696. [Google Scholar] [CrossRef]
- Minh, T.D.C.; Blake, D.R.; Galassetti, P.R. The Clinical Potential of Exhaled Breath Analysis for Diabetes Mellitus. Diabetes Res. Clin. Pract. 2012, 97, 195–205. [Google Scholar] [CrossRef] [Green Version]
- Wu, D.; Fan, Y.; Liu, S.; Woollam, M.D.; Sun, X.; Murao, E.; Zha, R.; Prakash, R.; Park, C.; Siegel, A.P.; et al. Loading-induced Antitumor Capability of Murine and Human Urine. FASEB J. 2020, 34, 7578–7592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kwak, J.; Grigsby, C.C.; Rizki, M.M.; Preti, G.; Köksal, M.; Josue, J.; Yamazaki, K.; Beauchamp, G.K. Differential Binding between Volatile Ligands and Major Urinary Proteins Due to Genetic Variation in Mice. Physiol. Behav. 2012, 107, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 1995, 57, 289–300. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grocki, P.; Woollam, M.; Wang, L.; Liu, S.; Kalra, M.; Siegel, A.P.; Li, B.-Y.; Yokota, H.; Agarwal, M. Chemometric Analysis of Urinary Volatile Organic Compounds to Monitor the Efficacy of Pitavastatin Treatments on Mammary Tumor Progression over Time. Molecules 2022, 27, 4277. https://doi.org/10.3390/molecules27134277
Grocki P, Woollam M, Wang L, Liu S, Kalra M, Siegel AP, Li B-Y, Yokota H, Agarwal M. Chemometric Analysis of Urinary Volatile Organic Compounds to Monitor the Efficacy of Pitavastatin Treatments on Mammary Tumor Progression over Time. Molecules. 2022; 27(13):4277. https://doi.org/10.3390/molecules27134277
Chicago/Turabian StyleGrocki, Paul, Mark Woollam, Luqi Wang, Shengzhi Liu, Maitri Kalra, Amanda P. Siegel, Bai-Yan Li, Hiroki Yokota, and Mangilal Agarwal. 2022. "Chemometric Analysis of Urinary Volatile Organic Compounds to Monitor the Efficacy of Pitavastatin Treatments on Mammary Tumor Progression over Time" Molecules 27, no. 13: 4277. https://doi.org/10.3390/molecules27134277





