A Rapid and Efficient Strategy for Quality Control of Clinopodii herba Encompassing Optimized Ultrasound-Assisted Extraction Coupled with Sensitive Variable Wavelength Detection
Abstract
:1. Introduction
2. Results and Discussion
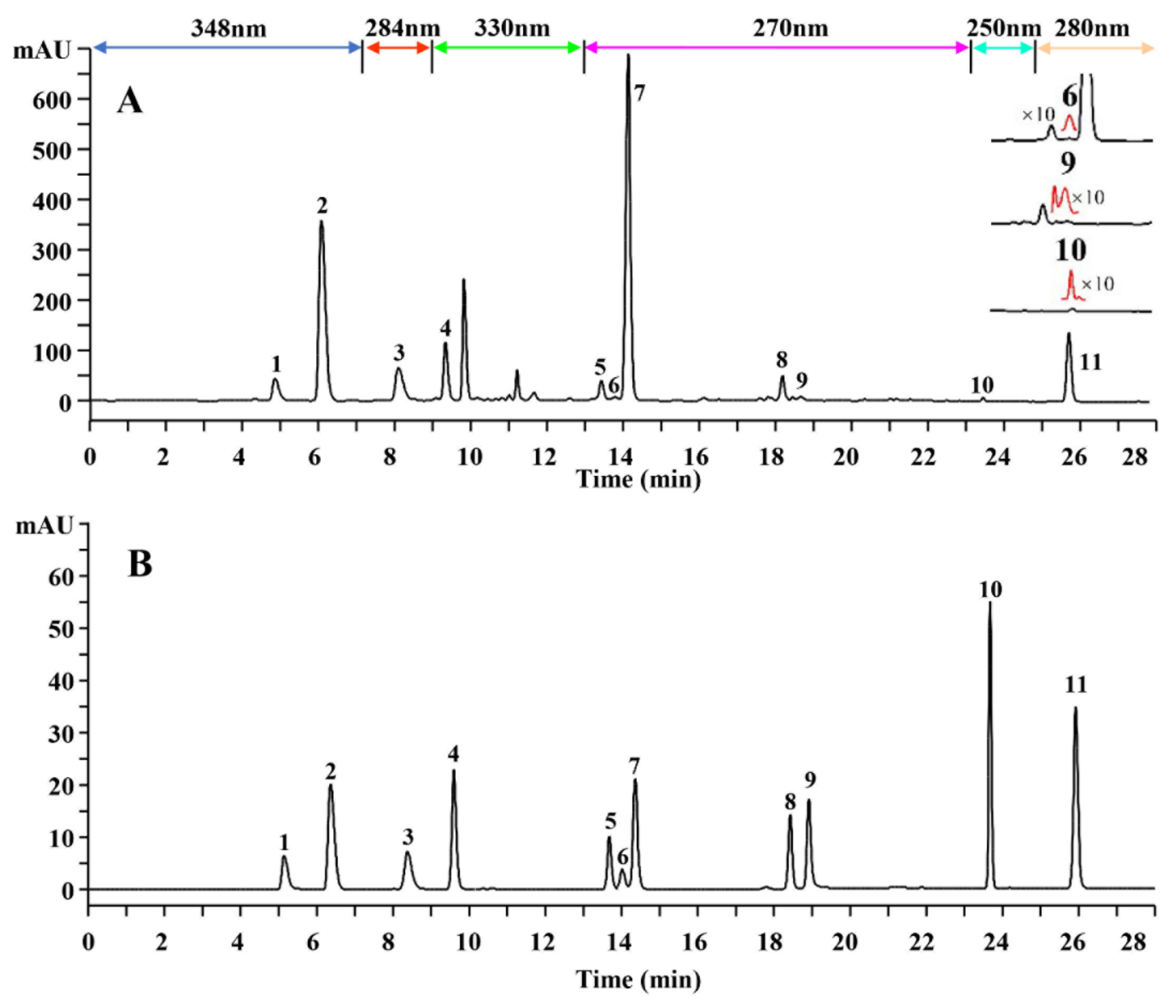
2.1. Selection of Markers of Quality Control
2.2. Optimization of UHPLC–DAD Conditions
2.3. Method Validation
2.3.1. Linearity, LOD and LOQ
2.3.2. Precision, Repeatability and Stability
2.3.3. Recovery
2.4. Single Factor Experiment
2.4.1. Optimization of the Proportion of Methanol–Water
2.4.2. Optimization of Liquid to Solid Ratio
2.4.3. Optimization of Extraction Time
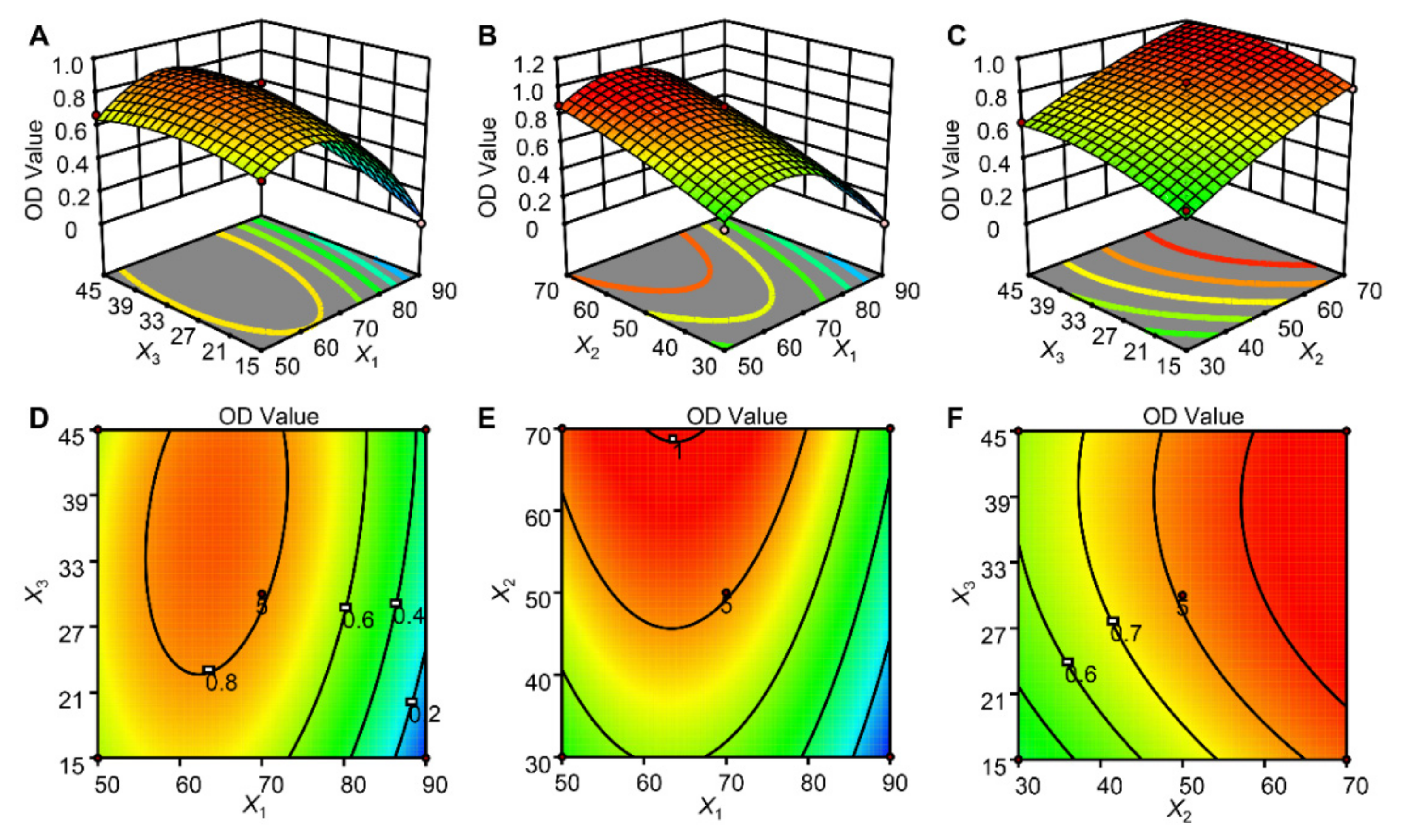
2.5. Optimization of UAE by RSM
2.5.1. Model Fitting and Statistical Analysis
2.5.2. Analysis of the Response Surface
2.5.3. Analysis of the Response Surface
2.6. Quantitative Analysis of CH
3. Materials and Methods
3.1. Reagents and Materials
3.2. Instrumentation and Chromatographic Conditions
3.3. Preparation of Solutions
3.3.1. Preparation of Standard Solutions
3.3.2. Preparation of Sample Solutions
3.4. Ultrasound-Assisted Extraction of CH and Preparations
3.5. Experimental Design
3.5.1. Single Factor Experimental Design
3.5.2. Box-Behnken Design
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Normile, D. The new face of traditional Chinese medicine. Science 2003, 299, 188–190. [Google Scholar] [CrossRef] [PubMed]
- Yao, C.L.; Zhang, J.Q.; Li, J.Y.; Wei, W.L.; Wu, S.F.; Guo, D.A. Traditional Chinese medicine (TCM) as a source of new anticancer drugs. Nat. Prod. Rep. 2021, 38, 1618–1633. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; David, B.; Tu, P.; Barbin, Y. Recent analytical approaches in quality control of traditional Chinese medicines—A review. Anal. Chim. Acta 2010, 657, 9–18. [Google Scholar] [PubMed]
- Xie, P.S.; Leung, A.Y. Understanding the traditional aspect of Chinese medicine in order to achieve meaningful quality control of Chinese materia medica. J. Chromatogr. A 2009, 1216, 1933–1940. [Google Scholar] [CrossRef]
- Li, H.; Wang, S.W.; Zhang, B.L.; Xie, Y.H.; Yang, Q.; Cao, W.; Wang, J.B. Simultaneous quantitative determination of 9 active components in traditional Chinese medicinal preparation ShuangDan oral liquid by RP-HPLC coupled with photodiode array detection. J. Pharm. Biomed. Anal. 2011, 56, 820–824. [Google Scholar] [CrossRef]
- Wang, H.; Jiang, Y.; Ding, M.; Li, J.; Hao, J.; He, J.; Wang, H.; Gao, X.; Chang, Y. Simultaneous determination and qualitative analysis of six types of components in Naoxintong capsule by miniaturized matrix solid-phase dispersion extraction coupled with ultra-high performance liquid chromatography with photodiode array detection and quadrupole time-of-flight mass spectrometry. J. Sep. Sci. 2018, 41, 2064–2084. [Google Scholar]
- Murata, T.; Sasaki, K.; Sato, K.; Yoshizaki, F.; Yamada, H.; Mutoh, H.; Umehara, K.; Miyase, T.; Warashina, T.; Aoshima, H.; et al. Matrix Metalloproteinase-2 Inhibitors from Clinopodium chinense var. parviflorum. J. Nat. Prod. 2009, 72, 1379–1384. [Google Scholar] [CrossRef]
- Zhong, M.; Sun, G.; Zhang, X.; Sun, G.; Xu, X.; Yu, S. A New Prenylated Naphthoquinoid from the Aerial Parts of Clinopodium chinense (Benth.) O. Kuntze. Molecules 2012, 17, 13910–13916. [Google Scholar] [CrossRef]
- Zhong, M.; Wu, H.; Zhang, X.; Sun, G.; Yu, S.; Xu, X. A new diterpene from Clinopodium chinense. Nat. Prod. Res. 2014, 28, 467–472. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Wu, H.F.; Ma, G.X.; Chen, R.C.; Long, H.L.; Zuo, Z.L.; Luo, Y.; Zhu, N.L.; Hou, B.; Xu, X.D.; et al. Clinoposides A–F: Meroterpenoids with protective effects on H9c2 cardiomyocyte from Clinopodium chinense. RSC Adv. 2016, 6, 7260–7266. [Google Scholar] [CrossRef]
- Zhu, Y.D.; Chen, R.C.; Wang, H.; Jiang, H.; Huang, X.L.; Zhang, M.L.; Li, L.Y.; Hu, Z.; Xu, X.D.; Wang, C.J.; et al. Two new flavonoid–triterpene saponin meroterpenoids from Clinopodium chinense and their protective effects against anoxia/reoxygenation-induced apoptosis in H9c2 cells. Fitoterapia 2018, 128, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Huang, Q.; Duan, X.; Han, L.; Peng, D. Protective effect of Clinopodium chinense (Benth.) O. Kuntze against abnormal uterine bleeding in female rats. J. Pharmacol. Sci. 2020, 143, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Liu, G.D.; Zhang, B.B.; Wang, S.; Ma, R.; Zhong, B.S.; He, B.; Liang, Y.; Wu, F.H. A new triterpenoid saponin from Clinopodium chinense (Benth.) O. Kuntze. Nat. Prod. Res. 2020, 30, 1001–1008. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.D.; Hong, J.Y.; Bao, F.D.; Xing, N.; Wang, L.T.; Sun, Z.H.; Luo, Y.; Jiang, H.; Xu, X.D.; Zhu, N.L.; et al. Triterpenoid saponins from Clinopodium chinense (Benth.) O. Kuntze and their biological activity. Arch. Pharmacal Res. 2017, 41, 1117–1130. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Wang, S.; Luan, H.; Tuerhong, D.; Lin, Y.; Liang, J.; Xiong, Y.; Rui, L.; Wu, F. Clinopodium chinense Attenuates Palmitic Acid-Induced Vascular Endothelial Inflammation and Insulin Resistance through TLR4-Mediated NF-κB and MAPK Pathways. Am. J. Chin. Med. 2019, 47, 97–117. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, F.H.; Su, J.B.; Ma, S.P. Study on antioxidation of active fraction from Clinopodium Chinese in vitro. Strait Pharm. J. 2012, 24, 17–20. [Google Scholar]
- Gao, Y.; Wang, Y.; Wang, K.; Zhu, J.; Li, G.; Tian, J.; Li, C.; Wang, Z.; Li, J.; Lee, A.W.; et al. Acute and a 28-day repeated-dose toxicity study of total flavonoids from Clinopodium chinense (Benth.) O. Ktze in mice and rats. Regul. Toxicol. Pharmacol. 2017, 91, 117–123. [Google Scholar] [CrossRef]
- Zhang, H.J.; Chen, R.C.; Sun, G.B.; Yang, L.P.; Zhu, Y.D.; Xu, X.D.; Sun, X.B. Protective effects of total flavonoids from Clinopodium chinense (Benth.) O. Ktze on myocardial injury in vivo and in vitro via regulation of Akt/Nrf2/HO-1 pathway. Phytomedicine 2018, 40, 88–97. [Google Scholar] [CrossRef]
- Committee Chinese Pharmacopoeia. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2020. [Google Scholar]
- Wu, W.B.; Zhao, C. Determing the content of hesperidin in Clinopodium polycephalum by HPLC. Res. Pract. Chin. Med. 2005, 19, 40–41. [Google Scholar]
- He, B.; Tian, J.; Li, C.H.; Ai, H.B. SPE-HPLC determination of buddlejasaponinsIVb in Duanxueliu capsules. Chin. J. Pharm. Anal. 2008, 28, 1316–1318. [Google Scholar]
- Huang, Y.; Yuan, X.H.; Liu, Z. HPLC Determination of didymin in duanxueliu Herb. J. Anhui Agric. Sci. 2014, 42, 10907–10908. [Google Scholar]
- Kang, N.; Gao, X.Y.; Fan, Q.L.; Lin, Y.; Xiao, W. Determination of Clinodiside A in Clinopodium herb by HPLC. J. China Prescr. Drug 2015, 13, 27–28. [Google Scholar]
- Lin, F.Y.; Sun, Y.X.; Chen, R.Y. Determination of Clinodiside A in Clinopodium oral liquids by HPLC. J. Pharm. Res. 2020, 39, 517–519. [Google Scholar]
- Ren, X.; Shao, X.X.; Li, X.X.; Jia, X.H.; Song, T.; Zhou, W.Y.; Wang, P.; Li, Y.; Wang, X.L.; Cui, Q.H.; et al. Identifying potential treatments of COVID-19 from Traditional Chinese Medicine (TCM) by using a data-driven approach. J. Ethnopharmacol. 2020, 258, 112932. [Google Scholar] [CrossRef]
- Esmaeili, F.; Hashemiravan, M.; Eshaghi, M.R.; Gandomi, H. Optimization of Aqueous Extraction Conditions of Inulin from the Arctium lappa L. Roots Using Ultrasonic Irradiation Frequency. J. Food Qual. 2021, 2021, 5520996. [Google Scholar] [CrossRef]
- Alara, O.R.; Abdurahman, N.H.; Ukaegbu, C.I. Extraction of phenolic compounds: A review. Curr. Res. Food Sci. 2021, 4, 200–214. [Google Scholar] [CrossRef]
- Vuong, Q.V.; Nguyen, V.T.; Thanh, D.T.; Bhuyan, D.J.; Goldsmith, C.D.; Sadeqzadeh, E.; Scarlett, C.J.; Bowyer, M.C. Optimization of ultrasound-assisted extraction conditions for euphol from the medicinal plant, Euphorbia tirucalli, using response surface methodology. Ind. Crop. Prod. 2015, 63, 197–202. [Google Scholar] [CrossRef]
- Jagadeesan, G.; Muniyand, K.; Lydia, M.A.; Nataraj, G.; Thamburaj, S.; Sathyanarayanan, S.; Thangaraj, P. Analysis of discrete and combined effect of solvent, extraction time, and extraction temperature on polyphenol compounds extraction from roxburgh fig (Ficus auriculata Lour.) fruit using response surface methodology. In Phytomedicine Research and Development; CRC Press: Boca Raton, FL, USA, 2020; pp. 27–37. [Google Scholar]
- Subramani, T.; Ganapathyswamy, H.; Sampathrajan, V.; Sundararajan, A. Optimization of extraction parameters to improve cottonseed milk yield and reduce gossypol levels using response surface methodology. J. Food Process. Preserv. 2021, 46, e16247. [Google Scholar] [CrossRef]
- Wang, K.H.; Li, G.Q.; Li, K.M.; Naumovski, V.R.; Chan, K. Optimisation of Pueraria isoflavonoids by response surface methodology using ultrasonic-assisted extraction. Food Chem. 2017, 231, 231–237. [Google Scholar] [CrossRef]
- Elboughdiri, N.; Ghernaout, D.; Kriaa, K.; Jamoussi, B. Enhancing the extraction of phenolic compounds from Juniper Berries using the box-behnken design. ACS Omega 2020, 5, 27990–28000. [Google Scholar] [CrossRef]
- Kowalska, I.; Adach, W.; Stochmal, A.; Olas, B. A comparison of the effects of apigenin and seven of its derivatives on selected biomarkers of oxidative stress and coagulation in vitro. Food Chem. Toxicol. 2020, 136, 111016. [Google Scholar] [CrossRef] [PubMed]
- Xie, M.J.; Yu, Y.T.; Zhu, Z.Y.; Deng, L.P.; Ren, B.; Zhang, M. Simultaneous determination of six main components in Bushen Huoxue prescription by HPLC-CAD. J. Pharm. Biomed. Anal. 2021, 201, 114087. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.X.; Huang, J.H.; Jiang, X.M.; Chen, Y.; Liu, Y.; Zeng, R.; Shehla, N.; Liu, Q.; Liao, D.F.; Guo, D.A.; et al. Quantitative and qualitative determination of LiuweiDihuang preparations by ultra-high-performance liquid chromatography in dual-wavelength fingerprinting mode and random forest. J. Sep. Sci. 2015, 38, 3720–3726. [Google Scholar] [CrossRef]
- Duan, L.; Guo, L.; Liu, K.; Liu, E.H.; Li, P. Characterization and classification of seven Citrus herbs by liquid chromatography–quadrupole time-of-flight mass spectrometry and genetic algorithm optimized support vector machines. J. Chromatogr. A 2014, 1339, 118–127. [Google Scholar] [CrossRef] [PubMed]
- Miladi, M.; Martins, A.A.; Mata, T.M.; Vegara, M.; Pérez-Infantes, M.; Remmani, R.; Ruiz-Canales, A.; Núñez-Gómez, D. Optimization of Ultrasound-Assisted Extraction of Spent Coffee Grounds Oil Using Response Surface Methodology. Processes 2021, 9, 2085. [Google Scholar] [CrossRef]
- Halim, R.; Gladman, B.; Danquah, M.K.; Webley, P.A. Oil extraction from microalgae for biodiesel production. Bioresour. Technol. 2011, 102, 178–185. [Google Scholar] [CrossRef]
- Mason, T.J.; Paniwnyk, L.; Lorimer, J.P. The uses of ultrasound in food technology. Ultrason. Sonochem. 1996, 3, 253–260. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, X.; Fan, E. Optimisation of ultrasound-assisted extraction of puerarin and total isoflavones from Puerariae Lobatae Radix (Pueraria lobata (Wild.) Ohwi) with response surface methodology. Phytochem. Anal. 2011, 23, 513–519. [Google Scholar] [CrossRef]
- Cheok, C.Y.; Chin, N.L.; Yusof, Y.A.; Talib, R.A.; Law, C.L. Optimization of total phenolic content extracted from Garcinia mangostana Linn. hull using response surface methodology versus artificial neural network. Ind. Crops Prod. 2012, 40, 247–253. [Google Scholar] [CrossRef]
| Analytes | Calibration Curves | Linear Range (μg/mL) | R2 | LOQ (μg/mL) | LOD (μg/mL) | Stability (RSD%, n = 6) | Precision (RSD%, n = 6) | Repeatability (RSD%, n = 6) | Average Recovery (%, n = 9) | Average Recovery (RSD%, n = 9) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Y = 13.984X − 3.0103 | 143.00–2.86 | 0.9997 | 0.0091 | 0.0046 | 0.85 | 0.75 | 2.10 | 98.52 | 0.62 |
| 2 | Y = 14.502X − 4.3899 | 1088.00–21.74 | 0.9996 | 0.0150 | 0.0076 | 0.20 | 0.72 | 1.54 | 99.09 | 1.10 |
| 3 | Y = 12.039X − 44.602 | 199.00–3.98 | 0.9995 | 0.0820 | 0.0270 | 1.24 | 0.74 | 1.97 | 101.74 | 0.87 |
| 4 | Y = 17.283X − 34.682 | 195.00–3.70 | 0.9996 | 0.0170 | 0.0065 | 0.66 | 0.78 | 1.82 | 102.62 | 0.87 |
| 5 | Y = 2.4643X − 1.4829 | 160.00–2.60 | 0.9995 | 0.0450 | 0.0180 | 0.45 | 0.70 | 1.57 | 98.51 | 0.42 |
| 6 | Y = 6.3739X − 3.0096 | 57.00–1.14 | 0.9996 | 0.2800 | 0.1400 | 1.97 | 2.89 | 2.52 | 100.04 | 0.55 |
| 7 | Y = 8.5706X + 1.4295 | 1076.00–21.52 | 0.9995 | 0.0160 | 0.0081 | 0.22 | 0.76 | 1.54 | 101.17 | 1.81 |
| 8 | Y = 20.834X − 0.4984 | 70.00–1.40 | 0.9995 | 0.0270 | 0.0130 | 0.65 | 0.94 | 1.08 | 98.28 | 1.03 |
| 9 | Y = 11.296X − 1.6736 | 63.00–1.26 | 0.9996 | 0.0410 | 0.0170 | 0.57 | 0.95 | 2.14 | 99.72 | 1.05 |
| 10 | Y = 14.782X − 4.4511 | 101.00–1.01 | 0.9998 | 0.0140 | 0.0070 | 2.48 | 1.86 | 2.22 | 101.31 | 1.40 |
| 11 | Y = 22.297X − 4.2959 | 100.00–2.00 | 0.9996 | 0.0240 | 0.0089 | 0.21 | 0.74 | 1.03 | 97.30 | 0.85 |
| Run | X1 (Methanol–Water Proportion, %) | X2 (Liquid to Solid Ratio, mL/g) | X3 (Extraction Time, Min) | Y = OD |
|---|---|---|---|---|
| 1 | −1 (50) | −1 (30) | 0 (35) | 0.4249 |
| 2 | 1 (90) | −1 (30) | 0 (35) | 0.0000 |
| 3 | −1 (50) | 1 (70) | 0 (35) | 0.8700 |
| 4 | 1 (90) | 1 (70) | 0 (35) | 0.4947 |
| 5 | −1 (50) | 0 (50) | −1 (20) | 0.6189 |
| 6 | 1 (90) | 0 (50) | −1 (20) | 0.0000 |
| 7 | −1 (50) | 0 (50) | 1 (50) | 0.6671 |
| 8 | 1 (90) | 0 (50) | 1 (50) | 0.3522 |
| 9 | 0 (70) | −1 (30) | −1 (20) | 0.4575 |
| 10 | 0 (70) | 1 (70) | −1 (20) | 0.8231 |
| 11 | 0 (70) | −1 (30) | 1 (50) | 0.6224 |
| 12 | 0 (70) | 1 (70) | 1 (50) | 0.9318 |
| 13 | 0 (70) | 0 (50) | 0 (35) | 0.8038 |
| 14 | 0 (70) | 0 (50) | 0 (35) | 0.8591 |
| 15 | 0 (70) | 0 (50) | 0 (35) | 0.7822 |
| 16 | 0 (70) | 0 (50) | 0 (35) | 0.8030 |
| 17 | 0 (70) | 0 (50) | 0 (35) | 0.7969 |
| Source | Sum of Square | DF | Mean Square | F Value | p Value | Significant |
|---|---|---|---|---|---|---|
| Model | 1.2900 | 9 | 0.1429 | 60.84 | <0.0001 | ** |
| X1 | 0.3758 | 1 | 0.3758 | 160.00 | <0.0001 | ** |
| X2 | 0.3259 | 1 | 0.3259 | 138.76 | <0.0001 | ** |
| X3 | 0.0568 | 1 | 0.0568 | 24.17 | 0.0017 | ** |
| X1X2 | 0.0006 | 1 | 0.0006 | 0.26 | 0.6246 | |
| X1X3 | 0.0231 | 1 | 0.0231 | 9.84 | 0.0165 | * |
| X2X3 | 0.0008 | 1 | 0.0008 | 0.34 | 0.5802 | |
| 0.4596 | 1 | 0.4596 | 195.64 | <0.0001 | ** | |
| 0.0041 | 1 | 0.0041 | 1.75 | 0.2277 | ||
| 0.0201 | 1 | 0.0201 | 8.55 | 0.0222 | * | |
| Residual | 0.0164 | 7 | 0.0023 | |||
| Lack of fit | 0.0130 | 3 | 0.0043 | 5.04 | 0.0760 | |
| Pure error | 0.0034 | 4 | 0.0009 | |||
| Cor Total | 1.3000 | 16 |
| No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| S1/June | 2.520 ± 0.045 | 10.006 ± 0.249 | 3.878 ± 0.090 | 4.609 ± 0.080 | 5.619 ± 0.134 | 0.126 ± 0.005 | 23.821 ± 0.557 | 0.122 ± 0.010 | 0.671 ± 0.018 | 0.060 ± 0.001 | 0.337 ± 0.008 |
| S1/July | 1.503 ± 0.098 | 11.545 ± 0.123 | 5.816 ± 0.089 | 7.119 ± 0.018 | 6.206 ± 0.075 | 0.122 ± 0.006 | 27.044 ± 0.297 | 0.048 ± 0.002 | 1.853 ± 0.037 | 0.067 ± 0.000 | 0.089 ± 0.000 |
| S1/August | 1.906 ± 0.093 | 9.543 ± 0.272 | 1.672 ± 0.138 | 4.462 ± 0.133 | 3.953 ± 0.118 | 0.256 ± 0.006 | 15.169 ± 0.434 | 0.046 ± 0.001 | 1.217 ± 0.036 | 0.051 ± 0.001 | 0.154 ± 0.009 |
| S2/June | 2.505 ± 0.127 | 18.100 ± 0.935 | 3.696 ± 0.186 | 5.272 ± 0.271 | 8.138 ± 0.443 | 0.172 ± 0.013 | 35.824 ± 1.670 | 0.184 ± 0.017 | 0.448 ± 0.045 | 0.055 ± 0.003 | 0.413 ± 0.020 |
| S2/July | 1.584 ± 0.088 | 23.221 ± 0.671 | 4.052 ± 0.234 | 6.413 ± 0.265 | 6.111 ± 0.387 | 0.106 ± 0.008 | 48.868 ± 1.355 | 0.311 ± 0.017 | 1.481 ± 0.050 | 0.074 ± 0.002 | 0.711 ± 0.035 |
| S2/August | 1.016 ± 0.062 | 5.474 ± 0.331 | 2.157 ± 0.094 | 3.575 ± 0.134 | 0.819 ± 0.066 | 1.128 ± 0.096 | 12.683 ± 0.687 | 0.562 ± 0.011 | 0.134 ± 0.011 | 0.054 ± 0.003 | 0.215 ± 0.013 |
| S3/June | 1.579 ± 0.010 | 8.954 ± 0.083 | 4.731 ± 0.194 | 2.635 ± 0.036 | 5.089 ± 0.059 | 0.090 ± 0.003 | 25.190 ± 0.224 | 0.306 ± 0.007 | 1.706 ± 0.016 | 0.043 ± 0.000 | 1.509 ± 0.019 |
| S3/July | 1.405 ± 0.166 | 8.348 ± 0.870 | 4.436 ± 0.330 | 5.75 ± 0.449 | 5.184 ± 0.158 | 0.209 ± 0.011 | 27.809 ± 2.967 | 0.116 ± 0.009 | 3.189 ± 0.383 | 0.052 ± 0.003 | 0.384 ± 0.042 |
| S3/August | 2.510 ± 0.153 | 7.620 ± 0.471 | 4.419 ± 0.275 | 4.322 ± 0.203 | 1.406 ± 0.124 | 4.675 ± 0.218 | 25.324 ± 1.638 | 0.652 ± 0.004 | 0.120 ± 0.009 | 0.052 ± 0.002 | 0.546 ± 0.036 |
| S4/June | 3.601 ± 0.045 | 17.075 ± 0.163 | 4.202 ± 0.027 | 6.759 ± 0.080 | 10.382 ± 0.13 | 0.208 ± 0.015 | 32.302 ± 0.280 | 0.169 ± 0.011 | 0.841 ± 0.012 | 0.052 ± 0.001 | 0.456 ± 0.008 |
| S4/July | 3.097 ± 0.085 | 15.702 ± 0.431 | 3.417 ± 0.074 | 7.303 ± 0.177 | 9.823 ± 0.230 | 0.253 ± 0.011 | 28.860 ± 0.745 | 0.066 ± 0.005 | 0.923 ± 0.020 | 0.059 ± 0.002 | 0.076 ± 0.001 |
| S4/August | 2.366 ± 0.026 | 10.156 ± 0.057 | 3.967 ± 0.023 | 6.189 ± 0.018 | 9.255 ± 0.082 | 0.710 ± 0.006 | 19.261 ± 0.090 | 0.100 ± 0.002 | 1.429 ± 0.022 | 0.046 ± 0.001 | 0.274 ± 0.005 |
| S5/June | 3.010 ± 0.101 | 15.289 ± 0.487 | 4.704 ± 0.119 | 7.916 ± 0.159 | 10.183 ± 0.222 | 0.138 ± 0.024 | 37.880 ± 1.148 | 0.123 ± 0.007 | 0.872 ± 0.094 | 0.065 ± 0.000 | 0.344 ± 0.010 |
| S5/July | 2.164 ± 0.053 | 14.735 ± 0.367 | 4.624 ± 0.111 | 8.774 ± 0.163 | 6.859 ± 0.075 | 0.161 ± 0.010 | 35.326 ± 0.867 | 0.072 ± 0.003 | 1.446 ± 0.022 | 0.070 ± 0.001 | 0.150 ± 0.047 |
| S5/August | 1.385 ± 0.068 | 8.834 ± 0.938 | 3.492 ± 0.366 | 6.162 ± 0.391 | 7.683 ± 0.049 | 0.747 ± 0.048 | 24.080 ± 2.453 | 0.092 ± 0.010 | 0.647 ± 0.067 | 0.062 ± 0.003 | 0.410 ± 0.047 |
| S6/June | 3.250 ± 0.146 | 20.325 ± 0.888 | 5.630 ± 0.214 | 5.728 ± 0.180 | 7.655 ± 0.376 | 0.115 ± 0.008 | 35.405 ± 1.527 | 0.161 ± 0.005 | 1.26 ± 0.031 | 0.051 ± 0.003 | 0.301 ± 0.012 |
| S6/July | 1.589 ± 0.025 | 17.057 ± 0.208 | 3.723 ± 0.047 | 5.411 ± 0.099 | 6.784 ± 0.033 | 0.138 ± 0.013 | 33.067 ± 0.381 | 0.131 ± 0.003 | 2.280 ± 0.020 | 0.063 ± 0.000 | 0.236 ± 0.003 |
| S6/August | 1.425 ± 0.020 | 8.137 ± 0.075 | 2.705 ± 0.012 | 4.673 ± 0.059 | 7.977 ± 0.146 | 0.395 ± 0.001 | 14.077 ± 0.150 | 0.100 ± 0.004 | 1.483 ± 0.014 | 0.063 ± 0.000 | 0.188 ± 0.002 |
| S7/June | 1.413 ± 0.025 | 9.459 ± 0.098 | 4.589 ± 0.058 | 4.060 ± 0.061 | 7.200 ± 0.098 | 0.102 ± 0.006 | 23.080 ± 0.269 | 0.089 ± 0.001 | 1.533 ± 0.031 | 0.055 ± 0.001 | 0.220 ± 0.004 |
| S7/July | 0.637 ± 0.015 | 6.241 ± 0.176 | 2.655 ± 0.062 | 3.788 ± 0.133 | 4.341 ± 0.118 | 0.115 ± 0.004 | 19.078 ± 0.519 | 0.091 ± 0.006 | 1.880 ± 0.048 | 0.045 ± 0.001 | 0.485 ± 0.011 |
| S7/August | 1.200 ± 0.063 | 9.643 ± 0.460 | 3.632 ± 0.106 | 5.167 ± 0.166 | 6.812 ± 0.143 | 0.502 ± 0.017 | 27.336 ± 1.267 | 0.162 ± 0.013 | 1.382 ± 0.068 | 0.064 ± 0.002 | 0.553 ± 0.022 |
| S8/June | 2.149 ± 0.112 | 18.056 ± 0.987 | 3.824 ± 0.162 | 6.046 ± 0.253 | 9.047 ± 0.199 | 0.308 ± 0.011 | 29.040 ± 1.603 | 0.145 ± 0.007 | 0.618 ± 0.027 | 0.063 ± 0.002 | 0.282 ± 0.017 |
| S8/July | 1.038 ± 0.019 | 9.945 ± 0.165 | 3.337 ± 0.124 | 4.835 ± 0.081 | 5.060 ± 0.087 | 0.205 ± 0.016 | 29.470 ± 0.434 | 0.095 ± 0.005 | 1.120 ± 0.043 | 0.067 ± 0.001 | 0.336 ± 0.007 |
| S8/August | 1.345 ± 0.029 | 8.745 ± 0.328 | 2.886 ± 0.064 | 5.338 ± 0.130 | 7.058 ± 0.102 | 0.579 ± 0.006 | 18.646 ± 0.682 | 0.073 ± 0.013 | 1.357 ± 0.062 | 0.056 ± 0.001 | 0.200 ± 0.010 |
| Independent Variables | Levels | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| Methanol–water proportion (X1) (%) | 50 | 70 | 90 |
| Liquid to solid ratio (X2) (mL/g) | 30 | 50 | 70 |
| Extraction time (X3) (min) | 20 | 35 | 50 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Song, X.; Shen, X.; Xiong, Y.; Liu, L.; Yang, Y.; Nian, S.; Liu, L. A Rapid and Efficient Strategy for Quality Control of Clinopodii herba Encompassing Optimized Ultrasound-Assisted Extraction Coupled with Sensitive Variable Wavelength Detection. Molecules 2022, 27, 4418. https://doi.org/10.3390/molecules27144418
Liu Y, Song X, Shen X, Xiong Y, Liu L, Yang Y, Nian S, Liu L. A Rapid and Efficient Strategy for Quality Control of Clinopodii herba Encompassing Optimized Ultrasound-Assisted Extraction Coupled with Sensitive Variable Wavelength Detection. Molecules. 2022; 27(14):4418. https://doi.org/10.3390/molecules27144418
Chicago/Turabian StyleLiu, Yao, Xiaojun Song, Xuebin Shen, Yuangen Xiong, Li Liu, Yuexi Yang, Sihui Nian, and Limin Liu. 2022. "A Rapid and Efficient Strategy for Quality Control of Clinopodii herba Encompassing Optimized Ultrasound-Assisted Extraction Coupled with Sensitive Variable Wavelength Detection" Molecules 27, no. 14: 4418. https://doi.org/10.3390/molecules27144418
APA StyleLiu, Y., Song, X., Shen, X., Xiong, Y., Liu, L., Yang, Y., Nian, S., & Liu, L. (2022). A Rapid and Efficient Strategy for Quality Control of Clinopodii herba Encompassing Optimized Ultrasound-Assisted Extraction Coupled with Sensitive Variable Wavelength Detection. Molecules, 27(14), 4418. https://doi.org/10.3390/molecules27144418





