Tetrahydropyridines’ Stereoselective Formation, How Lockdown Assisted in the Identification of the Features of Its Mechanism
Abstract
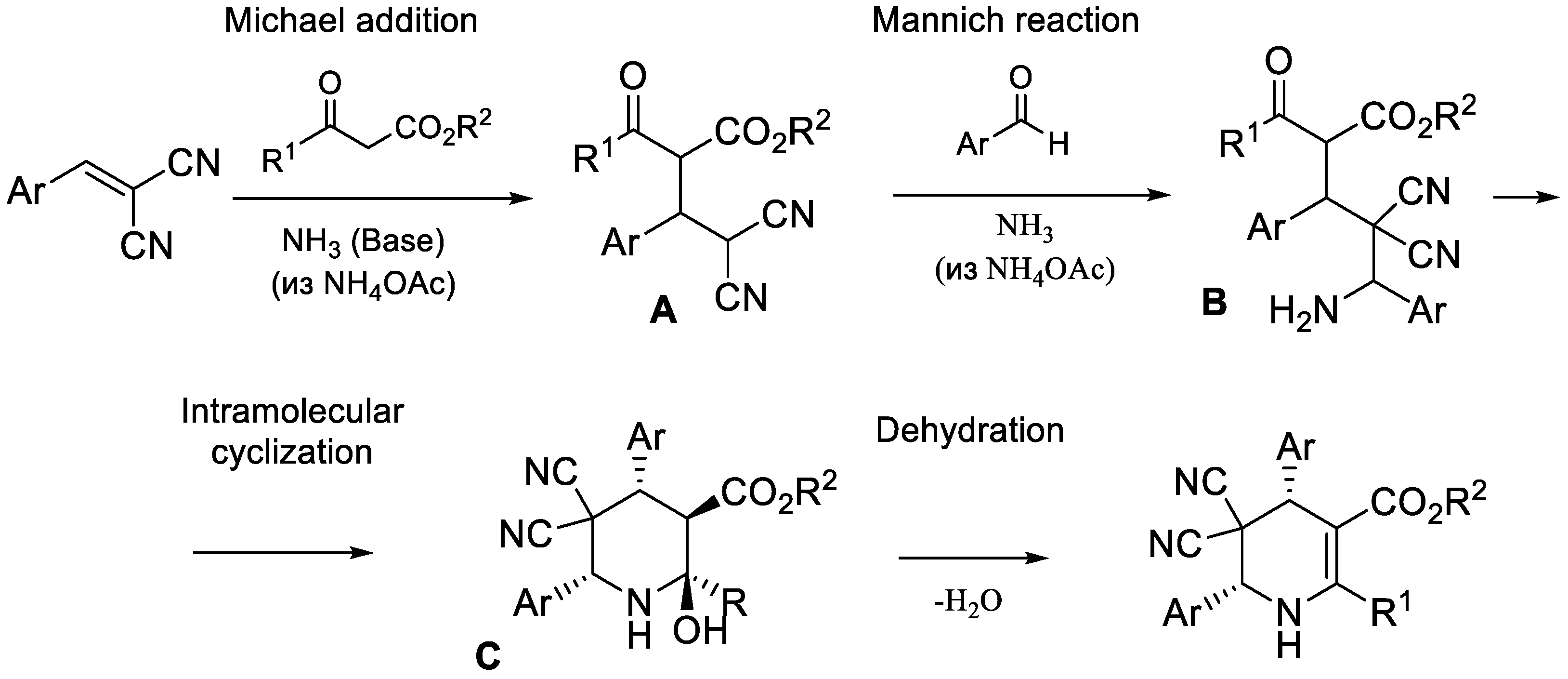
:1. Introduction
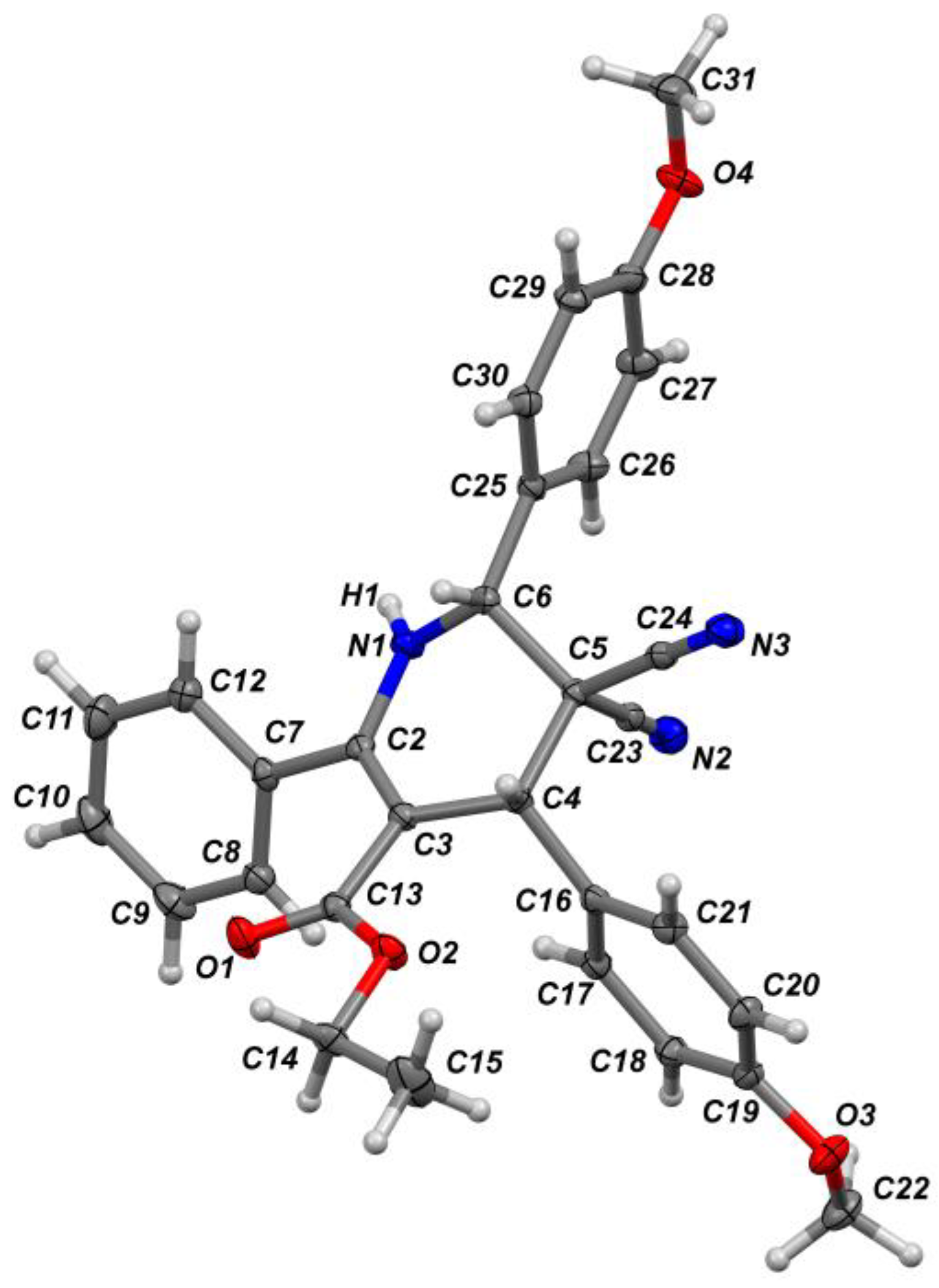
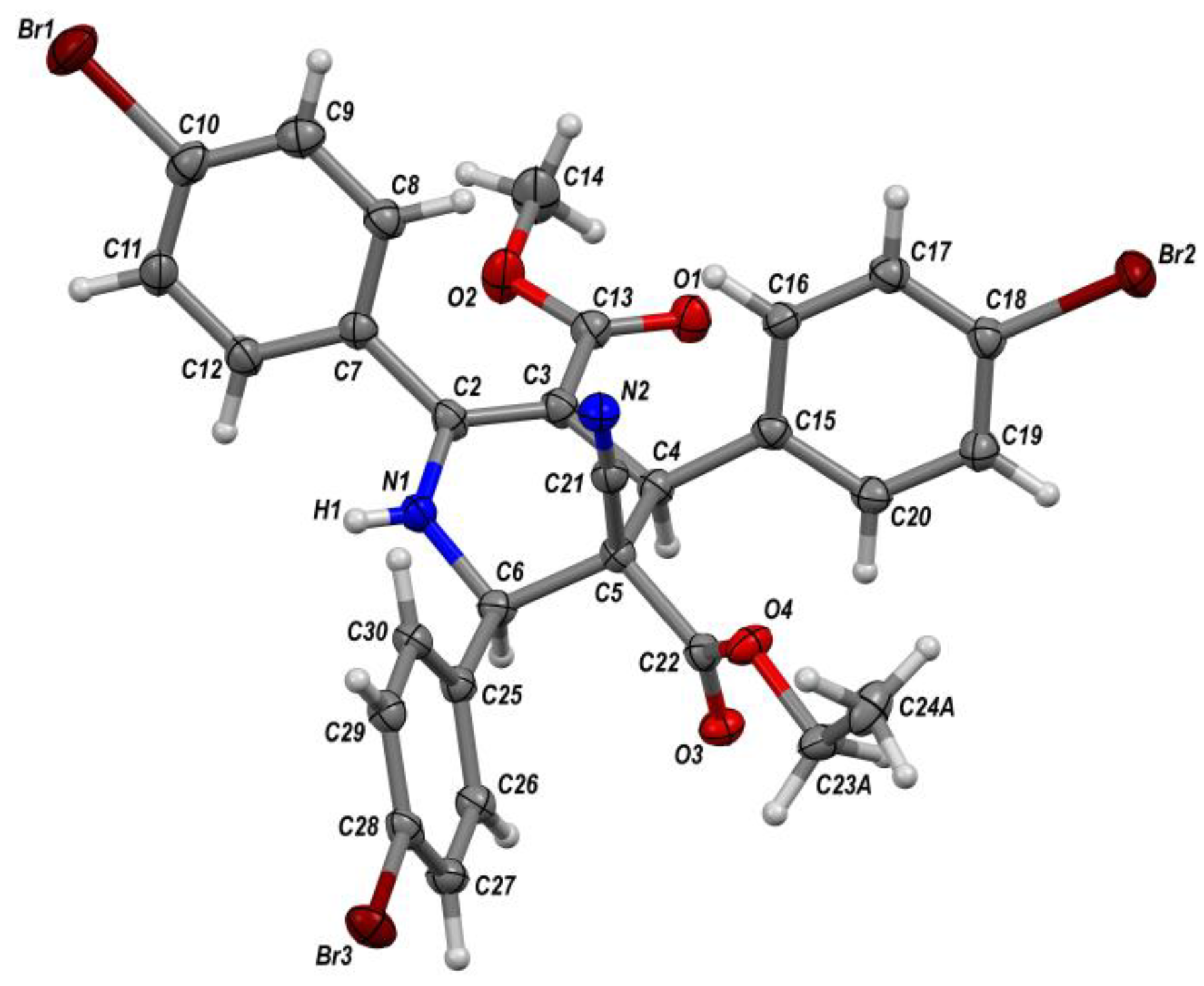
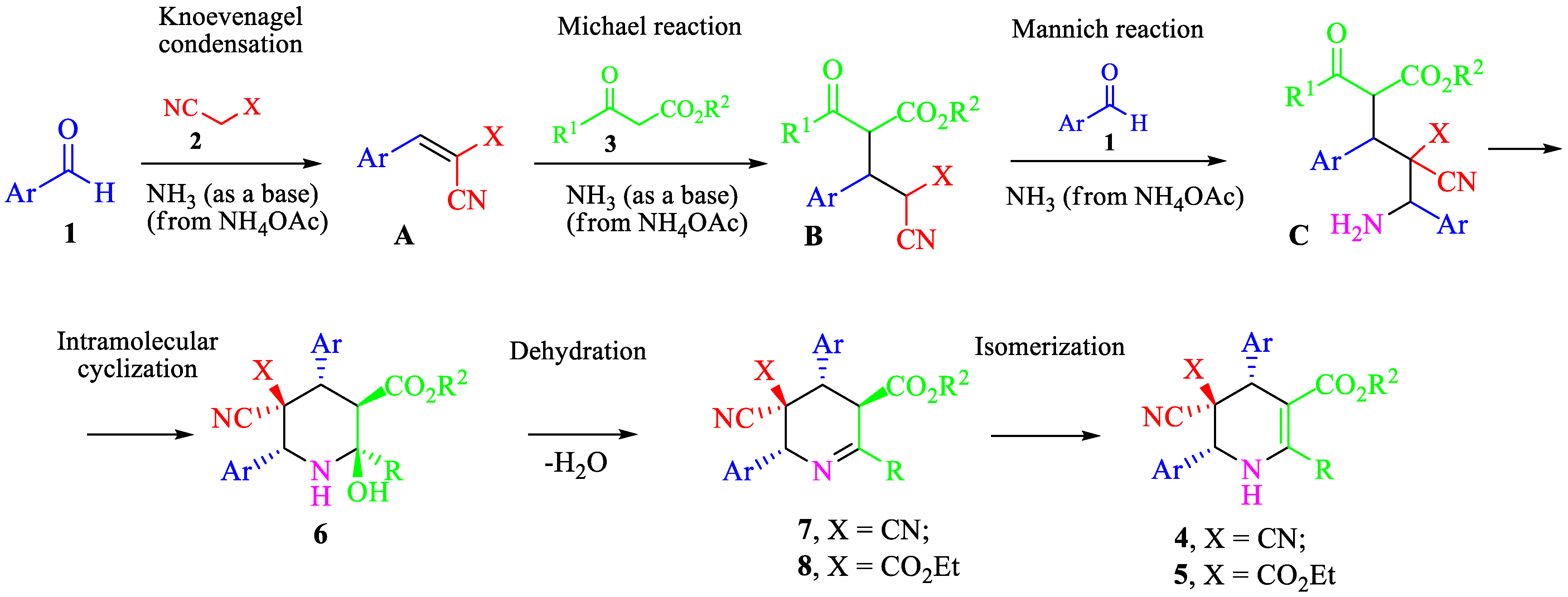
2. Results and Discussion
3. Experimental Procedure
3.1. General Information
3.2. DFT Calculations
3.3. X-ray Crystallographic Data and Refinement Details
3.4. Synthesis of 4–5
3.5. Synthesis of 6
3.6. Synthesis of 7
3.7. Synthesis of 8
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Vardanyan, R. Chapter 1—Introduction. In Piperidine-Based Drug Discovery; Vardanyan, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–82. [Google Scholar]
- Wang, W.; Hu, Y. Small molecule agents targeting the p53-MDM2 pathway for cancer therapy. Med. Res. Rev. 2012, 32, 1159–1196. [Google Scholar] [CrossRef] [PubMed]
- Petit, S.; Nallet, J.P.; Guillard, M.; Dreux, J.; Chermat, R.; Poncelet, M.; Bulach, C.; Simon, P.; Fontaine, C.; Barthelmebs, M.; et al. Synthèses et activités psychotropes de 3,4-diarylpipéridines. Corrélation structure-activité et recherche d’une activité antihypertensive. Eur. J. Med. Chem. 1991, 26, 19–32. [Google Scholar]
- Borza, I.; Domany, G. NR2B Selective NMDA Antagonists: The Evolution of the Ifenprodil-Type Pharmacophore. Curr. Top. Med. Chem. 2006, 6, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Mony, L.; Kew, J.N.; Gunthorpe, M.J.; Paoletti, P. Allosteric modulators of NR2B-containing NMDA receptors: Molecular mechanisms and therapeutic potential. Brit. J. Pharmacol. 2009, 157, 1301–1317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Gregor, V.E.; Ayida, B.K.; Winters, G.C.; Sun, Z.; Murphy, D.; Haley, G.; Bailey, D.; Froelich, J.M.; Fish, S.; et al. Synthesis and SAR of 3,5-diamino-piperidine derivatives: Novel antibacterial translation inhibitors as aminoglycoside mimetics. Bioorg. Med. Chem. Lett. 2007, 17, 1206–1210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, B.; Michael Crider, A.; Stables, J.P. Synthesis and structure–activity relationships of potential anticonvulsants based on 2-piperidinecarboxylic acid and related pharmacophores. Eur. J. Med. Chem. 2001, 36, 265–286. [Google Scholar] [CrossRef]
- Gitto, R.; De Luca, L.; Ferro, S.; Occhiuto, F.; Samperi, S.; De Sarro, G.; Russo, E.; Ciranna, L.; Costa, L.; Chimirri, A. Computational Studies to Discover a New NR2B/NMDA Receptor Antagonist and Evaluation of Pharmacological Profile. ChemMedChem 2008, 3, 1539–1548. [Google Scholar] [CrossRef]
- Tanaka, R.; Rubio, A.; Harn, N.K.; Gernert, D.; Grese, T.A.; Eishima, J.; Hara, M.; Yoda, N.; Ohashi, R.; Kuwabara, T.; et al. Design and synthesis of piperidine farnesyltransferase inhibitors with reduced glucuronidation potential. Bioorg. Med. Chem. 2007, 15, 1363–1382. [Google Scholar] [CrossRef]
- Kim, C.U.; Lew, W.; Williams, M.A.; Liu, H.; Zhang, L.; Swaminathan, S.; Bischofberger, N.; Chen, M.S.; Mendel, D.B.; Tai, C.Y.; et al. Influenza Neuraminidase Inhibitors Possessing a Novel Hydrophobic Interaction in the Enzyme Active Site: Design, Synthesis, and Structural Analysis of Carbocyclic Sialic Acid Analogues with Potent Anti-Influenza Activity. J. Am. Chem. Soc. 1997, 119, 681–690. [Google Scholar] [CrossRef]
- von Itzstein, M.; Wu, W.-Y.; Kok, G.B.; Pegg, M.S.; Dyason, J.C.; Jin, B.; Van Phan, T.; Smythe, M.L.; White, H.F.; Oliver, S.W.; et al. Rational design of potent sialidase-based inhibitors of influenza virus replication. Nature 1993, 363, 418–423. [Google Scholar] [CrossRef]
- Chand, P.; Kotian, P.L.; Dehghani, A.; El-Kattan, Y.; Lin, T.-H.; Hutchison, T.L.; Babu, Y.S.; Bantia, S.; Elliott, A.J.; Montgomery, J.A. Systematic Structure-Based Design and Stereoselective Synthesis of Novel Multisubstituted Cyclopentane Derivatives with Potent Antiinfluenza Activity. J. Med. Chem. 2001, 44, 4379–4392. [Google Scholar] [CrossRef] [PubMed]
- Jacob, G.S. Glycosylation inhibitors in biology and medicine. Curr. Opin. Struct. Biol. 1995, 5, 605–611. [Google Scholar] [CrossRef]
- Treadway, J.L.; Mendys, P.; Hoover, D.J. Glycogen phosphorylase inhibitors for treatment of type 2 diabetes mellitus. Expert Opin. Investig. Drugs 2001, 10, 439–454. [Google Scholar] [CrossRef] [PubMed]
- Groopman, J.E. Management of the Hematologic Complications of Human Immunodificiency Virus Infection. Rev. Infect. Dis. 1990, 12, 931–937. [Google Scholar] [CrossRef] [PubMed]
- Zitzmann, N.; Mehta, A.S.; Carrouée, S.; Butters, T.D.; Platt, F.M.; McCauley, J.; Blumberg, B.S.; Dwek, R.A.; Block, T.M. Imino sugars inhibit the formation and secretion of bovine viral diarrhea virus, a pestivirus model of hepatitis C virus: Implications for the development of broad spectrum anti-hepatitis virus agents. Proc. Natl. Acad. Sci. USA 1999, 96, 11878–11882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlsson, G.B.; Butters, T.D.; Dwek, R.A.; Platt, F.M. Effects of the imino sugar N-butyldeoxynojirimycin on the N-glycosylation of recombinant gp120. J. Biol. Chem. 1993, 268, 570–576. [Google Scholar] [CrossRef]
- Mochizuki, A.; Nakamoto, Y.; Naito, H.; Uoto, K.; Ohta, T. Design, synthesis, and biological activity of piperidine diamine derivatives as factor Xa inhibitor. Bioorg. Med. Chem. Lett. 2008, 18, 782–787. [Google Scholar] [CrossRef]
- Nishimura, Y.; Satoh, T.; Adachi, H.; Kondo, S.; Takeuchi, T.; Azetaka, M.; Fukuyasu, H.; Iizuka, Y. Synthesis and Antimetastatic Activity of l-Iduronic Acid-Type 1-N-Iminosugars. J. Med. Chem. 1997, 40, 2626–2633. [Google Scholar] [CrossRef]
- Sun, C.-W.; Wang, J.; Wu, Y.; Nan, S.-B.; Zhang, W.-G. Novel nitenpyram analogues with tetrahydropyridone-fixed cis-configuration: Synthesis, insecticidal activities, and molecular docking studies. Heterocycles 2013, 87, 1865–1880. [Google Scholar] [CrossRef]
- Brown, B.S.; Keddy, R.; Zheng, G.Z.; Schmidt, R.G.; Koenig, J.R.; McDonald, H.A.; Bianchi, B.R.; Honore, P.; Jarvis, M.F.; Surowy, C.S.; et al. Tetrahydropyridine-4-carboxamides as novel, potent transient receptor potential vanilloid 1 (TRPV1) antagonists. Bioorg. Med. Chem. 2008, 16, 8516–8525. [Google Scholar] [CrossRef]
- Misra, M.; Pandey, S.K.; Pandey, V.P.; Pandey, J.; Tripathi, R.; Tripathi, R.P. Organocatalyzed highly atom economic one pot synthesis of tetrahydropyridines as antimalarials. Bioorg. Med. Chem. 2009, 17, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Kozikowski, A.P.; Johnson, K.M.; Deschaux, O.; Bandyopadhyay, B.C.; Araldi, G.L.; Carmona, G.; Munzar, P.; Smith, M.P.; Balster, R.L.; Beardsley, P.M.; et al. Mixed Cocaine Agonist/Antagonist Properties of (+)-Methyl 4β-(4-Chlorophenyl)-1-methylpiperidine-3α-carboxylate, a Piperidine-Based Analog of Cocaine. J. Pharmacol. Exp. Ther. 2003, 305, 143–150. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, T.H.A.; Benneker, F.B.G.; Hoorn, H.J.; Picha, F. Process for Producing 4-arylpiperidine-3-carbinols and Related Compounds. Patent WO2000026187, 11 May 2000. [Google Scholar]
- Han, B.; Li, J.-L.; Ma, C.; Zhang, S.-J.; Chen, Y.-C. Organocatalytic Asymmetric Inverse-Electron-Demand Aza-Diels–Alder Reaction of N-Sulfonyl-1-aza-1,3-butadienes and Aldehydes. Angew. Chem. Int. Ed. 2008, 47, 9971–9974. [Google Scholar] [CrossRef] [PubMed]
- Rueping, M.; Antonchick, A.P. A Highly Enantioselective Brønsted Acid Catalyzed Reaction Cascade. Angew. Chem. Int. Ed. 2008, 47, 5836–5838. [Google Scholar] [CrossRef]
- Zhang, J.; Yang, W.-J.; Sun, J.; Yan, C.-G. Convenient Synthesis of Functionalized 6-Styryl-1,4,5,6-tetrahydropyridines through a Domino [2 + 2 + 2] Cycloaddition Reaction. Eur. J. Org. Chem. 2015, 2015, 7571–7582. [Google Scholar] [CrossRef]
- Han, R.-G.; Wang, Y.; Li, Y.-Y.; Xu, P.-F. Proline-Mediated Enantioselective Construction of Tetrahydropyridines via a Cascade Mannich-Type/Intramolecular Cyclization Reaction. Adv. Synth. Catal. 2008, 350, 1474–1478. [Google Scholar] [CrossRef]
- Clarke, P.A.; Santos, S.; Martin, W.H.C. Combining pot, atom and step economy (PASE) in organic synthesis. Synthesis of tetrahydropyran-4-ones. Green Chem. 2007, 9, 438–440. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, Y. Pot economy and one-pot synthesis. Chem. Sci. 2016, 7, 866–880. [Google Scholar] [CrossRef] [Green Version]
- Tietze, L.F.; Brasche, G.; Gerike, K. Domino Reactions in Organic Chemistry; Wiley: Weinheim, Germany, 2006. [Google Scholar]
- Tietze, L.F. Domino Reactions: Concepts for Efficient Organic Synthesis; Wiley: Weinheim, Germany, 2014. [Google Scholar]
- Zhu, J.P.; Bienayme, H. Multicomponent Reactions; Wiley: Weinheim, Germany, 2004. [Google Scholar]
- Dömling, A. Recent developments in isocyanide based multicomponent reactions in applied chemistry. Chem. Rev. 2006, 106, 17–89. [Google Scholar] [CrossRef]
- Dömling, A.; Ugi, I. Multicomponent reactions with isocyanides. Angew. Chemie Int. Ed. 2000, 39, 3168–3210. [Google Scholar] [CrossRef]
- Nair, V.; Rajesh, C.; Vinod, A.U.; Bindu, S.; Sreekanth, A.R.; Mathen, J.S.; Balagopal, L. Strategies for heterocyclic construction via novel multicomponent reactions based on isocyanides and nucleophilic carbenes. Acc. Chem. Res. 2003, 36, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.-J.; Mo, L.-P.; Zhang, Z.-H. Cerium Ammonium Nitrate-Catalyzed Multicomponent Reaction for Efficient Synthesis of Functionalized Tetrahydropyridines. ACS Comb. Sci. 2011, 13, 181–185. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, N.R.; Bahekar, S.P.; Sarode, P.B.; Zade, S.S.; Chandak, H.S. L-Proline nitrate: A recyclable and green catalyst for the synthesis of highly functionalized piperidines. RSC Adv. 2015, 5, 47053–47059. [Google Scholar] [CrossRef]
- Ismail, M.M.F.; Farrag, A.M.; Abou-El-Ela, D. Synthesis, anticancer screening, and in silico ADME prediction of novel 2-pyridones as Pim inhibitors. J. Heterocycl. Comp. 2020, 57, 3442–3460. [Google Scholar]
- Dyachenko, I.V.; Dyachenko, V.D.; Dorovatovskii, P.V.; Khrustalev, V.N.; Nenajdenko, V.G. Synthesis of functionalized tetrahydropyridones by the tandem Knoevenagel—Michael—intramolecular ammonolysis—alkylation reaction. Russ. Chem. Bull. 2021, 70, 2145–2155. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Dorofeeva, E.O.; Goloveshkin, A.S.; Egorov, M.P. Pseudo six-component stereoselective synthesis of 2,4,6-triaryl-3,3,5,5-tetracyanopiperidines. Mendeleev Commun. 2018, 28, 384–386. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Gorbunov, S.V.; Anisina, Y.E.; Egorov, M.P. Stereoselective multicomponent synthesis of (2RS, 6SR)-2,6-diaryl-3,3,5,5-tetracyanopiperidines. Russ. Chem. Bull. 2018, 67, 1534–1537. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Goloveshkin, A.S.; Ushakov, I.E.; Egorov, M.P. Four-component stereoselective synthesis of tetracyano-substituted piperidines. Res. Chem. Intermed. 2018, 44, 5623–5634. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Goloveshkin, A.S.; Dorofeeva, E.O.; Egorov, M.P. Highly diastereoselective four-component synthesis of polysubstituted 2-piperidinones with three and four stereogenic centers. Res. Chem. Intermed. 2020, 46, 1183–1199. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Elinson, M.N.; Minaeva, A.P.; Goloveshkin, A.S.; Hansford, K.A.; Egorov, M.P. One-pot five-component high diastereoselective synthesis of polysubstituted 2-piperidinones from aromatic aldehydes, nitriles, dialkyl malonates and ammonium acetate. Mol. Divers. 2020, 24, 1327–1342. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Karpenko, K.A.; Iliyasov, T.M.; Elinson, M.N.; Dorofeeva, E.O.; Fakhrutdinov, A.N.; Egorov, M.P. Diastereoselective multicomponent synthesis of (4RS,6SR)-4,6-diaryl-5,5-dicyano-2-methyl-1,4,5,6-tetrahydropyridine-3-carboxylates. Russ. Chem. Bull. 2018, 67, 2049–2053. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Iliyasov, T.M.; Karpenko, K.A.; Smirnov, V.A.; Ushakov, I.E.; Elinson, M.N. High diastereoselective four-component synthesis of polysubstituted 1,4,5,6-tetrahydropyridines. Chem. Heterocycl. Compd. 2021, 57, 929–933. [Google Scholar] [CrossRef]
- Nikishkin, N.I.; Huskens, J.; Verboom, W. Study on the Pd/C-Catalyzed (Retro-)Michael Addition Reaction of Activated Methylene Compounds to Electron-Poor Styrenes. Eur. J. Org. Chem. 2010, 2010, 6820–6823. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Feducovich, S.K.; Zaimovskaya, T.A.; Starikova, Z.A.; Belyakov, P.A.; Nikishin, G.I. Unexpected stereoselective sodium acetate catalyzed multicomponent cyclization of aryl aldehydes, malononitrile and acetone into cis-4-dicyanomethylene-2,6-diarylcyclohexane-1,1-dicarbonitriles. Tetrahedron Lett. 2007, 48, 6614–6619. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Egorov, M.P. The first electrocatalytic stereoselective multicomponent synthesis of cyclopropanecarboxylic acid derivatives. RSC Adv. 2015, 5, 98522–98526. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Ryzhkov, F.V. Catalysis of Cascade and Multicomponent Reactions of Carbonyl Compounds and C-H Acids by Electricity. Chem. Rec. 2016, 16, 1950–1964. [Google Scholar] [CrossRef]
- Elinson, M.N.; Feducovich, K.; Zaimovskaya, T.A.; Vereshchagin, A.N.; Nikishin, G.I. Stereoselective electrocatalytic transformations of malononitrile and aromatic aldehydes into (1R,5S,6R)*-4,4-dialkoxy-2-amino-6-aryl-1,5-dicyano-3-azabicyclo[3.1.0]hex-2-enes. Russ. Chem. Bull. 2005, 54, 673–677. [Google Scholar] [CrossRef]
- Elinson, N.M.; Vereshchagin, N.A.; Ryzkov, V.F. Electrochemical Synthesis of Heterocycles via Cascade Reactions. Curr. Org. Chem. 2017, 21, 1427–1439. [Google Scholar] [CrossRef]
- Elinson, M.N.; Vereshchagin, A.N.; Nasybullin, R.F.; Bobrovsky, S.I.; Ilovaisky, A.I.; Merkulova, V.M.; Bushmarinov, I.S.; Egorov, M.P. General approach to a spiro indole-3,1′-naphthalene tetracyclic system: Stereoselective pseudo four-component reaction of isatins and cyclic ketones with two molecules of malononitrile. RSC Adv. 2015, 5, 50421–50424. [Google Scholar] [CrossRef]
- Vereshchagin, A.N.; Elinson, M.N.; Anisina, Y.E.; Ryzhkov, F.V.; Novikov, R.A.; Egorov, M.P. PASE Pseudo-Four-Component Synthesis and Docking Studies of New 5-C-Substituted 2,4-Diamino-5H-Chromeno[2,3-b]pyridine-3-Carbonitriles. ChemistrySelect 2017, 2, 4593–4597. [Google Scholar] [CrossRef]
- Elinson, M.N.; Ryzhkov, F.V.; Vereshchagin, A.N.; Korshunov, A.D.; Novikov, R.A.; Egorov, M.P. ‘On-solvent’ new domino reaction of salicylaldehyde, malononitrile and 4-hydroxy-6-methylpyridin-2(1H)-one: Fast and efficient approach to medicinally relevant 4-pyridinyl-2-amino-4H-chromene scaffold. Mendeleev Commun. 2017, 27, 559–561. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian, Inc.: Wallingford, CT, USA. 2016. Available online: https://gaussian.com/citation/(accessed on 16 May 2022).
- Zhou, Z.; Yuan, J.; Yang, R. Efficient Knoevenagel Condensation Catalyzed by 2-Hydroxyethylammonium Acetate Under Solvent-Free Conditions at Room Temperature. Synth. Commun. 2009, 39, 2001–2007. [Google Scholar] [CrossRef]
- Gibadullina, N.N.; Latypova, D.R.; Nugumanov, T.R.; Spirikhin, L.V.; Dokichev, V.A. Synthesis of polyfunctionalized 1,1′-(α,ω-alkanediyl)bis(1,2,3,4-tetrahydropyridines). Chem. Heterocycl. Compd. 2017, 53, 1098–1102. [Google Scholar] [CrossRef]
- Sheldrick, G.M. CELL_NOW. Version 2008/4; Georg-August-Universität: Göttingen, Germany, 2008. [Google Scholar]
- Bruker. APEX-III; Bruker AXS Inc.: Madison, WI, USA, 2019. [Google Scholar]
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Cryst. 2015, A71, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Cryst. 2015, C71, 3–8. [Google Scholar]
- Flack, H.D. On enantiomorph-polarity estimation. Acta Cryst. 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Macrae, C.F.; Sovago, I.; Cottrell, S.J.; Galek, P.T.A.; McCabe, P.; Pidcock, E.; Platings, M.; Shields, G.P.; Stevens, J.S.; Towler, M.; et al. Mercury 4.0: From visualization to analysis, design and prediction. J. Appl. Cryst. 2020, 53, 226–235. [Google Scholar] [CrossRef] [Green Version]
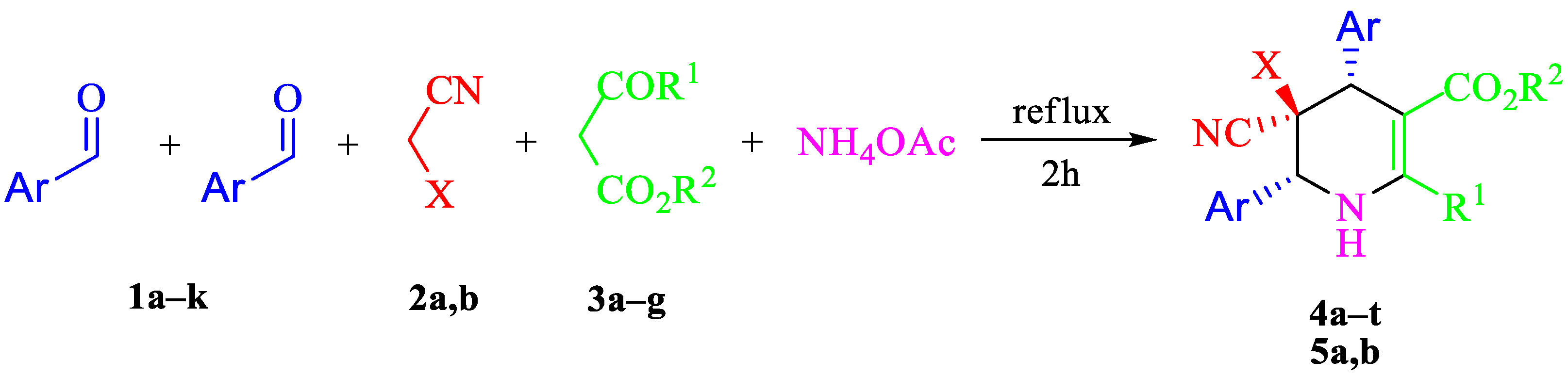
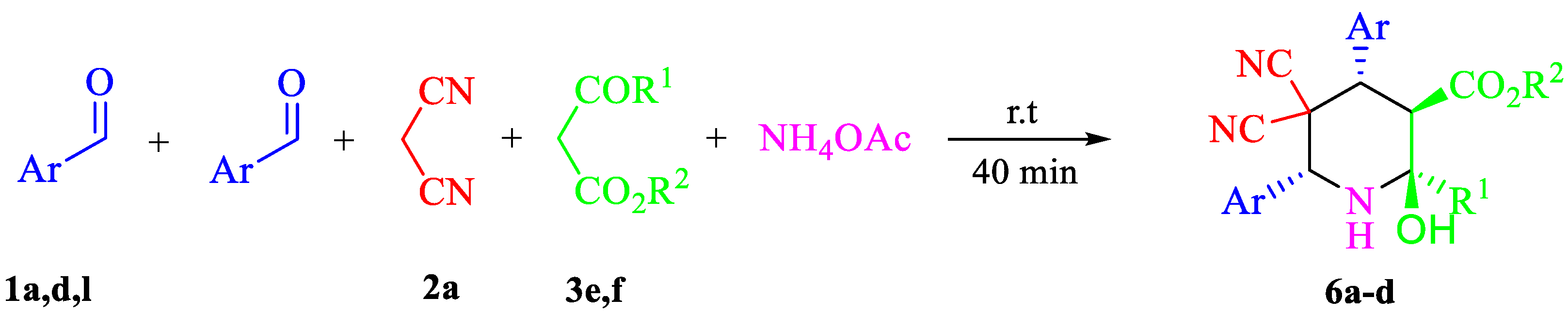
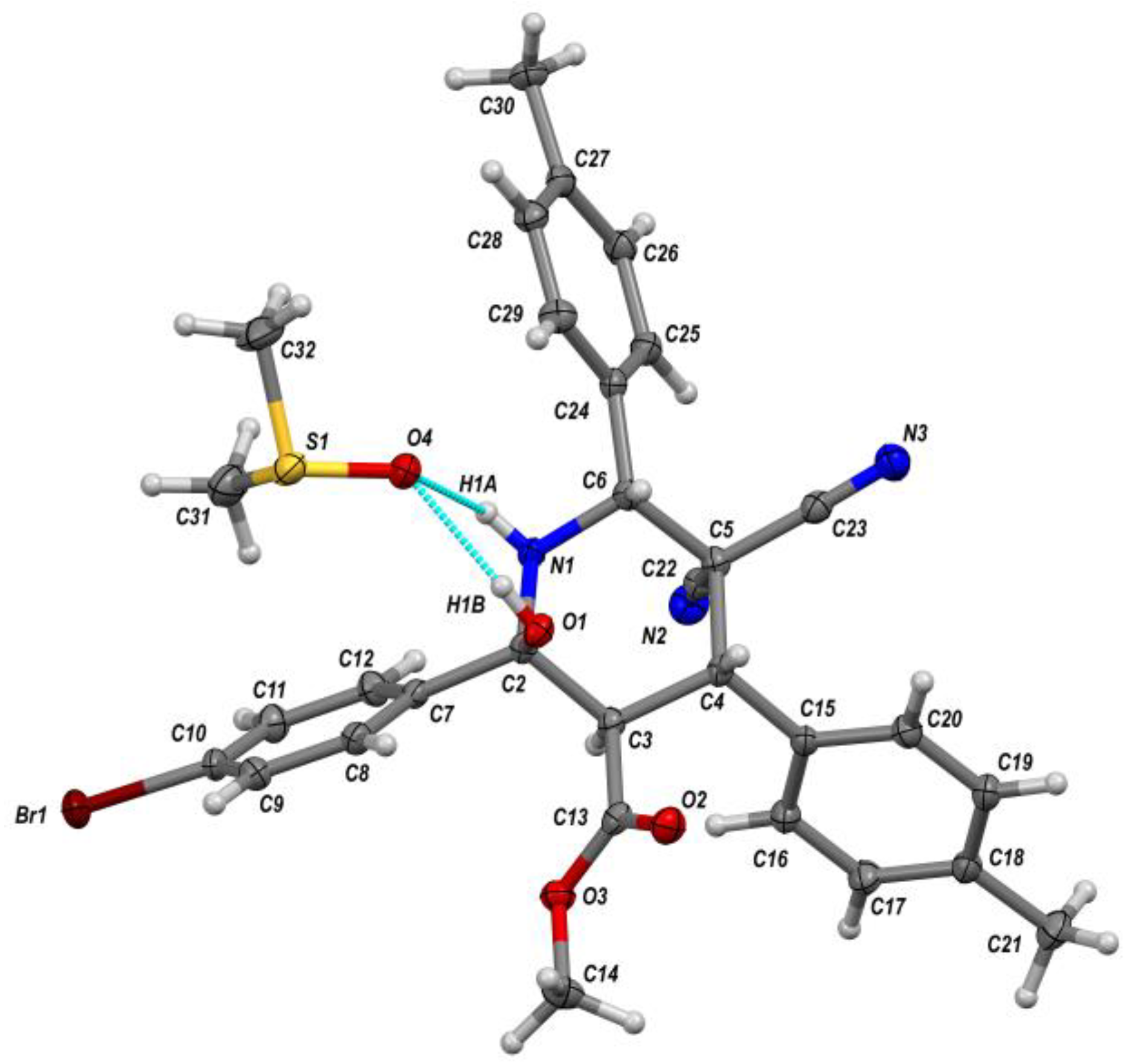

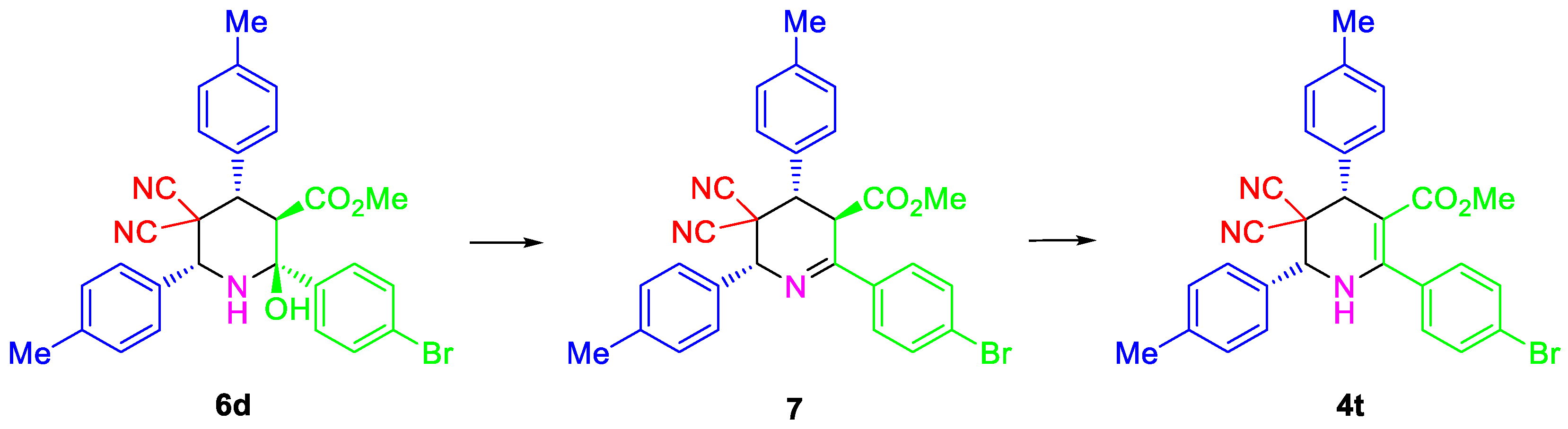
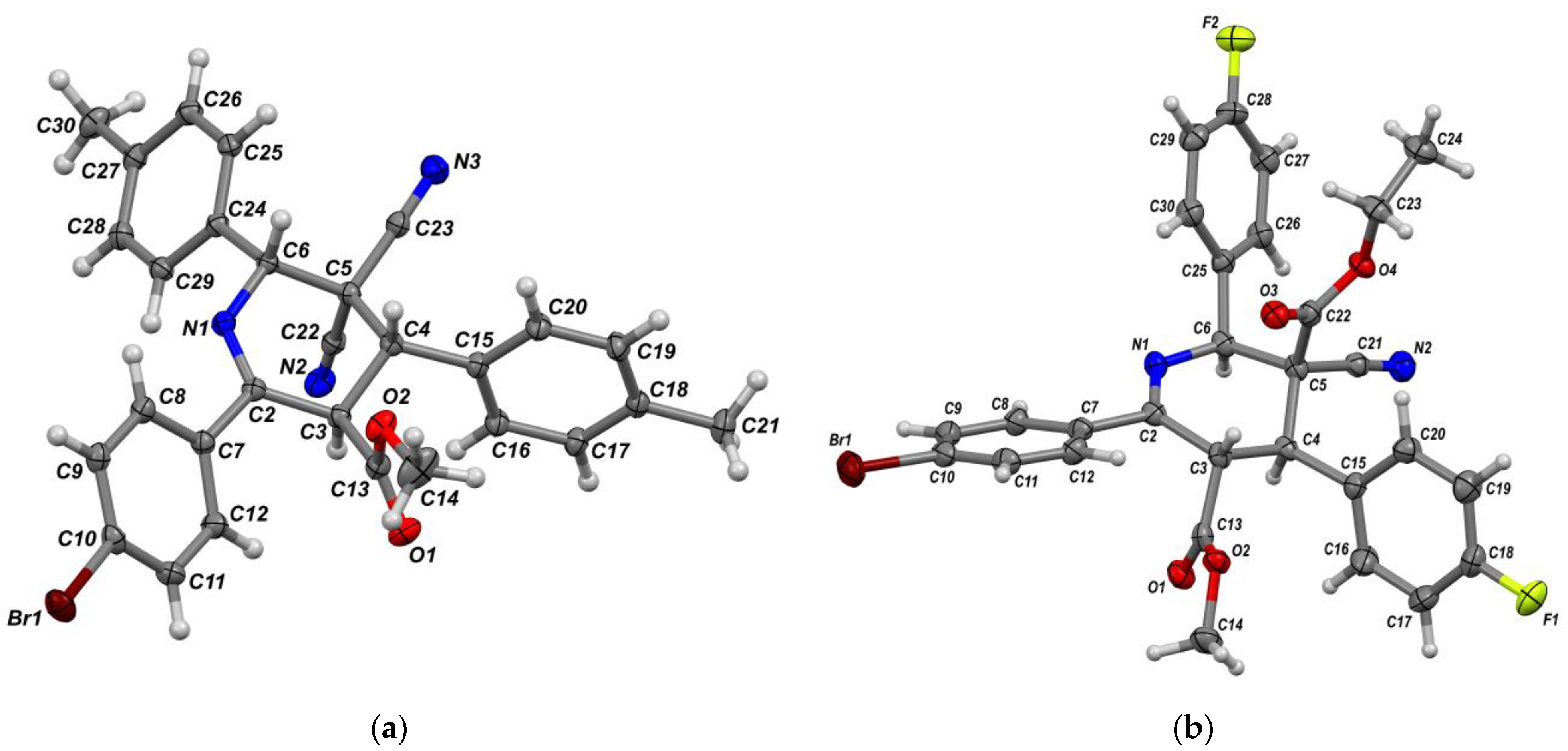
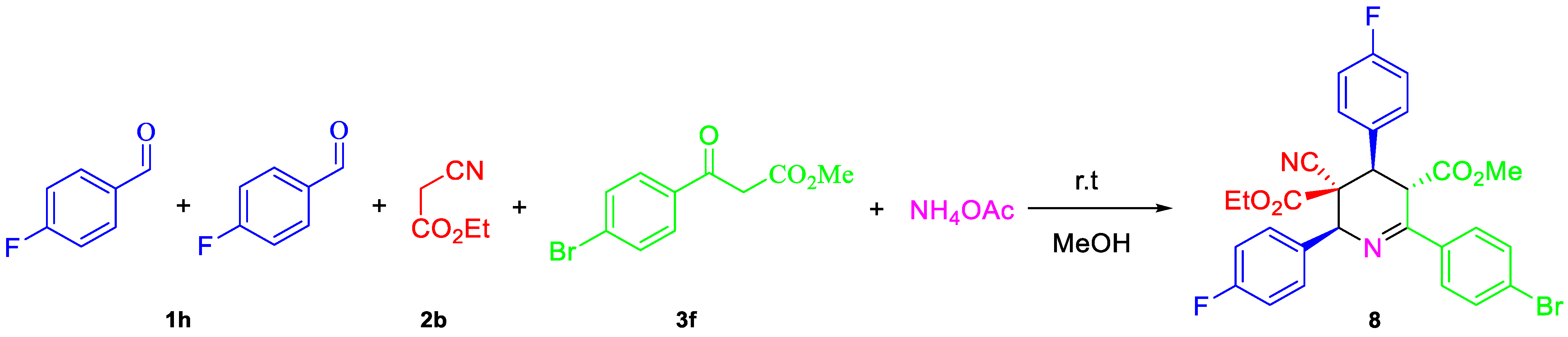
| Entry | Aldehyde | C-H Acid | C-H Acid | X | Ar | R1 | R2 | Product | Yield b |
|---|---|---|---|---|---|---|---|---|---|
| 1. | 1a | 2a | 3a | CN | C6H5 | Me | Me | 4a | 80 |
| 2. | 1b | 2a | 3a | CN | 2-MeC6H4 | Me | Me | 4b | 65 |
| 3. | 1c | 2a | 3a | CN | 3-MeC6H4 | Me | Me | 4c | 76 |
| 4. | 1d | 2a | 3a | CN | 4-MeC6H4 | Me | Me | 4d | 72 |
| 5. | 1e | 2a | 3a | CN | 3-FC6H4 | Me | Me | 4e | 71 |
| 6. | 1f | 2a | 3a | CN | 3-ClC6H4 | Me | Me | 4f | 68 |
| 7. | 1g | 2a | 3a | CN | 3-Py | Me | Me | 4g | 62 |
| 8. | 1a | 2a | 3b | CN | C6H5 | Me | Et | 4h | 86 |
| 9. | 1h | 2a | 3b | CN | 4-FC6H4 | Me | Et | 4i | 69 |
| 10. | 1i | 2a | 3b | CN | 4-NO2C6H4 | Me | Et | 4j | 63 |
| 11. | 1a | 2a | 3c | CN | C6H5 | Et | Me | 4k | 90 |
| 12. | 1d | 2a | 3c | CN | 4-MeC6H4 | Et | Me | 4l | 82 |
| 13. | 1j | 2a | 3c | CN | 4-BrC6H4 | Et | Me | 4m | 82 |
| 14. | 1i | 2a | 3c | CN | 4-NO2C6H4 | Et | Me | 4n | 58 |
| 15. | 1a | 2a | 3d | CN | C6H5 | C6H5 | Me | 4o | 76 |
| 16. | 1e | 2a | 3d | CN | 3-FC6H4 | C6H5 | Me | 4p | 52 |
| 17. | 1a | 2a | 3e | CN | C6H5 | C6H5 | Et | 4q | 58 |
| 18. | 1k | 2a | 3e | CN | 4-OMeC6H4 | C6H5 | Et | 4r | 44 |
| 19. | 1a | 2a | 3f | CN | C6H5 | 4-BrC6H4 | Me | 4s | 62 |
| 20. | 1d | 2a | 3f | CN | 4-MeC6H4 | 4-BrC6H4 | Me | 4t | 75 |
| 21. | 1j | 2b | 3f | CO2Et | 4-BrC6H4 | 4-BrC6H4 | Me | 5a | 73 |
| 22. | 1j | 2b | 3g | CO2Et | 4-BrC6H4 | 4-ClC6H4 | Me | 5b | 66 |
| Entry | Aldehyde | C-H Acid | R | R1 | R2 | Product | Yield b |
|---|---|---|---|---|---|---|---|
| 1 | 1a | 3e | H | C6H5 | Et | 6a | 72 |
| 2 | 1d | 3e | 4-Me | C6H5 | Et | 6b | 61 |
| 3 | 1l | 3e | 4-Cl | C6H5 | Et | 6c | 56 |
| 4 | 1d | 3f | 4-Me | 4-BrC6H4 | Me | 6d | 87 |
| Entry | AcOH/mol.eq. | Time, h | Tetrahydropyridine | Yield (%) b |
|---|---|---|---|---|
| 1 | 0 | 2 | - | - |
| 2 | 2 | 2 | 7 | 90 |
| 3 | 4 | 2 | 7 | 88 |
| 4 | 10 | 2 | 7/4t = 1:1 | 90 c |
| 5 | 10 | 4 | 7/4t = 1:1.5 | 92 c |
| 6 | 25 | 2 | 7/4t = 1:2 | 88 c |
| 7 | 50 | 2 | 4t | 92 |
| Structure | 4r | 5a | 6d | 7 | 8 |
|---|---|---|---|---|---|
| Empirical formula | C30H27N3O4 | C29H23Br3N2O4 | C29H26BrN3O3·C2H6OS | C29H24BrN3O2 | C29H23BrF2N2O4 |
| Formula weight | 493.54 | 703.22 | 622.56 | 526.42 | 581.40 |
| Crystal system | Monoclinic | Monoclinic | Monoclinic | Orthorhombic | Triclinic |
| Space group | P21/n | P21/c | P21 | P212121 | P |
| Unit cell parameters | |||||
| a, Å | 13.5215(6) | 14.1399(5) | 9.3382(4) | 8.9278(2) | 7.9773(3) |
| b, Å | 14.4873(6) | 13.7544(4) | 17.1583(8) | 14.0848(3) | 15.5602(6) |
| c, Å | 13.6402(6) | 14.6488(5) | 10.1005(5) | 19.5522(4) | 21.8741(8) |
| α, ° | 90 | 90 | 90 | 90 | 91.0048(12) |
| β, ° | 109.0602(13) | 107.0120(11) | 116.3107(12) | 90 | 91.2217(12) |
| γ, ° | 90 | 90 | 90 | 90 | 103.2089(14) |
| Volume, Å3 | 2525.49(19) | 2724.32(16) | 1450.72(12) | 2458.62(9) | 2642.06(17) |
| Z | 4 | 4 | 2 | 4 | 4 |
| Density (calcd.), g/cm3 | 1.298 | 1.715 | 1.425 | 1.422 | 1.462 |
| μ, mm−1 | 0.087 | 4.481 | 1.530 | 1.704 | 1.608 |
| F (000) | 1040 | 1392 | 644 | 1080 | 1184 |
| θ range, ° | 2.58–30.00 | 2.07–33.73 | 2.43–30.50 | 2.51–33.74 | 2.32–31.53 |
| Completeness to θmax | 0.999 | 1.000 | 1.000 | 0.999 | 0.995 |
| Index ranges | −19 < = h < = 15, −20 < = k < = 20, −19 < = l < = 19 | −13 < = h < = 13, −21 < = k < = 22, −30 < = l < = 29 | −13 < = h < = 13, −24 < = k < = 24, −14 < = l < = 14 | −13 < = h < = 13, −21 < = k < = 22, −30 < = l < = 29 | −11 < = h < = 11, −22 < = k < = 22, 0 < = l < = 32 |
| Reflections collected | 37323 | 90656 | 42307 | 80903 | 20648 |
| Independent reflections (R(int)) | 7344 [0.0737] | 10,873 [0.0808] | 8837 [0.0539] | 9808 [0.0411] | 20,648 [-] |
| Observed reflections (I > 2σ(I)) | 4852 | 6974 | 7570 | 8717 | 11,148 |
| Data, restraints, parameters | 7344, 0, 341 | 10,873, 1, 357 | 8837, 8, 386 | 9808, 0, 321 | 20,648, 0, 691 |
| Goodness of fit on F2 | 1.037 | 1.019 | 1.039 | 1.050 | 1.019 |
| Final R1, wR2 (I > 2σ(I)) | 0.0523, 0.0942 | 0.0491, 0.1042 | 0.0328, 0.0637 | 0.0295, 0.0667 | 0.0593, 0.0984 |
| Final R1, wR2 (all data) | 0.0951, 0.1171 | 0.0955, 0.1250 | 0.0454, 0.0693 | 0.0383, 0.0712 | 0.1452, 0.1219 |
| Absolute structure parameter | - | - | 0.016(6) | 0.388(6) | - |
| Largest diff. peak, hole, e/Å3 | 0.321, −0.282 | 0.993, −1.176 | 0.377, −0.488 | 0.348, −0.378 | 0.595, −0.861 |
| CCDC number | 1979309 | 2032519 | 1979186 | 2011646 | 2011600 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vereshchagin, A.N.; Iliyasov, T.M.; Karpenko, K.A.; Akchurin, R.N.; Minyaev, M.E. Tetrahydropyridines’ Stereoselective Formation, How Lockdown Assisted in the Identification of the Features of Its Mechanism. Molecules 2022, 27, 4367. https://doi.org/10.3390/molecules27144367
Vereshchagin AN, Iliyasov TM, Karpenko KA, Akchurin RN, Minyaev ME. Tetrahydropyridines’ Stereoselective Formation, How Lockdown Assisted in the Identification of the Features of Its Mechanism. Molecules. 2022; 27(14):4367. https://doi.org/10.3390/molecules27144367
Chicago/Turabian StyleVereshchagin, Anatoly N., Taigib M. Iliyasov, Kirill A. Karpenko, Radmir N. Akchurin, and Mikhail E. Minyaev. 2022. "Tetrahydropyridines’ Stereoselective Formation, How Lockdown Assisted in the Identification of the Features of Its Mechanism" Molecules 27, no. 14: 4367. https://doi.org/10.3390/molecules27144367






