Forensic Analysis of Textile Synthetic Fibers Using a FT-IR Spectroscopy Approach
Abstract
:1. Introduction
2. Materials and Methods
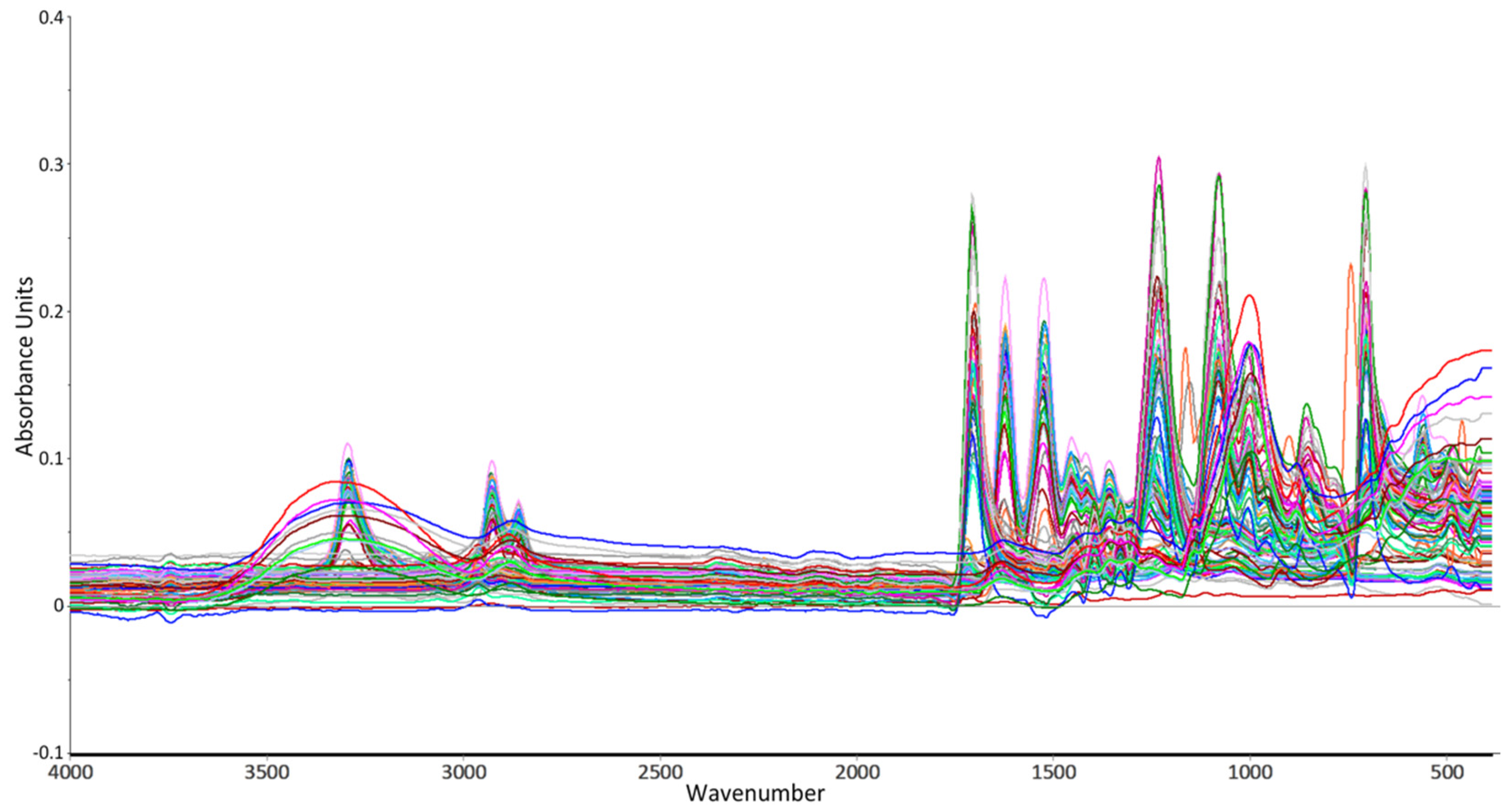
2.1. Sampling and FT-IR Spectroscopy
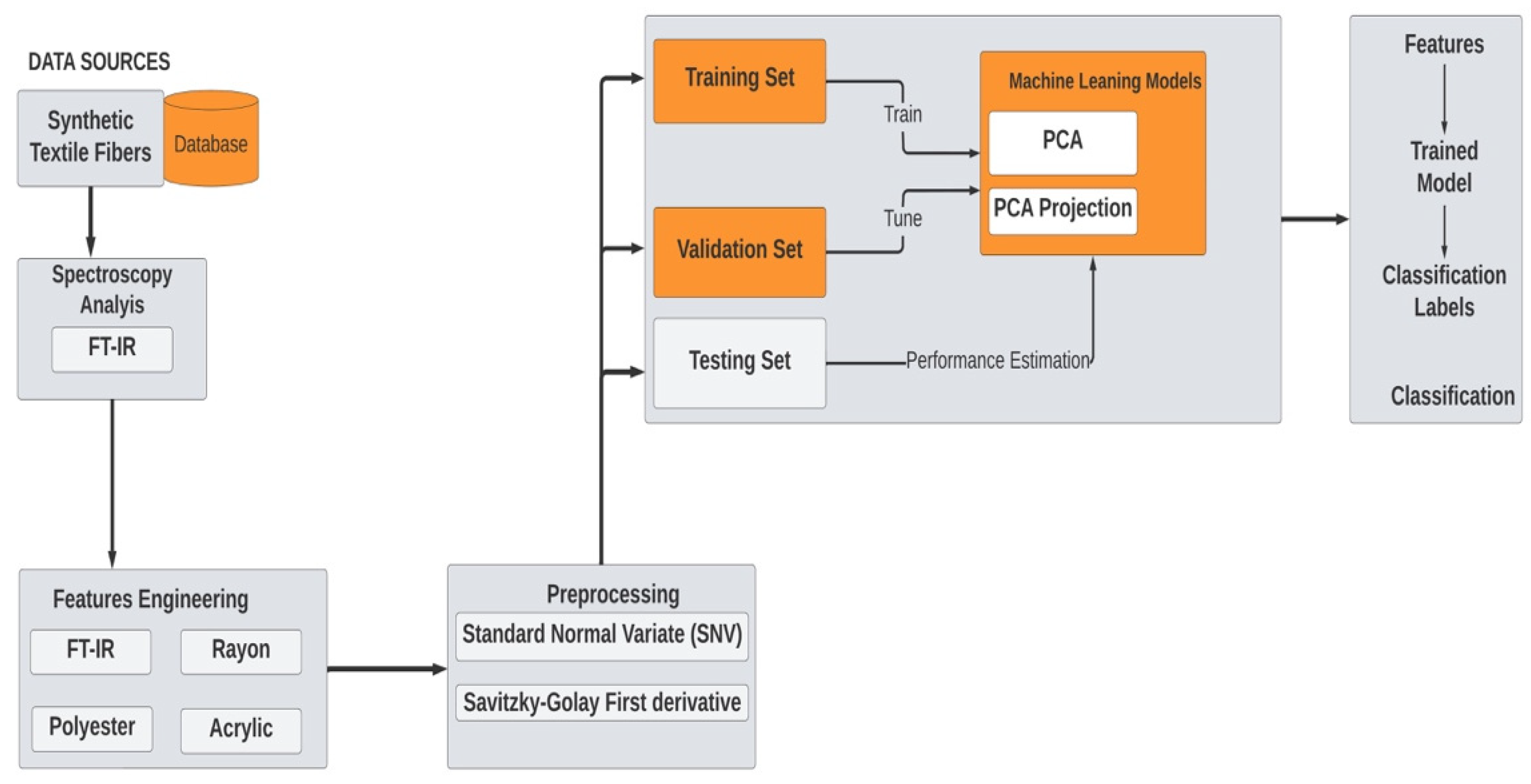
2.2. Data Analysis and Chemometrics
2.2.1. Methodology–Software Package
2.2.2. Data Selection
2.2.3. Data Cleaning
2.2.4. Data Characterization
- 26 acrylic datasets: 19 training, 7 tests;
- 12 rayon datasets: 9 training, 3 tests;
- 48 nylon datasets: 34 training, 14 tests;
- 52 polyester datasets: 37 training, 15 tests.
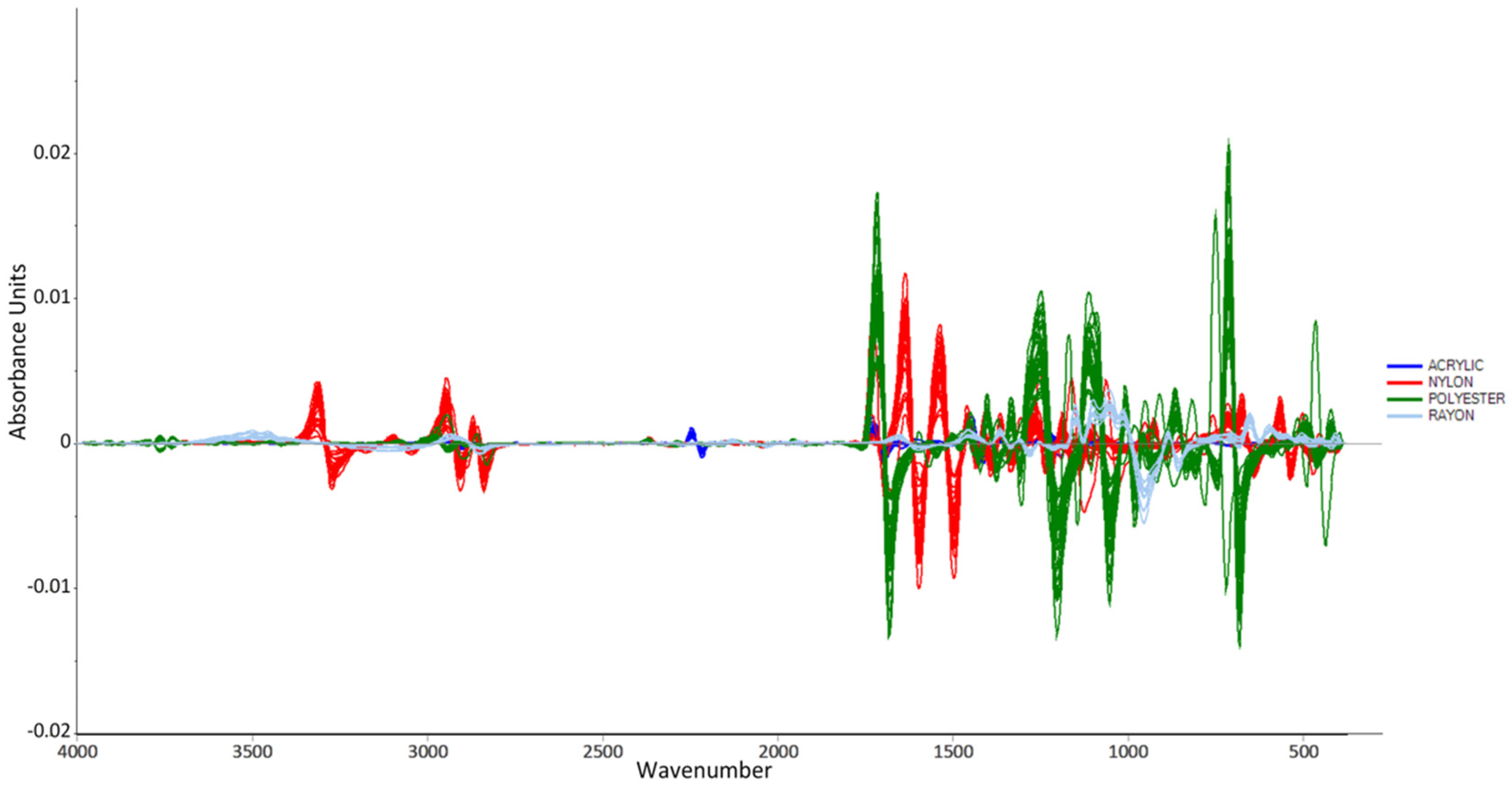
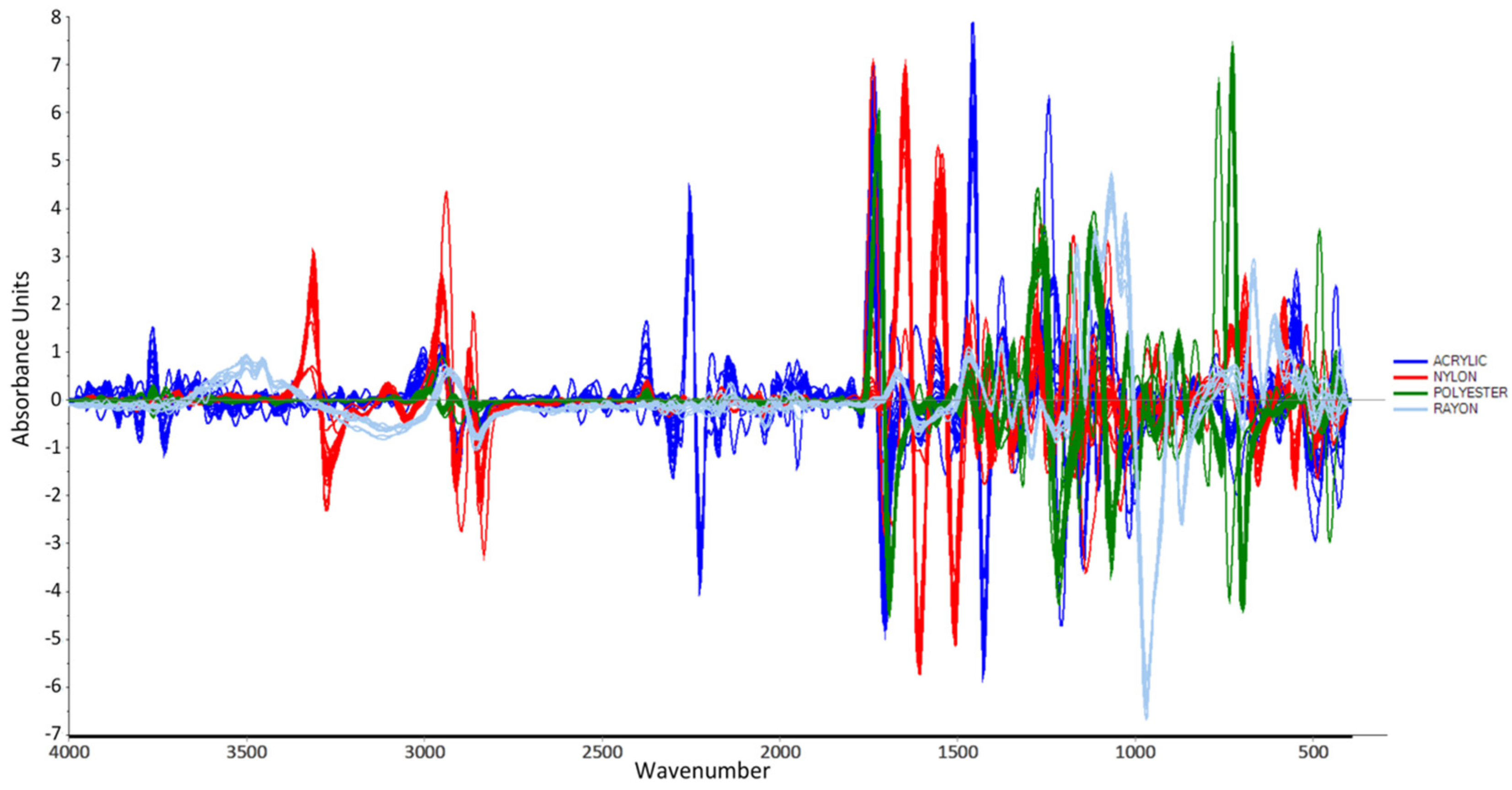
2.2.5. Data Preprocessing
3. Results and Discussion
3.1. Classification Models
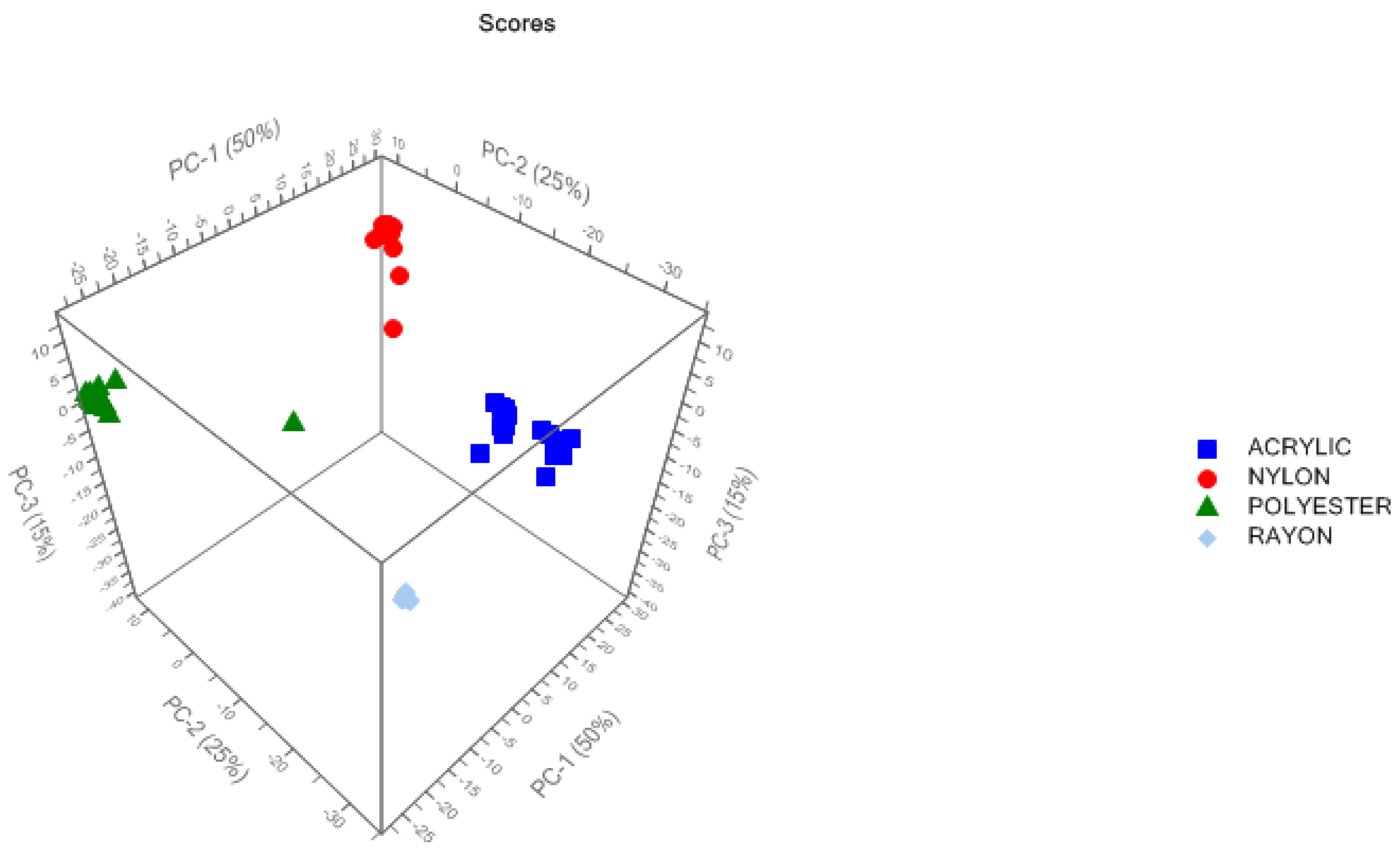
3.1.1. PCA
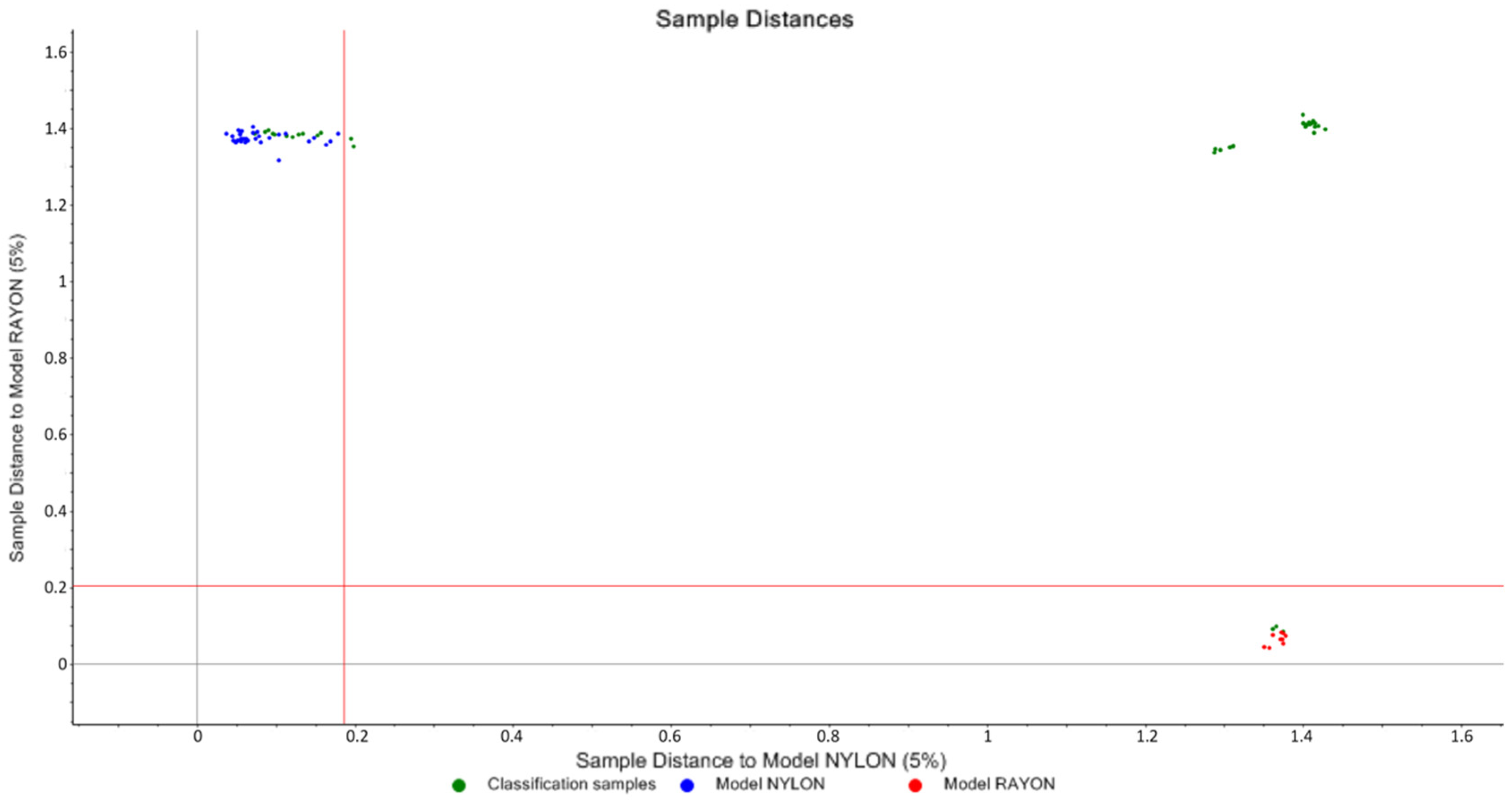
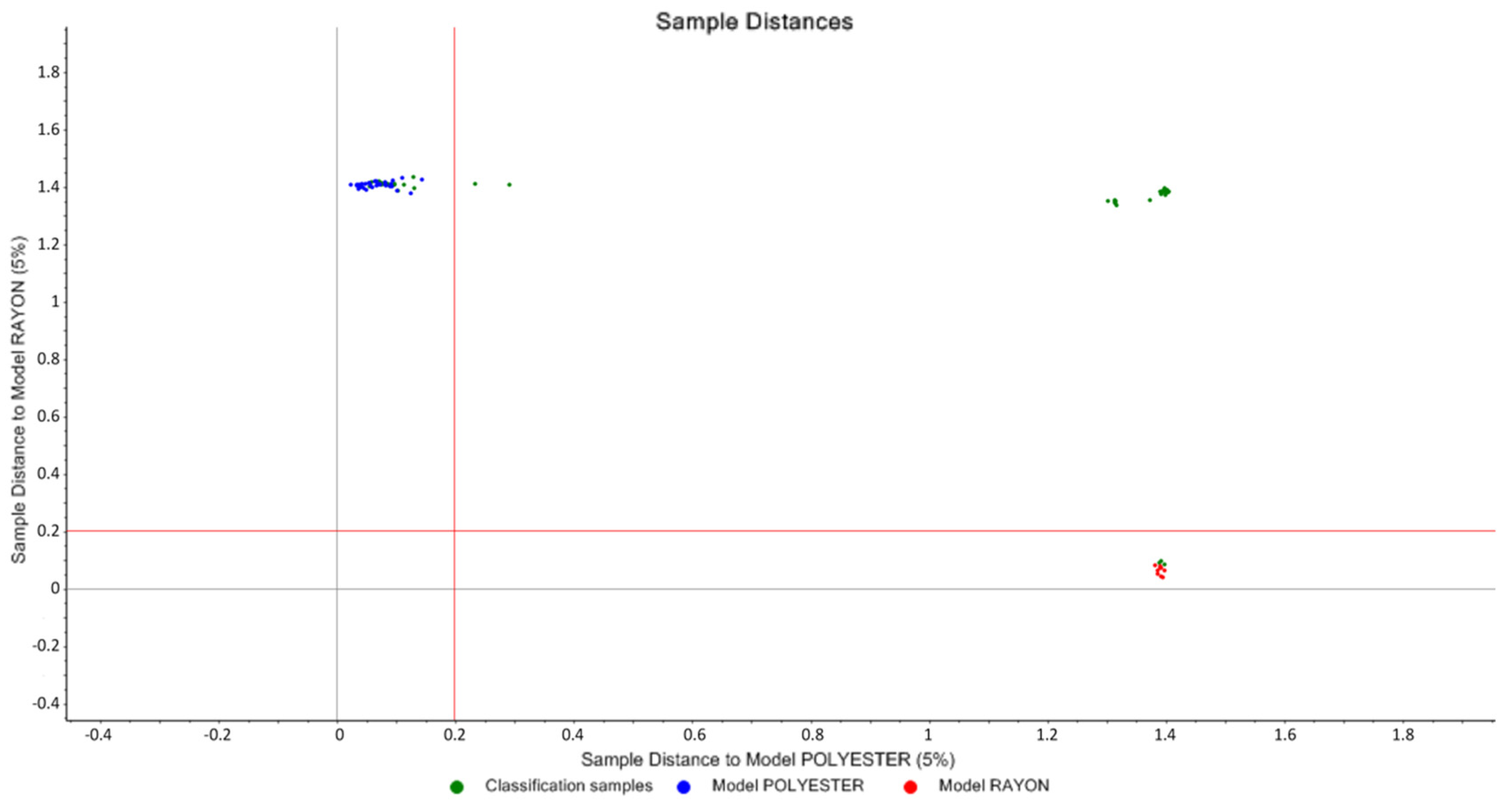
3.1.2. SIMCA and Cooman’s Plot
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Goodpaster, J.V.; Liszewski, E.A. Forensic analysis of dyed textile fibers. Anal. Bioanal. Chem. 2009, 394, 2009–2018. [Google Scholar] [CrossRef]
- Farah, S.; Kunduru, K.R.; Tsach, T.; Bentolila, A.; Domb, A.J. Forensic comparison of synthetic fibers. Polym. Adv. Technol. 2015, 26, 785–796. [Google Scholar] [CrossRef]
- Hu, C.; Zhu, J.; Mei, H.; Shi, H.; Guo, H.; Zhang, G.; Wang, P.; Lu, L.; Zheng, X. A sensitive HPLC-MS/MS method for the analysis of fiber dyes. Forensic Chem. 2018, 11, 1–6. [Google Scholar] [CrossRef]
- Schotman, T.G.; Xu, X.; Rodewijk, N.; van der Weerd, J. Application of dye analysis in forensic fibre and textile examination: Case examples. Forensic Sci. Int. 2017, 278, 338–350. [Google Scholar] [CrossRef] [PubMed]
- Causin, V.; Marega, C.; Schiavone, S.; Della Guardia, V.; Marigo, A. Forensic analysis of acrylic fibers by pyrolysis–gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 2006, 75, 43–48. [Google Scholar] [CrossRef]
- Lim, S.J.; Park, Y.-K.; Kim, H.; Kwon, J.; Moon, H.M.; Lee, Y.; Watanabe, A.; Teramae, N.; Ohtani, H.; Kim, Y.-M. Selective solvent extraction and quantification of synthetic microfibers in textile laundry wastewater using pyrolysis-gas chromatography/mass spectrometry. Chem. Eng. J. 2022, 434, 134653. [Google Scholar] [CrossRef]
- Powell, R.; Collins, P.; Horsley, G.; Coumbaros, J.; van Bronswijk, W. Enhancing the evidential value of textile fibres Part 2: Application of a database-driven fibre comparison strategy to a cold-case investigation. Forensic Sci. Int. 2021, 325, 110894. [Google Scholar] [CrossRef]
- Zieba-Palus, J.; Borusiewicz, R.; Kunicki, M. PRAXIS—Combined μ-Raman and μ-XRF spectrometers in the examination of forensic samples. Forensic Sci. Int. 2008, 175, 1–10. [Google Scholar] [CrossRef]
- Zapata, F.; Ortega-Ojeda, F.E.; García-Ruiz, C. Forensic examination of textile fibres using Raman imaging and multivariate analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 268, 120695. [Google Scholar] [CrossRef]
- Peets, P.; Kaupmees, K.; Vahur, S.; Leito, I. Reflectance FT-IR spectroscopy as a viable option for textile fiber identification. Herit. Sci. 2019, 7, 93. [Google Scholar] [CrossRef]
- Meleiro, P.P.; García-Ruiz, C. Spectroscopic techniques for the forensic analysis of textile fibers. Appl. Spectrosc. Rev. 2015, 51, 278–301. [Google Scholar] [CrossRef]
- Lepot, L.; De Wael, K.; Gason, F.; Gilbert, B. Application of Raman spectroscopy to forensic fibre cases. Sci. Justice 2008, 48, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Zięba-Palus, J.; Wąs-Gubała, J. An investigation into the use of micro-Raman spectroscopy for the analysis of car paints and single textile fibres. J. Mol. Struct. 2010, 993, 127–133. [Google Scholar] [CrossRef]
- Nayak, R.; Houshyar, S.; Khandual, A.; Padhye, R.; Fergusson, S. Identification of natural textile fibres. In Handbook of Natural Fibres; Elsevier: Amsterdam, The Netherlands, 2020; pp. 503–534. [Google Scholar] [CrossRef]
- Peets, P.; Leito, I.; Pelt, J.; Vahur, S. Identification and classification of textile fibres using ATR-FT-IR spectroscopy with chemometric methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2017, 173, 175–181. [Google Scholar] [CrossRef]
- Široká, B.; Široký, J.; Bechtold, T. Application of ATR-FT-IR Single-Fiber Analysis for the Identification of a Foreign Polymer in Textile Matrix. Int. J. Polym. Anal. Charact. 2011, 16, 259–268. [Google Scholar] [CrossRef]
- Garside, P.; Wyeth, P. Identification of Cellulosic Fibres by FTIR Spectroscopy—Thread and Single Fibre Analysis by Attenuated Total Reflectance. Stud. Conserv. 2003, 48, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Schüürmann, G.; Funar-Timofei, S. Multilinear regression and comparative molecular field analysis (CoMFA) of azo dye−fiber affinities. 2. inclusion of solution-phase molecular orbital descriptors. J. Chem. Inf. Comput. Sci. 2003, 43, 1502–1512. [Google Scholar] [CrossRef]
- Tokutake, S.; Dozono, T.; Ishizawa, H.; Koyama, S.; Miki, T. Prompt quantitative analysis of fiber mixtures based on infrared absorption features. In Proceedings of the SICE Annual Conference 2010, Taipei, Taiwan, 18–21 August 2010; IEEE: Manhattan, NY, USA, 2010. [Google Scholar]
- Broda, J.; Ślusarczyk, C.; Fabia, J.; Demsar, A. Formation and properties of polypropylene/stearic acid composite fibers. Text. Res. J. 2015, 86, 64–71. [Google Scholar] [CrossRef]
- Măluțan, T.; Marta, U.C. Structural changes in hemp fibers following temperature, humidity and UV or gamma-ray radiation exposure. Cellul. Chem. Technol. 2016, 50, 31–39. [Google Scholar]
- Sheikh, J.; Bramhecha, I. Multifunctional modification of linen fabric using chitosan-based formulations. Int. J. Biol. Macromol. 2018, 118, 896–902. [Google Scholar] [CrossRef]
- Neves, A.C.D.O.; Soares, G.M.; de Morais, S.C.; da Costa, F.S.L.; Porto, D.L.; de Lima, K.M.G. Dissolution testing of isoniazid, rifampicin, pyrazinamide and ethambutol tablets using near-infrared spectroscopy (NIRS) and multivariate calibration. J. Pharm. Biomed. Anal. 2012, 57, 115–119. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, E.O.; Silva, A.M.S.; Fragoso, W.D.; Pasquini, C.; Pimentel, M.F. Determination of degree of polymerization of insulating paper using near infrared spectroscopy and multivariate calibration. Vib. Spectrosc. 2010, 52, 154–157. [Google Scholar] [CrossRef]
- Masithoh, R.E.; Yuliyanda, I. NIR reflectance spectroscopy and SIMCA for classification of crops flour. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 3rd International Symposium on Agricultural and Biosystem Engineering, South Sulawesi, Indonesia, 6–8 August 2019; IOP Publishing: Bristol, UK, 2019; Volume 355. [Google Scholar] [CrossRef]
- Schneider, R.C.; Kovar, K.-A. Analysis of ecstasy tablets: Comparison of reflectance and transmittance near infrared spectroscopy. Forensic Sci. Int. 2003, 134, 187–195. [Google Scholar] [CrossRef]
- Rismiwandira, K.; Roosmayanti, F.; Pahlawan, M.F.R.; Masithoh, R.E. Application of Fourier Transform Near-Infrared (FT-NIR) spectroscopy for detection of adulteration in palm sugar. In IOP Conference Series: Earth and Environmental Science, Proceedings of the 2nd International Conference on Sustainable Agriculture for Rural Development 2020, Purwokerto, Indonesia, 20 October 2020; Volume 653, IOP Publishing: Bristol, UK, 2020. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, L.; Ding, Q.; Wang, R. Textile Fiber Identification Using Near-Infrared Spectroscopy and Pattern Recognition. Autex Res. J. 2019, 19, 201–209. [Google Scholar] [CrossRef] [Green Version]
- Jalalvand, A.R. Chemometrics in investigation of small molecule-biomacromolecule interactions: A review. Int. J. Biol. Macromol. 2021, 181, 478–493. [Google Scholar] [CrossRef]
- Arora, T.; Verma, R.; Kumar, R.; Chauhan, R.; Kumar, B.; Sharma, V. Chemometrics based ATR-FTIR spectroscopy method for rapid and non-destructive discrimination between eyeliner and mascara traces. Microchem. J. 2021, 164, 106080. [Google Scholar] [CrossRef]
- Gładysz, M.; Król, M.; Kościelniak, P. Differentiation of red lipsticks using the attenuated total reflection technique supported by two chemometric methods. Forensic Sci. Int. 2017, 280, 130–138. [Google Scholar] [CrossRef]
- Liang, J.; Frazier, J.; Benefield, V.; Chong, N.S.; Zhang, M. Forensic Fiber Analysis by Thermal Desorption/Pyrolysis-Direct Analysis in Real Time-Mass Spectrometry. Anal. Chem. 2019, 92, 1925–1933. [Google Scholar] [CrossRef]
- McManus, C.E.; McMillan, N.J.; Dowe, J.; Bell, J. Diamonds Certify Themselves: Multivariate Statistical Provenance Analysis. Minerals 2020, 10, 916. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, V. Forensic analysis of cigarette ash using ATR-FTIR spectroscopy and chemometric methods. Microchem. J. 2022, 178, 107406. [Google Scholar] [CrossRef]
- Sharma, V.; Bhardwaj, S.; Kumar, R. On the spectroscopic investigation of Kohl stains via ATR-FTIR and multivariate analysis: Application in forensic trace evidence. Vib. Spectrosc. 2019, 101, 81–91. [Google Scholar] [CrossRef]
- Chauhan, R.; Kumar, R.; Sharma, V. Soil forensics: A spectroscopic examination of trace evidence. Microchem. J. 2018, 139, 74–84. [Google Scholar] [CrossRef]
- Han, B.; Vial, J.; Sakamoto, S.; Sablier, M. Identification of traditional East Asian handmade papers through the multivariate data analysis of pyrolysis-GC/MS data. Analyst 2018, 144, 1230–1244. [Google Scholar] [CrossRef] [PubMed]
- Ruffin, C.; King, R.L. The analysis of hyperspectral data using Savitzky-Golay filtering-Theoretical basis (part 1). In IGARSS’99 Proceedings: Remote Sensing of the System Earth—A Challenge for the 21st Century, Proceedings of the IEEE 1999 International Geoscience and Remote Sensing Symposium, Congress Centrum Hamburg, Hamburg, Germany, 28 June–2 July 1999; IEEE: Manhattan, NY, USA, 1999. [Google Scholar]
- Sandak, J.; Sandak, A.; Meder, R. Assessing Trees, Wood and Derived Products with near Infrared Spectroscopy: Hints and Tips. J. Near Infrared Spectrosc. 2016, 24, 485–505. [Google Scholar] [CrossRef] [Green Version]

| Sample-Class | RAYON | NYLON | ACRYLIC | POLYESTER |
|---|---|---|---|---|
| SYN_NYLON_106 | ✓ | |||
| SYN_NYLON_117 | ||||
| SYN_NYLON_118 | ✓ | |||
| SYN_NYLON_119 | ✓ | |||
| SYN_NYLON_120 | ||||
| SYN_NYLON_17 | ✓ | |||
| SYN_NYLON_109 | ✓ | |||
| SYN_NYLON_110 | ✓ | |||
| SYN_NYLON_111 | ✓ | |||
| SYN_NYLON_112 | ✓ | |||
| SYN_NYLON_113 | ✓ | |||
| SYN_NYLON_114 | ✓ | |||
| SYN_NYLON_115 | ✓ | |||
| SYN_NYLON_116 | ✓ | |||
| SYN_POLYESTER_165 | ✓ | |||
| SYN_POLYESTER_175 | ✓ | |||
| SYN_POLYESTER_176 | ✓ | |||
| SYN_POLYESTER_177 | ✓ | |||
| SYN_POLYESTER_178 | ✓ | |||
| SYN_POLYESTER_179 | ✓ | |||
| SYN_POLYESTER_180 | ✓ | |||
| SYN_POLYESTER_166 | ✓ | |||
| SYN_POLYESTER_167 | ||||
| SYN_POLYESTER_168 | ✓ | |||
| SYN_POLYESTER_169 | ✓ | |||
| SYN_POLYESTER_170 | ✓ | |||
| SYN_POLYESTER_171 | ✓ | |||
| SYN_POLYESTER_172 | ✓ | |||
| SYN_POLYESTER_174 | ||||
| SYN_RAYON_19 | ✓ | |||
| SYN_RAYON_19 | ✓ | |||
| SYN_RAYON_19 | ✓ | |||
| SYN_ACRYLIC_24 | ✓ | |||
| SYN_ACRYLIC_26 | ✓ | |||
| SYN_ACRYLIC_27 | ✓ | |||
| SYN_ACRYLIC_28 | ✓ | |||
| SYN_ACRYLIC_29 | ✓ | |||
| SYN_ACRYLIC_30 | ✓ | |||
| SYN_ACRYLIC_31 | ✓ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aljannahi, A.; Alblooshi, R.A.; Alremeithi, R.H.; Karamitsos, I.; Ahli, N.A.; Askar, A.M.; Albastaki, I.M.; Ahli, M.M.; Modak, S. Forensic Analysis of Textile Synthetic Fibers Using a FT-IR Spectroscopy Approach. Molecules 2022, 27, 4281. https://doi.org/10.3390/molecules27134281
Aljannahi A, Alblooshi RA, Alremeithi RH, Karamitsos I, Ahli NA, Askar AM, Albastaki IM, Ahli MM, Modak S. Forensic Analysis of Textile Synthetic Fibers Using a FT-IR Spectroscopy Approach. Molecules. 2022; 27(13):4281. https://doi.org/10.3390/molecules27134281
Chicago/Turabian StyleAljannahi, Abdulrahman, Roudha Abdulla Alblooshi, Rashed Humaid Alremeithi, Ioannis Karamitsos, Noora Abdulkarim Ahli, Asma Mohammed Askar, Ikhlass Mohammed Albastaki, Mohamed Mahmood Ahli, and Sanjay Modak. 2022. "Forensic Analysis of Textile Synthetic Fibers Using a FT-IR Spectroscopy Approach" Molecules 27, no. 13: 4281. https://doi.org/10.3390/molecules27134281
APA StyleAljannahi, A., Alblooshi, R. A., Alremeithi, R. H., Karamitsos, I., Ahli, N. A., Askar, A. M., Albastaki, I. M., Ahli, M. M., & Modak, S. (2022). Forensic Analysis of Textile Synthetic Fibers Using a FT-IR Spectroscopy Approach. Molecules, 27(13), 4281. https://doi.org/10.3390/molecules27134281




_Karamitsos.jpg)


