Lanthanide Molecular Species Generated Fe3O4@SiO2-TbDPA Nanosphere for the Efficient Determination of Nitrite
Abstract
:1. Introduction
2. Experimental Section
2.1. Reagents and Materials
2.2. Characterization
2.3. Preparation of Fe3O4@SiO2-NH2 Nanospheres
2.4. Preparation of Fe3O4@SiO2-DPA Nanospheres
2.5. Fabrication of Terbium Hybrid Materials (Fe3O4@SiO2-TbDPA)
2.6. Optical Studies
3. Results and Discussion
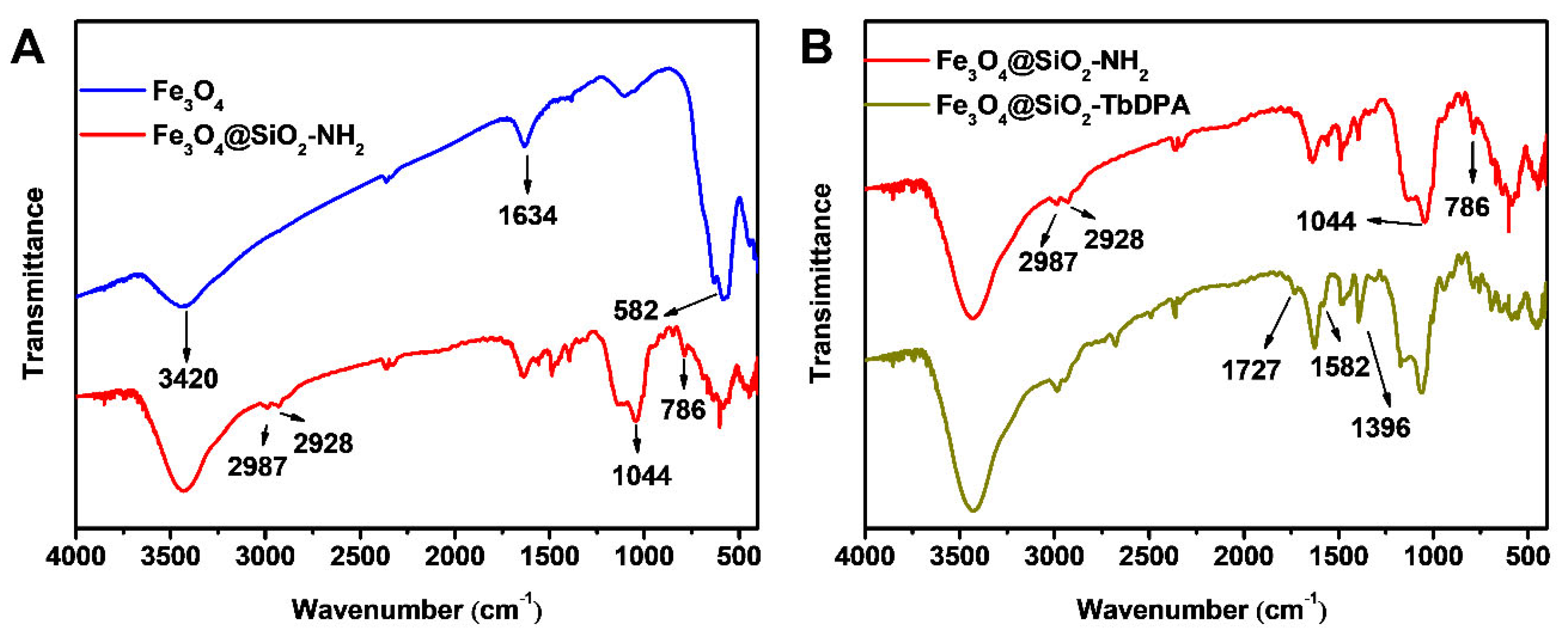
3.1. FT-IR Analysis
3.2. UV-Vis Analysis
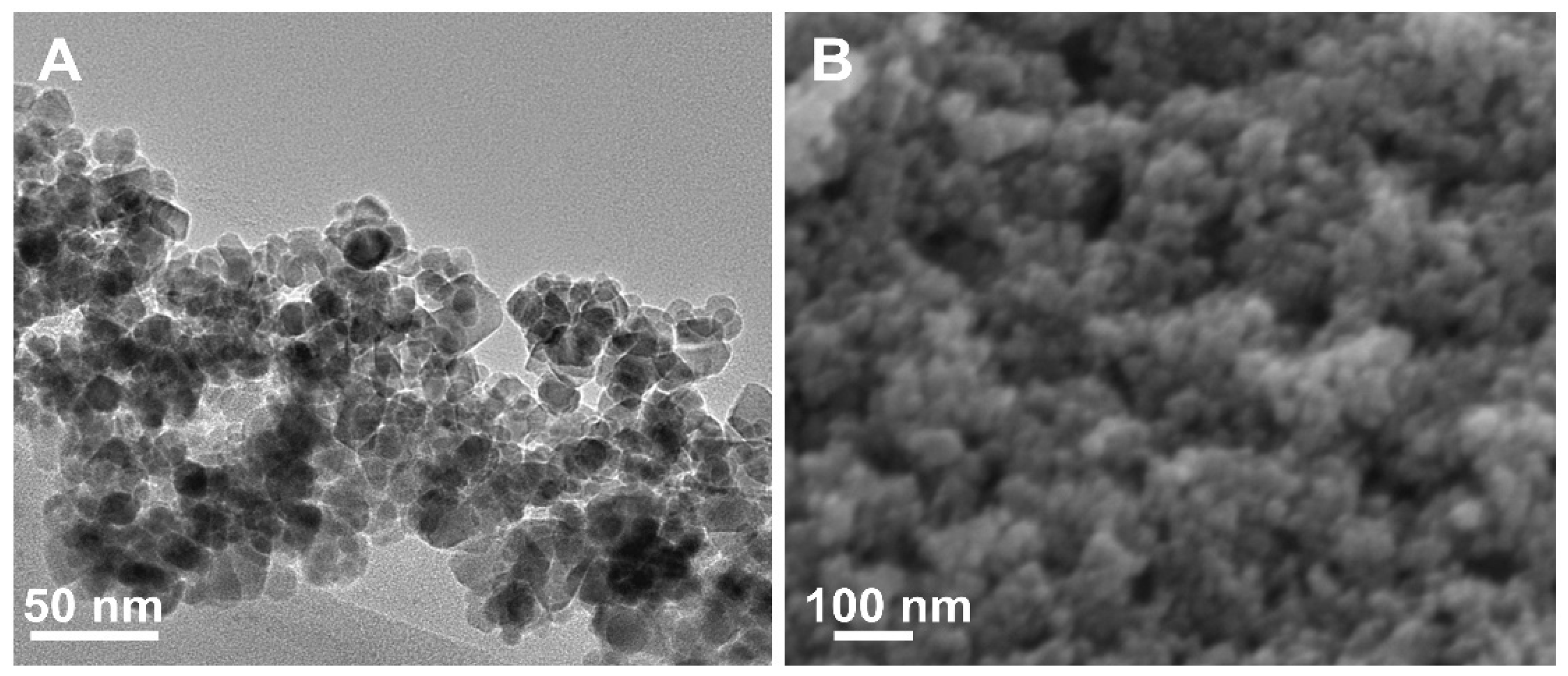
3.3. Morphological Analysis
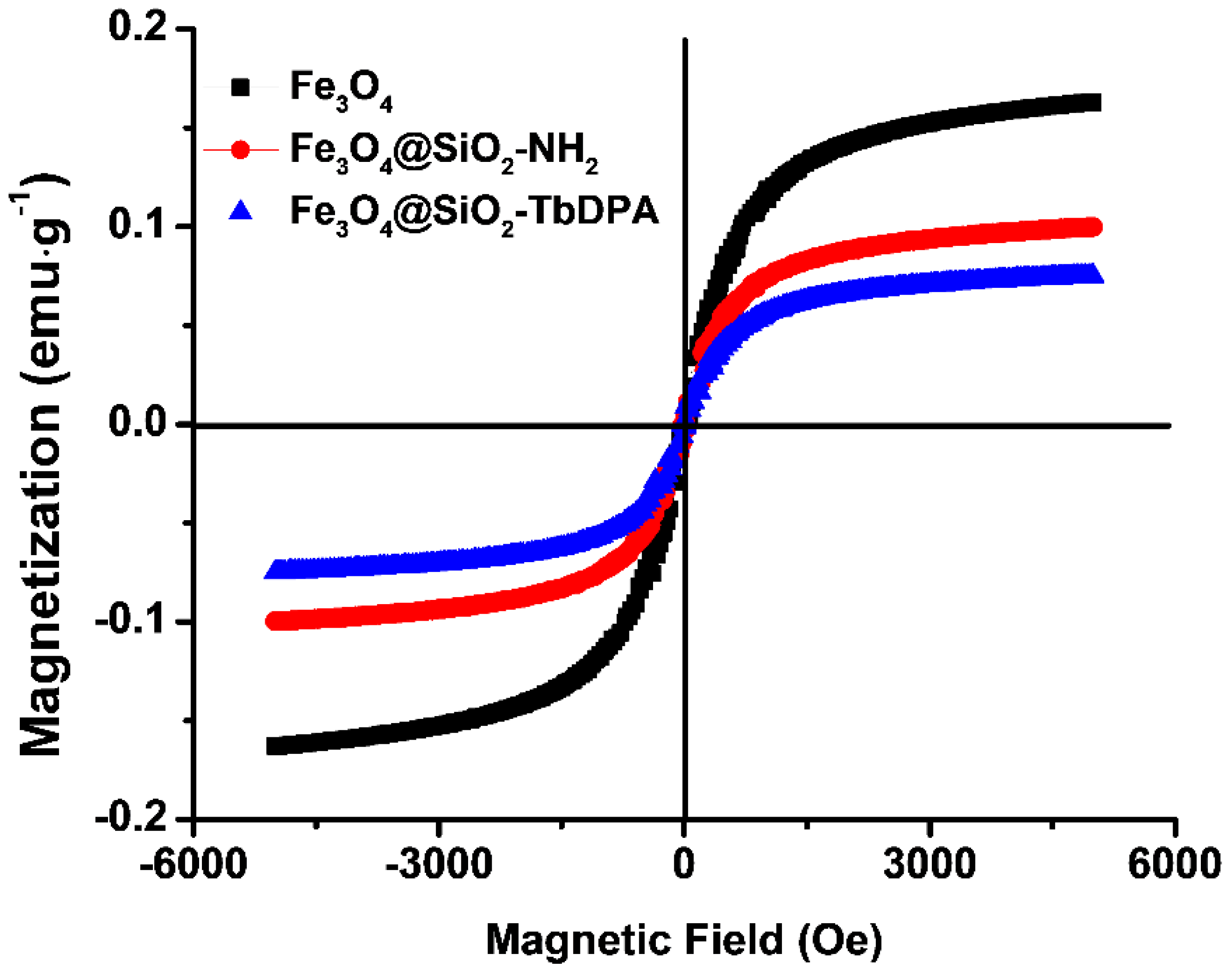
3.4. Magnetic Properties
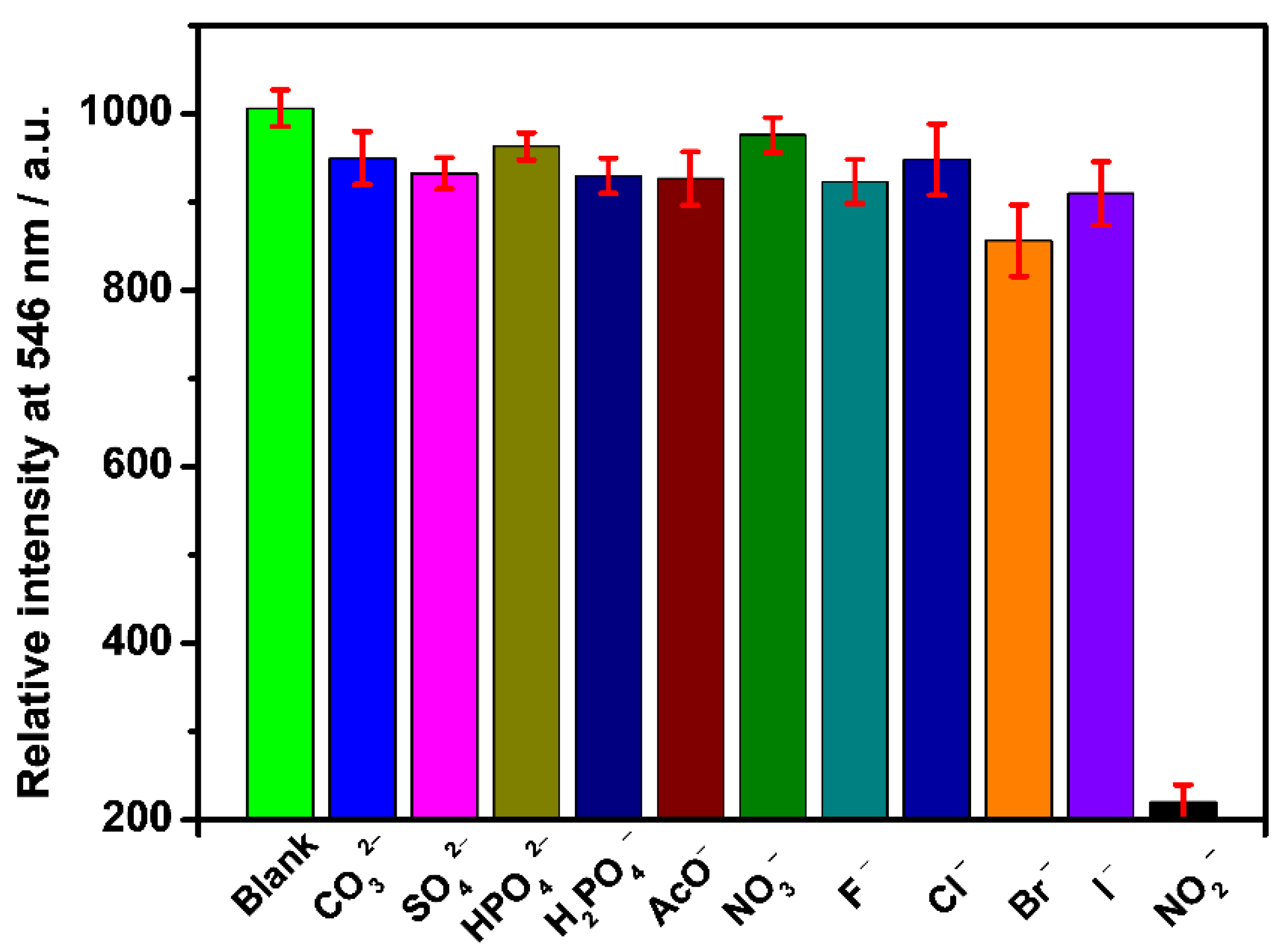
3.5. Selective and Sensitive Detecting NO2−
3.6. Detection of Nitrite Ions in Tap Water Sample
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Li, W.; Shi, Y.; Hu, X.; Li, Z.; Huang, X.; Holmes, M.; Gong, Y.; Shi, J.; Zou, X. Visual detection of nitrite in sausage based on a ratiometric fluorescent system. Food Control 2019, 106, 106704. [Google Scholar] [CrossRef]
- Feng, Z.; Li, Z.; Zhang, X.; Shi, Y.; Zhou, N. Nitrogen-doped carbon quantum dots as fluorescent probes for sensitive and selective detection of nitrite. Molecules 2017, 22, 2061. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, H.; Han, Z.; Wang, M.; Li, Y.; Zhou, T.; Shi, W.; Cheng, P. A water-stable terbium metal–organic framework as a highly sensitive fluorescent sensor for nitrite. Inorg. Chem. Front. 2020, 7, 3379–3385. [Google Scholar] [CrossRef]
- Ma, Z.; Li, J.; Hu, X.; Cai, Z.; Dou, X. Ultrasensitive, specific, and rapid fluorescence turn-on nitrite sensor enabled by precisely modulated fluorophore binding. Adv. Sci. 2020, 7, 2002991. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Pang, S.; He, L.; Nugen, S.R. Highly sensitive and selective detection of nitrite ions using Fe3O4@SiO2/Au magnetic nanoparticles by surface-enhanced Raman spectroscopy. Biosens. Bioelectron. 2016, 85, 726–733. [Google Scholar] [CrossRef] [Green Version]
- Jayawardane, B.M.; Wei, S.; McKelvie, I.D.; Kolev, S.D. Microfluidic paper-based analytical device for the determination of nitrite and nitrate. Anal. Chem. 2014, 86, 7274–7279. [Google Scholar] [CrossRef]
- Rahim, A.; Santos, L.S.S.; Barros, S.B.A.; Kubota, L.T.; Landers, R.; Gushikem, Y. Electrochemical detection of nitrite in meat and water samples using a mesoporous carbon ceramic SiO2/C electrode modified with in situ generated manganese(II) phthalocyanine. Electroanalysis 2014, 26, 541–547. [Google Scholar] [CrossRef]
- Matteo, V.D.; Esposito, E. Methods for the determination of nitrite by high-performance liquid chromatography with electrochemical detection. J. Chromatogr. A 1997, 789, 213–219. [Google Scholar] [CrossRef]
- Wang, B.; Zheng, S.; Huang, Y.; Wang, Y.; Zhu, Z.; Ma, R.; Zhao, Y.; Yin, X.; Su, J.; Xiong, J.; et al. Novel GC/Py/GC/IRMS-based method for isotope measurements of nitrate and nitrite. I: Converting nitrate to benzyl nitrate for delta(18)O analysis. Anal. Chem. 2020, 92, 12216–12225. [Google Scholar] [CrossRef]
- Moravský, L.; Troška, P.; Klas, M.; Masár, M.; Matejčík, Š. Determination of nitrites and nitrates in plasma-activated deionized water by microchip capillary electrophoresis. Contrib. Plasm. Phys. 2020, 60, e202000014. [Google Scholar] [CrossRef]
- Vilian, A.T.E.; Umapathi, R.; Hwang, S.K.; Huh, Y.S.; Han, Y.K. Pd-Cu nanospheres supported on Mo2C for the electrochemical sensing of nitrites. J. Hazard. Mater. 2021, 408, 124914. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Tong, C. Dual-emission fluorescent probe for the simultaneous detection of nitrite and mercury(II) in environmental water samples based on the Tb3+-modified carbon quantum dot/3-aminophenylboronic acid hybrid. Anal. Chem. 2020, 92, 8859–8866. [Google Scholar] [CrossRef]
- Zheng, X.J.; Liang, R.P.; Li, Z.J.; Zhang, L.; Qiu, J.D. One-step, stabilizer-free and green synthesis of Cu nanoclusters as fluorescent probes for sensitive and selective detection of nitrite ions. Sens. Actuators B Chem. 2016, 230, 314–319. [Google Scholar] [CrossRef]
- Wang, L.; Jana, J.; Chung, J.S.; Choi, W.M.; Hur, S.H. Designing an intriguingly fluorescent N, B-doped carbon dots based fluorescent probe for selective detection of NO2(−) ions. Spectrochim. Acta. Part A Molecul. Biomol. Spectrosc. 2022, 268, 120657. [Google Scholar] [CrossRef]
- Gu, J.; Zhang, F.; Zheng, Z.; Li, X.; Deng, R.; Zhou, Z.; Ma, L.; Liu, W.; Wang, Q. Establishment of a new molecular model for mercury determination verified by single crystal X-ray diffraction, spectroscopic analysis and biological potentials. Chin. Chem. Lett. 2021, 32, 87–91. [Google Scholar] [CrossRef]
- He, W.M.; Zhou, Z.; Han, Z.; Li, S.; Zhou, Z.; Ma, L.F.; Zang, S.Q. Ultrafast size expansion and turn-on luminescence of atomically precise silver clusters by hydrogen sulfide. Angew. Chem. Int. Ed. 2021, 60, 8505–8509. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.Q.; Sun, W.J.; Wang, Q.M. Exploration of sulfur-containing nanoparticles: Synthesis, microstructure analysis, and sensing potential. Inorg. Chem. 2022, 61, 4159–4170. [Google Scholar] [CrossRef]
- Paderni, D.; Giorgi, L.; Fusi, V.; Formica, M.; Ambrosi, G.; Micheloni, M. Chemical sensors for rare earth metal ions. Coordin. Chem. Rev. 2021, 429, 213639. [Google Scholar] [CrossRef]
- Na, M.; Zhang, S.; Liu, J.; Ma, S.; Han, Y.; Wang, Y.; He, Y.; Chen, H.; Chen, X. Determination of pathogenic bacteria-Bacillus anthrax spores in environmental samples by ratiometric fluorescence and test paper based on dual-emission fluorescent silicon nanoparticles. J. Hazard. Mater. 2020, 386, 121956. [Google Scholar] [CrossRef]
- Aleem, A.R.; Liu, J.; Wang, J.; Wang, J.; Zhao, Y.; Wang, Y.; Wang, Y.; Wang, W.; Rehman, F.U.; Kipper, M.J.; et al. Selective sensing of Cu2+ and Fe3+ ions with vis-excitation using fluorescent Eu3+-induced aggregates of polysaccharides (EIAP) in mammalian cells and aqueous systems. J. Hazard. Mater. 2020, 399, 122991. [Google Scholar] [CrossRef]
- Wang, J.; Li, D.; Ye, Y.; Qiu, Y.; Liu, J.; Huang, L.; Liang, B.; Chen, B. A Fluorescent metal-organic framework for food real-time visual monitoring. Adv. Mater. 2021, 33, e2008020. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fang, L.; Jiang, K.; He, H.; Yang, Y.; Cui, Y.; Li, B.; Qian, G. Nanoscale fluorescent metal-organic framework composites as a logic platform for potential diagnosis of asthma. Biosens. Bioelectron. 2019, 130, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Yan, B. Lanthanide-functionalized metal-organic framework hybrid systems to create multiple luminescent centers for chemical sensing. Acc. Chem. Res. 2017, 50, 2789–2798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yuan, J. Responsive metal complex probes for time-gated luminescence biosensing and imaging. Acc. Chem. Res. 2020, 53, 1316–1329. [Google Scholar] [CrossRef]
- Ren, J.; Niu, Z.; Ye, Y.; Tsai, C.; Liu, S.; Liu, Q.; Huang, X.; Nafady, A.; Ma, S. Second-sphere interaction promoted turn-on fluorescence for selective sensing of organic amines in a Tb(III) framework. Angew. Chem. Int. Ed. 2021, 60, 23705–23712. [Google Scholar] [CrossRef]
- Sun, Z.; Huang, H.; Zhang, R.; Yang, X.; Yang, H.; Li, C.; Zhang, Y.; Wang, Q. Activatable rare earth near-infrared-II fluorescence ratiometric nanoprobes. Nano Lett. 2021, 21, 6576–6583. [Google Scholar] [CrossRef]
- Koo, T.M.; Ko, M.J.; Park, B.C.; Kim, M.S.; Kim, Y.K. Fluorescent detection of dipicolinic acid as a biomarker in bacterial spores employing terbium ion-coordinated magnetite nanoparticles. J. Hazard. Mater. 2021, 408, 124870. [Google Scholar] [CrossRef]
- Wang, Q.; Tan, C.; Chen, H.; Tamiaki, H. A new fluoride luminescence quencher based on a nanostructured covalently bonded terbium hybrid material. J. Phys. Chem. C 2010, 114, 13879–13883. [Google Scholar] [CrossRef]
- Tan, C.; Wang, Q. Reversible terbium luminescent polyelectrolyte hydrogels for detection of H2PO4− and HSO4− in water. Inorg. Chem. 2011, 50, 2953–2956. [Google Scholar] [CrossRef]
- Li, X.; Gu, J.; Zhou, Z.; Ma, L.; Tang, Y.; Gao, J.; Wang, Q. New lanthanide ternary complex system in electrospun nanofibers: Assembly, physico-chemical property and sensor application. Chem. Eng. J. 2019, 358, 67–73. [Google Scholar] [CrossRef]
- Zhou, Z.; Li, X.; Tang, Y.; Zhang, C.C.; Fu, H.; Wu, N.; Ma, L.; Gao, J.; Wang, Q. Oxidative deoximation reaction induced recognition of hypochlorite based on a new fluorescent lanthanide-organic framework. Chem. Eng. J. 2018, 351, 364–370. [Google Scholar] [CrossRef]
- Zhu, C.; Zhang, M.; Qiao, Y.; Xiao, G.; Zhang, G.F.; Chen, Y. Fe3O4/TiO2 core/shell nanotubes: Synthesis and magnetic and electromagnetic wave absorption characteristics. J. Phys. Chem. C 2010, 114, 16229–16235. [Google Scholar] [CrossRef]
- Chen, M.; Zheng, Y.; Gao, J.; Li, C.; Yu, C.; Wang, Q. Fluorometric determination of dopamine by using a terbium (III) inorganic-organic network. Microchim. Acta 2017, 184, 2275–2280. [Google Scholar] [CrossRef]
- Zhang, J.L.; Srivastava, R.S.; Misra, R.D.K. Core-shell magnetite nanoparticles surface encapsulated with smart stimuli-responsive polymer: Synthesis, characterization, and LCST of viable drug-targeting delivery system. Langmuir 2007, 23, 6342–6351. [Google Scholar] [CrossRef]
- Sundrarajan, M.; Ramalakshmi, M. Novel cubic magnetite nanoparticle synthesis using room temperature ionic liquid. E-J. Chem. 2012, 9, 1070–1076. [Google Scholar] [CrossRef]
- Du, N.; Xu, Y.; Zhang, H.; Zhai, C.; Yang, D. Selective synthesis of Fe2O3 and Fe3O4 nanowires via a single precursor: A general method for metal oxide nanowires. Nanoscale Res. Lett. 2010, 5, 1295–1300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, N.; Tang, Y.; Zeng, M.; Gao, J.; Lu, X.; Zheng, Y. Design of hybrid inorganic-organic nanosensor based on Fe3O4 as the core and recovery features. J. Lumin. 2018, 202, 502–507. [Google Scholar] [CrossRef]
- Zhao, X.; He, X.; Hou, A.; Cheng, C.; Wang, X.; Yue, Y.; Wu, Z.; Wu, H.; Liu, B.; Li, H.; et al. Growth of Cu2O nanoparticles on two-dimensional Zr-ferrocene-metal-organic framework nanosheets for photothermally enhanced chemodynamic antibacterial therapy. Inorg. Chem. 2022, 61, 9328–9338. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Samiei, M.; Joo, S.; Anzaby, M.; Hanifehpour, Y.; Nasrabadi, H.; Davaran, S. Synthesis, characterization and in vitro studies of doxorubicin-loaded magnetic nanoparticles grafted to smart copolymers on A549 lung cancer cell line. J. NanoBiotechnol. 2012, 10, 46. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Gao, P.; Wang, R.; Zhu, C.; Wang, L.; Cao, M.; Jin, H. Porous Fe3O4/SnO2 core/shell nanorods: Synthesis and electromagnetic properties. J. Phys. Chem. C 2009, 113, 10061–10064. [Google Scholar] [CrossRef]
- Bai, R.B.; Wang, J.F.; Wang, D.G.; Cui, J.J.; Zhang, Y.Q. Recovery of lithium from high Mg/Li ratio salt-lake brines using ion-exchange with NaNTf2 and TBP. Hydrometallurgy 2022, 213, 105914. [Google Scholar] [CrossRef]
- Liu, Z.; Mao, H. Towards fluorescent magnetic core shell composites for nitrite optical sensing. J. Lumin. 2017, 190, 179–187. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Z.; Li, B.; Zhang, L.; Fan, D.; Ma, H. Recyclable magnetic mesoporous nanocomposite with improved sensing performance toward nitrite. ACS Appl. Mater. Interfaces 2016, 8, 12344–12351. [Google Scholar] [CrossRef] [PubMed]
- Jia, J.; Lu, W.; Li, L.; Jiao, Y.; Gao, Y.F.; Shuang, S. Orange luminescent carbon dots as fluorescent probe for detection of nitrite. Chin. J. Anal. Chem. 2019, 47, 560–566. [Google Scholar] [CrossRef]
- Jin, L.; Wang, Y.; Liu, F.; Yu, S.; Gao, Y.; Zhang, J. The determination of nitrite by a graphene quantum dot fluorescence quenching method without sample pretreatment. Luminescence 2018, 33, 289–296. [Google Scholar] [CrossRef]
- Deng, Y.; Qian, J.; Zhou, Y.; Niu, Y. Preparation of N/S doped carbon dots and their application in nitrite detection. RSC Adv. 2021, 11, 10922–10928. [Google Scholar] [CrossRef]
- Hao, X.; Liang, Y.; Zhen, H.; Sun, X.; Liu, X.; Li, M.; Shen, A.; Yang, Y. Fast and sensitive fluorescent detection of nitrite based on an amino-functionalized MOFs of UiO-66-NH2. J. Solid State Chem. 2020, 287, 121323. [Google Scholar] [CrossRef]
- Qiao, G.X.; Liu, L.; Hao, X.X.; Zheng, J.K.; Liu, W.Q.; Gao, J.W.; Zhang, C.C.; Wang, Q.M. Signal transduction from small particles: Sulfur nanodots featuring mercury sensing, cell entry mechanism and in vitro tracking performance. Chem. Eng. J. 2020, 382, 122907. [Google Scholar] [CrossRef]
- Wu, N.T.; Wen, Q.; Wang, Q. Single optical sensor to multiple functions: Ratiometric sensing for SO32− and dual signal determination for copper (II). Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 249, 119219. [Google Scholar] [CrossRef]
- Wen, Q.; Zheng, Y.H.; Liu, W.Q.; Wang, Q.M. Stepwise assembly protocols for the rational design of lanthanide functionalized carbon dots-hydrogel and its sensing evaluation. J. Fluores. 2021, 31, 695–702. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.; Wen, Q.; Chen, J.; Sun, W.; Zheng, Y.; Long, C.; Wang, Q. Lanthanide Molecular Species Generated Fe3O4@SiO2-TbDPA Nanosphere for the Efficient Determination of Nitrite. Molecules 2022, 27, 4431. https://doi.org/10.3390/molecules27144431
Li X, Wen Q, Chen J, Sun W, Zheng Y, Long C, Wang Q. Lanthanide Molecular Species Generated Fe3O4@SiO2-TbDPA Nanosphere for the Efficient Determination of Nitrite. Molecules. 2022; 27(14):4431. https://doi.org/10.3390/molecules27144431
Chicago/Turabian StyleLi, Xiangqian, Qin Wen, Jiannian Chen, Wenjie Sun, Yuhui Zheng, Chenggang Long, and Qianming Wang. 2022. "Lanthanide Molecular Species Generated Fe3O4@SiO2-TbDPA Nanosphere for the Efficient Determination of Nitrite" Molecules 27, no. 14: 4431. https://doi.org/10.3390/molecules27144431




