Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats
Abstract
:1. Introduction
2. Results
2.1. Selected General Nutritional Parameters and Biochemical Parameters
2.2. Sterols
2.2.1. F. esculentum Freeze-Dried Grains and Sprouts
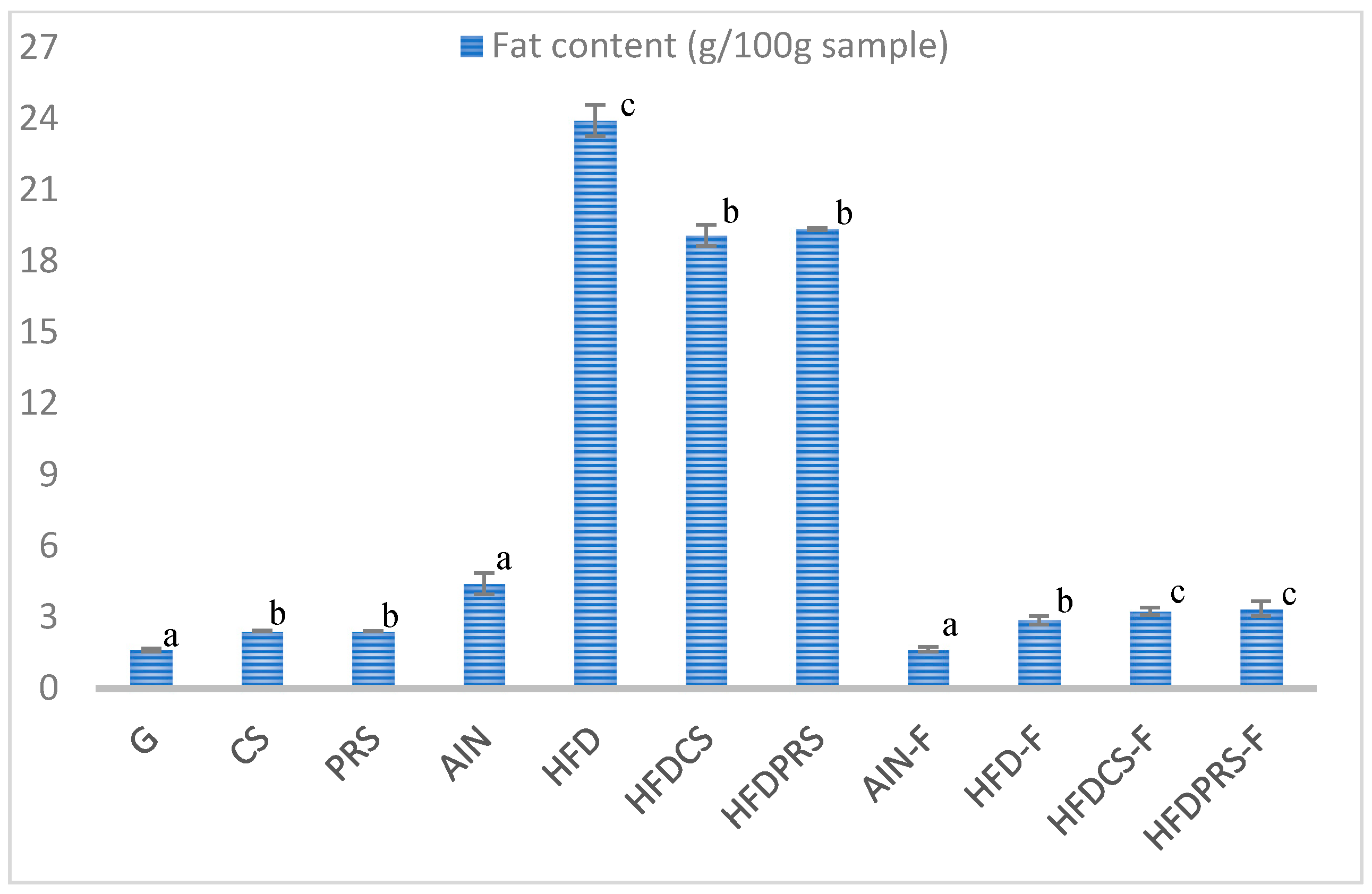
2.2.2. Experimental Diets
2.2.3. Serum
2.2.4. Liver
2.2.5. Feces
2.3. Fatty Acids
2.3.1. F. esculentum Freeze-Dried Grains, Sprouts and Experimental Diets
2.3.2. Serum
2.3.3. Liver
2.3.4. Feces
3. Discussion
3.1. Sterols
3.2. Fatty Acids
4. Materials and Methods
4.1. Animals
4.1.1. Experimental Diets
Buckwheat Seeds
4.1.2. Experimental Design
4.1.3. Biochemical Parameters
4.2. Determination of Crude Fats
4.3. Fat Extraction
4.3.1. Fat Extraction from Freeze-Dried Seeds; the Freeze-Dried Control Sprouts; Freeze-Dried Sprouts Rich in Probiotics; Experimental Diet; Feces
4.3.2. Hepatic-Fat Extraction
4.3.3. Serum
4.4. Analysis of the Fatty Acid Composition of: Freeze-Dried Seeds, Freeze-Dried Control Sprouts, Lyophilized Sprouts Rich in Probiotics, Experimental Diet, Liver, Feces
4.5. Analysis of Fatty Acid Composition of Serum
4.6. Analysis of the Composition of Sterols: Freeze-Dried Seeds, Freeze-Dried Control Sprouts, Lyophilized Sprouts Rich in Probiotics, Experimental Diet, Liver, Serum, Feces
4.7. Statistical Analysis
5. Conclusions
- (1)
- It can be observed that the modification changed the content of individual compounds contained in the raw material, i.e., buckwheat. This is especially noticeable for the fatty acids C18:1, C18:2, C18:3n-3;
- (2)
- Changes were noticed in the lipid profile by comparing the HFD and HFDPRS groups. In the HFDPRS group, a lower value of the non-HDL and LDL-C index was noticed.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Clatici, V.G.; Voicu, C.; Voaides, C.; Rosenu, A.; Icriverzi, M.; Jurcoane, S. Diseases of Civilization—Cancer, Diabetes, Obesity and Acne—The Implication of Milk, IGF-1 and MTORC1. Maedica (Bucur) 2018, 13, 273–281. [Google Scholar] [CrossRef]
- de Jong, A.; Plat, J.; Mensink, R.P. Metabolic Effects of Plant Sterols and Stanols (Review). J. Nutr. Biochem. 2003, 14, 362–369. [Google Scholar] [CrossRef]
- Kopeć, A.; Nowacka, E.; Piątkowska, E.; Leszczyńska, T. Charakterystyka i Prozdrowtne Właściwości Steroli Roślinnych [Characteristics and Pro-Health Properties of Plant Sterols]. ŻYWNOŚĆ. Nauka. Technologia. Jakość 2011, 3, 5–14. [Google Scholar]
- Lagarda, M.J.; García-Llatas, G.; Farré, R. Analysis of Phytosterols in Foods. J Pharm. Biomed. Anal. 2006, 41, 1486–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zawadzka, A.; Kobus-Cisowska, J.; Stachowiak, B. Bioaktywne metabolity gryki (Fagopyrum Mill.) [Buckwheat bioactive metabolites (Fagopyrum Mill.)]. Zagadnienia Doradz. Rol. 2021, 103, 58–66. [Google Scholar]
- Krochmal-Marczak, B.; Sawicka, B.; Tobiasz-Salach, R.; Bienia, B. Wartość Prozdrowotna i Znaczenie Gospodarcze Gryki (Fagopyrum esculentum M.) [Health-Promoting Value and Economic Importance of Buckwheat (Fagopyrum esculentum M.)]. In Rośliny Zielarskie, Kosmetyki Naturalne i Żywność Funkcjonalne; Państwowa Wyższa Szkoła Zawodowa im. Stanisława Pigonia w Krośnie, Krosno 2016: Krosno-Wrocław, Poland, 2017; pp. 260–271. [Google Scholar]
- Zhang, G.; Xu, Z.; Gao, Y.; Huang, X.; Zou, Y.; Yang, T. Effects of Germination on the Nutritional Properties, Phenolic Profiles, and Antioxidant Activities of Buckwheat. J. Food Sci. 2015, 80, H1111–H1119. [Google Scholar] [CrossRef] [PubMed]
- Zarzecka, K.; Gugała, M.; Mystkowska, I. Wartość odżywcza i prozdrowotna gryki siewnej [Nutritional and pro-health value of buckwheat]. Probl. Hig. Epidemiol. 2015, 96, 410–413. [Google Scholar]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Endrizzi, I.; Framondino, V.; Ciaghi, R.; Gasperi, F.; Gallerani, G.; Calò, D.G.; Montanari, A. Toward a new fruit juice line with a high healthy power. Ingred. Aliment. 2006, 5, 11–17. [Google Scholar]
- Bevilacqua, A.; Corbo, M.R.; Sinigaglia, M. Selection of Yeasts as Starter Cultures for Table Olives: A Step-by-Step Procedure. Front. Microbiol. 2012, 3, 194. [Google Scholar] [CrossRef] [Green Version]
- FAO. WHO Guidelines for the Evaluation of Probiotics in Food; Probiotic in foods. In Health and Nutritional Properties and Guidelines for Evaluation; Food and Agriculture Organization of the United Nations/World Health Organization: London, ON, Canada, 2006; pp. 1–11. [Google Scholar]
- Pala, V.; Sieri, S.; Berrino, F.; Vineis, P.; Sacerdote, C.; Palli, D.; Masala, G.; Panico, S.; Mattiello, A.; Tumino, R.; et al. Yogurt Consumption and Risk of Colorectal Cancer in the Italian European Prospective Investigation into Cancer and Nutrition Cohort. Int. J. Cancer 2011, 129, 2712–2719. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Bastida, J.A.; Zieliński, H. Buckwheat as a Functional Food and Its Effects on Health. J. Agric. Food Chem. 2015, 63, 7896–7913. [Google Scholar] [CrossRef] [PubMed]
- Świeca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Bochnak, J.; Złotek, U.; Baraniak, B. Lactobacillus Plantarum 299V Improves the Microbiological Quality of Legume Sprouts and Effectively Survives in These Carriers during Cold Storage and in Vitro Digestion. PLoS ONE 2018, 13, e0207793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swieca, M.; Kordowska-Wiater, M.; Pytka, M.; Gawlik-Dziki, U.; Seczyk, L.; Złotek, U.; Kapusta, I. Nutritional and Pro-Health Quality of Lentil and Adzuki Bean Sprouts Enriched with Probiotic Yeast Saccharomyces Cerevisiae Var. Boulardii. LWT 2019, 100, 220–226. [Google Scholar] [CrossRef]
- Child, P.; Kuksis, A. Investigation of the Role of Micellar Phospholipid in the Preferential Uptake of Cholesterol over Sitosterol by Dispersed Rat Jejunal Villus Cells. Biochem. Cell Biol. 1986, 64, 847–853. [Google Scholar] [CrossRef] [PubMed]
- Meguro, S.; Higashi, K.; Hase, T.; Honda, Y.; Otsuka, A.; Tokimitsu, I.; Itakura, H. Solubilization of Phytosterols in Diacylglycerol versus Triacylglycerol Improves the Serum Cholesterol-Lowering Effect. Eur. J. Clin. Nutr. 2001, 55, 513–517. [Google Scholar] [CrossRef] [Green Version]
- Raguindin, P.F.; Adam Itodo, O.; Stoyanov, J.; Dejanovic, G.M.; Gamba, M.; Asllanaj, E.; Minder, B.; Bussler, W.; Metzger, B.; Muka, T.; et al. A Systematic Review of Phytochemicals in Oat and Buckwheat. Food Chem. 2021, 338, 127982. [Google Scholar] [CrossRef]
- Yang, N.; Li, Y.M.; Zhang, K.; Jiao, R.; Ma, K.Y.; Zhang, R.; Ren, G.; Chen, Z.-Y. Hypocholesterolemic Activity of Buckwheat Flour Is Mediated by Increasing Sterol Excretion and Down-Regulation of Intestinal NPC1L1 and ACAT2. J. Funct. Foods 2014, 6, 311–318. [Google Scholar] [CrossRef]
- Liu, J.; Song, Y.; Zhao, Q.; Wang, Y.; Li, C.; Zou, L.; Hu, Y. Effects of Tartary Buckwheat Protein on Gut Microbiome and Plasma Metabolite in Rats with High-Fat Diet. Foods 2021, 10, 2457. [Google Scholar] [CrossRef]
- Ryan, J.J.; Hanes, D.A.; Schafer, M.B.; Mikolai, J.; Zwickey, H. Effect of the Probiotic Saccharomyces Boulardii on Cholesterol and Lipoprotein Particles in Hypercholesterolemic Adults: A Single-Arm, Open-Label Pilot Study. J. Altern. Complement. Med. 2015, 21, 288–293. [Google Scholar] [CrossRef] [Green Version]
- Ntanios, F.Y.; Jones, P.J.; Frohlich, J.J. Dietary Sitostanol Reduces Plaque Formation but Not Lecithin Cholesterol Acyl Transferase Activity in Rabbits. Atherosclerosis 1998, 138, 101–110. [Google Scholar] [CrossRef]
- Lu, K.; Lee, M.H.; Hazard, S.; Brooks-Wilson, A.; Hidaka, H.; Kojima, H.; Ose, L.; Stalenhoef, A.F.; Mietinnen, T.; Bjorkhem, I.; et al. Two Genes That Map to the STSL Locus Cause Sitosterolemia: Genomic Structure and Spectrum of Mutations Involving Sterolin-1 and Sterolin-2, Encoded by ABCG5 and ABCG8, Respectively. Am. J. Hum. Genet. 2001, 69, 278–290. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, S.B. Plant Sterols and Stanols: Their Role in Health and Disease. J. Clin. Lipidol. 2008, 2, S11–S19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary Heart Disease: Seven Dietary Factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]
- Đurendić-Brenesel, M.; Popović, T.; Pilija, V.; Arsić, A.; Milić, M.; Kojić, D.; Jojić, N.; Milić, N. Hypolipidemic and Antioxidant Effects of Buckwheat Leaf and Flower Mixture in Hyperlipidemic Rats. Bosn. J. Basic Med. Sci. 2013, 13, 100–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klug, L.; Daum, G. Yeast Lipid Metabolism at a Glance. FEMS Yeast Res. 2014, 14, 369–388. [Google Scholar] [CrossRef] [Green Version]
- Kot, A.; Błażejak, S.; Kurcz, A.; Gientka, I. Drożdże Jako Potencjalne Źródło Tłuszczu Mikrobiologicznego [Yeast as a potential source of microbial fat]. Postępy Mikrobiol. [Adv. Microbiol.] 2015, 54, 364–373. [Google Scholar]
- Patel, A.; Karageorgou, D.; Rova, E.; Katapodis, P.; Rova, U.; Christakopoulos, P.; Matsakas, L. An Overview of Potential Oleaginous Microorganisms and Their Role in Biodiesel and Omega-3 Fatty Acid-Based Industries. Microorganisms 2020, 8, 434. [Google Scholar] [CrossRef] [Green Version]
- Molska, M.; Reguła, J.; Rudzińska, M.; Świeca, M. Fatty Acids Profile, Atherogenic and Thrombogenic Health Lipid Indices of Lyophilized Buckwheat Sprouts Modified with the Addition of Saccharomyces Cerevisiae Var. Boulardii. Acta Sci. Pol. Technol. Aliment. 2020, 19, 483–490. [Google Scholar] [CrossRef]
- Alves-Bezerra, M.; Cohen, D.E. Triglyceride Metabolism in the Liver. Compr. Physiol. 2017, 8, 1–8. [Google Scholar] [CrossRef]
- Zhukova, N.V.; Novgorodtseva, T.P.; Denisenko, Y.K. Effect of the Prolonged High-Fat Diet on the Fatty Acid Metabolism in Rat Blood and Liver. Lipids Health Dis. 2014, 13, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Zhang, Q.; Zhang, Y.; Yao, Z.; Song, P.; Wei, L.; Zhao, G.; Yan, Z. Effect of Tartary Buckwheat, Rutin, and Quercetin on Lipid Metabolism in Rats during High Dietary Fat Intake. Food Sci. Nutr. 2020, 8, 199–213. [Google Scholar] [CrossRef]
- Ellegård, L.; Bosaeus, I.; Andersson, H. Will Recommended Changes in Fat and Fibre Intake Affect Cholesterol Absorption and Sterol Excretion? An Ileostomy Study. Eur. J. Clin. Nutr. 2000, 54, 306–313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reeves, P.G.; Nielsen, F.H.; Fahey, G.C. AIN-93 Purified Diets for Laboratory Rodents: Final Report of the American Institute of Nutrition Ad Hoc Writing Committee on the Reformulation of the AIN-76A Rodent Diet. J. Nutr. 1993, 123, 1939–1951. [Google Scholar] [CrossRef] [PubMed]
- Dembińska-Kieć, A.; Naskalski, J.W.; Solnica, B. Diagnostyka Laboratoryjna z Elementami Biochemii Klinicznej-A. Dembińska [Laboratory Diagnostics with Elements of Clinical Biochemistry], 4th ed.; Edra Urban & Partner: Worcław, Poland, 2021. [Google Scholar]
- Official Methods of Analysis Program. Available online: https://www.aoac.org/scientific-solutions/standards-and-official-methods/ (accessed on 2 June 2022).
- Folch, J.; Lees, M.; Stanley Stanley, G.H. A Simple Method for the Isolation and Purification of Total Lipides from Animal Tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Mopuri, R.; Kalyesubula, M.; Rosov, A.; Edery, N.; Moallem, U.; Dvir, H. Improved Folch Method for Liver-Fat Quantification. Front. Vet. Sci. 2021, 7, 594853. [Google Scholar] [CrossRef]
- Muzsik, A.; Bajerska, J.; Jeleń, H.H.; Gaca, A.; Chmurzynska, A. Associations between Fatty Acid Intake and Status, Desaturase Activities, and FADS Gene Polymorphism in Centrally Obese Postmenopausal Polish Women. Nutrients 2018, 10, 1068. [Google Scholar] [CrossRef] [Green Version]
- AOCS Official Method Ce 2b-11. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details?productId=112493 (accessed on 24 April 2022).
- AOCS Official Method Ch 6-91. Available online: https://myaccount.aocs.org/PersonifyEbusiness/Store/Product-Details/productId/114625 (accessed on 26 April 2022).

| Groups | n | Parameters | |||
|---|---|---|---|---|---|
| Consumed Diet (g) | FER * | Initial Weight (g) | Weight Gain (g) | ||
| AIN | 8 | 970.31 ± 102.20 b | 0.20 ± 0.02 a | 188.88 ± 23.71 a | 190.00 ± 26.27 b |
| HFD | 8 | 669.06 ± 64.68 a | 0.20 ± 0.03 a | 191.63 ± 12.21 a | 134.88 ± 24.47 a |
| HFDCS | 8 | 674.88 ± 29.25 a | 0.25 ± 0.02 b | 187.50 ± 14.36 a | 171.50 ± 9.68 b |
| HFDPRS | 8 | 671.74 ± 15.03 a | 0.26 ± 0.02 b | 187.13 ± 15.09 a | 176.75 ± 14.27 b |
| Parameters | AIN | HFD | HFDCS | HFDPRS |
|---|---|---|---|---|
| ALT (U/L) | 26.30 ± 7.68 a | 26.24 ± 10.38 a | 24.74 ± 8.09 a | 24.54 ± 4.27 a |
| AST (U/L) | 104.73 ± 24.27 a | 101.54 ± 56.28 a | 96.56 ± 23.58 a | 117.91 ± 45.75 a |
| TCH (mg/dL) | 66.86 ± 3.13 a | 79.25 ± 10.86 b | 70.35 ± 6.94 a | 70.19 ± 8.10 ab |
| HDL-C (mg/dL) | 60.30 ± 2.83 a | 64.74 ± 8.37 a | 61.64 ± 5.83 a | 62.41 ± 7.79 a |
| Non-HDL (mg/dL) | 7.17 ± 1.46 a | 14.51 ± 5.57 b | 8.71 ± 3.98 a | 7.78 ± 3.00 a |
| LDL-C (mg/dL) | 7.29 ± 1.86 a | 16.13 ± 4.27 b | 11.14 ± 2.88 a | 10.04 ± 2.26 a |
| CRP (mg/L) | 0.31 ± 0.03 a | 0.68 ± 0.39 b | 0.30 ± 0.01 a | 0.30 ± 0.01 a |
| GLU (mg/dL) | 7.41 ± 2.25 ab | 4.68 ± 2.29 a | 6.85 ± 2.10 ab | 8.21 ± 2.28 b |
| TAG (mg/dL) | 96.49 ± 19.36 b | 53.69 ± 15.51 a | 63.54 ± 17.26 a | 66.54 ± 8.57 a |
| Sample | Cholesterol | Brassicasterol | Campesterol | Campestanol | Stigmasterol | β-Sitosterol | Sitostanol | Δ5-Avenasterol | α-Amryin | 5,24-Stigmasta-Dienol | Δ7-Stigmasterol | Cycloartanol | 24-Methylene-cycloartanol | Total Sterols |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G | ND | 0.30 ± 0.09 a | 1.85 ± 0.42 a | 0.14 ± 0.03 a | 0.35 ± 0.05 a | 13.7 ± 1.80 a | 0.40 ± 0.06 a | 2.51 ± 0.19 b | 0.42 ± 0.09 a | 0.14 ± 0.02 a | 0.34 ± 0.09 a | 0.20 ± 0.03 a | 0.34 ± 0.03 a | 20.81 ± 1.93 a |
| CS | ND | 0.30 ± 0.08 a | 1.97 ± 0.47 a | 0.17 ± 0.04 a | 1.52 ± 0.28 b | 12.8 ± 1.24 a | 0.40 ± 0.09 a | 2.31 ± 0.30 ab | 0.35 ± 0.17 a | 0.14 ± 0.06 a | 0.41 ± 0.08 a | 0.16 ± 0.03 a | 0.26 ± 0.05 a | 20.85 ± 1.88 a |
| PRS | ND | 0.31 ± 0.08 a | 1.69 ± 0.36 a | 0.16 ± 0.04 a | 1.48 ± 0.26 b | 11.67 ± 1.47 a | 0.38 ± 0.07 a | 2.00 ± 0.36 a | 0.31 ± 0.11 a | 0.18 ± 0.05 a | 0.32 ± 0.07 a | 0.20 ± 0.07 a | 0.30 ± 0.09 a | 19.00 ± 2.18 a |
| AIN | ND | 76.96 ± 4.52 a | 459.43 ± 0.81 d | 16.21 ± 0.11 a | 101.03 ± 0.42 d | 1093.56 ± 9.11 d | 70.09 ± 0.12 d | 44.89 ± 3.30 c | ND | 93.11 ± 1.97 c | 57.06 ± 1.51 c | 25.20 ± 0.11 c | 64.92 ± 4.79 c | 2102.40 ± 12.74 d |
| HFD | ND | ND | 38.46 ± 1.82 a | ND | 4.37 ± 0.23 a | 35.10 ± 1.23 a | 2.19 ± 0.05 a, | 2.28 ± 0.18 a | ND | 3.30 ± 0.21 a | 2.78 ± 0.07 a | 5.11 ± 0.42 a | 4.29 ± 0.35 a | 97.86 ± 0.97 a |
| HFDCS | ND | ND | 65.94 ± 3.26 b | ND | 10.48 ± 0.74 b | 108.99 ± 1.44 b | 4.12 ± 0.17 b | 14.71 ± 0.48 b | ND | 7.43 ± 0.05 b | 8.85 ± 0.14 b | 18.92 ± 0.51 b | 6.99 ± 0.49 a | 246.42 ± 5.57 b |
| HFDPRS | ND | ND | 96.68 ± 5.63 c | ND | 16.06 ± 0.11 c | 179.40 ± 3.01 c | 9.43 ± 0.32 c | 20.71 ± 0.38 b | ND | 8.76 ± 0.04 b | 10.75 ± 0.04 b | 30.96 ± 0.64 d | 21.90 ± 1.56 b | 394.64 ± 0.59 c |
| AIN-S | 49.00 ± 1.31 b | ND | ND | ND | ND | ND | 1.23 ± 0.18 b | ND | ND | ND | ND | ND | ND | 47.78 ± 3.06 b |
| HFD-S | 26.58 ± 1.04 a | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 26.58 ± 3.58 a |
| HFDCS-S | 19.38 ± 1.10 a | ND | ND | ND | ND | ND | 0.64 ± 0.05 a | ND | ND | ND | ND | ND | ND | 20.03 ± 5.43 a |
| HFDPRS-S | 25.45 ± 0.52 a | ND | ND | ND | ND | ND | 0.67 ± 0.03 a | ND | ND | ND | ND | ND | ND | 26.12 ± 3.71 a |
| AIN-L | 28.4 ± 2.04 ab | ND | 0.35 ± 0.08 b | 0.11 ± 0.00 c | 0.07 ± 0.00 bc | 0.57 ± 0.05 c | 0.01 ± 0.00 a, | 0.08 ± 0.00 bc | ND | ND | ND | ND | ND | 29.64 ± 2.06 ab |
| HFD-L | 32.32 ± 2,76 b | ND | 0.14 ± 0.04 a | 0.04 ± 0.00 a | 0.09 ± 0.02 c | 0.17 ± 0.02 a | ND | 0.09 ± 0.01 c | ND | ND | ND | ND | ND | 32.84 ± 2.75 b |
| HFDCS-L | 31.20 ± 3.14 b | ND | 0.20 ± 0.07 a | 0.07 ± 0.01 b | 0.04 ± 0.01 a | 0.40 ± 0.07 b | 0.01 ± 0.00 a | 0.05 ± 0.01 a | ND | ND | ND | ND | ND | 31.93 ± 3.11 b |
| HFDPRS-L | 24.68 ± 1.96 a | ND | 0.13 ± 0.05 a, | 0.07 ± 0.01 b | 0.05 ± 0.01 ab | 0.38 ± 0.03 b | ND | 0.07 ± 0.01 b | ND | ND | ND | ND | ND | 25.30 ± 1.98 a |
| AIN-F | 15.45 ± 2.25 a | 12.81 ± 1.81 b | 0.64 ± 0.01 a | ND | ND | 5.27 ± 0.37 ab | 1.56 ± 0.11 b | 0.11 ± 0.00 a | ND | ND | 0.97 ± 0.04 b, | 0.78 ± 0.08 ab | 1.38 ± 0.15 b | 38.96 ± 4.64 a |
| HFD-F | 52.04 ± 1.73 b | 5.40 ± 0.88 a | 2.37 ± 0.11 b | 0.83 ± 0.08 a | 1.33 ± 0.23 c | 5.55 ± 0.07 b | 1.12 ± 0.03 a | 0.33 ± 0.03 b | ND | 1.52 ± 0.02 c | 0.62 ± 0.03 b | 0.48 ± 0.06 a | 1.14 ± 0.22 ab | 72.72 ± 3.29 b |
| HFDCS-F | 13.53 ± 0.85 a | 26.98 ± 1.00 d | 5.16 ± 0.19 d | 1.00 ± 0.00 b | 0.45 ± 0.01 ab | 5.77 ± 0.08 b | 2.10 ± 0.14 c | 1.97 ± 0.04 d | 0.55 ± 0.14 a | 1.15 ± 0.04 b | 1.15 ± 0.01 c | 1.02 ± 0.15 b | 1.45 ± 0.11 b | 62.27 ± 1.92 b |
| HFDPRS-F | 9.47± 0.00 a | 20.13 ± 0.17 c | 3.15 ± 0.23 c | ND | 0.60 ± 0.04 b | 4.53 ± 0.07 a | 1.39 ± 0.10 ab | 1.35 ± 0.00 c | 0.44 ± 0.04 a | 0.69 ± 0.02 a | 0.65 ± 0.03 a | 0.73 ± 0.07 ab | 0.78 ± 0.01 a | 43.90 ± 0.26 a |
| Fatty Acids | G | CS | PRS | AIN | HFD | HFDCS | HFDPRS | AIN-S | HFD-S | HFDCS-S | HFDPRS-S | AIN-L | HFD-L | HFDCS-L | HFDPRS-L | AIN-F | HFD-F | HFDCS-F | HFDPRS-F |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C10:0 | ND | ND | ND | ND | ND | ND | ND | 46.38 ± 3.12 b | 34.99 ± 2.34 a | 32.62 ± 2.21 a | 32.11 ± 2.49 a | ND | ND | ND | ND | ND | ND | ND | ND |
| C14:0 | ND | 0.72 ± 0.02 a | 1.53 ± 0.06 c | 5.49 ± 0.15 a | 9.77 ± 0.39 b | 9.90 ± 0.24 b | 9.56 ± 0.30 b | 454.36 ± 3.09 c | 366.97 ± 5.75 a | 385.64 ± 5.81 b | 389.95 ± 4.97 b | 2.13 ± 0.16 ab | 1.82 ± 0.05 a | 2.51 ± 0.21 b | 2.30 ± 0.17 ab | 18.48 ± 0.00 c | 2.41 ± 0.23 a | 4.47 ± 0.54 b | 3.88 ± 0.69 b |
| C 16:0 | 127.82 ± 0.20 c | 59.83 ± 0.11 a | 89.91 ± 0.10 b | 89.79 ± 0.93 a | 195.29 ± 0.35 b | 204.26 ± 0.25 d | 199.41 ± 0.32 c | 121.31 ± 4.06 c | 88.18 ± 4.50 a | 86.36 ± 4.77 a | 96.37 ± 2.93 b | 149.26 ± 4.63 a | 140.19 ± 6.48 a | 166.31 ± 3.27 b | 162.09 ± 2.68 b | 132.61 ± 0.00 c | 48.26 ± 0.86 a | 69.25 ± 4.13 b | 62.95 ± 7.22 b |
| C18:0 | 22.52 ± 0.19 c | 10.83 ± 0.19 a | 16.63 ± 0.46 b | 44.69 ± 0.33 a | 111.89 ± 0.32 b | 118.23 ± 0.79 c | 111.89 ± 0.39 b | 84.95 ± 3.07 a | 92.13 ± 2.37 b | 85.62 ± 3.78 a | 93.16 ± 4.31 b | 133.68 ± 3.62 bc | 94.07 ± 4.37 a | 131.25 ± 3.49 b | 141.15 ± 0.86 c | 176.63 ± 0.00 c | 81.72 ± 1.57 a | 120.24 ± 9.68 b | 123.28 ± 4.72 b |
| C20:0 | 11.66 ± 0.38 b | 7.92 ± 0.08 a | 12.36 ± 0.14 b | 2.95 ± 0.15 c | 1.59 ± 0.07 a | 2.14 ± 0.05 b | 2.01 ± 0.06 b | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 3.82 ± 0.00 a | 4.05 ± 0.00 b |
| C22:0 | 13.86 ± 0.27 b | 9.74 ± 0.23 a | 15.08 ± 0.12 c | 8.50 ± 0.52 b | 0.76 ± 0.05 a | 1.46 ± 0.11 a | 1.49 ± 0.05 a | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 1.91 ± 0.00 a | 3.97 ± 0.00 b |
| C23:0 | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 130.27 ± 2.77 a | 142.33 ± 2.33 b | 151.05 ± 1.80 b | 169.71 ± 4.79 c | ND | ND | ND | ND |
| C24:0 | 7.47 ± 0.34 a | 7.16 ± 0.09 a | 10.38 ± 0.00 b | 2.96 ± 0.06 c | 0.31 ± 0.02 a | 0.64 ± 0.00 b | 0.59 ± 0.01 b | 1.99 ± 0.19 b | 1.47 ± 0.16 a | 1.79 ± 0.19 b | 1.51 ± 0.10 a | ND | ND | ND | ND | ND | ND | ND | ND |
| C14:1 | ND | ND | ND | ND | ND | ND | ND | 22.58 ± 2.05 b | 20.65 ± 0.46 b | 17.98 ± 2.06 a | 16.56 ± 1.90 a | ND | ND | ND | ND | ND | ND | ND | ND |
| C16:1 | ND | 1.59 ± 0.09 a | 2.24 ± 0.05 b | 1.75 ± 0.05 a | 13.63 ± 0.32 b | 13.64 ± 0.51 b | 13.29 ± 0.77 b | 11.83 ± 0.53 b | 3.71 ± 0.28 a | 4.08 ± 0.37 a | 4.04 ± 0.25 a | 4.23 ± 0.64 a | 12.75 ± 0.02 b | 5.19 ± 0.40 a | 4.87 ± 0.49 a | ND | 1.82 ± 0.25 b | 1.07 ± 0.24 ab | 0.66 ± 0.53 a |
| C18:1 | 313.47 ± 0.32 c | 169.33 ± 2.13 a | 262.34 ± 0.44 b | 369.55 ± 0.35 d | 322.09 ± 0.23 a | 349.13 ± 0.82 c | 339.84 ± 0.44 b | 91.88 ± 3.73 c | 78.77 ± 2.44 ab | 75.32 ± 4.11 a | 83.52 ± 3.36 b | 174.32 ± 8.08 b | 112.57 ± 5.33 a | 180.56 ± 0.38 b | 170.17 ± 1.05 b | 40.76 ± 0.00 a | 91.88 ± 1.86 d | 74.96 ± 4.68 c | 56.72 ± 4.63 b |
| C18:1 trans | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | ND | 30.98 ± 0.00 c | 10.89 ± 1.00 ab | 10.99 ± 1.51 b | 8.21 ± 0.97 a |
| C20:1 | 23.3 ± 0.07 b | 13.89 ± 0.69 a | 22.24 ± 0.28 b | 1.97 ± 0.09 a | 4.91 ± 0.14 b | 6.24 ± 0.03 d | 5.77 ± 0.00 c | 3.58 ± 0.07 b | 3.26 ± 0.24 a | 3.46 ± 0.27 ab | 3.95 ± 0.14 c | 2.51 ± 0.31 b | ND | 2.19 ± 0.08 b | 2.21 ± 0.09 b | 18.48 ± 0.00 c | 1.68 ± 0.23 a | 3.81 ± 0.23 b | 3.53 ± 0.38 b |
| C 18:2 | 316.06 ± 0.89 c | 174.21 ± 1.89 a | 270.89 ± 0.67 b | 621.37 ± 1.90 c | 132.19 ± 0.38 a | 149.76 ± 0.05 b | 148.24 ± 0.19 b | 107.35 ± 4.07 c | 61.21 ± 1.73 a | 59.51 ± 3.73 a | 68.78 ± 2.53 b | 110.52 ± 7.09 a | 109.07 ± 3.73 ab | 126.39 ± 6.46 b | 122.07 ± 2.50 ab | 16.85 ± 0.00 d | 7.91 ± 0.53 b | 9.86 ± 0.79 c | 6.48 ± 0.29 a |
| C18:3n-3 | 19.92 ± 0.10 c | 9.67 ± 0.43 a | 14.09 ± 0.08 b | 3.54 ± 0.01 a | 5.51 ± 0.23 b | 5.78 ± 0.19 b | 5.24 ± 0.12 b | 1.91 ± 0.21 b | 1.14 ± 0.15 a | 1.31 ± 0.22 a | 1.75 ± 0.10 b | 1.65 ± 0.32 b | ND | 1.39 ± 0.25 b | 1.21 ± 0.15 b | ND | ND | ND | ND |
| C18:3n-6 | ND | ND | ND | ND | ND | ND | ND | 2.17 ± 0.17 c | 1.63 ± 0.15 b | 1.20 ± 0.14 a | 1.55 ± 0.12 b | 2.59 ± 0.12 a | 2.78 ± 0.09 ab | 2.85 ± 0.21 ab | 3.13 ± 0.20 b | ND | ND | ND | ND |
| C20:3n-6 | ND | ND | ND | ND | ND | ND | ND | 1.55 ± 0.11 b | 1.05 ± 0.10 a | 1.50 ± 0.22 b | 1.49 ± 0.10 b | ND | ND | ND | ND | ND | ND | ND | ND |
| C20:4n-6 | ND | ND | ND | ND | ND | ND | ND | 136.18 ± 5.08 b | 137.77 ± 3.54 b | 118.35 ± 4.35 a | 139.44 ± 4.23 b | ND | ND | ND | ND | ND | ND | ND | ND |
| C22:5 | ND | ND | ND | ND | ND | ND | ND | 1.17 ± 0.06 a | 1.76 ± 0.15 c | 1.50 ± 0.15 b | 1.86 ± 0.11 c | ND | ND | ND | ND | ND | ND | ND | ND |
| C22:6 | ND | ND | ND | ND | ND | ND | ND | 4.45 ± 0.35 a | 10.55 ± 0.34 d | 7.69 ± 0.46 b | 8.85 ± 0.50 c | ND | ND | ND | ND | ND | ND | ND | ND |
| G | CS | PRS | AIN | HFD | HFDCS | HFDPRS | AIN-S | HFD-S | HFDCS-S | HFDPRS-S | AIN-L | HFD-L | HFDCS-L | HFDPRS-L | AIN-F | HFD-F | HFDCS-F | HFDPRS-F | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SFA | 183.33 ± 0.06 c | 96.20 ± 0.12 a | 145.89 ± 0.87 b | 154.38 ± 0.38 a | 319.61 ± 1.19 b | 336.63 ± 2.76 c | 324.95 ± 1.13 b | 708.99 ± 5.81 c | 583.74 ± 9.35 a | 592.03 ± 10.64 a | 613.10 ± 6.82 b | 415.34 ± 9.96 b | 378.41 ± 13.23 a | 451.12 ± 7.95 c | 475.25 ± 5.95 c | 327.72 ± 0.00 c | 132.39 ± 1.91 a | 199.69 ± 6.67 b | 198.13 ± 4.96 b |
| MUFA | 336.77 ± 0.25 c | 184.81 ± 2.91 a | 286.82 ± 0.21 b | 373.27 ± 0.48 d | 340.63 ± 0.70 a | 369.01 ± 1.35 c | 358.90 ± 1.21 b | 129.87 ± 3.27 c | 106.39 ± 2.24 b | 100.84 ± 4.86 a | 108.07 ± 3.54 b | 181.06 ± 8.91 b | 125.32 ± 5.35 a | 187.94 ± 0.11 b | 177.25 ± 1.43 b | 90.22 ± 0.00 b | 106.27 ± 3.16 c | 90.83 ± 5.99 b | 69.12 ± 5.89 a |
| PUFA | 335.98 ± 0.99 c | 183.88 ± 1.46 a | 284.98 ± 0.75 b | 624.91 ± 1.90 c | 137.70 ± 0.61 a | 155.54 ± 0.24 b | 153.48 ± 0.07 b | 254.78 ± 4.78 c | 215.11 ± 3.70 b | 191.06 ± 4.67 a | 223.72 ± 5.58 b | 114.76 ± 7.10 a | 111.85 ± 3.82 a | 130.63 ± 6.85 b | 126.41 ± 2.55 ab | 16.85 ± 0.00 d | 7.91 ± 0.53 b | 9.86 ± 0.79 c | 6.48 ± 0.29 a |
| Component | AIN-93M | HFD 1 | HFDCS 2 | HFDPRS 3 |
|---|---|---|---|---|
| Caseine | 140 | 140 | 140 | 140 |
| Soybean oil (rapeseed) | 40 | 40 | 40 | 40 |
| Wheat starch | 622.5 | 422.5 | 422.5 | 422.5 |
| Potato starch | 50 | 50 | 50 | 50 |
| Lard | - | 200 | 200 | 200 |
| Saccharose | 100 | 100 | 100 | 100 |
| Mineral mix | 35 | 35 | 35 | 35 |
| Vitamin mix | 10 | 10 | 10 | 10 |
| Choline | 2.5 | 2.5 | 2.5 | 2.5 |
| Control sprouts | - | - | 300 | - |
| Probiotic-rich sprouts | - | - | - | 300 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Molska, M.; Reguła, J.; Grygier, A.; Muzsik-Kazimierska, A.; Rudzińska, M.; Gramza-Michałowska, A. Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats. Molecules 2022, 27, 4394. https://doi.org/10.3390/molecules27144394
Molska M, Reguła J, Grygier A, Muzsik-Kazimierska A, Rudzińska M, Gramza-Michałowska A. Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats. Molecules. 2022; 27(14):4394. https://doi.org/10.3390/molecules27144394
Chicago/Turabian StyleMolska, Marta, Julita Reguła, Anna Grygier, Agata Muzsik-Kazimierska, Magdalena Rudzińska, and Anna Gramza-Michałowska. 2022. "Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats" Molecules 27, no. 14: 4394. https://doi.org/10.3390/molecules27144394
APA StyleMolska, M., Reguła, J., Grygier, A., Muzsik-Kazimierska, A., Rudzińska, M., & Gramza-Michałowska, A. (2022). Effect of the Addition of Buckwheat Sprouts Modified with the Addition of Saccharomyces cerevisiae var. boulardii to an Atherogenic Diet on the Metabolism of Sterols, Stanols and Fatty Acids in Rats. Molecules, 27(14), 4394. https://doi.org/10.3390/molecules27144394










