Effects of Preparation Conditions on the Efficiency of Visible-Light-Driven Hydrogen Generation Based on Ni(II)-Modified Cd0.25Zn0.75S Photocatalysts
Abstract
:1. Introduction
2. Results and Discussion
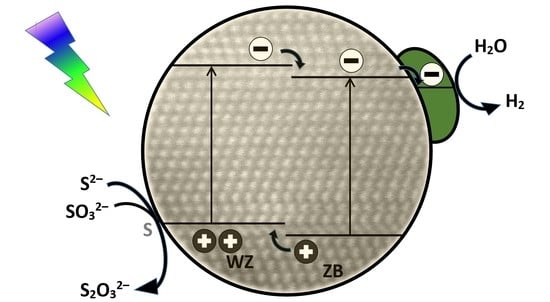
2.1. Photocatalytic H2 Production
2.1.1. Ni(II) and Pt Modified Catalysts
2.1.2. Effects of Ammonia as Complexing Agent
2.2. Characterization of Catalysts
2.3. Stability of Catalysts
3. Materials and Methods
3.1. Materials
3.2. Photocatalyst Preparation
3.3. Characterization
3.4. Photochemical Experiments
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cai, C.; Worden, R.H.; Wolff, G.A.; Bottrell, S.; Wang, D.; Li, X. Origin of sulfur rich oils and H2S in Tertiary lacustrine sections of the Jinxian Sag, Bohai Bay Basin, China. Appl. Geochem. 2005, 20, 1427–1444. [Google Scholar] [CrossRef]
- Rezazadeh, R.; Rezvantalab, S. Investigation of Inlet Gas Streams Effect on the Modified Claus Reaction Furnace. Adv. Chem. Eng. Sci. 2013, 3, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Cheremisinoff, N.; Rosenfeld, P.; Davletshin, A.R. Responsible Care: A New Strategy for Pollution Prevention and Waste Reduction through Environmental Management, Chapter 5, Refineries. In Responsible Care: A New Strategy for Pollution Prevention and Waste Reduction through Environmental Management; Elsevier: Houston, TX, USA, 2008; pp. 236–316. [Google Scholar]
- Gary, J.H.; Handwerk, J.H.; Kaiser, M.J.; Geddes, D. Petroleum Refining: Technology and Economics, 5th ed.; CRC Press: Boca Raton, FL, USA, 2007; ISBN 9780203907924. [Google Scholar] [CrossRef]
- Kalyanasundaram, K.; Borgarello, E.; Gráutzel, M. Visible Light Induced Water Cleavage in CdS Dispersions Loaded with Pt and RuO2, Hole Scavenging by RuO2. Helv. Chim. Acta 1981, 64, 362–366. [Google Scholar] [CrossRef]
- Borgarello, E.; Serpone, N.; Grätzel, M.; Pelizzetti, E. Photodecomposition of H2S in aqueous alkaline media catalyzed by RuO2-loaded alumina in the presence of cadmium sulfide. Application of the inter-particle electron transfer mechanism. Inorg. Chim. Acta 1986, 112, 197–201. [Google Scholar] [CrossRef]
- Buehler, N.; Meier, K.; Reber, J.F. Photochemical hydrogen production with cadmium sulfide suspensions. J. Phys. Chem. 1984, 88, 3261–3268. [Google Scholar] [CrossRef]
- Zhang, Y.; Heo, Y.-J.; Lee, J.-W.; Lee, J.-H.; Bajgai, J.; Lee, K.-J.; Park, S.-J. Photocatalytic Hydrogen Evolution via Water Splitting: A Short Review. Catalysts 2018, 8, 655. [Google Scholar] [CrossRef] [Green Version]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Kudo, A. Development of photocatalyst materials for water splitting. Int. J. Hydrogen Energy 2006, 31, 197–202. [Google Scholar] [CrossRef]
- Matsuoka, M.; Kitano, M.; Takeuchi, M.; Tsujimaru, K.; Anpo, M.; Thomas, J.M. Photocatalysis for new energy production. Catal. Today 2007, 122, 51–61. [Google Scholar] [CrossRef]
- Zhu, J.; Zäch, M. Nanostructured materials for photocatalytic hydrogen production. Curr. Opin. Colloid Interface Sci. 2009, 14, 260–269. [Google Scholar] [CrossRef]
- Acar, C.; Dincer, I.; Zamfirescu, C. A review on selected heterogeneous photocatalysts for hydrogen production. Int. J. Energy Res. 2014, 38, 1903–1920. [Google Scholar] [CrossRef]
- Gong, B.; Lu, Y.; Wu, P.; Huang, Z.; Zhu, Y.; Dang, Z.; Zhu, N.; Lu, G.; Huang, J. Enhanced photocatalytic activity over Cd0.5Zn0.5S with stacking fault structure combined with Cu2+ modified carbon nanotubes. Appl. Surf. Sci. 2016, 365, 280–290. [Google Scholar] [CrossRef]
- Lu, Q.; Yu, Y.; Ma, Q.; Chen, B.; Zhang, H. 2D Transition-Metal-Dichalcogenide-Nanosheet-Based Composites for Photocatalytic and Electrocatalytic Hydrogen Evolution Reactions. Adv. Mater. 2016, 28, 1917–1933. [Google Scholar] [CrossRef]
- Rahman, A.; Khan, M.M. Chalcogenides as photocatalysts. New J. Chem. 2021, 45, 19622–19635. [Google Scholar] [CrossRef]
- Khan, M.M. Chapter 7—Chalcogenides for visible light-induced photocatalysis. In Micro and Nano Technologies; Nayak, A.K., Sahu, N.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 185–195. ISBN 978-0-12-823018-3. [Google Scholar]
- Guo, L.; Jing, D.; Liu, M.; Chen, Y.; Shen, S.; Shi, J.; Zhang, K. Functionalized nanostructures for enhanced photocatalytic performance under solar light. Beilstein J. Nanotechnol. 2014, 5, 994–1004. [Google Scholar] [CrossRef] [Green Version]
- Joe, J.; Yang, H.; Bae, C.; Shin, H. Metal chalcogenides on silicon photocathodes for efficient water splitting: A mini overview. Catalysts 2019, 9, 149. [Google Scholar] [CrossRef] [Green Version]
- Stroyuk, A.L.; Raevskaya, A.E.; Korzhak, A.V.; Kotenko, I.E.; Glebov, E.M.; Plyusnin, V.F.; Kuchmii, S.Y. Photocatalytic production of hydrogen in systems based on CdxZn1–xS/Ni0 nanostructures. Theor. Exp. Chem. 2009, 45, 12–22. [Google Scholar] [CrossRef]
- Liu, X.; Liang, X.; Wang, P.; Huang, B.; Qin, X.; Zhang, X.; Dai, Y. Highly efficient and noble metal-free NiS modified MnxCd1-xS solid solutions with enhanced photocatalytic activity for hydrogen evolution under visible light irradiation. Appl. Catal. B Environ. 2017, 203, 282–288. [Google Scholar] [CrossRef]
- Saraswathi Amma, B.; Manzoor, K.; Ramakrishna, K.; Pattabi, M. Synthesis and optical properties of CdS/ZnS coreshell nanoparticles. Mater. Chem. Phys. 2008, 112, 789–792. [Google Scholar] [CrossRef]
- Lyubina, T.P.; Kozlova, E.A. New photocatalysts based on cadmium and zinc sulfides for hydrogen evolution from aqueous Na2S-Na2SO3 solutions under irradiation with visible light. Kinet. Catal. 2012, 53, 188–196. [Google Scholar] [CrossRef]
- Xing, C.; Zhang, Y.; Yan, W.; Guo, L. Band structure-controlled solid solution of Cd1-xZnxS photocatalyst for hydrogen production by water splitting. Int. J. Hydrogen Energy 2006, 31, 2018–2024. [Google Scholar] [CrossRef]
- Koca, A. Photocatalytic hydrogen production by direct sun light from sulfide/sulfite solution. Int. J. Hydrogen Energy 2002, 27, 363–367. [Google Scholar] [CrossRef]
- Chan, C.-C.; Chang, C.-C.; Hsu, C.-H.; Weng, Y.-C.; Chen, K.-Y.; Lin, H.-H.; Huang, W.-C.; Cheng, S.-F. Efficient and stable photocatalytic hydrogen production from water splitting over ZnxCd1–xS solid solutions under visible light irradiation. Int. J. Hydrogen Energy 2014, 39, 1630–1639. [Google Scholar] [CrossRef]
- Fodor, L.; Solymosi, B.; Horváth, O. Investigation of Hydrogen Production from Alkaline Sulfide Solution with Nanosized CdS/ZnS-PdS Photocatalyst of Various Compositions. J. Nanosci. Nanotechnol. 2018, 19, 509–515. [Google Scholar] [CrossRef]
- Li, X.; Xue, F.; Li, N.; Wei, X.; Liu, H.; Zhou, J.; Lyu, B.; Liu, M. One-Pot Hydrothermal Synthesis of MoS2/Zn0.5Cd0.5S Heterojunction for Enhanced Photocatalytic H2 Production. Front. Chem. 2020, 8, 779. [Google Scholar] [CrossRef]
- De, G.C.; Roy, A.M.; Bhattacharya, S.S. Effect of n-Si on the photocatalytic production of hydrogen by Pt-loaded CdS and CdS/ZnS catalyst. Int. J. Hydrogen Energy 1996, 21, 19–23. [Google Scholar] [CrossRef]
- Zhang, Y.; Peng, Z.; Guan, S.; Fu, X. Novel β-NiS film modified CdS nanoflowers heterostructure nanocomposite: Extraordinarily highly efficient photocatalysts for hydrogen evolution. Appl. Catal. B Environ. 2018, 224, 1000–1008. [Google Scholar] [CrossRef]
- Qin, Z.; Xue, F.; Chen, Y.; Shen, S.; Guo, L. Spatial charge separation of one-dimensional Ni2P-Cd0.9Zn0.1S/g-C3N4 heterostructure for high-quantum-yield photocatalytic hydrogen production. Appl. Catal. B Environ. 2017, 217, 551–559. [Google Scholar] [CrossRef]
- Li, L.; Wu, J.; Liu, B.; Liu, X.; Li, C.; Gong, Y.; Huang, Y.; Pan, L. NiS sheets modified CdS/reduced graphene oxide composite for efficient visible light photocatalytic hydrogen evolution. Catal. Today 2018, 315, 110–116. [Google Scholar] [CrossRef]
- Li, C.; Wang, H.; Naghadeh, S.B.; Zhang, J.Z.; Fang, P. Visible light driven hydrogen evolution by photocatalytic reforming of lignin and lactic acid using one-dimensional NiS/CdS nanostructures. Appl. Catal. B Environ. 2018, 227, 229–239. [Google Scholar] [CrossRef]
- Guan, S.; Fu, X.; Zhang, Y.; Peng, Z. β-NiS modified CdS nanowires for photocatalytic H2 evolution with exceptionally high efficiency. Chem. Sci. 2018, 9, 1574–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, X.; Sun, H.; Zhang, H.; Tu, W. One-pot hydrothermal synthesis of CdS/NiS photocatalysts for high H2 evolution from water under visible light. Int. J. Hydrogen Energy 2017, 42, 11199–11205. [Google Scholar] [CrossRef]
- Xu, Y.; Tu, W.; Yin, S.; Kraft, M.; Zhang, Q.; Xu, R. Self-template synthesis of CdS/NiSx heterostructured nanohybrids for efficient photocatalytic hydrogen evolution. Dalt. Trans. 2017, 46, 10650–10656. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Guo, L. NiS modified CdS pyramids with stacking fault structures: Highly efficient and stable photocatalysts for hydrogen production from water. Int. J. Hydrogen Energy 2017, 42, 23995–24005. [Google Scholar] [CrossRef]
- Li, N.; Zhou, B.; Guo, P.; Zhou, J.; Jing, D. Fabrication of noble-metal-free Cd0.5Zn0.5S/NiS hybrid photocatalyst for efficient solar hydrogen evolution. Int. J. Hydrogen Energy 2013, 38, 11268–11277. [Google Scholar] [CrossRef]
- Chen, M.; Wu, P.; Zhu, Y.; Yang, S.; Lu, Y.; Lin, Z. Enhanced photocatalytic H2 production activity of CdZnS with stacking faults structure assisted by ethylenediamine and NiS. Int. J. Hydrogen Energy 2018, 43, 10938–10949. [Google Scholar] [CrossRef]
- Liu, M.; Jing, D.; Zhou, Z.; Guo, L. Twin-induced one-dimensional homojunctions yield high quantum efficiency for solar hydrogen generation. Nat. Commun. 2013, 4, 2278. [Google Scholar] [CrossRef]
- Huang, H.-B.; Fang, Z.-B.; Yu, K.; Lü, J.; Cao, R. Visible-light-driven photocatalytic H2 evolution over CdZnS nanocrystal solid solutions: Interplay of twin structures, sulfur vacancies and sacrificial agents. J. Mater. Chem. A 2020, 8, 3882–3891. [Google Scholar] [CrossRef]
- Mersel, M.A.; Fodor, L.; Pekker, P.; Jakab, M.; Makó, É.; Horváth, O. Effects of preparation conditions on the efficiency of visible-light-driven hydrogen generation based on Cd0.25Zn0.75S photocatalysts. Catalysts 2021, 11, 1534. [Google Scholar] [CrossRef]
- Xue, F.; Fu, W.; Liu, M.; Wang, X.; Wang, B.; Guo, L. Insight into Cd0.9Zn0.1S solid-solution nanotetrapods: Growth mechanism and their application for photocatalytic hydrogen production. Int. J. Hydrogen Energy 2016, 41, 20455–20464. [Google Scholar] [CrossRef]
- Lingampalli, S.R.; Roy, A.; Ikram, M.; Rao, C.N.R. Visible-light induced hydrogen generation with ZnO/NiO/Cd1−xZnxS (x = 0.0, 0.2) heterostructures. Chem. Phys. Lett. 2014, 610–611, 316–320. [Google Scholar] [CrossRef]
- Roy, A.; Lingampalli, S.R.; Saha, S.; Rao, C.N.R. Effects of morphology and surface area of the oxide nanostructures on the visible–light induced generation of hydrogen in ZnO(TiO2)/Cd1−xZnxS and ZnO(TiO2)/Pt/Cd1−xZnxS heterostructures (x = 0.0, 0.2). Chem. Phys. Lett. 2015, 637, 137–142. [Google Scholar] [CrossRef]
- Li, Q.; Meng, H.; Zhou, P.; Zheng, Y.; Wang, J.; Yu, J.; Gong, J. Zn1-xCdxS solid solutions with controlled bandgap and enhanced visible-light photocatalytic H2-production activity. ACS Catal. 2013, 3, 882–889. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, Z.; Wang, Y.; Xu, R. Doped Solid Solution: (Zn0.95Cu0.05)1−xCdxS Nanocrystals with High Activity for H2 Evolution from Aqueous Solutions under Visible Light. J. Phys. Chem. C 2008, 112, 17635–17642. [Google Scholar] [CrossRef]
- Taqui-Khan, M.; Bhardwaj, R.; Bhardwaj, C. Photodecomposition of H2S by silver doped cadmium sulfide and mixed sulfides with ZnS. Int. J. Hydrogen Energy 1988, 13, 7–10. [Google Scholar] [CrossRef]
- Villa, K.; Domènech, X.; García-Pérez, U.M.; Peral, J. Optimization of the experimental conditions of hydrogen production by the Pt–(CdS/ZnS) system under visible light illumination. RSC Adv. 2016, 6, 36681–36688. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Chen, J.; Li, N.; Zheng, J.; Zhao, J.; Zhu, Z. Enhanced photocatalytic H2-production activity of CdxZn1−xS nanocrystals by surface loading MS (M = Ni, Co, Cu) species. Appl. Surf. Sci. 2012, 259, 118–123. [Google Scholar] [CrossRef]
- Yao, L.; Wei, D.; Ni, Y.; Yan, D.; Hu, C. Surface localization of CdZnS quantum dots onto 2D g-C3N4 ultrathin microribbons: Highly efficient visible light-induced H2-generation. Nano Energy 2016, 26, 248–256. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Bish, D.L.; Post, J.E. Modern Powder Diffraction; The Mineralogical Society of America: Washington, DC, USA, 1989. [Google Scholar]
- Zhang, Y.; Hu, H.; Kang, W.; Qiu, G.; Liang, R.; Deng, L.; Yuan, H. Enhancing hydrogen evolution by photoelectrocatalysis of water splitting over a CdS flowers-loaded TiO2 nanotube array film on the Ti foil substrate. Ceram. Int. 2020, 46, 17606–17613. [Google Scholar] [CrossRef]









| Band-Gap Energy/eV | ||
|---|---|---|
| Ni Content (%) | HT Treated (Ni-B) | HT Untreated (Ni-S) |
| 0 | 2.59 ± 0.02 | 2.68 ± 0.03 |
| 0.1 | 2.59 ± 0.02 | 2.73 ± 0.03 |
| 0.2 | 2.58 ± 0.02 | 2.72 ± 0.03 |
| 0.3 | 2.70 ± 0.03 | |
| 0.4 | 2.70 ± 0.03 | |
| 0.5 | 2.57 ± 0.02 | 2.65 ± 0.03 |
| 1.0 | 2.57 ± 0.02 | |
| 2.0 | 2.61 ± 0.02 * | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mersel, M.-A.; Fodor, L.; Pekker, P.; Makó, É.; Horváth, O. Effects of Preparation Conditions on the Efficiency of Visible-Light-Driven Hydrogen Generation Based on Ni(II)-Modified Cd0.25Zn0.75S Photocatalysts. Molecules 2022, 27, 4296. https://doi.org/10.3390/molecules27134296
Mersel M-A, Fodor L, Pekker P, Makó É, Horváth O. Effects of Preparation Conditions on the Efficiency of Visible-Light-Driven Hydrogen Generation Based on Ni(II)-Modified Cd0.25Zn0.75S Photocatalysts. Molecules. 2022; 27(13):4296. https://doi.org/10.3390/molecules27134296
Chicago/Turabian StyleMersel, Maali-Amel, Lajos Fodor, Péter Pekker, Éva Makó, and Ottó Horváth. 2022. "Effects of Preparation Conditions on the Efficiency of Visible-Light-Driven Hydrogen Generation Based on Ni(II)-Modified Cd0.25Zn0.75S Photocatalysts" Molecules 27, no. 13: 4296. https://doi.org/10.3390/molecules27134296
APA StyleMersel, M.-A., Fodor, L., Pekker, P., Makó, É., & Horváth, O. (2022). Effects of Preparation Conditions on the Efficiency of Visible-Light-Driven Hydrogen Generation Based on Ni(II)-Modified Cd0.25Zn0.75S Photocatalysts. Molecules, 27(13), 4296. https://doi.org/10.3390/molecules27134296