Preparation of Polycarbonate-ZnO Nanocomposite Films: Surface Investigation after UV Irradiation
Abstract
:1. Introduction
2. Experimental
2.1. Materials and Methods
2.2. Synthesis of PC-ZnO Nanocomposite Films
3. Results and Discussion
3.1. XRD Characterization of ZnO NPs, and PC-ZnO Films
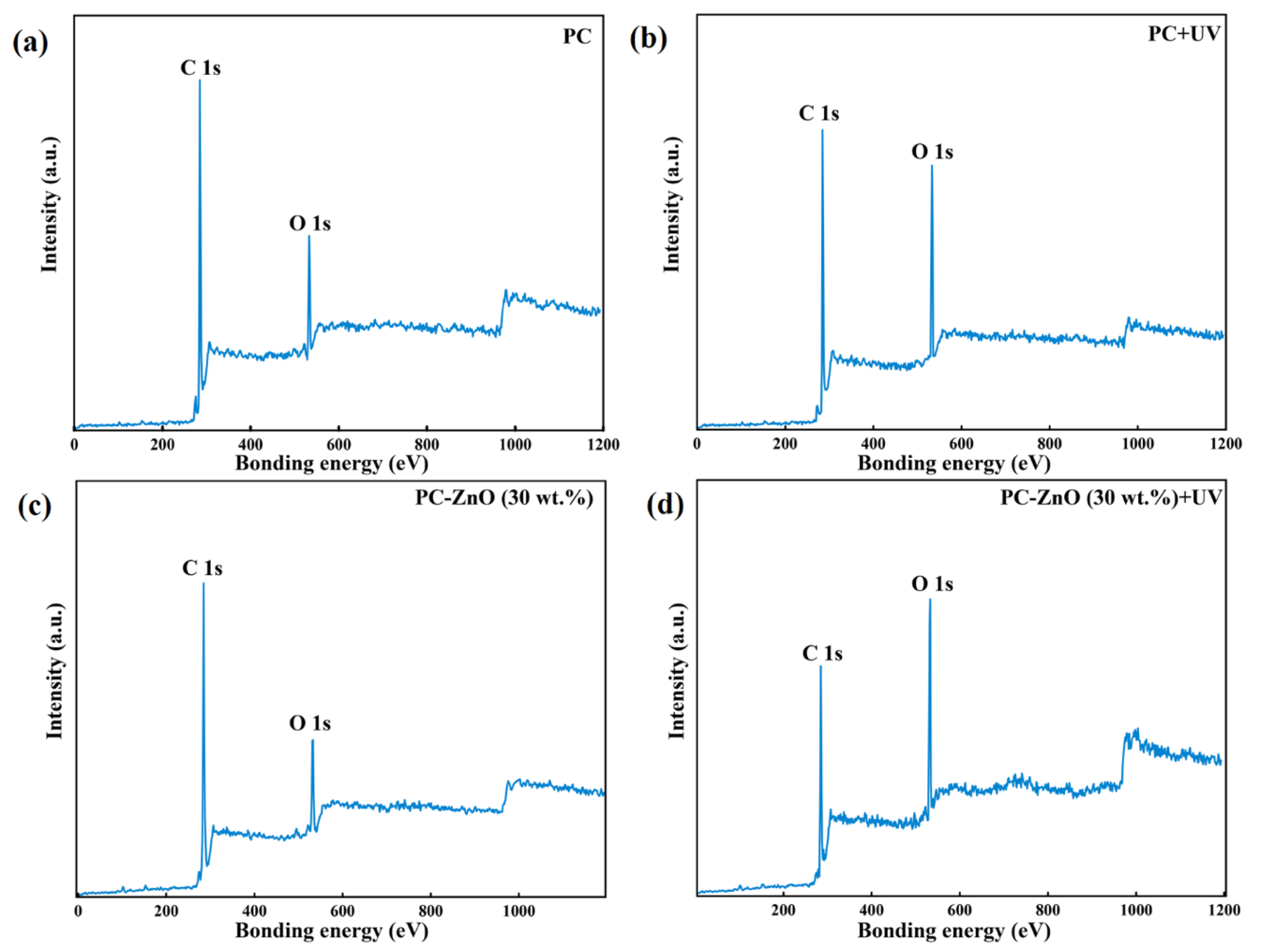
3.2. X-ray Photoelectron Spectroscopy (XPS) Analysis
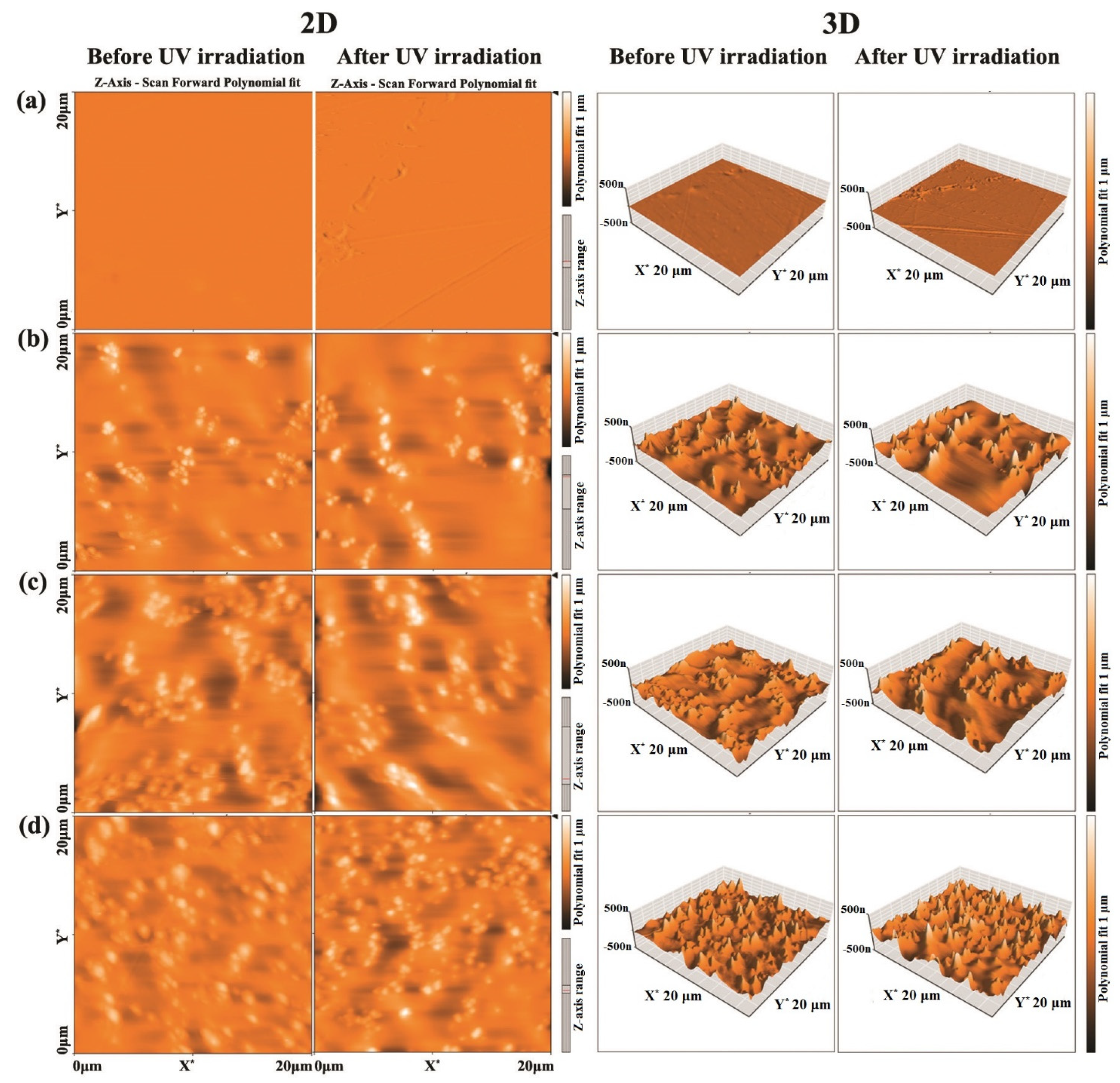
3.3. AFM Measurements
3.4. Surface Wettability
3.5. Microhardness
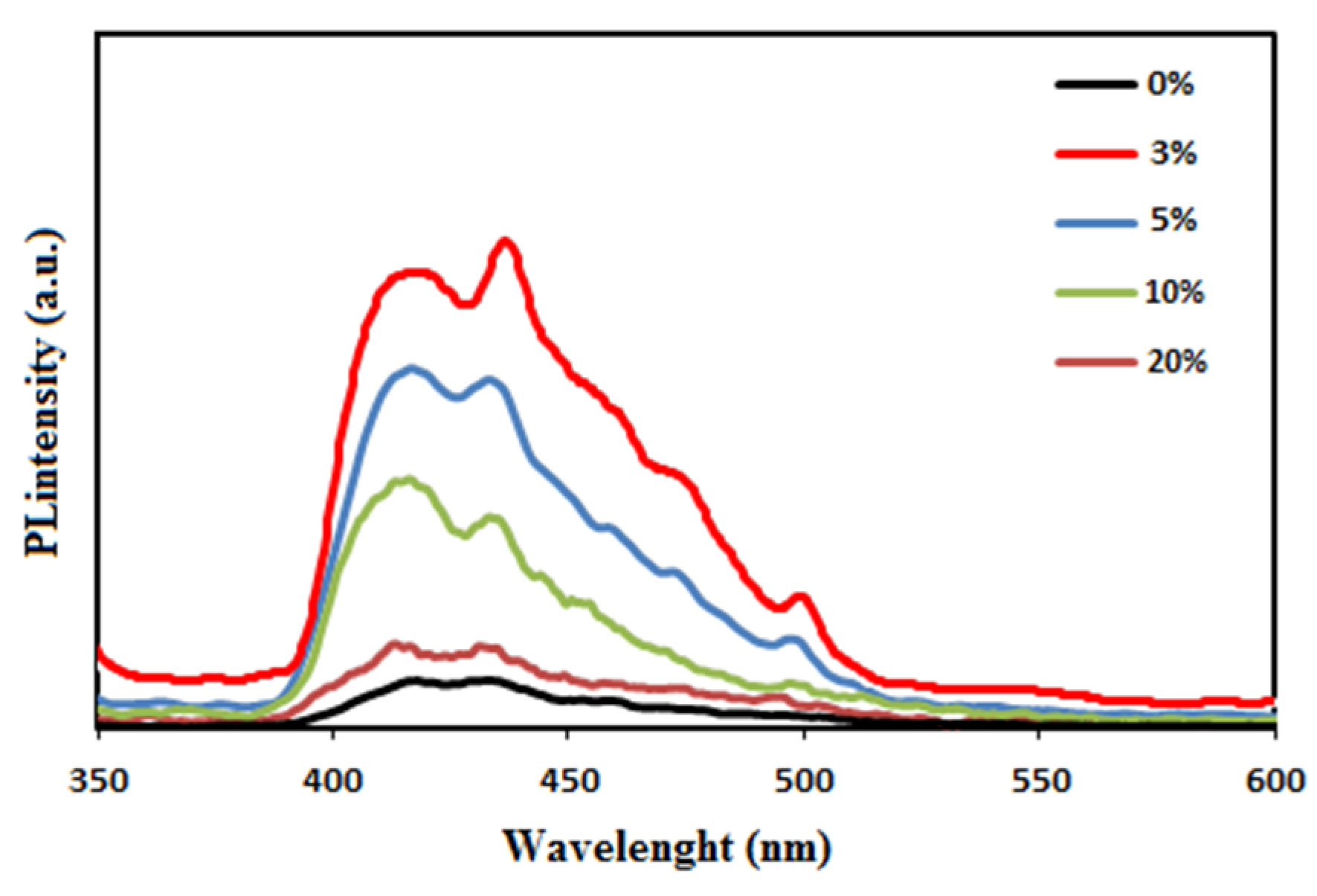
3.6. Photoluminescence Studies
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Harito, C.; Bavykin, D.V.; Yuliarto, B.; Dipojono, H.K.; Walsh, F.C. Polymer nanocomposites having a high filler content: Synthesis, structures, properties, and applications. Nanoscale 2019, 11, 4653–4682. [Google Scholar] [CrossRef] [PubMed]
- Radzuan, N.A.M.; Sulong, A.B.; Sahari, J. A review of electrical conductivity models for conductive polymer composite. Int. J. Hydrogen Energy 2017, 42, 9262–9273. [Google Scholar] [CrossRef]
- Radzuan, N.A.M.; Zakaria, M.Y.; Sulong, A.B.; Sahari, J. The effect of milled carbon fibre filler on electrical conductivity in highly conductive polymer composites. Compos. B Eng. 2017, 110, 153–160. [Google Scholar] [CrossRef]
- Arash, B.; Wang, Q.; Varadan, V.K. Mechanical properties of carbon nanotube/polymer composites. Sci. Rep. 2014, 4, 6479. [Google Scholar] [CrossRef]
- Arjomandi, J.; Keramat Irad Mossa, N.; Jaleh, B. Electrochemical synthesis and In situ spectroelectrochemistry of conducting NMPy-TiO2 and ZnO polymer nanocomposites for Li secondary battery applications. J. Appl. Polym. Sci. 2015, 132, 41526. [Google Scholar] [CrossRef]
- Jaleh, B.; Shahbazi, N.; Jabbari, A. Optical and Thermal Properties of Polycarbonate-TiO2 Nanocomposite Film. Synth. React. Inorg. Met.-Org. Nano-Met. Chem. 2016, 46, 602–607. [Google Scholar] [CrossRef]
- Abdulkadir, A.; Sarker, T.; He, Q.; Guo, Z.; Wei, S. Mössbauer spectroscopy of polymer nanocomposites. In Spectroscopy of Polymer Nanocomposites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 393–409. [Google Scholar]
- Zhai, S.; Zhang, P.; Xian, Y.; Zeng, J.; Shi, B. Effective thermal conductivity of polymer composites: Theoretical models and simulation models. Int. J. Heat Mass Transf. 2018, 117, 358–374. [Google Scholar] [CrossRef]
- Dhillon, A.; Kumar, D. Recent advances and perspectives in polymer-based nanomaterials for Cr (VI) removal. In New Polymer Nanocomposites for Environmental Remediation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 29–46. [Google Scholar]
- Abbasi, S.; Carreau, P.J.; Derdouri, A. Flow induced orientation of multiwalled carbon nanotubes in polycarbonate nanocomposites: Rheology, conductivity and mechanical properties. Polymer 2010, 51, 922–935. [Google Scholar] [CrossRef] [Green Version]
- Cho, B.-G.; Hwang, S.-H.; Park, M.; Park, J.K.; Park, Y.-B.; Chae, H.G. The effects of plasma surface treatment on the mechanical properties of polycarbonate/carbon nanotube/carbon fiber composites. Compos. B Eng. 2019, 160, 436–445. [Google Scholar] [CrossRef]
- Aden, M.; Roesner, A.; Olowinsky, A. Optical characterization of polycarbonate: Influence of additives on optical properties. J. Polym. Sci. Part B Polym. Phys. 2010, 48, 451–455. [Google Scholar] [CrossRef]
- Eskandari, M.; Liavali, M.N.; Malekfar, R.; Taboada, P. Investigation of Optical Properties of Polycarbonate/TiO2/ZnO Nanocomposite: Experimental and DFT Calculations. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5283–5292. [Google Scholar] [CrossRef]
- Charde, S.J.; Sonawane, S.S.; Rathod, A.P.; Sonawane, S.H.; Shimpi, N.G.; Parate, V.R. Copper-doped zinc oxide nanoparticles: Influence on thermal, thermo mechanical, and tribological properties of polycarbonate. Polym. Compos. 2018, 39, E1398–E1406. [Google Scholar] [CrossRef]
- Khutia, M.; Joshi, G.M.; Deshmukh, K.; Pandey, M. Optimization of dielectric constant of polycarbonate/polystyrene modified blend by ceramic metal oxide. Polym. Plast. Technol. Eng. 2015, 54, 383–389. [Google Scholar] [CrossRef]
- Delavar, M.; Bakeri, G.; Hosseini, M. Fabrication of polycarbonate mixed matrix membranes containing hydrous manganese oxide and alumina nanoparticles for heavy metal decontamination: Characterization and comparative study. Chem. Eng. Res. Des. 2017, 120, 240–253. [Google Scholar] [CrossRef]
- Jaleh, B.; Mohammadtaheri, S.; Shahbazi, N.; Azizian, S. Influence of O2 Plasma on Surface Properties of PC-TiO2 Nanocomposite Film. Int. J. Polym. Anal. Charact. 2015, 20, 733–742. [Google Scholar] [CrossRef]
- Qu, C.; Hu, J.; Liu, X.; Li, Z.; Ding, Y. Morphology and mechanical properties of polyimide films: The effects of UV irradiation on microscale surface. Materials 2017, 10, 1329. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaleh, B.; Nasri, A.; Shahbazi, N.; Nikfarjad, H. Surface properties of UV irradiated CR-39 polymer before and after chemical etching and registration of fingerprints on CR-39. Radiat. Meas. 2017, 101, 22–28. [Google Scholar] [CrossRef]
- Jaleh, B.; Shahbazi, N. Surface properties of UV irradiated PC-TiO2 nanocomposite film. Appl. Surf. Sci. 2014, 313, 251–258. [Google Scholar] [CrossRef]
- Senatova, S.L.; Senatov, F.S.; Kuznetsov, D.V.; Stepashkin, A.A.; Issi, J.P. Effect of UV-radiation on structure and properties of PP nanocomposites. J. Alloys Compd. 2017, 707, 304–309. [Google Scholar] [CrossRef]
- Król, A.; Pomastowski, P.; Rafińska, K.; Railean-Plugaru, V.; Buszewski, B. Zinc oxide nanoparticles: Synthesis, antiseptic activity and toxicity mechanism. Adv. Colloid Interface Sci. 2017, 249, 37–52. [Google Scholar] [CrossRef]
- Mirzaei, H.; Darroudi, M. Zinc oxide nanoparticles: Biological synthesis and biomedical applications. Ceram. Int. 2017, 43, 907–914. [Google Scholar] [CrossRef]
- Pu, W.-F.; Yuan, C.-D.; Wang, X.-C.; Sun, L.; Zhao, R.-K.; Song, W.-J.; Li, X.-F. The wettability alteration and the effect of initial rock wettability on oil recovery in surfactant-based enhanced oil recovery processes. J. Dispers. Sci. Technol. 2016, 37, 602–611. [Google Scholar] [CrossRef]
- Tian, H.; Wang, M. Molecular dynamics for ion-tuned wettability in oil/brine/rock systems. AIP Adv. 2017, 7, 125017. [Google Scholar] [CrossRef]
- Marmur, A.; Della Volpe, C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 5, 3–8. [Google Scholar] [CrossRef] [Green Version]
- Farazin, J.; Pirgholi-Givi, G.; Azizian-Kalandaragh, Y. Wettability measurement, optical characteristics, and investigation of the quantum confinement effect of ZnS-scotch tape nanocomposite films prepared by successive ionic layer adsorption and reaction (SILAR) method. Phys. B Condens. Matter 2019, 564, 94–103. [Google Scholar] [CrossRef]
- Naghdi, S.; Jaleh, B.; Ehsani, A. Electrophoretic deposition of graphene oxide on aluminum: Characterization, low thermal annealing, surface and anticorrosive properties. Bull. Chem. Soc. Jpn. 2015, 88, 722–728. [Google Scholar] [CrossRef]
- Majidi, S.; Jaleh, B.; Mohazzab, B.F.; Eslamipanah, M.; Moradi, A. Wettability of Graphene Oxide/Zinc Oxide Nanocomposite on Aluminum Surface Switching by UV Irradiation and Low Temperature Annealing. J. Inorg. Organomet. Polym. Mater. 2020, 30, 3073–3083. [Google Scholar] [CrossRef]
- Jaleh, B.; Etivand, E.S.; Mohazzab, B.F.; Nasrollahzadeh, M.; Varma, R.S. Improving wettability: Deposition of TiO2 nanoparticles on the O2 plasma activated polypropylene membrane. Int. J. Mol. Sci. 2019, 20, 3309. [Google Scholar] [CrossRef] [Green Version]
- Mohammed, M.; Rozyanty, R.; Mohammed, A.M.; Osman, A.F.; Adam, T.; Dahham, O.S.; Hashim, U.; Noriman, N.Z.; Betar, B.O. Fabrication and characterization of zinc oxide nanoparticle-treated kenaf polymer composites for weather resistance based on a solar UV radiation. BioResources 2018, 13, 6480–6496. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Dalvand, R.; Mahmud, S.; Rouhi, J.; Ooi, C.H.R. Well-aligned ZnO nanoneedle arrays grown on polycarbonate substrates via electric field-assisted chemical method. Mater. Lett. 2015, 146, 65–68. [Google Scholar] [CrossRef]
- Dhapte, V.; Gaikwad, N.; More, P.V.; Banerjee, S.; Dhapte, V.V.; Kadam, S.; Khanna, P.K. Transparent ZnO/polycarbonate nanocomposite for food packaging application. Nanocomposites 2015, 1, 106–112. [Google Scholar] [CrossRef]
- Gaur, M.S.; Rathore, B.S. Structural and thermal properties of swift heavy ion beam irradiated polycarbonate/zinc oxide nanocomposites. J. Therm. Anal. Calorim. 2015, 119, 1105–1112. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Wang, G.Y.; Zhang, X.Z.; Yu, D.P. Study on the microstructure and growth mechanism of electrochemical deposited ZnO nanowires. J. Cryst. Growth 2006, 287, 89–93. [Google Scholar] [CrossRef]
- Leprince-Wang, Y.; Yacoubi-Ouslim, A.; Wang, G.Y. Structure study of electrodeposited ZnO nanowires. Microelectron. J. 2005, 36, 625–628. [Google Scholar] [CrossRef]
- Moustaghfir, A.; Rivaton, A.; Tomasella, E.; Mailhot, B.; Cellier, J.; Jacquet, M.; Gardette, J.L. Photostabilization of polycarbonate by ZnO coatings. J. Appl. Polym. Sci. 2005, 95, 380–385. [Google Scholar] [CrossRef]
- Gong, L.; Liu, Y.; Jiang, L.; Liu, F. Study on the adhesive mechanism between the Ga doped ZnO thin film and the polycarbonate substrate. Mater. Sci. Semicond. Process. 2017, 66, 105–108. [Google Scholar] [CrossRef]
- Venkateswarlu, K.; Bose, A.C.; Rameshbabu, N. X-ray peak broadening studies of nanocrystalline hydroxyapatite by Williamson-Hall analysis. Phys. B Condens. Matter 2010, 405, 4256–4261. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Jaleh, B.; Jabbari, A. Synthesis, characterization and catalytic activity of graphene oxide/ZnO nanocompsite. RSC Adv. 2014, 4, 36713–36720. [Google Scholar] [CrossRef]
- Khranovskyy, V.; Ekblad, T.; Yakimova, R.; Hultman, L. Surface morphology effects on the light-controlled wettability of ZnO nanostructures. Appl. Surf. Sci. 2012, 258, 8146–8152. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Chen, H.; Zhong, J.; Saraf, G.; Lu, Y. Fast and reversible wettability transitions on ZnO nanostructures. J. Electron. Mater. 2007, 36, 895–899. [Google Scholar] [CrossRef]
- Chand, P.; Gaur, A.; Kumar, A. Structural and optical properties of ZnO nanoparticles synthesized at different pH values. J. Alloys Compd. 2012, 539, 174–178. [Google Scholar] [CrossRef]
- Liang, Z.; Yu, X.; Lei, B.; Liu, P.; Mai, W. Novel blue-violet photoluminescence from sputtered ZnO thin films. J. Alloys Compd. 2011, 509, 5437–5440. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Deepa, M.; Bahadur, N.; Goyat, M.S. Influence of Fe doping on nanostructures and photoluminescence of sol-gel derived ZnO. Mater. Chem. Phys. 2009, 114, 194–198. [Google Scholar] [CrossRef]
- Zhou, J.; Wang, Y.; Zhao, F.; Wang, Y.; Zhang, Y.; Yang, L. Photoluminescence of ZnO nanoparticles prepared by a novel gel-template combustion process. J. Lumin. 2006, 119, 248–252. [Google Scholar] [CrossRef]
- Wang, Z.; Lin, C.; Liu, X.; Li, G.; Luo, Y.; Quan, Z.; Xiang, H.; Lin, J. Tunable photoluminescent and cathodoluminescent properties of ZnO and ZnO: Zn phosphors. J. Phys. Chem. B 2006, 110, 9469–9476. [Google Scholar] [CrossRef]
- Man, M.T.; Kim, J.-H.; Jeong, M.S.; Do, A.-T.T.; Lee, H.S. Oriented ZnO nanostructures and their application in photocatalysis. J. Lumin. 2017, 185, 17–22. [Google Scholar] [CrossRef]
- Musa, I.; Massuyeau, F.; Faulques, E.; Nguyen, T.-P. Investigations of optical properties of MEH-PPV/ZnO nanocomposites by photoluminescence spectroscopy. Synth. Met. 2012, 162, 1756–1761. [Google Scholar] [CrossRef]
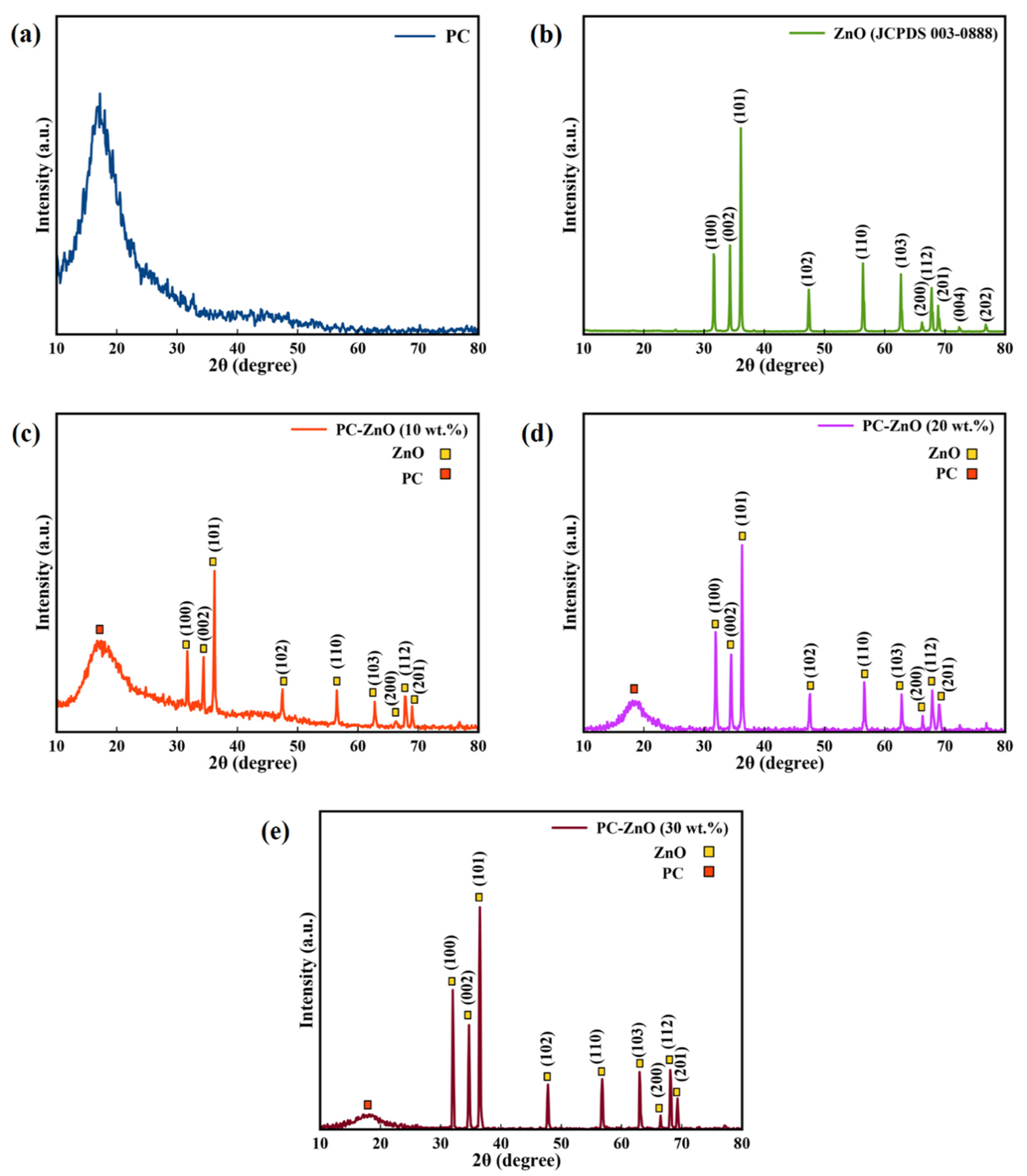


| Sample | 2θ | hkl | dhkl (Å) | D (nm) | Lattice Parameters | ||
|---|---|---|---|---|---|---|---|
| c (Å) | a (Å) | c/a | |||||
| Pure ZnO | 31.77 34.42 | 100 002 | 2.814 2.603 | 36 | 5.207 | 3.250 | 1.602 |
| PC-ZnO (10 wt.%) | 31.70 | 100 | 2.812 | 29 | 5.189 | 3.255 | 1.594 |
| 34.53 | 002 | 2.594 | |||||
| PC-ZnO (20 wt.%) | 31.90 34.50 | 100 002 | 2.29 2.60 | 29 | 5.202 | 3.248 | 1.601 |
| PC-ZnO (30 wt.%) | 32.04 | 100 | 2.79 | 37 | 5.164 | 3.222 | 1.602 |
| 34.70 | 002 | 2.58 | |||||
| Sample | C | O | O/C |
|---|---|---|---|
| PC | 69.50% | 30.50% | 0.48 |
| PC + UV | 50.69% | 49.31% | 0.97 |
| PC-ZnO (30 wt.%) | 71.70% | 28.30% | 0.40 |
| PC-ZnO (30 wt.%) + UV | 42.67% | 57.33% | 1.34 |
| Sample | C–C/C–H | C–O | C=O | O–C=O |
|---|---|---|---|---|
| PC | 90.00% | 7.80% | 2.20% | - |
| PC + UV | 72.65% | 9.64% | 11.40% | 6.31% |
| PC-ZnO (30 wt.%) | 87.40% | 3.80% | 8.80% | - |
| PC-ZnO (30 wt.%) + UV | 61.97% | 10.80% | 15.15% | 12.08% |
| Sample | C=O | C–O |
|---|---|---|
| PC | 63.26% | 36.74% |
| PC + UV | 58.00% | 42.00% |
| PC-ZnO (30 wt.%) | 63.84% | 36.16% |
| PC-ZnO (30 wt.%) + UV | 50.60% | 49.39% |
| PC-ZnO (wt.%) | Before UV Irradiation | After UV Irradiation | ||
|---|---|---|---|---|
| Sa (nm) | Sq (nm) | Sa (nm) | Sq (nm) | |
| 0 | 1.7 | 2.9 | 2.7 | 5.0 |
| 10 | 29.5 | 43.1 | 44.5 | 64.4 |
| 20 | 54.6 | 80.3 | 68.1 | 92.0 |
| 30 | 71.1 | 93.3 | 79.5 | 100.1 |
| Sample | WCA (°) |
|---|---|
| PC, PC-ZnO | 91, 90 |
| PC, PC-ZnO 4 h irradiation | 58, 44 |
| PC, PC-ZnO 8 h irradiation | 41, 28 |
| PC, PC-ZnO 10 h irradiation | 32, 25 |
| PC, PC-ZnO 12 h irradiation | 31, 20 |
| PC, PC-ZnO 15 h irradiation | 27, 17 |
| Sample | Microhardness (HV) |
|---|---|
| PC | 11.5 |
| PC-ZnO (20 wt.%) | 12.7 |
| PC-ZnO (30 wt.%) | 14.33 |
| PC + UV | 11.4 |
| PC-ZnO (20 wt.%) + UV | 13.7 |
| PC-ZnO (30 wt.%) + UV | 20.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jaleh, B.; Hamzehi, S.; Sepahvand, R.; Azizian, S.; Eslamipanah, M.; Golbedaghi, R.; Meidanchi, A.; Fausto, R. Preparation of Polycarbonate-ZnO Nanocomposite Films: Surface Investigation after UV Irradiation. Molecules 2022, 27, 4448. https://doi.org/10.3390/molecules27144448
Jaleh B, Hamzehi S, Sepahvand R, Azizian S, Eslamipanah M, Golbedaghi R, Meidanchi A, Fausto R. Preparation of Polycarbonate-ZnO Nanocomposite Films: Surface Investigation after UV Irradiation. Molecules. 2022; 27(14):4448. https://doi.org/10.3390/molecules27144448
Chicago/Turabian StyleJaleh, Babak, Sara Hamzehi, Reza Sepahvand, Saeid Azizian, Mahtab Eslamipanah, Reza Golbedaghi, Alireza Meidanchi, and Rui Fausto. 2022. "Preparation of Polycarbonate-ZnO Nanocomposite Films: Surface Investigation after UV Irradiation" Molecules 27, no. 14: 4448. https://doi.org/10.3390/molecules27144448
APA StyleJaleh, B., Hamzehi, S., Sepahvand, R., Azizian, S., Eslamipanah, M., Golbedaghi, R., Meidanchi, A., & Fausto, R. (2022). Preparation of Polycarbonate-ZnO Nanocomposite Films: Surface Investigation after UV Irradiation. Molecules, 27(14), 4448. https://doi.org/10.3390/molecules27144448








