Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress
Abstract
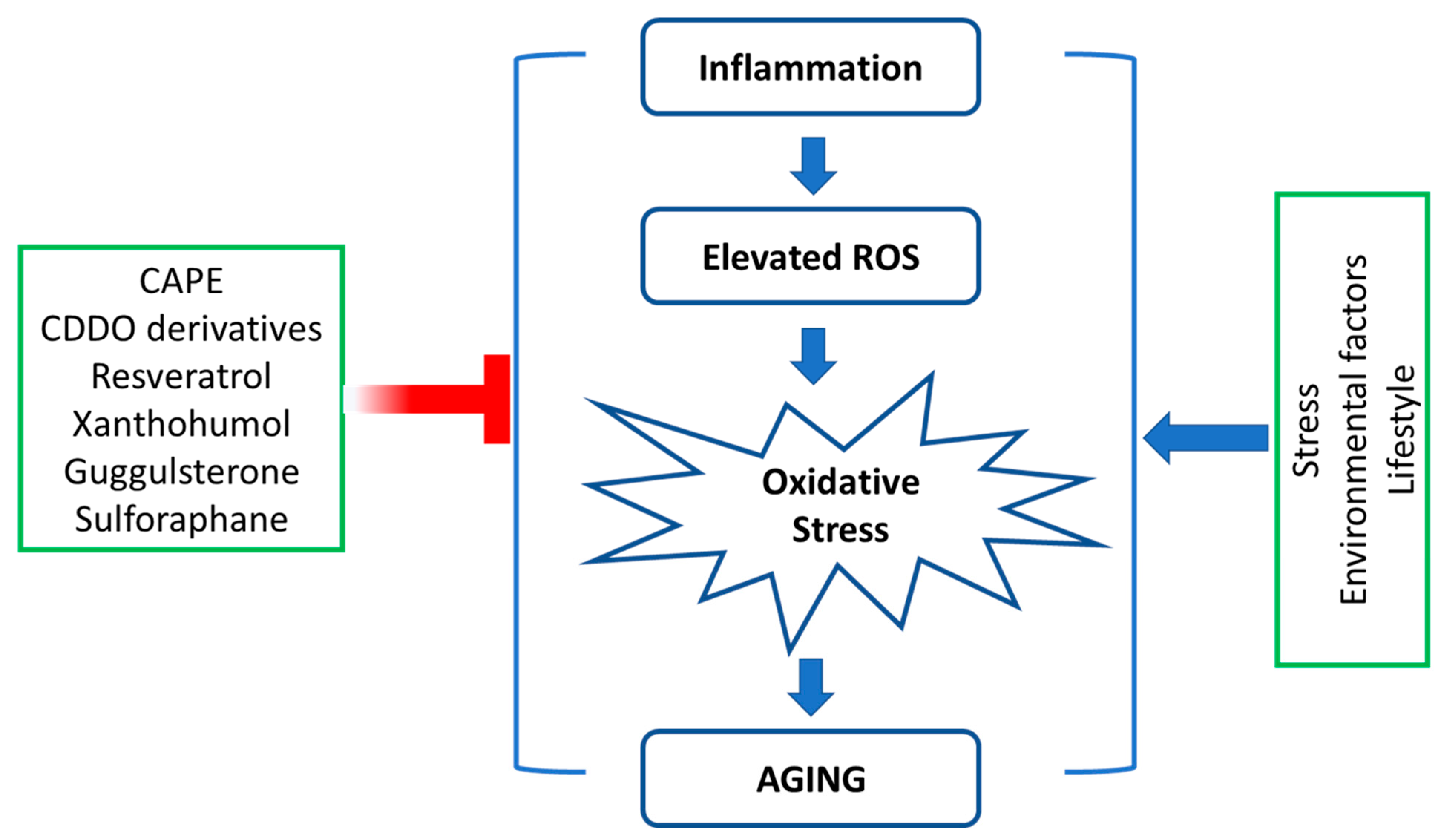
1. Introduction
2. Autophagy and Aging
3. Oxidative Stress and Aging
4. Antiaging Compounds
4.1. CDDO Derivatives
4.2. Caffeic Acid Phenethyl Ester (CAPE)
4.3. Xanthohumol
4.4. Guggulsterone
4.5. Resveratrol
4.6. Sulforaphane
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harraan, D. Aging: A Theory Based on Free Radical and Radiation Chemistry; University of California: Berkeley, CA, USA, 1955. [Google Scholar]
- Monti, D.; Ostan, R.; Borelli, V.; Castellani, G.; Franceschi, C. Inflammaging and human longevity in the omics era. Mech. Ageing Dev. 2017, 165, 129–138. [Google Scholar] [CrossRef]
- Rattan, S.I.S. Theories of biological aging: Genes, proteins, and free radicals. Free Radic. Res. 2006, 40, 1230–1238. [Google Scholar] [CrossRef]
- Edrey, Y.H.; Salmon, A.B. Revisiting an age-old question regarding oxidative stress. Free Radic. Biol. Med. 2014, 71, 368–378. [Google Scholar] [CrossRef]
- Schöttker, B.; Saum, K.-U.; Jansen, E.H.J.M.; Boffetta, P.; Trichopoulou, A.; Holleczek, B.; Dieffenbach, A.K.; Brenner, H. Oxidative stress markers and all-cause mortality at older age: A population-based cohort study. J. Gerontol. Ser. A 2014, 70, 518–524. [Google Scholar] [CrossRef]
- Scrivo, A.; Bourdenx, M.; Pampliega, O.; Cuervo, A.M. Selective autophagy as a potential therapeutic target for neurodegenerative disorders. Lancet Neurol. 2018, 17, 802–815. [Google Scholar] [CrossRef]
- Condello, M.; Pellegrini, E.; Caraglia, M.; Meschini, S. Targeting autophagy to overcome human diseases. Int. J. Mol. Sci. 2019, 20, 725. [Google Scholar] [CrossRef]
- Djajadikerta, A.; Keshri, S.; Pavel, M.; Prestil, R.; Ryan, L.; Rubinsztein, D.C. Autophagy induction as a therapeutic strategy for neurodegenerative diseases. J. Mol. Biol. 2020, 432, 2799–2821. [Google Scholar] [CrossRef]
- Mputhia, Z.; Hone, E.; Tripathi, T.; Sargeant, T.; Martins, R.; Bharadwaj, P. Autophagy modulation as a treatment of amyloid diseases. Molecules 2019, 24, 3372. [Google Scholar] [CrossRef]
- Fang, E.F.; Hou, Y.; Palikaras, K.; Adriaanse, B.A.; Kerr, J.S.; Yang, B.; Lautrup, S.; Hasan-Olive, M.M.; Caponio, D.; Dan, X.; et al. Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of alzheimer’s disease. Nat. Neurosci. 2019, 22, 401–412. [Google Scholar] [CrossRef]
- Cabreiro, F.; Au, C.; Leung, K.-Y.; Vergara-Irigaray, N.; Cochemé, H.M.; Noori, T.; Weinkove, D.; Schuster, E.; Greene, N.D.; Gems, D. Metformin retards aging in c. elegans by altering microbial folate and methionine metabolism. Cell 2013, 153, 228–239. [Google Scholar] [CrossRef]
- Partridge, L.; Fuentealba, M.; Kennedy, B.K. The quest to slow ageing through drug discovery. Nat. Rev. Drug Discov. 2020, 19, 513–532. [Google Scholar] [CrossRef]
- Madeo, F.; Eisenberg, T.; Pietrocola, F.; Kroemer, G. Spermidine in health and disease. Science 2018, 359, eaan2788. [Google Scholar] [CrossRef] [PubMed]
- Eisenberg, T.; Knauer, H.; Schauer, A.; Büttner, S.; Ruckenstuhl, C.; Carmona-Gutierrez, D.; Ring, J.; Schroeder, S.; Magnes, C.; Antonacci, L.; et al. Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 2009, 11, 1305–1314. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xiao, D.; Burton-Freeman, B.M.; Edirisinghe, I. Chemical changes of bioactive phytochemicals during thermal processing. Food Sci. 2016. [Google Scholar] [CrossRef]
- Liu, R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013, 78, A18–A25. [Google Scholar] [CrossRef]
- Martinez, K.B.; Mackert, J.D.; McIntosh, M.K. Polyphenols and intestinal health. In Nutrition and Functional Foods for Healthy Aging; Elsevier: Amsterdam, The Netherlands, 2017; pp. 191–210. [Google Scholar]
- Süntar, I.; Yakıncı, Ö.F. Potential risks of phytonutrients associated with high-dose or long-term use. In Phytonutrients in Food; Elsevier: Amsterdam, The Netherlands, 2020; pp. 137–155. [Google Scholar]
- Zhang, Y.J.; Gan, R.Y.; Li, S.; Zhou, Y.; Li, A.N.; Xu, D.P.; Li, H.B. Antioxidant Phytochemical for the Prevention and Treatment of Chronic Disease. Molecules 2015, 20, 21138–21156. [Google Scholar] [CrossRef]
- Pietta, P.-G. Flavonoids as Antioxidants. J. Nat. Prod. 2000, 63, 1035–1042. [Google Scholar] [CrossRef]
- Li, Y.; Guo, Y.; Wang, X.; Yu, X.; Duan, W.; Hong, K.; Wang, J.; Han, H.; Li, C. Trehalose decreases mutant SOD1 expression and alleviates motor deficiency in early but not end-stage amyotrophic lateral sclerosis in a SOD1-G93A mouse model. Neuroscience 2015, 298, 12–25. [Google Scholar] [CrossRef]
- Menzies, F.M.; Fleming, A.; Caricasole, A.; Bento, C.F.; Andrews, S.P.; Ashkenazi, A.; Füllgrabe, J.; Jackson, A.; Sanchez, M.J.; Karabiyik, C.; et al. Autophagy and neurodegeneration: Pathogenic mechanisms and therapeutic opportunities. Neuron 2017, 93, 1015–1034. [Google Scholar] [CrossRef]
- Giampieri, F.; Afrin, S.; Forbes-Hernandez, T.Y.; Gasparrini, M.; Cianciosi, D.; Reboredo-Rodriguez, P.; Varela-Lopez, A.; Quiles, J.L.; Battino, M. Autophagy in Human Health and Disease: Novel Therapeutic Opportunities. Antioxid. Redox Signal. 2019, 30, 577–634. [Google Scholar] [CrossRef]
- Ray, P.D.; Huang, B.-W.; Tsuji, Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell. Signal. 2012, 24, 981–990. [Google Scholar] [CrossRef]
- Phaniendra, A.; Jestadi, D.B.; Periyasamy, L. Free radicals: Properties, sources, targets, and their implication in various diseases. Indian J. Clin. Biochem. 2015, 30, 11–26. [Google Scholar] [CrossRef]
- Thoma, A.; Akter-Miah, T.; Reade, R.L.; Lightfoot, A.P. Targeting reactive oxygen species (ROS) to combat the age-related loss of muscle mass and function. Biogerontology 2020, 21, 475–484. [Google Scholar] [CrossRef]
- Ryan, M.J.; Dudash, H.J.; Docherty, M.; Geronilla, K.B.; Baker, B.A.; Haff, G.G.; Cutlip, R.G.; Alway, S.E. Vitamin E and C supplementation reduces oxidative stress, improves antioxidant enzymes and positive muscle work in chronically loaded muscles of aged rats. Exp. Gerontol. 2010, 45, 882–895. [Google Scholar] [CrossRef]
- Lim, J.J.; Zurinah, W.N.W.; Mouly, V.; Norwahidah, A.K. Tocotrienol-Rich Fraction (TRF) Treatment Promotes Proliferation Capacity of Stress-Induced Premature Senescence Myoblasts and Modulates the Renewal of Satellite Cells: Microarray Analysis. Oxidative Med. Cell. Longev. 2019, 2019, 9141343. [Google Scholar] [CrossRef]
- Baumann, C.; Lees, S.; Otis, J.; Rogers, R. Muscular strength is unaffected by short-term resveratrol supplementation in aged mouse muscle. Int. J. Clin. Exp. Physiol. 2014, 1, 253. [Google Scholar] [CrossRef]
- Bosutti, A.; Degens, H. The impact of resveratrol and hydrogen peroxide on muscle cell plasticity shows a dose-dependent interaction. Sci. Rep. 2015, 5, 8093. [Google Scholar] [CrossRef]
- Brenner, M.; Hearing, V.J. The protective role of melanin against UV damage in human skin. Photochem. Photobiol. 2008, 84, 539–549. [Google Scholar] [CrossRef]
- Gunn, D.A.; De Craen, A.J.M.; Dick, J.L.; Tomlin, C.C.; Van Heemst, D.; Catt, S.D.; Griffiths, T.; Ogden, S.; Maier, A.; Murray, P.G.; et al. Facial appearance reflects human familial longevity and cardiovascular disease risk in healthy individuals. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2013, 68, 145–152. [Google Scholar] [CrossRef]
- Gunn, D.A.; Larsen, L.A.; Lall, J.S.; Rexbye, H.; Christensen, K. Mortality is written on the face. J. Gerontol. Ser. A Biomed. Sci. Med. Sci. 2016, 71, 72–77. [Google Scholar] [CrossRef]
- Waaijer, M.E.; Goldeck, D.; Gunn, D.A.; van Heemst, D.; Westendorp, R.G.; Pawelec, G.; Maier, A.B. Are skin senescence and immunosenescence linked within individuals? Aging Cell 2019, 18, e12956. [Google Scholar] [CrossRef] [PubMed]
- Waaijer, M.E.C.; Parish, W.E.; Strongitharm, B.H.; Van Heemst, D.; Slagboom, P.E.; De Craen, A.J.M.; Sedivy, J.M.; Westendorp, R.G.J.; Gunn, D.A.; Maier, A.B. The number of p16INK4a positive cells in human skin reflects biological age. Aging Cell 2012, 11, 722–725. [Google Scholar] [CrossRef]
- Yang, Z.; Klionsky, D.J. An overview of the molecular mechanism of autophagy. Autophagy Infect. Immun. 2009, 335, 1–32. [Google Scholar]
- Wollert, T. Autophagy. Curr. Biol. 2019, 29, R671–R677. [Google Scholar] [CrossRef]
- Mizushima, N. Autophagy: Process and function. Genes Dev. 2007, 21, 2861–2873. [Google Scholar] [CrossRef]
- Rubinsztein, D.C.; Shpilka, T.; Elazar, Z. Mechanisms of autophagosome biogenesis. Curr. Biol. 2012, 22, R29–R34. [Google Scholar] [CrossRef]
- Nakamura, S.; Yoshimori, T. New insights into autophagosome–Lysosome fusion. J. Cell Sci. 2017, 130, 1209–1216. [Google Scholar] [CrossRef]
- Jung, C.H.; Jun, C.B.; Ro, S.-H.; Kim, Y.-M.; Otto, N.M.; Cao, J.; Kundu, M.; Kim, D.-H. ULK-atg13-FIP200 complexes mediate mtor signaling to the autophagy machinery. Mol. Biol. Cell 2009, 20, 1992–2003. [Google Scholar] [CrossRef]
- Chan, E.Y.; Kir, S.; Tooze, S.A. siRNA screening of the kinome identifies ULK1 as a multidomain modulator of autophagy. J. Biol. Chem. 2007, 282, 25464–25474. [Google Scholar] [CrossRef]
- Zachari, M.; Ganley, I.G. The mammalian ULK1 complex and autophagy initiation. Essays Biochem. 2017, 61, 585–596. [Google Scholar]
- Hardie, D.G. AMPK and autophagy get connected. EMBO J. 2011, 30, 634–635. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef]
- Cohen-Kaplan, V.; Livneh, I.; Avni, N.; Fabre, B.; Ziv, T.; Kwon, Y.T.; Ciechanover, A. p62- and ubiquitin-dependent stress-induced autophagy of the mammalian 26S proteasome. Proc. Natl. Acad. Sci. USA 2016, 113, E7490–E7499. [Google Scholar] [CrossRef]
- Moscat, J.; Diaz-Meco, M.T. p62 at the crossroads of autophagy, apoptosis, and cancer. Cell 2009, 137, 1001–1004. [Google Scholar] [CrossRef]
- Lau, A.; Wang, X.-J.; Zhao, F.; Villeneuve, N.F.; Wu, T.; Jiang, T.; Sun, Z.; White, E.; Zhang, D.D. A noncanonical mechanism of nrf2 activation by autophagy deficiency: Direct interaction between keap1 and p62. Mol. Cell. Biol. 2010, 30, 3275–3285. [Google Scholar] [CrossRef]
- Sahu, R.; Kaushik, S.; Clement, C.C.; Cannizzo, E.S.; Scharf, B.; Follenzi, A.; Potolicchio, I.; Nieves, E.; Cuervo, A.M.; Santambrogio, L. Microautophagy of cytosolic proteins by late endosomes. Dev. Cell 2011, 20, 131–139. [Google Scholar] [CrossRef]
- Bandyopadhyay, U.; Kaushik, S.; Varticovski, L.; Cuervo, A.M. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol. Cell. Biol. 2008, 28, 5747–5763. [Google Scholar] [CrossRef]
- Tan, C.-C.; Yu, J.-T.; Tan, M.-S.; Jiang, T.; Zhu, X.-C.; Tan, L. Autophagy in aging and neurodegenerative diseases: Implications for pathogenesis and therapy. Neurobiol. Aging 2014, 35, 941–957. [Google Scholar] [CrossRef]
- Matecic, M.; Smith, D.L., Jr.; Pan, X.; Maqani, N.; Bekiranov, S.; Boeke, J.D.; Smith, J.S. A microarray-based genetic screen for yeast chronological aging factors. PLoS Genet. 2010, 6, e1000921. [Google Scholar] [CrossRef]
- Simonsen, A.; Cumming, R.C.; Brech, A.; Isakson, P.; Schubert, D.R.; Finley, K.D. Promoting basal levels of autophagy in the nervous system enhances longevity and oxidant resistance in adult drosophila. Autophagy 2008, 4, 176–184. [Google Scholar] [CrossRef]
- Tóth, M.L.; Sigmond, T.; Borsos, E.; Barna, J.; Erdélyi, P.; Takács-Vellai, K.; Orosz, L.; Kovács, A.L.; Csikós, G.; Sass, M.; et al. Longevity pathways converge on autophagy genes to regulate life span in caenorhabditis elegans. Autophagy 2008, 4, 330–338. [Google Scholar] [CrossRef]
- Harding, T.M.; Hefner-Gravink, A.; Thumm, M.; Klionsky, D.J. Genetic and phenotypic overlap between autophagy and the cytoplasm to vacuole protein targeting pathway. J. Biol. Chem. 1996, 271, 17621–17624. [Google Scholar] [CrossRef]
- Hanada, T.; Noda, N.N.; Satomi, Y.; Ichimura, Y.; Fujioka, Y.; Takao, T.; Inagaki, F.; Ohsumi, Y. The atg12-atg5 conjugate has a novel E3-like activity for protein lipidation in autophagy. J. Biol. Chem. 2007, 282, 37298–37302. [Google Scholar] [CrossRef]
- Cadwell, K.; Liu, J.Y.; Brown, S.L.; Miyoshi, H.; Loh, J.; Lennerz, J.K.; Kishi, C.; Kc, W.; Carrero, J.A.; Hunt, S.; et al. A key role for autophagy and the autophagy gene atg16l1 in mouse and human intestinal paneth cells. Nature 2008, 456, 259–263. [Google Scholar] [CrossRef]
- Hara, T.; Nakamura, K.; Matsui, M.; Yamamoto, A.; Nakahara, Y.; Suzuki-Migishima, R.; Yokoyama, M.; Mishima, K.; Saito, I.; Okano, H.; et al. Suppression of basal autophagy in neural cells causes neurodegenerative disease in mice. Nature 2006, 441, 885–889. [Google Scholar] [CrossRef]
- Komatsu, M.; Waguri, S.; Ueno, T.; Iwata, J.; Murata, S.; Tanida, I.; Ezaki, J.; Mizushima, N.; Ohsumi, Y.; Uchiyama, Y.; et al. Impairment of starvation-induced and constitutive autophagy in atg7-deficient mice. J. Cell Biol. 2005, 169, 425–434. [Google Scholar] [CrossRef]
- Wang, R.C.; Wei, Y.; An, Z.; Zou, Z.; Xiao, G.; Bhagat, G.; White, M.; Reichelt, J.; Levine, B. Akt-mediated regulation of autophagy and tumorigenesis through beclin 1 phosphorylation. Science 2012, 338, 956–959. [Google Scholar] [CrossRef]
- Johnson, S.; Rabinovitch, P.S.; Kaeberlein, M. mTOR is a key modulator of ageing and age-related disease. Nature 2013, 493, 338–345. [Google Scholar] [CrossRef]
- Liu, G.Y.; Sabatini, D.M. mTOR at the nexus of nutrition, growth, ageing and disease. Nat. Rev. Mol. Cell Biol. 2020, 21, 183–203. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. mTOR signaling in aging and neurodegeneration: At the crossroad between metabolism dysfunction and impairment of autophagy. Neurobiol. Dis. 2015, 84, 39–49. [Google Scholar] [CrossRef]
- Stavoe, A.K.H.; Holzbaur, E.L.F. Axonal autophagy: Mini-review for autophagy in the CNS. Neurosci. Lett. 2019, 697, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Naseri, N.N.; Wang, H.; Guo, J.; Sharma, M.; Luo, W. The complexity of tau in alzheimer’s disease. Neurosci. Lett. 2019, 705, 183–194. [Google Scholar] [CrossRef] [PubMed]
- Cuervo, A.M.; Stefanis, L.; Fredenburg, R.; Lansbury, P.T.; Sulzer, D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science 2004, 305, 1292–1295. [Google Scholar] [CrossRef] [PubMed]
- Issa, A.-R.; Sun, J.; Petitgas, C.; Mesquita, A.; Dulac, A.; Robin, M.; Mollereau, B.; Jenny, A.; Chérif-Zahar, B.; Birman, S. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced parkinson disease-like symptoms in the Drosophila brain. Autophagy 2018, 14, 1898–1910. [Google Scholar] [CrossRef]
- Ikeda, T.; Sporn, M.; Honda, T.; Gribble, G.W.; Kufe, N. The novel triterpenoid CDDO and its derivatives induce apoptosis by disruption of intracellular redox balance. Cancer Res. 2003, 63, 5551–5558. [Google Scholar]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive oxygen species in metabolic and inflammatory signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef]
- Hiona, A.; Leeuwenburgh, C. The role of mitochondrial DNA mutations in aging and sarcopenia: Implications for the mitochondrial vicious cycle theory of aging. Exp. Gerontol. 2008, 43, 24–33. [Google Scholar] [CrossRef]
- Wang, C.-H.; Wu, S.-B.; Wu, Y.-T.; Wei, Y.-H. Oxidative stress response elicited by mitochondrial dysfunction: Implication in the pathophysiology of aging. Exp. Biol. Med. 2013, 238, 450–460. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhou, T.; Ziegler, A.C.; Dimitrion, P.; Zuo, L. Oxidative stress in neurodegenerative diseases: From molecular mechanisms to clinical applications. Oxid. Med. Cell. Longev. 2017, 2017, 2525967. [Google Scholar] [CrossRef] [PubMed]
- Liguori, I.; Russo, G.; Curcio, F.; Bulli, G.; Aran, L.; Della-Morte, D.; Gargiulo, G.; Testa, G.; Cacciatore, F.; Bonaduce, D.; et al. Oxidative stress, aging, and diseases. Clin. Interv. Aging 2018, 13, 757–772. [Google Scholar] [CrossRef] [PubMed]
- DiLoreto, R.; Murphy, C.T. The cell biology of aging. Mol. Biol. Cell 2015, 26, 4524–4531. [Google Scholar] [CrossRef]
- Rossi, D.J.; Jamieson, C.H.; Weissman, I.L. Stems cells and the pathways to aging and cancer. Cell 2008, 132, 681–696. [Google Scholar] [CrossRef]
- Goodell, M.A.; Rando, T.A. Stem cells and healthy aging. Science 2015, 350, 1199–1204. [Google Scholar] [CrossRef]
- de Haan, G.; Lazare, S.S. Aging of hematopoietic stem cells. Blood 2018, 131, 479–487. [Google Scholar] [CrossRef]
- Blasco, M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef]
- Ito, K.; Hirao, A.; Arai, F.; Matsuoka, S.; Takubo, K.; Hamaguchi, I.; Nomiyama, K.; Hosokawa, K.; Sakurada, K.; Nakagata, N.; et al. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 2004, 431, 997–1002. [Google Scholar] [CrossRef]
- Kasai, S.; Shimizu, S.; Tatara, Y.; Mimura, J.; Itoh, K. Regulation of Nrf2 by mitochondrial reactive oxygen species in physiology and pathology. Biomolecules 2020, 10, 320. [Google Scholar] [CrossRef]
- Hayes, J.D.; Dinkova-Kostova, A.T. The Nrf2 regulatory network provides an interface between redox and intermediary metabolism. Trends Biochem. Sci. 2014, 39, 199–218. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, F.; Dixit, D.; Sharma, V.; Kumar, A.; Joshi, S.D.; Sarkar, C.; Sen, E. Nrf2-driven TERT regulates pentose phosphate pathway in glioblastoma. Cell Death Dis. 2016, 7, e2213. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.B.; Pandita, R.K.; Eskiocak, U.; Ly, P.; Kaisani, A.; Kumar, R.; Cornelius, C.; Wright, W.E.; Pandita, T.K.; Shay, J.W. Targeting of Nrf2 induces DNA damage signaling and protects colonic epithelial cells from ionizing radiation. Proc. Natl. Acad. Sci. USA 2012, 109, E2949–E2955. [Google Scholar] [CrossRef] [PubMed]
- Jayakumar, S.; Pal, D.; Sandur, S.K. Nrf2 facilitates repair of radiation induced DNA damage through homologous recombination repair pathway in a ROS independent manner in cancer cells. Mutat. Res. Mol. Mech. Mutagen. 2015, 779, 33–45. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Abramov, A.Y. The emerging role of Nrf2 in mitochondrial function. Free Radic. Biol. Med. 2015, 88, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Hoeijmakers, J.H. DNA damage, aging, and cancer. N. Engl. J. Med. 2009, 361, 1475–1485. [Google Scholar] [CrossRef]
- Kapeta, S.; Chondrogianni, N.; Gonos, E.S. Nuclear erythroid factor 2-mediated proteasome activation delays senescence in human fibroblasts. J. Biol. Chem. 2010, 285, 8171–8184. [Google Scholar] [CrossRef]
- Ayeleso, T.B.; Matumba, M.G.; Mukwevho, E. Oleanolic acid and its derivatives: Biological activities and therapeutic potential in chronic diseases. Molecules 2017, 22, 1915. [Google Scholar] [CrossRef]
- Castellano, J.M.; Ramos-Romero, S.; Perona, J.S. Oleanolic acid: Extraction, characterization and biological activity. Nutrients 2022, 14, 623. [Google Scholar] [CrossRef]
- Mathis, B.J.; Cui, T. CDDO and its role in chronic diseases. Drug Discov. Mother Nat. 2016, 929, 291–314. [Google Scholar]
- Tran, T.A.; McCoy, M.K.; Sporn, M.B.; Tansey, M.G. The synthetic triterpenoid CDDO-methyl ester modulates microglial activities, inhibits TNF production, and provides dopaminergic neuroprotection. J. Neuroinflamm. 2008, 5, 14. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.; Yore, M.M.; Sporn, M.B. Triterpenoids and rexinoids as multifunctional agents for the prevention and treatment of cancer. Nat. Cancer 2007, 7, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Dinkova-Kostova, A.T.; Liby, K.T.; Stephenson, K.K.; Holtzclaw, W.D.; Gao, X.; Suh, N.; Williams, C.; Risingsong, R.; Honda, T.; Gribble, G.W.; et al. Extremely potent triterpenoid inducers of the phase 2 response: Correlations of protection against oxidant and inflammatory stress. Proc. Natl. Acad. Sci. USA 2005, 102, 4584–4589. [Google Scholar] [CrossRef] [PubMed]
- Liby, K.; Hock, T.; Yore, M.M.; Suh, N.; Place, A.E.; Risingsong, R.; Williams, C.R.; Royce, D.B.; Honda, T.; Honda, Y.; et al. The synthetic triterpenoids, CDDO and CDDO-imidazolide, are potent inducers of heme oxygenase-1 and Nrf2/ARE signaling. Cancer Res. 2005, 65, 4789–4798. [Google Scholar] [CrossRef]
- Lapillonne, H.; Konopleva, M.; Tsao, T.; Gold, D.; McQueen, T.; Sutherland, R.L.; Madden, T.; Andreeff, M. Activation of peroxisome proliferator-activated receptor γ by a novel synthetic triterpenoid 2-cyano-3, 12-dioxooleana-1, 9-dien-28-oic acid induces growth arrest and apoptosis in breast cancer cells. Cancer Res. 2003, 63, 5926–5939. [Google Scholar]
- Yates, M.S.; Kwak, M.-K.; Egner, P.A.; Groopman, J.D.; Bodreddigari, S.; Sutter, T.R.; Baumgartner, K.J.; Roebuck, B.; Liby, K.T.; Yore, M.M.; et al. Potent protection against aflatoxin-induced tumorigenesis through induction of Nrf2-regulated pathways by the triterpenoid 1-[2-cyano-3-,12-dioxooleana-1,9(11)-dien-28-oyl]imidazole. Cancer Res. 2006, 66, 2488–2494. [Google Scholar] [CrossRef]
- Place, A.E.; Suh, N.; Williams, C.R.; Risingsong, R.; Honda, T.; Honda, Y.; Gribble, G.W.; Leesnitzer, L.M.; Stimmel, J.B.; Willson, T.M.; et al. The novel synthetic triterpenoid, CDDO-imidazolide, inhibits inflammatory response and tumor growth in vivo. Clin. Cancer Res. 2003, 9, 2798–2806. [Google Scholar]
- Wang, Y.-Y.; Yang, Y.-X.; Zhe, H.; He, Z.-X.; Zhou, S.-F. Bardoxolone methyl (CDDO-Me) as a therapeutic agent: An update on its pharmacokinetic and pharmacodynamic properties. Drug Des. Dev. Ther. 2014, 8, 2075–2088. [Google Scholar]
- Albini, A.; Noonan, D.M.; Ferrari, N. Molecular pathways for cancer angioprevention. Clin. Cancer Res. 2007, 13, 4320–4325. [Google Scholar] [CrossRef]
- Jackson, S.J.; Singletary, K.W.; Venema, R.C. Sulforaphane suppresses angiogenesis and disrupts endothelial mitotic progression and microtubule polymerization. Vasc. Pharmacol. 2007, 46, 77–84. [Google Scholar] [CrossRef]
- Asakage, M.; Tsuno, N.H.; Kitayama, J.; Tsuchiya, T.; Yoneyama, S.; Yamada, J.; Okaji, Y.; Kaisaki, S.; Osada, T.; Takahashi, K.; et al. Sulforaphane induces inhibition of human umbilical vein endothelial cells proliferation by apoptosis. Angiogenesis 2006, 9, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Bertl, E.; Bartsch, H.; Gerhauser, C. Inhibition of angiogenesis and endothelial cell functions are novel sulforaphane-mediated mechanisms in chemoprevention. Mol. Cancer Ther. 2006, 5, 575–585. [Google Scholar] [CrossRef] [PubMed]
- Vannini, N.; Lorusso, G.; Cammarota, R.; Barberis, M.; Noonan, D.M.; Sporn, M.B.; Albini, A. The synthetic oleanane triterpenoid, CDDO-methyl ester, is a potent antiangiogenic agent. Mol. Cancer Ther. 2007, 6, 3139–3146. [Google Scholar] [CrossRef]
- Tashiro, K.; Shishido, M.; Fujimoto, K.; Hirota, Y.; Yo, K.; Gomi, T.; Tanaka, Y. Age-related disruption of autophagy in dermal fibroblasts modulates extracellular matrix components. Biochem. Biophys. Res. Commun. 2014, 443, 167–172. [Google Scholar] [CrossRef]
- Rittié, L.; Fisher, G.J. Natural and sun-induced aging of human skin. Cold Spring Harb. Perspect. Med. 2015, 5, a015370. [Google Scholar] [CrossRef] [PubMed]
- Kammeyer, A.; Luiten, R.M. Oxidation events and skin aging. Ageing Res. Rev. 2015, 21, 16–29. [Google Scholar] [CrossRef]
- Itakura, E.; Mizushima, N. Characterization of autophagosome formation site by a hierarchical analysis of mammalian atg proteins. Autophagy 2010, 6, 764–776. [Google Scholar] [CrossRef]
- Ryu, Y.S.; Kang, K.A.; Piao, M.J.; Ahn, M.J.; Yi, J.M.; Bossis, G.; Hyun, Y.-M.; Park, C.O.; Hyun, J.W. Particulate matter-induced senescence of skin keratinocytes involves oxidative stress-dependent epigenetic modifications. Exp. Mol. Med. 2019, 51, 1–14. [Google Scholar] [CrossRef]
- Choi, J.K.; Oh, H.-M.; Lee, S.; Park, J.-W.; Khang, D.; Lee, S.W.; Lee, W.S.; Rho, M.-C.; Kim, S.-H. Oleanolic acid acetate inhibits atopic dermatitis and allergic contact dermatitis in a murine model. Toxicol. Appl. Pharmacol. 2013, 269, 72–80. [Google Scholar] [CrossRef]
- Kim, Y.J.; Lee, J.E.; Jang, H.S.; Hong, S.Y.; Lee, J.B.; Park, S.Y.; Hwang, J.S. Oleanolic acid protects the skin from particulate matter-induced aging. Biomol. Ther. 2021, 29, 220–226. [Google Scholar] [CrossRef]
- Bankova, V. Chemical diversity of propolis makes it a valuable source of new biologically active compounds. J. ApiProduct ApiMedical Sci. 2009, 1, 23–28. [Google Scholar] [CrossRef]
- Chen, H.-C.; Chen, J.-H.; Chang, C.; Shieh, C.-J. Optimization of ultrasound-accelerated synthesis of enzymatic caffeic acid phenethyl ester by response surface methodology. Ultrason. Sonochem. 2011, 18, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Widjaja, A.; Yeh, T.H.; Ju, Y.H. Enzymatic synthesis of caffeic acid phenethyl ester. J. Chin. Inst. Chem. Eng. 2008, 39, 413–418. [Google Scholar] [CrossRef]
- Chen, H.-C.; Ju, H.-Y.; Twu, Y.-K.; Chen, J.-H.; Chang, C.-M.J.; Liu, Y.-C.; Chang, C.; Shieh, C.-J. Optimized enzymatic synthesis of caffeic acid phenethyl ester by RSM. New Biotechnol. 2010, 27, 89–93. [Google Scholar] [CrossRef]
- Ha, S.H.; Van Anh, T.; Lee, S.H.; Koo, Y.-M. Effect of ionic liquids on enzymatic synthesis of caffeic acid phenethyl ester. Bioprocess Biosyst. Eng. 2012, 35, 235–240. [Google Scholar] [CrossRef]
- Wang, X.; Stavchansky, S.; Bowman, P.D.; Kerwin, S.M. Cytoprotective effect of caffeic acid phenethyl ester (CAPE) and catechol ring-fluorinated CAPE derivatives against menadione-induced oxidative stress in human endothelial cells. Bioorganic Med. Chem. 2006, 14, 4879–4887. [Google Scholar] [CrossRef]
- Van Acker, S.A.B.E.; Van Den Berg, D.-J.; Tromp, M.N.J.L.; Griffioen, D.H.; Van Bennekom, W.P.; Van Der Vijgh, W.J.F.; Bast, A. Structural aspects of antioxidant activity of flavonoids. Free Radic. Biol. Med. 1996, 20, 331–342. [Google Scholar] [CrossRef]
- Tomiyama, R.; Takakura, K.; Takatou, S.; Le, T.M.; Nishiuchi, T.; Nakamura, Y.; Konishi, T.; Matsugo, S.; Hori, O. 3,4-dihydroxybenzalacetone and caffeic acid phenethyl ester induce preconditioning ER stress and autophagy in SH-SY5Y cells. J. Cell. Physiol. 2017, 233, 1671–1684. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol extract of propolis and its constituent caffeic acid phenethyl ester inhibit breast cancer cells proliferation in inflammatory microenvironment by inhibiting TLR4 signal pathway and inducing apoptosis and autophagy. BMC Complement. Altern. Med. 2017, 17, 417. [Google Scholar] [CrossRef]
- Vijayakurup, V.; Carmela, S.; Carmelo, D.; Corrado, T.; Srinivas, P.; Gopala, S. Phenethyl caffeate benzo[kl]xanthene lignan with DNA interacting properties induces DNA damage and apoptosis in colon cancer cells. Life Sci. 2012, 91, 1336–1344. [Google Scholar] [CrossRef]
- Si, H.; Liu, D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014, 25, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Havermann, S.; Chovolou, Y.; Humpf, H.-U.; Wätjen, W. Caffeic Acid Phenethylester Increases Stress Resistance and Enhances Lifespan in Caenorhabditis elegans by Modulation of the Insulin-Like DAF-16 Signalling Pathway. PLoS ONE 2014, 9, e100256. [Google Scholar] [CrossRef] [PubMed]
- Eşrefoğlu, M.; Gül, M.; Ateş, B.; Erdoğan, A. The effects of caffeic acid phenethyl ester and melatonin on age-related vascular remodeling and cardiac damage. Fundam. Clin. Pharmacol. 2010, 25, 580–590. [Google Scholar] [CrossRef]
- Elwood, J.M.; Jopson, J. Melanoma and sun exposure: An overview of published studies. Int. J. Cancer 1997, 73, 198–203. [Google Scholar] [CrossRef]
- Shin, E.J.; Jo, S.; Choi, H.-K.; Choi, S.; Byun, S.; Lim, T.-G. Caffeic acid phenethyl ester inhibits UV-induced MMP-1 expression by targeting histone acetyltransferases in human skin. Int. J. Mol. Sci. 2019, 20, 3055. [Google Scholar] [CrossRef] [PubMed]
- Ozguner, F.; Altinbas, A.; Ozaydin, M.; Dogan, A.; Vural, H.; Kisioglu, A.N.; Cesur, G.; Yildirim, N.G. Mobile phone-induced myocardial oxidative stress: Protection by a novel antioxidant agent caffeic acid phenethyl ester. Toxicol. Ind. Health 2005, 21, 223–230. [Google Scholar] [CrossRef]
- Serarslan, G.; Altuğ, E.; Kontas, T.; Atik, E.; Avci, G. Caffeic acid phenetyl ester accelerates cutaneous wound healing in a rat model and decreases oxidative stress. Clin. Exp. Dermatol. Exp. Dermatol. 2007, 32, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Kavakli, H.S.; Alici, O.; Koca, C.; Ilhan, A.; Isik, B. Caffeic acid phenethyl ester decreases oxidative stress index in blunt spinal cord injury in rats. Hong Kong J. Emerg. Med. 2010, 17, 250–255. [Google Scholar] [CrossRef]
- Olczyk, P.; Komosińska-Vassev, K.; Winsz-Szczotka, K.; Stojko, J.; Klimek, K.; Koźma, E.M. Propolis Induces Chondroitin/Dermatan Sulphate and Hyaluronic Acid Accumulation in the Skin of Burned Wound. Evid.-Based Complement. Altern. Med. 2013, 2013, 290675. [Google Scholar] [CrossRef]
- Yasui, N.; Nishiyama, E.; Juman, S.; Negishi, H.; Miki, T.; Yamori, Y.; Ikeda, K. Caffeic acid phenethyl ester suppresses oxidative stress in 3T3-L1 adipocytes. J. Asian Nat. Prod. Res. 2013, 15, 1189–1196. [Google Scholar] [CrossRef]
- Jiang, C.-H.; Sun, T.-L.; Xiang, D.-X.; Wei, S.-S.; Li, W.-Q. Anticancer activity and mechanism of xanthohumol: A prenylated flavonoid from hops (Humulus lupulus L.). Front. Pharmacol. 2018, 9, 530. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Hansen, P.E.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological profile of xanthohumol, a prenylated flavonoid from hops (Humulus lupulus). Molecules 2015, 20, 754–779. [Google Scholar] [CrossRef] [PubMed]
- Fernández-García, C.; Rancan, L.; Paredes, S.D.; Montero, C.; de la Fuente, M.; Vara, E.; Tresguerres, J.A.F. Xanthohumol exerts protective effects in liver alterations associated with aging. Eur. J. Nutr. 2019, 58, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Karabín, M.; Hudcová, T.; Jelínek, L.; Dostálek, P. Biologically active compounds from hops and prospects for their use. Compr. Rev. Food Sci. Food Saf. 2016, 15, 542–567. [Google Scholar] [CrossRef] [PubMed]
- Rancán, L.; Paredes, S.D.; García, I.; Muñoz, P.; García, C.; de Hontanar, G.L.; de la Fuente, M.; Vara, E.; Tresguerres, J.A. Protective effect of xanthohumol against age-related brain damage. J. Nutr. Biochem. 2017, 49, 133–140. [Google Scholar] [CrossRef]
- Sasazawa, Y.; Kanagaki, S.; Tashiro, E.; Nogawa, T.; Muroi, M.; Kondoh, Y.; Osada, H.; Imoto, M. Xanthohumol Impairs Autophagosome Maturation through Direct Inhibition of Valosin-Containing Protein. ACS Chem. Biol. 2012, 7, 892–900. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef]
- Plazar, J.; Žegura, B.; Lah, T.T.; Filipič, M. Protective effects of xanthohumol against the genotoxicity of benzo (a) pyrene (BaP), 2-amino-3-methylimidazo [4,5-f] quinoline (IQ) and tert-butyl hydroperoxide (t-BOOH) in HepG2 human hepatoma cells. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2007, 632, 1–8. [Google Scholar] [CrossRef]
- Dorn, C.; Massinger, S.; Wuzik, A.; Heilmann, J.; Hellerbrand, C. Xanthohumol suppresses inflammatory response to warm ischemia–Reperfusion induced liver injury. Exp. Mol. Pathol. 2013, 94, 10–16. [Google Scholar] [CrossRef]
- Dietz, B.M.; Kang, Y.-H.; Liu, G.; Eggler, A.L.; Yao, P.; Chadwick, L.R.; Pauli, G.F.; Farnsworth, N.R.; Mesecar, A.D.; van Breemen, A.R.B.; et al. Xanthohumol isolated from Humulus lupulus inhibits menadione-induced DNA damage through induction of quinone reductase. Chem. Res. Toxicol. 2005, 18, 1296–1305. [Google Scholar] [CrossRef]
- Harikumar, K.B.; Kunnumakkara, A.B.; Ahn, K.S.; Anand, P.; Krishnan, S.; Guha, S.; Aggarwal, B.B. Modification of the cysteine residues in IκBα kinase and NF-κB (p65) by xanthohumol leads to suppression of NF-κB–regulated gene products and potentiation of apoptosis in leukemia cells. Blood J. Am. Soc. Hematol. 2009, 113, 2003–2013. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.-S.; Lim, J.; Gal, J.; Kang, J.C.; Kim, H.J.; Kang, B.Y.; Choi, H.J. Anti-inflammatory activity of xanthohumol involves heme oxygenase-1 induction via NRF2-ARE signaling in microglial BV2 cells. Neurochem. Int. 2011, 58, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Philips, N.; Samuel, M.; Arena, R.; Chen, Y.-J.; Conte, J.; Natarajan, P.; Natrajan, P.; Haas, G.; Gonzalez, S. Direct inhibition of elastase and matrixmetalloproteinases and stimulation of biosynthesis of fibrillar collagens, elastin, and fibrillins by xanthohumol. J. Cosmet. Sci. 2010, 61, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Bokelmann, J.M. Medicinal Herbs in Primary Care-E-Book: An Evidence-Guided Reference for Healthcare Providers; Elsevier Health Sciences: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Dietz, B.M.; Hagos, G.K.; Eskra, J.N.; Wijewickrama, G.T.; Anderson, J.R.; Nikolic, D.; Guo, J.; Wright, B.; Chen, S.N.; Pauli, G.F.; et al. Differential regulation of detoxification enzymes in hepatic and mammary tissue by hops (H umulus lupulus) in vitro and in vivo. Mol. Nutr. Food Res. 2013, 57, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Sarup, P.; Bala, S.; Kamboj, S. Pharmacology and phytochemistry of oleo-gum resin of commiphora wightii (Guggulu). Scientifica 2015, 2015, 138039. [Google Scholar] [CrossRef]
- Deng, R. Therapeutic effects of guggul and its constituent guggulsterone: Cardiovascular benefits. Cardiovasc. Drug Rev. 2007, 25, 375–390. [Google Scholar] [CrossRef]
- Satyavati, G.V.; Dwarakanath, C.; Tripathi, S.N. Experimental studies on the hypocholesterolemic effect of Commiphora mukul Engl.(guggul). Indian J. Med. Res. 1969, 57, 1950–1962. [Google Scholar]
- Chander, R.; Rizvi, F.; Khanna, A.K.; Pratap, R. Cardioprotective activity of synthetic guggulsterone (E and Z-isomers) in isoproterenol induced myocardial ischemia in rats: A comparative study. Indian J. Clin. Biochem. 2003, 18, 71–79. [Google Scholar] [CrossRef]
- Urizar, N.L.; Liverman, A.B.; Dodds, D.T.; Silva, F.V.; Ordentlich, P.; Yan, Y.; Gonzalez, F.J.; Heyman, R.A.; Mangelsdorf, D.J.; Moore, D.D. A natural product that lowers cholesterol as an antagonist ligand for FXR. Science 2002, 296, 1703–1706. [Google Scholar] [CrossRef]
- Rayalam, S.; Della-Fera, M.A.; Ambati, S.; Boyan, B.; Baile, C.A. Enhanced effects of guggulsterone plus 1,25(OH)2D3 on 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2007, 364, 450–456. [Google Scholar] [CrossRef]
- Miller, C.N.; Samuels, J.S.; Azhar, Y.; Parmar, A.; Shashidharamurthy, R.; Rayalam, S. Guggulsterone activates adipocyte beiging through direct effects on 3T3-L1 adipocytes and indirect effects mediated through RAW264.7 macrophages. Medicines 2019, 6, 22. [Google Scholar] [CrossRef] [PubMed]
- Wenz, T. Mitochondria and PGC-1alpha in aging and age-associated diseases. J. Aging Res. 2011, 2011, 810619. [Google Scholar] [CrossRef] [PubMed]
- Rader, D.J. ATVB in focus: Novel approaches to the treatment of dyslipidemia. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 480–481. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Huang, L.; Zhao, A.; Lew, J.-L.; Yu, J.; Sahoo, S.; Meinke, P.T.; Royo, I.; Peláez, F.; Wright, S.D. Guggulsterone is a farnesoid X receptor antagonist in coactivator association assays but acts to enhance transcription of bile salt export pump. J. Biol. Chem. 2003, 278, 10214–10220. [Google Scholar] [CrossRef]
- Koo, J.-H.; Rhee, K.-S.; Koh, H.-W.; Jang, H.-Y.; Park, B.-H.; Park, J.-W. Guggulsterone inhibits melanogenesis in B16 murine melanoma cells by downregulating tyrosinase expression. Int. J. Mol. Med. 2012, 30, 974–978. [Google Scholar] [CrossRef]
- Sarfaraz, S.; Siddiqui, I.A.; Syed, D.N.; Afaq, F.; Mukhtar, H. Guggulsterone modulates MAPK and NF-kappaB pathways and inhibits skin tumorigenesis in SENCAR mice. Carcinogenesis 2008, 29, 2011–2018. [Google Scholar] [CrossRef]
- Diaz-Gerevini, G.T.; Repossi, G.; Dain, A.; Tarres, M.C.; Das, U.N.; Eynard, A.R. Beneficial action of resveratrol: How and why? Nutrition 2016, 32, 174–178. [Google Scholar] [CrossRef]
- Boo, Y.C. Human skin lightening efficacy of resveratrol and its analogs: From in vitro studies to cosmetic applications. Antioxidants 2019, 8, 332. [Google Scholar] [CrossRef]
- Cui, H.; Kong, Y.; Zhang, H. Oxidative stress, mitochondrial dysfunction, and aging. J. Signal Transduct. 2012, 2012, 646354. [Google Scholar] [CrossRef]
- Tung, B.T.; Rodriguez-Bies, E.; Thanh, H.N.; Le-Thi-Thu, H.; Navas, P.; Sanchez, V.M.; López-Lluch, G. Organ and tissue-dependent effect of resveratrol and exercise on antioxidant defenses of old mice. Aging Clin. Exp. Res. 2015, 27, 775–783. [Google Scholar] [CrossRef]
- Aguilar-Alonso, P.; Vera-López, O.; Brambila-Colombres, E.; Segura-Badilla, O.; Avalos-López, R.; Lazcano-Hernández, M.; Navarro-Cruz, A.R. Evaluation of Oxidative Stress in Cardiomyocytes during the Aging Process in Rats Treated with Resveratrol. Oxidative Med. Cell. Longev. 2018, 2018, 1390483. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Yang, J.; Zhou, M.; Zhang, Y.; Liu, Y.; Hou, P.; Zeng, X.; Yi, L.; Mi, M. Resveratrol attenuates endothelial oxidative injury by inducing autophagy via the activation of transcription factor EB. Nutr. Metab. 2019, 16, 42. [Google Scholar] [CrossRef] [PubMed]
- Morselli, E.; Mariño, G.; Bennetzen, M.V.; Eisenberg, T.; Megalou, E.; Schroeder, S.; Cabrera, S.; Bénit, P.; Rustin, P.; Criollo, A.; et al. Spermidine and resveratrol induce autophagy by distinct pathways converging on the acetylproteome. J. Cell Biol. 2011, 192, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.G.; Rogina, B.; Lavu, S.; Howitz, K.; Helfand, S.L.; Tatar, M.; Sinclair, D. Sirtuin activators mimic caloric restriction and delay ageing in metazoans. Nature 2004, 430, 686–689. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A.; Pearson, K.J.; Price, N.L.; Jamieson, H.A.; Lerin, C.; Kalra, A.; Prabhu, V.V.; Allard, J.S.; Lopez-Lluch, G.; Lewis, K.; et al. Resveratrol improves health and survival of mice on a high-calorie diet. Nature 2006, 444, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Quan, J.I.; Kinghorn, K.J.; Bjedov, I. Genetics and pharmacology of longevity: The road to therapeutics for healthy aging. Adv. Genet. 2015, 90, 1–101. [Google Scholar]
- Bakker, G.C.; Van Erk, M.J.; Pellis, L.; Wopereis, S.; Rubingh, C.M.; Cnubben, N.H.; Kooistra, T.; Van Ommen, B.; Hendriks, H.F. An antiinflammatory dietary mix modulates inflammation and oxidative and metabolic stress in overweight men: A nutrigenomics approach. Am. J. Clin. Nutr. 2010, 91, 1044–1059. [Google Scholar] [CrossRef]
- Yiu, E.M.; Tai, G.; Peverill, R.E.; Lee, K.J.; Croft, K.D.; Mori, T.A.; Scheiber-Mojdehkar, B.; Sturm, B.; Praschberger, M.; Vogel, A.P.; et al. An open-label trial in friedreich ataxia suggests clinical benefit with high-dose resveratrol, without effect on frataxin levels. J. Neurol. 2015, 262, 1344–1353. [Google Scholar] [CrossRef]
- Brasnyó, P.; Molnar, G.A.; Mohás, M.; Markó, L.; Laczy, B.; Cseh, J.; Mikolás, E.; Szijártó, I.A.; Mérei, A.; Halmai, R.; et al. Resveratrol improves insulin sensitivity, reduces oxidative stress and activates the Akt pathway in type 2 diabetic patients. Br. J. Nutr. 2011, 106, 383–389. [Google Scholar] [CrossRef]
- Yoshino, J.; Conte, C.; Fontana, L.; Mittendorfer, B.; Imai, S.-I.; Schechtman, K.B.; Gu, C.; Kunz, I.; Fanelli, F.R.; Patterson, B.W.; et al. Resveratrol supplementation does not improve metabolic function in nonobese women with normal glucose tolerance. Cell Metab. 2012, 16, 658–664. [Google Scholar] [CrossRef]
- Van Der Made, S.M.; Plat, J.; Mensink, R.P. Resveratrol does not influence metabolic risk markers related to cardiovascular health in overweight and slightly obese subjects: A randomized, placebo-controlled crossover trial. PLoS ONE 2015, 10, e0118393. [Google Scholar] [CrossRef] [PubMed]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.P.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—Safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G.; et al. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef] [PubMed]
- Boocock, D.J.; Faust, G.E.S.; Patel, K.R.; Schinas, A.M.; Brown, V.A.; Ducharme, M.P.; Booth, T.D.; Crowell, J.A.; Perloff, M.; Gescher, A.J.; et al. Phase I dose escalation pharmacokinetic study in healthy volunteers of resveratrol, a potential cancer chemopreventive agent. Cancer Epidemiol. Biomark. Prev. 2007, 16, 1246–1252. [Google Scholar] [CrossRef]
- Sikdar, S.; Papadopoulou, M.; Dubois, J. What do we know about sulforaphane protection against photoaging? J. Cosmet. Dermatol. 2016, 15, 72–77. [Google Scholar] [CrossRef]
- Gómez-Linton, D.R.; Alavez, S.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Konigsberg, M.; Pérez-Flores, L.J. Some naturally occurring compounds that increase longevity and stress resistance in model organisms of aging. Biogerontology 2019, 20, 583–603. [Google Scholar] [CrossRef]
- Santín-Márquez, R.; Alarcón-Aguilar, A.; López-Diazguerrero, N.E.; Chondrogianni, N.; Königsberg, M. Sulforaphane—Role in aging and neurodegeneration. GeroScience 2019, 41, 655–670. [Google Scholar] [CrossRef]
- Wang, M.; Zhu, J.-Y.; Chen, S.; Qing, Y.; Wu, D.; Lin, Y.-M.; Luo, J.-Z.; Han, W.; Li, Y.-Q. Effects of co-treatment with sulforaphane and autophagy modulators on uridine 5′-diphospho-glucuronosyltransferase 1A isoforms and cytochrome P450 3A4 expression in Caco-2 human colon cancer cells. Oncol. Lett. 2014, 8, 2407–2416. [Google Scholar] [CrossRef]
- Alumkal, J.J.; Slottke, R.; Schwartzman, J.; Cherala, G.; Munar, M.; Graff, J.N.; Beer, T.M.; Ryan, C.W.; Koop, D.R.; Gibbs, A.; et al. A phase II study of sulforaphane-rich broccoli sprout extracts in men with recurrent prostate cancer. Investig. New Drugs 2015, 33, 480–489. [Google Scholar] [CrossRef]
- Guerrero-Beltrán, C.E.; Calderón-Oliver, M.; Pedraza-Chaverri, J.; Chirino, Y.I. Protective effect of sulforaphane against oxidative stress: Recent advances. Exp. Toxicol. Pathol. 2012, 64, 503–508. [Google Scholar] [CrossRef]
- Herman-Antosiewicz, A.; Johnson, D.E.; Singh, S.V. Sulforaphane causes autophagy to inhibit release of cytochrome c and apoptosis in human prostate cancer cells. Cancer Res. 2006, 66, 5828–5835. [Google Scholar] [CrossRef]
- Gan, N.; Wu, Y.-C.; Brunet, M.; Garrido, C.; Chung, F.-L.; Dai, C.; Mi, L. Sulforaphane activates heat shock response and enhances proteasome activity through up-regulation of Hsp27. J. Biol. Chem. 2010, 285, 35528–35536. [Google Scholar] [CrossRef] [PubMed]
- Hariton, F.; Xue, M.; Rabbani, N.; Fowler, M.; Thornalley, P.J. Sulforaphane Delays Fibroblast Senescence by Curbing Cellular Glucose Uptake, Increased Glycolysis, and Oxidative Damage. Oxidative Med. Cell. Longev. 2018, 2018, 5642148. [Google Scholar] [CrossRef] [PubMed]
- Herzig, S.; Shaw, R.J. AMPK: Guardian of metabolism and mitochondrial homeostasis. Nat. Rev. Mol. Cell Biol. 2018, 19, 121–135. [Google Scholar] [CrossRef] [PubMed]
- Degtyarev, M.; De Mazière, A.; Orr, C.; Lin, J.; Lee, B.B.; Tien, J.Y.; Prior, W.W.; van Dijk, S.; Wu, H.; Gray, D.C.; et al. Akt inhibition promotes autophagy and sensitizes PTEN-null tumors to lysosomotropic agents. J. Cell Biol. 2008, 183, 101–116. [Google Scholar] [CrossRef]
- Su, Y.; Qu, Y.; Zhao, F.; Li, H.; Mu, D.; Li, X. Regulation of autophagy by the nuclear factor kappaB signaling pathway in the hippocampus of rats with sepsis. J. Neuroinflamm. 2015, 12, 116. [Google Scholar] [CrossRef]
- Tang, Z.; Hu, B.; Zang, F.; Wang, J.; Zhang, X.; Chen, H. Nrf2 drives oxidative stress-induced autophagy in nucleus pulposus cells via a Keap1/Nrf2/p62 feedback loop to protect intervertebral disc from degeneration. Cell Death Dis. 2019, 10, 510. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taylor, E.; Kim, Y.; Zhang, K.; Chau, L.; Nguyen, B.C.; Rayalam, S.; Wang, X. Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress. Molecules 2022, 27, 4396. https://doi.org/10.3390/molecules27144396
Taylor E, Kim Y, Zhang K, Chau L, Nguyen BC, Rayalam S, Wang X. Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress. Molecules. 2022; 27(14):4396. https://doi.org/10.3390/molecules27144396
Chicago/Turabian StyleTaylor, Elizabeth, Yujin Kim, Kaleb Zhang, Lenne Chau, Bao Chieu Nguyen, Srujana Rayalam, and Xinyu Wang. 2022. "Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress" Molecules 27, no. 14: 4396. https://doi.org/10.3390/molecules27144396
APA StyleTaylor, E., Kim, Y., Zhang, K., Chau, L., Nguyen, B. C., Rayalam, S., & Wang, X. (2022). Antiaging Mechanism of Natural Compounds: Effects on Autophagy and Oxidative Stress. Molecules, 27(14), 4396. https://doi.org/10.3390/molecules27144396





_Wang.png)
