Purification and Identification of Flavonoid Molecules from Rosa setate x Rosa rugosa Waste Extracts and Evaluation of Antioxidant, Antiproliferative and Antimicrobial Activities
Abstract
:1. Introduction
2. Results and Discussion
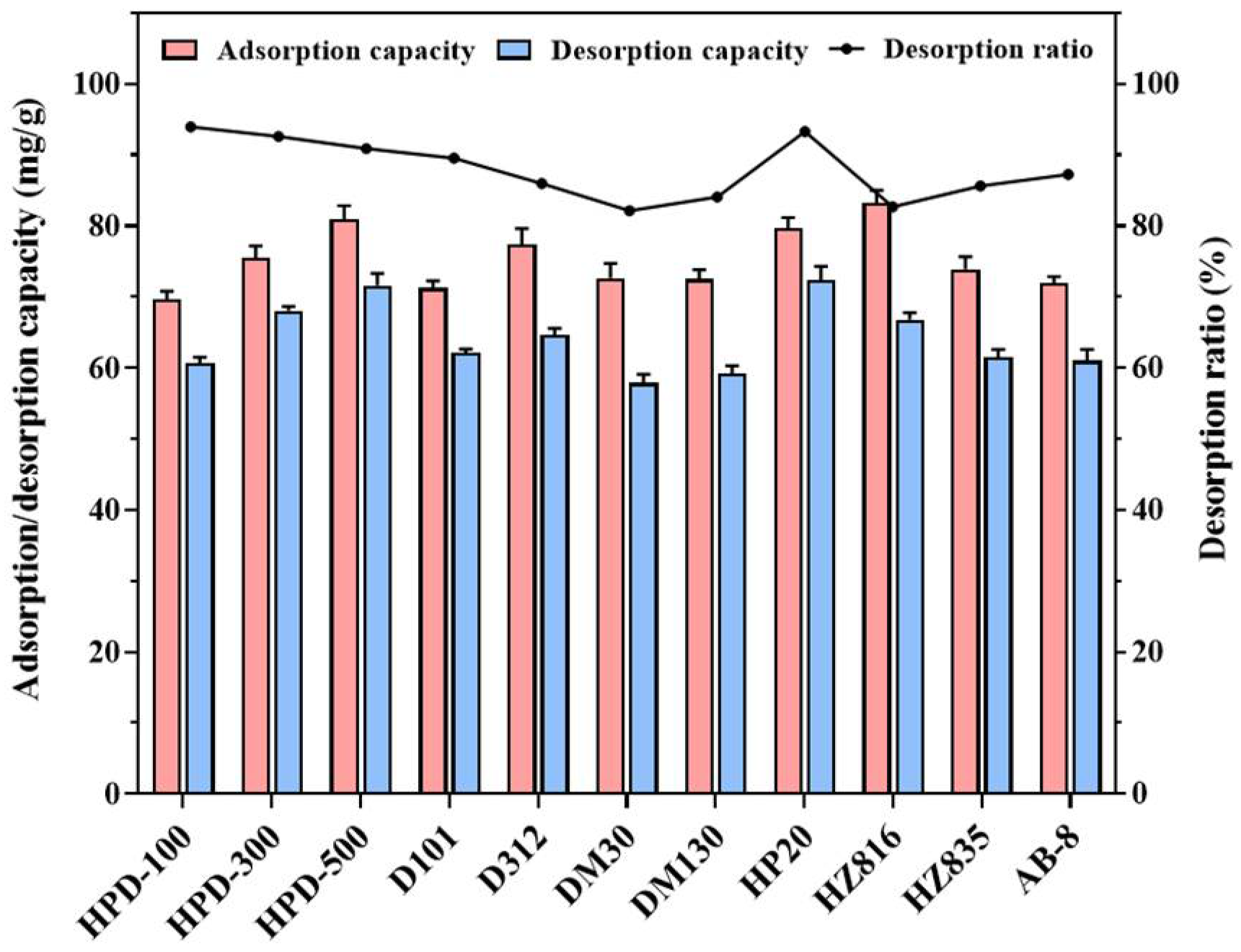
2.1. Static and Dynamic Screening of MARs
2.2. Resin Column Chromatography
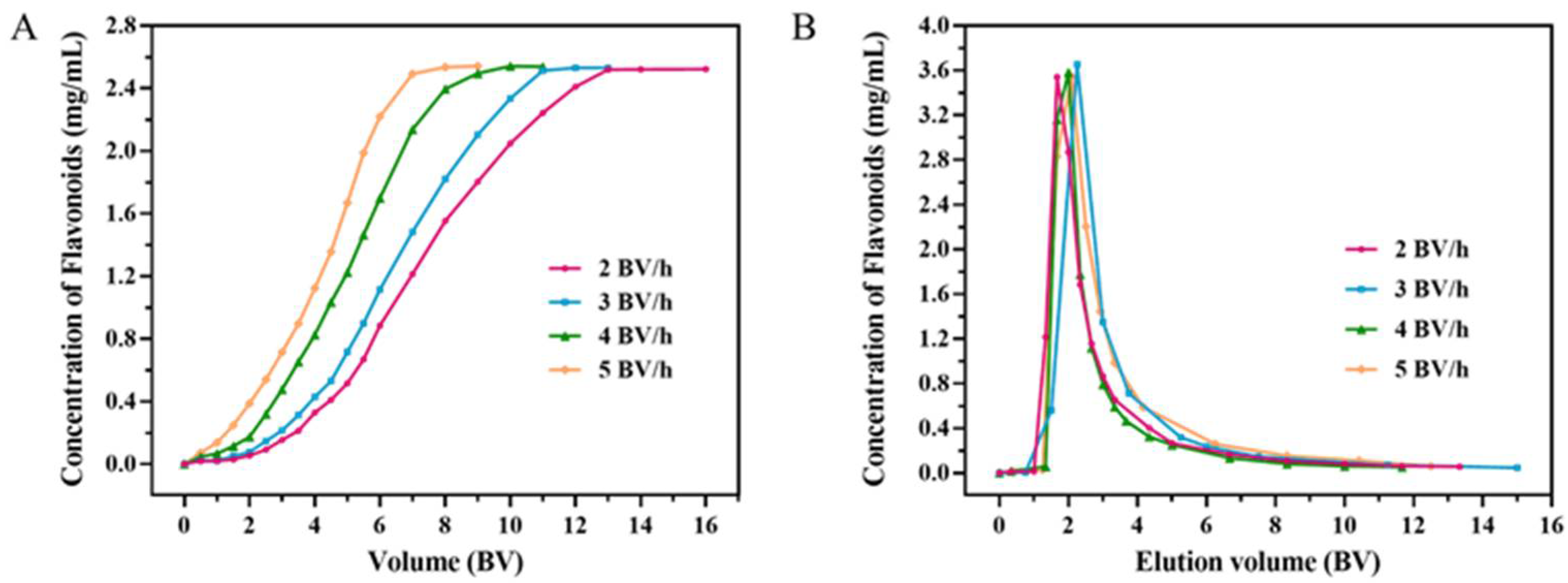
2.2.1. Dynamic Breakthrough Curves
2.2.2. Effect of Ethanol Concentration
2.2.3. Dynamic Desorption Curves
2.3. Identification of Flavonoids by UPLC-QTOF-MS
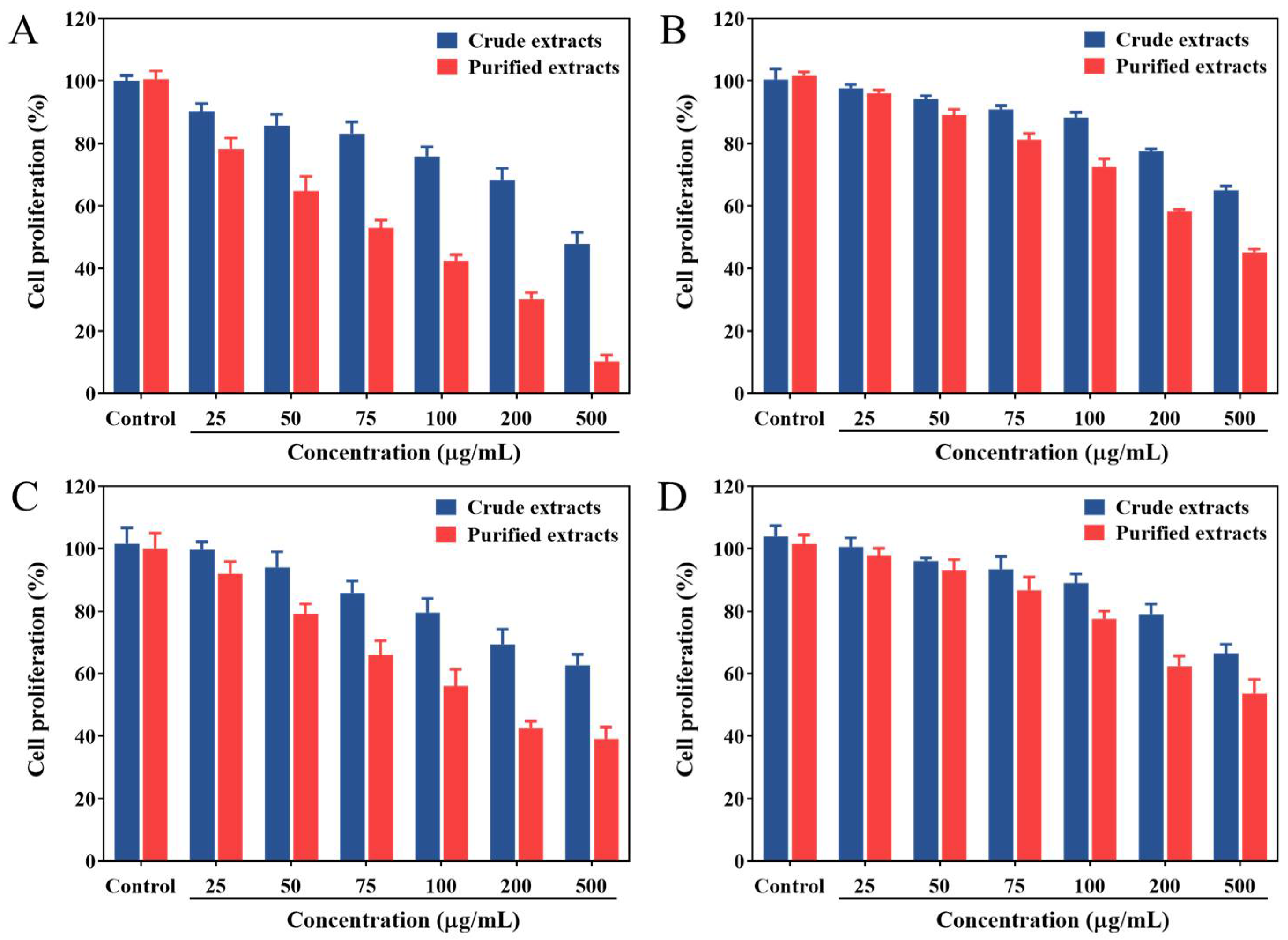
2.4. Antiproliferative Activity
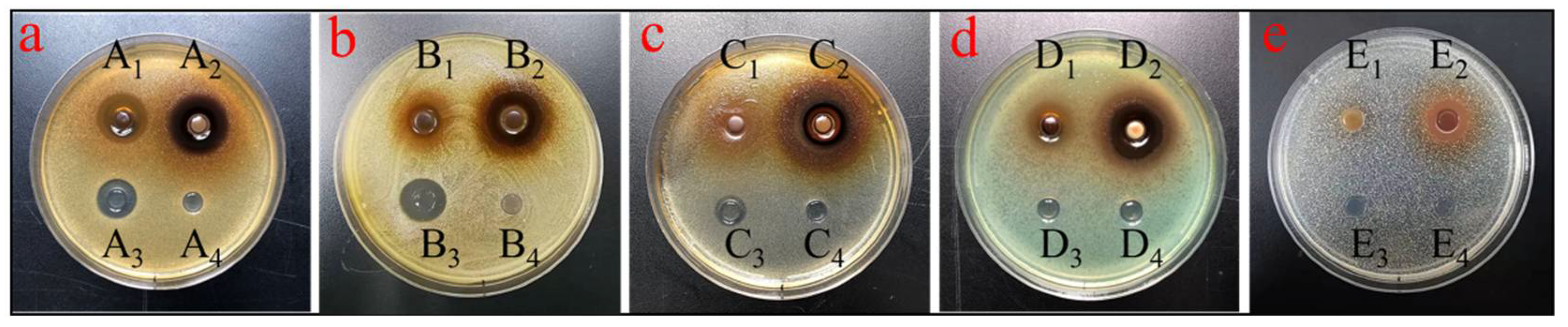
2.5. Antimicrobial Activity
3. Materials and Methods
3.1. Reagents and Chemicals
3.2. Preparation of Crude Total Flavonoid Extracts
3.3. Determination of Total Flavonoid Contents
3.4. Purification of Flavonoids by MARs
3.4.1. Pretreatment of MARs
3.4.2. Screening of MARs
3.4.3. Dynamic Analysis of MARs
3.4.4. Procedures for Chromatographic Purification
3.5. UHPLC-ESI-QTOF-MS/MS Analysis
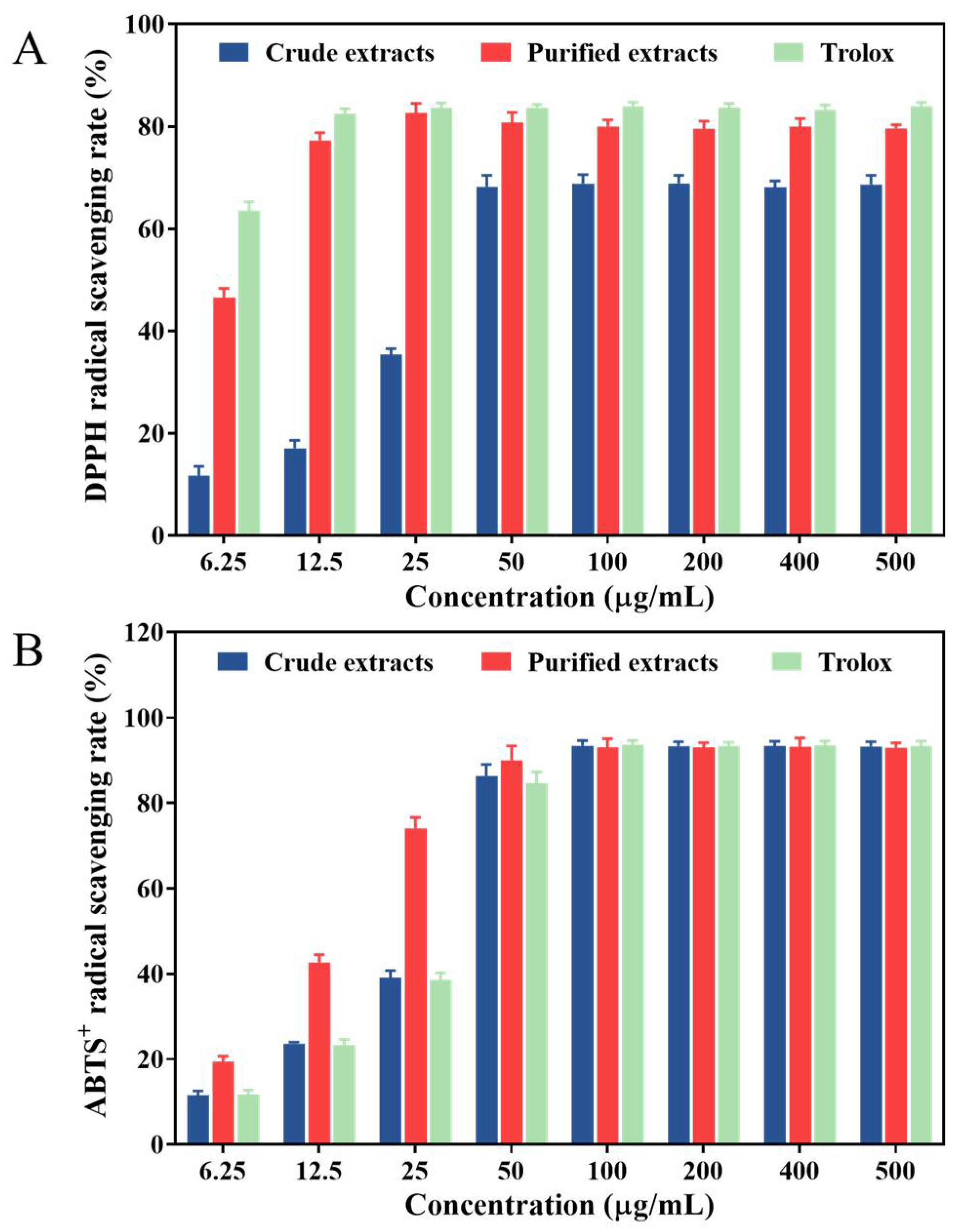
3.6. In Vitro Antioxidant Capacity
3.6.1. DPPH• Scavenging Assay
3.6.2. ABTS•+ Scavenging Assay
3.7. Cell Culture and Antiproliferative Activity Assay
3.8. Assay for Antimicrobial Activity
3.8.1. Inhibition Zone Diameter Determination
3.8.2. Measurement of Minimum Inhibition Concentration
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Liu, Y.; Zhi, D.J.; Wang, X.; Fei, D.Q.; Zhang, Z.X.; Wu, Z.R.; Li, Y.; Chen, P.; Li, H.Y. Kushui rose (R. Setate x R. Rugosa) decoction exerts antitumor effects in C. elegans by downregulating Ras/MAPK pathway and resisting oxidative stress. Int. J. Mol. Med. 2018, 42, 1411–1417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ng, T.B.; He, J.S.; Niu, S.M.; Zhao, L.; Pi, Z.F.; Shao, W.; Liu, F. A gallic acid derivative and polysaccharides with antioxidative activity from rose (Rosa rugosa) flowers. J. Pharm. Pharmacol. 2004, 56, 537–545. [Google Scholar] [CrossRef] [PubMed]
- Jung, H.-J.; Nam, J.-H.; Choi, J.; Lee, K.-T.; Park, H.-J. 19α-Hydroxyursane-type triterpenoids: Antinociceptive anti-inflammatory principles of the roots of Rosa rugosa. Biol. Pharm. Bull. 2005, 28, 101–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, Y.H.; Jung, M.G.; Kang, H.B.; Choi, K.C.; Haam, S.; Jun, W.; Kim, Y.J.; Cho, H.Y.; Yoon, H.G. Effect of anti-histone acetyltransferase activity from Rosa rugosa Thunb. (Rosaceae) extracts on androgen receptor-mediated transcriptional regulation. J. Ethnopharmacol. 2008, 118, 412–417. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Q.; Feng, H.F.; Song, J.X.; Chen, L.X.; Xu, Z.Z.; Xia, W.; Zhang, W.Q. Structural elucidation and immunomodulatory activity of a neutral polysaccharide from the Kushui rose (Rosa setate x Rosa rugosa) waste. Carbohydr. Polym. 2020, 232, 115804. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Li, W.; Zhang, Y.; Shi, L.; Xu, Z.; Xia, W.; Zhang, W. Structure characteristics, hypoglycemic and immunomodulatory activities of pectic polysaccharides from Rosa setate x Rosa rugosa waste. Carbohydr. Polym. 2021, 253, 117190. [Google Scholar] [CrossRef]
- Slavov, A.; Kiyohara, H.; Yamada, H. Immunomodulating pectic polysaccharides from waste rose petals of Rosa damascena Mill. Int. J. Biol. Macromol. 2013, 59, 192–200. [Google Scholar] [CrossRef]
- Slavov, A.; Denev, P.; Panchev, I.; Shikov, V.; Nenov, N.; Yantcheva, N.; Vasileva, I. Combined recovery of polysaccharides and polyphenols from Rosa damascena wastes. Ind. Crop. Prod. 2017, 100, 85–94. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Shen, N.; Wang, T.; Gan, Q.; Liu, S.; Wang, L.; Jin, B. Plant flavonoids: Classification, distribution, biosynthesis, and antioxidant activity. Food Chem. 2022, 383, 132531. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.; Yan, L.; Shi, Z.; Wang, L.; Shan, L.; Efferth, T. Hepatoprotective and anti-inflammatory effects of total flavonoids of Qu Zhi Ke (peel of Citrus changshan-huyou) on non-alcoholic fatty liver disease in rats via modulation of NF-κB and MAPKs. Phytomedicine 2019, 64, 153082. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Xiao, M.; Zou, X.; Tang, J.; Huang, B.; Xue, H. Extraction and separation of flavonoids from Malus hupehensis using high-speed countercurrent chromatography based on deep eutectic solvent. J. Chromatogr. A 2021, 1641, 461998. [Google Scholar] [CrossRef]
- Chong, K.Y.; Stefanova, R.; Zhang, J.; Brooks, M.S.-L. Aqueous two-phase extraction of bioactive compounds from haskap leaves (Lonicera caerulea): Comparison of salt/ethanol and sugar/propanol systems. Sep. Purif. Technol. 2020, 252, 117399. [Google Scholar] [CrossRef]
- Quan, T.; Wang, D.; Yang, L.; Liu, S.; Tao, Y.; Wang, J.; Deng, L.; Kang, X.; Zhang, K.; Xia, Z.; et al. Effective extraction methods based on hydrophobic deep eutectic solvent coupled with functional molecularly imprinted polymers: Application on quercetagetin extraction from natural medicine and blood. Microchem. J. 2022, 174, 107076. [Google Scholar] [CrossRef]
- Conidi, C.; Cassano, A.; Drioli, E. Membrane diafiltration for enhanced purification of biologically active compounds from goji berries extracts. Sep. Purif. Technol. 2022, 282, 119991. [Google Scholar] [CrossRef]
- Song, L.; Liu, P.; Yan, Y.; Huang, Y.; Bai, B.; Hou, X.; Zhang, L. Supercritical CO2 fluid extraction of flavonoid compounds from Xinjiang jujube (Ziziphus jujuba Mill.) leaves and associated biological activities and flavonoid compositions. Ind. Crop. Prod. 2019, 139, 111508. [Google Scholar] [CrossRef]
- Syafni, N.; Devi, S.; Zimmermann-Klemd, A.M.; Reinhardt, J.K.; Danton, O.; Gründemann, C.; Hamburger, M. Immunosuppressant flavonoids from Scutellaria baicalensis. Biomed. Pharmacother. 2021, 144, 112326. [Google Scholar] [CrossRef]
- Cheng, X.; Cheng, Y.; Zhang, N.; Zhao, S.; Cui, H.; Zhou, H. Purification of flavonoids from Carex meyeriana Kunth based on AHP and RSM: Composition analysis, antioxidant, and antimicrobial activity. Ind. Crop. Prod. 2020, 157, 112900. [Google Scholar] [CrossRef]
- Chen, X.; Wang, H.; Huang, X.; Xia, S.; Chen, C.; Nie, Q.; Nie, S. Efficient enrichment of total flavonoids from kale (Brassica oleracea L. var. acephala L.) extracts by NKA-9 resin and antioxidant activities of flavonoids extract In Vitro. Food Chem. 2022, 374, 131508. [Google Scholar] [PubMed]
- Li, C.; Zheng, Y.; Wang, X.; Feng, S.; Di, D. Simultaneous separation and purification of flavonoids and oleuropein from Olea europaea L. (olive) leaves using macroporous resin. J. Sci. Food Agric. 2011, 91, 2826–2834. [Google Scholar] [CrossRef] [PubMed]
- Zou, S.-P.; Liu, M.; Wang, Q.-L.; Xiong, Y.; Niu, K.; Zheng, Y.-G.; Shen, Y.-C. Preparative separation of echinocandin B from Aspergillus nidulans broth using macroporous resin adsorption chromatography. J. Chromatogr. B 2015, 978, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Choucry, M.A.; Shalabi, A.A.; El Halawany, A.M.; El-Sakhawy, F.S.; Zaiter, A.; Morita, H.; Chaimbault, P.; Abdel-Sattar, E. New pregnane glycosides isolated from Caralluma hexagona Lavranos as inhibitors of α-glucosidase, pancreatic lipase, and aAdvanced glycation end products formation. ACS Omega 2021, 6, 18881–18889. [Google Scholar] [CrossRef]
- Wu, P.; Li, F.; Zhang, J.; Yang, B.; Ji, Z.; Chen, W. Phytochemical compositions of extract from peel of hawthorn fruit, and its antioxidant capacity, cell growth inhibition, and acetylcholinesterase inhibitory activity. BMC Complement. Altern. Med. 2017, 17, 151. [Google Scholar] [CrossRef] [Green Version]
- Zhao, P.; Qi, C.; Wang, G.; Dai, X.; Hou, X. Enrichment and purification of total flavonoids from Cortex Juglandis Mandshuricae extracts and their suppressive effect on carbon tetrachloride-induced hepatic injury in Mice. J. Chromatogr. B. 2015, 1007, 8–17. [Google Scholar] [CrossRef]
- Sun, L.; Guo, Y.; Fu, C.; Li, J.; Li, Z. Simultaneous separation and purification of total polyphenols, chlorogenic acid and phlorizin from thinned young apples. Food Chem. 2013, 136, 1022–1029. [Google Scholar] [CrossRef]
- Abdulla, R.; Mansur, S.; Lai, H.; Ubul, A.; Sun, G.; Huang, G.; Aisa, H.A. Qualitative analysis of polyphenols in macroporous resin pretreated Pomegranate husk extract by HPLC-QTOF-MS. Phytochem. Anal. 2017, 28, 465–473. [Google Scholar] [CrossRef]
- Cendrowski, A.; Scibisz, I.; Kieliszek, M.; Kolniak-Ostek, J.; Mitek, M. UPLC-PDA-Q/TOF-MS Profile of polyphenolic compounds of liqueurs from rose petals (Rosa rugosa). Molecules 2017, 22, 1832. [Google Scholar] [CrossRef] [Green Version]
- Gu, D.; Yang, Y.; Bakri, M.; Chen, Q.; Xin, X.; Aisa, H.A. A LC/QTOF-MS/MS application to investigate chemical compositions in a fraction with protein tyrosine phosphatase 1B inhibitory activity from Rosa rugosa flowers. Phytochem. Anal. 2013, 24, 661–670. [Google Scholar] [CrossRef]
- Xu, H.; Wang, Y.; Yuan, F.; Gao, Y. Polyphenols in chinese Kushui rose (Rosa sertata × Rosa rugosa) leaves. Acta Aliment. 2018, 47, 433–442. [Google Scholar] [CrossRef] [Green Version]
- Kumar, N.; Bhandari, P.; Singh, B.; Bari, S.S. Antioxidant activity and ultra-performance LC-electrospray ionization-quadrupole time-of-flight mass spectrometry for phenolics-based fingerprinting of Rose species: Rosa damascena, Rosa bourboniana and Rosa brunonii. Food Chem. Toxicol. 2009, 47, 361–367. [Google Scholar] [CrossRef] [PubMed]
- Cendrowski, A.; Ścibisz, I.; Mitek, M.; Kieliszek, M.; Kolniak-Ostek, J. Profile of the phenolic compounds of Rosa rugosa petals. J. Food Qual. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Lawal, U.; Leong, S.W.; Shaari, K.; Ismail, I.S.; Khatib, A.; Abas, F. α-Glucosidase inhibitory and antioxidant activities of different Ipomoea aquatica cultivars and LC-MS/MS profiling of the active cultivar. J. Food Biochem. 2017, 41, e12303. [Google Scholar] [CrossRef]
- Alamed, J.; Chaiyasit, W.; McClements, D.J.; Decker, E.A. Relationships between free radical scavenging and antioxidant activity in foods. J. Agric. Food Chem. 2009, 57, 2969–2976. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Tian, W.; Yang, C.; Shi, W.; Cao, P.; Long, J.; Xiao, L.; Wu, Y.; Liang, J.; Li, X.; et al. Identification of flavonoids in Plumula nelumbinis and evaluation of their antioxidant properties from different habitats. Ind. Crop. Prod. 2019, 127, 36–45. [Google Scholar] [CrossRef]
- Nowak, R.; Olech, M.; Pecio, L.; Oleszek, W.; Los, R.; Malm, A.; Rzymowska, J. Cytotoxic, antioxidant, antimicrobial properties and chemical composition of rose petals. J. Sci. Food Agric. 2014, 94, 560–567. [Google Scholar] [CrossRef]
- Mao, S.; Wang, K.; Lei, Y.; Yao, S.; Lu, B.; Huang, W. Antioxidant synergistic effects of Osmanthus fragrans flowers with green tea and their major contributed antioxidant compounds. Sci. Rep. 2017, 7, 46501. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Wang, D.; Liu, Z. Synergistic, additive and antagonistic effects of Potentilla fruticosa combined with EGb761 on antioxidant capacities and the possible mechanism. Ind. Crop. Prod. 2015, 67, 227–238. [Google Scholar] [CrossRef]
- Zhu, M.-Z.; Wu, W.; Jiao, L.-L.; Yang, P.-F.; Guo, M.-Q. Analysis of flavonoids in Lotus (Nelumbo nucifera) leaves and their antioxidant activity using macroporous resin chromatography coupled with LC-MS/MS and cntioxidant biochemical assays. Molecules 2015, 20, 10553–10565. [Google Scholar] [CrossRef] [Green Version]
- Hashidoko, Y. The phytochemistry of Rosa rugosa. Phytochemistry 1996, 43, 535–549. [Google Scholar] [CrossRef]
- Ge, L.; Li, J.; Wan, H.; Zhang, K.; Wu, W.; Zou, X.; Wu, S.; Zhou, B.; Tian, J.; Zeng, X. Novel flavonoids from Lonicera japonica flower buds and validation of their anti-hepatoma and hepatoprotective activity in vitro studies. Ind. Crop. Prod. 2018, 125, 114–122. [Google Scholar] [CrossRef]
- Hong, J.; Hu, J.-Y.; Liu, J.-H.; Zhou, Z.; Zhao, A.-F. In Vitro antioxidant and antimicrobial activities of flavonoids from Panax notoginseng flowers. Nat. Prod. Res. 2014, 28, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhang, H.; Chen, L.; Xia, W.; Zhang, W. Phytochemical profiles and antioxidant activities of different varieties of Chimonanthus Praecox. Ind. Crop. Prod. 2016, 85, 11–21. [Google Scholar] [CrossRef]
- Hou, M.; Zhang, L. Adsorption/desorption characteristics and chromatographic purification of polyphenols from Vernonia patula (Dryand.) Merr. using macroporous adsorption resin. Ind. Crop. Prod. 2021, 170, 113729. [Google Scholar] [CrossRef]
- Dinh, T.V.; Saravana, P.S.; Woo, H.C.; Chun, B.S. Ionic liquid-assisted subcritical water enhances the extraction of phenolics from brown seaweed and its antioxidant activity. Sep. Purif. Technol. 2018, 196, 287–299. [Google Scholar] [CrossRef]
- Yin, L.; Han, H.; Zheng, X.; Wang, G.; Li, Y.; Wang, W. Flavonoids analysis and antioxidant, antimicrobial, and anti-inflammatory activities of crude and purified extracts from Veronicastrum latifolium. Ind. Crop. Prod. 2019, 137, 652–661. [Google Scholar] [CrossRef]
- Jia, G.; Lu, X. Enrichment and purification of madecassoside and asiaticoside from Centella asiatica extracts with macroporous resins. J. Chromatogr. A 2008, 1193, 136–141. [Google Scholar] [CrossRef]

| Peak | tR a (min) | [M-H]− (m/z) | Formula | MS/MS Fragment Ion (m/z) | Tentative Identification |
|---|---|---|---|---|---|
| 1 | 8.900 | 625.1449 | C27H30O17 | 300 | Quercetin-3,4′-di-O-diglucoside |
| 2 | 9.350 | 625.1462 | C27H30O17 | 445, 300 | Quercetin-3-O-sophoroside |
| 3 | 12.168 | 609.1463 | C27H30O16 | 285, 284 | Kaempferol-3,4′-di-O-diglucoside |
| 4 | 12.701 | 639.1613 | C28H32O17 | 477, 315, 314, 300, 299 | Isorhamnetin-3-O-sophoroside |
| 5 | 12.951 | 609.1479 | C27H30O16 | 489, 429, 327, 284 | Kaempferol-3-O-sophoroside |
| 6 | 14.902 | 463.0921 | C21H20O12 | 301, 300, 271, 255 | Hyperoside b |
| 7 | 15.486 | 609.1476 | C27H30O16 | 301, 300, 271 | Rutin b |
| 8 | 16.253 | 463.0924 | C21H20O12 | 301, 300, 271, 255 | Isoquercitrin b |
| 9 | 17.920 | 433.0799 | C20H18O11 | 301, 300, 271, 255 | Quercetin-3-O-pentoside |
| 10 | 19.187 | 615.1020 | C28H24O16 | 463, 301, 179, 151 | Quercetin-3-O-(6″-galloyl)-glucoside |
| 11 | 19.437 | 579.1440 | C26H28O15 | 284, 255, 227 | Kaempferol-3,7-di-O-rhamnoside |
| 12 | 21.138 | 433.0796 | C20H18O11 | 301, 300, 271, 255 | Quercetin-3-O-pentoside |
| 13 | 22.589 | 447.0962 | C21H20O11 | 285, 284, 255, 227 | Astragalin |
| 14 | 22.789 | 447.0974 | C21H20O11 | 301, 300, 271, 151 | Quercitrin |
| Experimental Strains | Crude Extracts | Purified Extracts | Kanamycin | ||
|---|---|---|---|---|---|
| ZOI a (mm) 50 mg/mL | MIC b (mg/mL) | ZOI a (mm) 50 mg/mL | MIC b (mg/mL) | ZOI a (mm) 50 μg/mL | |
| Staphylococcus aureus | 16.8 ± 0.24 | 12.5 | 20.7 ± 0.4 | 1.5625 | 17.2 ± 0.29 |
| Staphylococcus epidermidis | 11.9 ± 0.15 | 12.5 | 18.9 ± 0.1 | 1.5625 | 16.9 ± 0.33 |
| Escherichia coli | 9.5 ± 0.38 | 25.0 | 13.8 ± 0.23 | 6.25 | 11.9 ± 0.26 |
| Pseudomonas aeruginosa | 12.2 ± 0.13 | 12.5 | 16.4 ± 0.22 | 3.125 | - |
| Candida albicans | - | - | 11.2 ± 0.17 | 12.5 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, M.; Xu, J.; Zhang, H.; Xia, W.; Li, W.; Zhang, W. Purification and Identification of Flavonoid Molecules from Rosa setate x Rosa rugosa Waste Extracts and Evaluation of Antioxidant, Antiproliferative and Antimicrobial Activities. Molecules 2022, 27, 4379. https://doi.org/10.3390/molecules27144379
Wu M, Xu J, Zhang H, Xia W, Li W, Zhang W. Purification and Identification of Flavonoid Molecules from Rosa setate x Rosa rugosa Waste Extracts and Evaluation of Antioxidant, Antiproliferative and Antimicrobial Activities. Molecules. 2022; 27(14):4379. https://doi.org/10.3390/molecules27144379
Chicago/Turabian StyleWu, Mengqi, Jingying Xu, Hui Zhang, Wei Xia, Wei Li, and Wenqing Zhang. 2022. "Purification and Identification of Flavonoid Molecules from Rosa setate x Rosa rugosa Waste Extracts and Evaluation of Antioxidant, Antiproliferative and Antimicrobial Activities" Molecules 27, no. 14: 4379. https://doi.org/10.3390/molecules27144379







