The Application of Carbon Nanomaterials in Sensing, Imaging, Drug Delivery and Therapy for Gynecologic Cancers: An Overview
Abstract
:1. Introduction
2. Sensing
3. Imaging
4. Drug Delivery
5. Therapy
6. Biotoxicity
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hashemipour, M.; Boroumand, H.; Mollazadeh, S.; Tajiknia, V.; Nourollahzadeh, Z.; Borj, M.R.; Pourghadamyari, H.; Rahimian, N.; Hamblin, M.R.; Mirzaei, H. Exosomal microRNAs and exosomal long non-coding RNAs in gynecologic cancers. Gynecol. Oncol. 2021, 161, 314–327. [Google Scholar] [CrossRef] [PubMed]
- Kalliala, I.; Athanasiou, A.; Veroniki, A.A.; Salanti, G.; Efthimiou, O.; Raftis, N.; Bowden, S.; Paraskevaidi, M.; Aro, K.; Arbyn, M.; et al. Incidence and mortality from cervical cancer and other malignancies after treatment of cervical intraepithelial neoplasia: A systematic review and meta-analysis of the literature. Ann. Oncol. 2020, 31, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, Z.; He, Y.; Ma, G.; Deng, Y.; Chen, Y.; Zhou, Y.; Xu, X.; Li, X.; Du, Y. Years of life lost due to premature death and their trends in people with malignant neoplasm of female genital organs in Shanghai, China during 1995–2018: A population based study. BMC Public Health 2020, 20, 1489. [Google Scholar] [CrossRef] [PubMed]
- Pourhanifeh, M.H.; Darvish, M.; Tabatabaeian, J.; Fard, M.R.; Mottaghi, R.; Azadchehr, M.J.; Jahanshahi, M.; Sahebkar, A.; Mirzaei, H. Therapeutic role of curcumin and its novel formulations in gynecological cancers. J. Ovarian Res. 2020, 13, 130. [Google Scholar] [CrossRef]
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alharbi, K.S.; Afzal, M.; Alotaibi, N.H.; Yasir, M.; Elmowafy, M.; Alshehri, S. Novel nanotechnology approaches for diagnosis and therapy of breast, ovarian and cervical cancer in female: A review. J. Drug Deliv. Sci. Technol. 2020, 61, 102198. [Google Scholar] [CrossRef]
- Chaurasiya, S.; Mishra, V. Biodegradable nanoparticles as theranostics of ovarian cancer: An overview. J. Pharm. Pharmacol. 2018, 70, 435–449. [Google Scholar] [CrossRef] [Green Version]
- Jayson, G.C.; Kerbel, R.; Ellis, L.M.; Harris, A.L. Antiangiogenic therapy in oncology: Current status and future directions. Lancet 2016, 388, 518–529. [Google Scholar] [CrossRef]
- Jain, R.K. Normalizing tumor vasculature with anti-angiogenic therapy: A new paradigm for combination therapy. Nat. Med. 2001, 7, 987–989. [Google Scholar] [CrossRef]
- Haupt, K.; Rangel, P.X.M.; Bui, B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020, 120, 9554–9582. [Google Scholar] [CrossRef]
- Kievit, F.M.; Zhang, M. Cancer Nanotheranostics: Improving Imaging and Therapy by Targeted Delivery Across Biological Barriers. Adv. Mater. 2011, 23, H217. [Google Scholar] [CrossRef] [Green Version]
- Mukerjee, A.; Ranjan, A.P.; Vishwanatha, J. Combinatorial Nanoparticles for Cancer Diagnosis and Therapy. Curr. Med. Chem. 2012, 19, 3714–3721. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.; Khan, A.; Khalid, K.; Naeem, M.; Khalil, A.A.K.; Khan, K.; Qasim, M. Nanotechnology, A Tool for Diagnostics and Treatment of Cancer. Curr. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef] [PubMed]
- Bayda, S.; Adeel, M.; Tuccinardi, T.; Cordani, M.; Rizzolio, F. The History of Nanoscience and Nanotechnology: From Chemical–Physical Applications to Nanomedicine. Molecules 2020, 25, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, F.d.S.; dos Santos Pereira, L.N.; de Costa e Silva, T.P.; de Sousa Luz, R.A.; Mendes, A.N. Development of nano-particulate systems with action in breast and ovarian cancer: Nanotheragnostics. J. Drug Target. 2019, 27, 732. [Google Scholar] [CrossRef]
- Chen, J.; Fan, T.; Xie, Z.; Zeng, Q.; Xue, P.; Zheng, T.; Chen, Y.; Luo, X.; Zhang, H. Advances in nanomaterials for photody-namic therapy applications: Status and challenges. Biomaterials 2020, 237, 119827. [Google Scholar] [CrossRef]
- Mauro, N.; Utzeri, M.; Varvarà, P.; Cavallaro, G. Functionalization of Metal and Carbon Nanoparticles with Potential in Cancer Theranostics. Molecules 2021, 26, 3085. [Google Scholar] [CrossRef]
- Curcio, M.; Farfalla, A.; Saletta, F.; Valli, E.; Pantuso, E.; Nicoletta, F.P.; Iemma, F.; Vittorio, O.; Cirillo, G. Functionalized Carbon Nanostructures Versus Drug Resistance: Promising Scenarios in Cancer Treatment. Molecules 2020, 25, 2102. [Google Scholar] [CrossRef]
- Garg, B.; Sung, C.-H.; Ling, Y.-C. Graphene-based nanomaterials as molecular imaging agents. WIREs Nanomed. Nanobiotechnol. 2015, 7, 737–758. [Google Scholar] [CrossRef]
- Maiti, D.; Tong, X.; Mou, X.; Yang, K. Carbon-Based Nanomaterials for Biomedical Applications: A Recent Study. Front. Pharmacol. 2019, 9, 1401. [Google Scholar] [CrossRef]
- Biju, V. Chemical modifications and bioconjugate reactions of nanomaterials for sensing, imaging, drug delivery and therapy. Chem. Soc. Rev. 2013, 43, 744–764. [Google Scholar] [CrossRef] [PubMed]
- Kearns, O.; Camisasca, A.; Giordani, S. Hyaluronic Acid-Conjugated Carbon Nanomaterials for Enhanced Tumour Targeting Ability. Molecules 2021, 27, 48. [Google Scholar] [CrossRef] [PubMed]
- Albert, K.; Hsu, H.-Y. Carbon-Based Materials for Photo-Triggered Theranostic Applications. Molecules 2016, 21, 1585. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.; Revia, R.A.; Zhang, M. Graphene Quantum Dots and Their Applications in Bioimaging, Biosensing, and Therapy. Adv. Mater. 2019, 33, e1904362. [Google Scholar] [CrossRef]
- Curell, A.; Balibrea, J.M. Finding Lymph Nodes with Carbon Nanoparticle Suspension Injection. JAMA Netw. Open 2022, 5, e227759. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Q.; Zhou, S. Carbon-based hybrid nanogels: A synergistic nanoplatform for combined biosensing, bioimaging, and responsive drug delivery. Chem. Soc. Rev. 2018, 47, 4198–4232. [Google Scholar] [CrossRef]
- Shen, C.-L.; Liu, H.-R.; Lou, Q.; Wang, F.; Liu, K.-K.; Dong, L.; Shan, C.-X. Recent progress of carbon dots in targeted bi-oimaging and cancer. Theranostics 2022, 12, 2860. [Google Scholar] [CrossRef]
- Nocito, G.; Calabrese, G.; Forte, S.; Petralia, S.; Puglisi, C.; Campolo, M.; Esposito, E.; Conoci, S. Carbon Dots as Promising Tools for Cancer Diagnosis and Therapy. Cancers 2021, 13, 1991. [Google Scholar] [CrossRef]
- Ansari, L.; Hallaj, S.; Hallaj, T.; Amjadi, M. Doped-carbon dots: Recent advances in their biosensing, bioimaging and therapy applications. Colloids Surf. B Biointerfaces 2021, 203, 111743. [Google Scholar] [CrossRef]
- Mohajeri, M.; Behnam, B.; Sahebkar, A. Biomedical applications of carbon nanomaterials: Drug and gene delivery potentials. J. Cell. Physiol. 2018, 234, 298–319. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Rai, D.B.; Jangid, A.K.; Kulhari, H. A Review of Theranostics Applications and Toxicities of Carbon Nanomaterials. Curr. Drug Metab. 2019, 20, 506–532. [Google Scholar] [CrossRef] [PubMed]
- Bura, C.; Mocan, T.; Grapa, C.; Mocan, L. Carbon Nanotubes-Based Assays for Cancer Detection and Screening. Pharmaceutics 2022, 14, 781. [Google Scholar] [CrossRef] [PubMed]
- Samadi Pakchin, P.; Fathi, M.; Ghanbari, H.; Saber, R.; Omidi, Y. A novel electrochemical immunosensor for ultrasensitive detection of CA125 in ovarian cancer. Biosens. Bioelectron. 2020, 153, 112029. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Chen, R.; Wu, X.; Shi, N.; Duan, T.; Xu, K.; Huang, T. Fluorescence turn-on immunosensing of HE4 biomarker and ovarian cancer cells based on target-triggered metal-enhanced fluorescence of carbon dots. Anal. Chim. Acta 2021, 1187, 339160. [Google Scholar] [CrossRef] [PubMed]
- Chandra, P.; Noh, H.-B.; Pallela, R.; Shim, Y.-B. Ultrasensitive detection of drug resistant cancer cells in biological matrixes using an amperometric nanobiosensor. Biosens. Bioelectron. 2015, 70, 418–425. [Google Scholar] [CrossRef]
- Hasanzadeh, M.; Mohammadzadeh, A.; Jafari, M.; Habibi, B. Ultrasensitive immunoassay of glycoprotein 125 (CA 125) in untreated human plasma samples using poly (CTAB-chitosan) doped with silver nanoparticles. Int. J. Biol. Macromol. 2018, 120, 2048–2064. [Google Scholar] [CrossRef]
- Geim, A.K. Graphene: Status and Prospects. Science 2009, 324, 1530–1534. [Google Scholar] [CrossRef] [Green Version]
- Balandin, A.A.; Ghosh, S.; Bao, W.; Calizo, I.; Teweldebrhan, D.; Miao, F.; Lau, C.N. Superior thermal conductivity of sin-gle-layer graphene. Nano Lett. 2008, 8, 902. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Piner, R.D.; Kohlhaas, K.A.; Kleinhammes, A.; Jia, Y.; Wu, Y.; Nguyen, S.T.; Ruoff, R.S. Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 2007, 45, 1558–1565. [Google Scholar] [CrossRef]
- Tripathy, S.; Gangwar, R.; Supraja, P.; Rao, A.N.; Vanjari, S.R.K.; Singh, S.G. Graphene Doped Mn2 O3 Nanofibers as a Facile Electroanalytical DNA Point Mutation Detection Platform for Early Diagnosis of Breast/Ovarian Cancer. Electroanalysis 2018, 30, 2110–2120. [Google Scholar] [CrossRef]
- Rusling, J.F.; Sotzing, G.; Papadimitrakopoulosa, F. Designing nanomaterial-enhanced electrochemical immunosensors for cancer biomarker proteins. Bioelectrochemistry 2009, 76, 189–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, K.; Zhang, J.; Yang, G.; Wang, C.; Zhu, J.-J. Direct electrochemistry and electrocatalysis of hemoglobin based on poly(diallyldimethylammonium chloride) functionalized graphene sheets/room temperature ionic liquid composite film. Electrochem. Commun. 2010, 12, 402–405. [Google Scholar] [CrossRef]
- Li, M.; Zhang, M.; Ge, S.; Yan, M.; Yu, J.; Huang, J.; Liu, S. Ultrasensitive electrochemiluminescence immunosensor based on nanoporous gold electrode and Ru-AuNPs/graphene as signal labels. Sensor. Actuat. B 2013, 181, 50. [Google Scholar] [CrossRef]
- Fan, Y.; Shi, S.; Ma, J.; Guo, Y. Smartphone-based electrochemical system with multi-walled carbon nanotubes/thionine/gold nanoparticles modified screen-printed immunosensor for cancer antigen 125 detection. Microchem. J. 2021, 174, 107044. [Google Scholar] [CrossRef]
- Samadi Pakchin, P.; Ghanbari, H.; Saber, R.; Omidi, Y. Electrochemical immunosensor based on chitosan-gold nanoparti-cle/carbon nanotube as a platform and lactate oxidase as a label for detection of CA125 oncomarker. Biosens. Bioelectron. 2018, 122, 68. [Google Scholar] [CrossRef]
- Büyüktiryaki, S.; Say, R.; Denizli, A.; Ersöz, A. Phosphoserine imprinted nanosensor for detection of Cancer Antigen 125. Talanta 2017, 167, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Wang, J.; Zhao, X.; Chen, H.; Xu, H.; Bai, L.; Wang, W.; Yang, H.; Wei, D.; Yuan, B. Highly sensitive electrochemical immunosensor for the simultaneous detection of multiple tumor markers for signal amplification. Talanta 2021, 226, 122133. [Google Scholar] [CrossRef]
- Sangili, A.; Kalyani, T.; Chen, S.-M.; Nanda, A.; Jana, S.K. Label-Free Electrochemical Immunosensor Based on One-Step Electrochemical Deposition of AuNP-RGO Nanocomposites for Detection of Endometriosis Marker CA 125. ACS Appl. Bio Mater. 2020, 3, 7620. [Google Scholar] [CrossRef]
- Abdurhman, A.A.M.; Zhang, Y.; Zhang, G.; Wang, S. Hierarchical nanostructured noble metal/metal oxide/graphene-coated carbon fiber: In situ electrochemical synthesis and use as microelectrode for real-time molecular detection of cancer cells. Anal. Bioanal. Chem. 2015, 407, 8129–8136. [Google Scholar] [CrossRef]
- Chowdhury, A.K.M.R.H.; Tan, B.; Venkatakrishnan, K. SERS-Active 3D Interconnected Nanocarbon Web toward Non-plasmonic in Vitro Sensing of HeLa Cells and Fibroblasts. ACS Appl. Mater. Inter. 2018, 10, 35715. [Google Scholar] [CrossRef]
- Yuan, F.; Li, S.; Fan, Z.; Meng, X.; Fan, L.; Yang, S. Shining carbon dots: Synthesis and biomedical and optoelectronic appli-cations. Nano Today 2016, 11, 565. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Deng, H.; Xiong, X.; Zhang, H.; Liang, T.; Li, C. An aptamer biosensor for CA125 quantification in human serum based on upconversion luminescence resonance energy transfer. Microchem. J. 2020, 161, 105761. [Google Scholar] [CrossRef]
- Hamd-Ghadareh, S.; Salimi, A.; Fathi, F.; Bahrami, S. An amplified comparative fluorescence resonance energy transfer immunosensing of CA125 tumor marker and ovarian cancer cells using green and economic carbon dots for bio-applications in labeling, imaging and sensing. Biosens. Bioelectron. 2017, 96, 308–316. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Akhtar, N.; Jalal, N.; Hussain, I.; Szmigielski, R.; Hayat, M.Q.; Ahmad, H.B.; El-Said, W.A.; Yang, M.; Janjua, H.A. Carbon-dot wrapped ZnO nanoparticle-based photoelectrochemical sensor for selective monitoring of H2O2 released from cancer cells. Mikrochim. Acta 2019, 186, 127. [Google Scholar] [CrossRef]
- Gong, X.; Wang, H.; Liu, Y.; Hu, Q.; Gao, Y.; Yang, Z.; Shuang, S.; Dong, C. A di-functional and label-free carbon-based chem-nanosensor for real-time monitoring of pH fluctuation and quantitative determining of Curcumin. Anal. Chim. Acta 2019, 1057, 132–144. [Google Scholar] [CrossRef] [PubMed]
- Miao, Q.; Xie, C.; Zhen, X.; Lyu, Y.; Duan, H.; Liu, X.; Jokerst, J.V.; Pu, K. Molecular afterglow imaging with bright, biodegradable polymer nanoparticles. Nat. Biotechnol. 2017, 35, 1102–1110. [Google Scholar] [CrossRef]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Meng, H.-M.; Liu, H.; Kuai, H.; Peng, R.; Mo, L.; Zhang, X.-B. Aptamer-integrated DNA nanostructures for biosensing, bioimaging and cancer therapy. Chem. Soc. Rev. 2016, 45, 2583–2602. [Google Scholar] [CrossRef]
- Kostarelos, K.; Bianco, A.; Prato, M. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 2009, 4, 627–633. [Google Scholar] [CrossRef]
- Huang, Q.; Lin, X.; Li, F.; Weng, W.; Lin, L.; Hu, S. Synthesis and Applications of Carbon Dots. Prog. Chem. 2015, 27, 1604. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, J.; Chen, B.; Bao, Y.; Luo, C.; Luo, Y.; Li, T.; Lv, J.; Cheng, X. Sentinel Lymph Node Biopsy Mapped with Carbon Nanoparticle Suspensions in Patients with Breast Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 2022, 12, 1032. [Google Scholar] [CrossRef]
- Chen, X.; Bai, J.; Yuan, G.; Zhang, L.; Ren, L. One-pot preparation of nitrogen-doped carbon dots for sensitive and selective detection of Ag+ and glutathione. Microchem. J. 2021, 165, 106156. [Google Scholar] [CrossRef]
- Hashemi, F.; Heidari, F.; Mohajeri, N.; Mahmoodzadeh, F.; Zarghami, N. Fluorescence Intensity Enhancement of Green Carbon Dots: Synthesis, Characterization and Cell Imaging. Photochem. Photobiol. 2020, 96, 1032–1040. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Zhang, S.; Liu, R.; Qiu, J.; Zhao, L.; Wang, S.; Li, J.; Sang, Y.; Jiang, H.; Liu, H. Prolonged fluorescence lifetime of carbon quantum dots by combining with hydroxyapatite nanorods for bio-applications. Nanoscale 2016, 9, 2162–2171. [Google Scholar] [CrossRef]
- Asgari, M.; Khanahmad, H.; Motaghi, H.; Farzadniya, A.; Mehrgardi, M.A.; Shokrani, P. Aptamer modified nanoprobe for multimodal fluorescence/magnetic resonance imaging of human ovarian cancer cells. Appl. Phys. A 2021, 127, 47. [Google Scholar] [CrossRef]
- Guo, J.; Liu, D.; Filpponen, I.; Johansson, L.-S.; Malho, J.-M.; Quraishi, S.; Liebner, F.; Santos, H.A.; Rojas, O.J. Photolumi-nescent Hybrids of Cellulose Nanocrystals and Carbon Quantum Dots as Cytocompatible Probes for in Vitro Bioimaging. Biomacromolecules 2017, 18, 2045. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Zeng, G.; Xin, Q.; Yang, J.; Zeng, C.; Tang, K.; Yang, S.; Tang, X. Carbon nanoparticles suspension injection for photothermal therapy of xenografted human thyroid carcinoma in vivo. MedComm 2020, 1, 202–210. [Google Scholar] [CrossRef]
- Shang, J.; Gu, J.; Wang, W.; Nie, X.; Han, Q.; Wang, K. Potential role for carbon nanoparticles identification and preservation in situ of parathyroid glands during total thyroidectomy and central compartment node dissection. J. Clin. Oncol. 2015, 33, e17048. [Google Scholar] [CrossRef]
- Xie, P.; Xin, Q.; Yang, S.-T.; He, T.; Huang, Y.; Zeng, G.; Ran, M.; Tang, X. Skeleton labeled 13C-carbon nanoparticles for the imaging and quantification in tumor drainage lymph nodes. Int. J. Nanomed. 2017, 12, 4891–4899. [Google Scholar] [CrossRef] [Green Version]
- Tian, Y.; Yang, P.; Lin, Y.; Hu, Y.; Deng, H.; Ma, W.; Guo, H.; Liu, Y.; Zhang, Z.; Ding, P.; et al. Assessment of Carbon Nanoparticle Suspension Lymphography–Guided Distal Gastrectomy for Gastric Cancer. JAMA Netw. Open 2022, 5, e227739. [Google Scholar] [CrossRef]
- Lu, C.; Wang, L.; Gao, Q. Chylous ascites with lymphatic leakage localization: Technical aspects and clinical applications. BMC Surg. 2022, 22, 158. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Sun, C.; Chang, Q.; Wang, Y.; Chen, Y.; Wang, Q.; Li, Z.; Niu, L. Contrast-enhanced ultrasound–based nomogram for predicting malignant involvements among sonographically indeterminate/suspicious cervical lymph nodes in patients with differentiated thyroid carcinoma. Ultrasound Med. Biol. 2022, 48, 1579–1589. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Lin, Q.; Chen, G.; Lu, J.; Zeng, Y.; Chen, X.; Yan, J. Sentinel Lymph Node Detection Using Carbon Nanoparticles in Patients with Early Breast Cancer. PLoS ONE 2015, 10, e0135714. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Zhaode, B.; Bu, Z.; Wu, A.; Wu, X.; Shan, F.; Ji, X.; Zhang, Y.; Xing, Z.; Ji, J. Clinical study of harvesting lymph nodes with carbon nanoparticles in advanced gastric cancer: A prospective randomized trial. World J. Surg. Oncol. 2016, 14, 88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hezhu, Z.; Chen, X.; Zhang, H.; Chen, L.; Zhou, S.; Wu, K.; Wang, Z.; Kong, L.; Zhuang, H. Carbon nanoparticle-guided central lymph node dissection in clinically node-negative patients with papillary thyroid carcinoma. Head Neck 2015, 38, 840–845. [Google Scholar] [CrossRef]
- Lei, J.; Zhong, J.; Jiang, K.; Li, Z.; Gong, R.; Zhu, J. Skip lateral lymph node metastasis leaping over the central neck com-partment in papillary thyroid carcinoma. Oncotarget 2017, 8, 27022. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Xie, P.; Yang, S.-T.; Zhang, X.; Zeng, G.; Xin, Q.; Tang, X.-H. Carbon nanoparticles suspension injection for the delivery of doxorubicin: Comparable efficacy and reduced toxicity. Mater. Sci. Eng. C 2018, 92, 416–423. [Google Scholar] [CrossRef]
- Xie, P.; Yang, S.-T.; He, T.; Yang, S.; Tang, X.-H. Bioaccumulation and Toxicity of Carbon Nanoparticles Suspension Injection in Intravenously Exposed Mice. Int. J. Mol. Sci. 2017, 18, 2562. [Google Scholar] [CrossRef] [Green Version]
- Rungruang, B.; Olawaiye, A.B. Comprehensive surgical staging for endometrial cancer. Rev. Obstet. Gynecol. 2012, 5, 28–34. [Google Scholar]
- Wang, Y.; Dan, Z.; Yuan, G.; Zhang, G.; Liu, S.; Zhang, Y.; Li, B. Detection of sentinel lymph node in laparoscopic surgery for uterine cervical cancer using carbon nanoparticles. J. Surg. Oncol. 2020, 122, 934. [Google Scholar] [CrossRef]
- Ya, X.; Qian, W.; Huiqing, L.; Haixiao, W.; Weiwei, Z.; Jing, B.; Lei, C.; Jianping, Y.; Shuping, Y.; Jiaya, M.; et al. Role of carbon nanoparticle suspension in sentinel lymph node biopsy for early-stage cervical cancer: A prospective study. BJOG Int. J. Obstet. Gynaecol. 2020, 128, 890–898. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wu, L.Y.; Cheng, M.; Bai, P.; Lei, C.Z.; Li, N.; Zhang, G.Y.; Zhao, D.; Li, B. Comparison Study of Laparoscopic Sentinel Lymph Node Mapping in Endometrial Carcinoma Using Carbon Nanoparticles and Lymphatic Pathway Verification. J. Minim. Invasive Gynecol. 2018, 26, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.Y.; Wang, Z.Q.; Liang, S.C.; Hou, H.Y.; Chen, D.B.; Wang, J.L. Sentinel lymph node mapping with carbon nanoparticles in endometrial cancer. Eur. J. Gynaecol. Oncol. 2020, 41, 408. [Google Scholar]
- Liu, Z.; Tabakman, S.; Welsher, K.; Dai, H. Carbon nanotubes in biology and medicine: In vitro and in vivo detection, imaging and drug delivery. Nano Res. 2009, 2, 85–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panwar, N.; Soehartono, A.M.; Chan, K.K.; Zeng, S.; Xu, G.; Qu, J.; Coquet, P.; Yong, K.-T.; Chen, X. Nanocarbons for Biology and Medicine: Sensing, Imaging, and Drug Delivery. Chem. Rev. 2019, 119, 9559–9656. [Google Scholar] [CrossRef] [PubMed]
- Shirani, M.P.; Rezaei, B.; Khayamian, T.; Dinari, M.; Karami, K.; Mehri-Lighvan, Z.; Shamili, F.H.; Ramazani, M.; Alibolandi, M. Folate receptor-targeted multimodal fluorescence mesosilica nanoparticles for imaging, delivery palladium complex and in vitro G-quadruplex DNA interaction. J. Biomol. Struct. Dyn. 2017, 36, 4156–4169. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-W.; Chung, P.-W.; Slowing, I.; Tsunoda, M.; Yeung, E.S.; Lin, V.S.-Y. Structurally Ordered Mesoporous Carbon Nanoparticles as Transmembrane Delivery Vehicle in Human Cancer Cells. Nano Lett. 2008, 8, 3724–3727. [Google Scholar] [CrossRef] [Green Version]
- Zhu, S.; Zhen, H.; Li, Y.; Wang, P.; Huang, X.; Shi, P. PEGylated graphene oxide as a nanocarrier for podophyllotoxin. J. Nanoparticle Res. 2014, 16, 2530. [Google Scholar] [CrossRef]
- Di Santo, R.; Digiacomo, L.; Palchetti, S.; Palmieri, V.; Perini, G.; Pozzi, D.; Papi, M.; Caracciolo, G. Microfluidic manufacturing of surface-functionalized graphene oxide nanoflakes for gene delivery. Nanoscale 2019, 11, 2733–2741. [Google Scholar] [CrossRef]
- Sengupta, A.; Mezencev, R.; McDonald, J.F.; Prausnitz, M.R. Delivery of siRNA to ovarian cancer cells using laser-activated carbon nanoparticles. Nanomedicine 2015, 10, 1775–1784. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.; Zhang, M.; Lu, H.; Liang, D.; Huang, Y.; Xia, Y.; Hu, Y.; Hu, S.; Wang, J.; Yi, X.; et al. Triple Stimuli-Responsive Magnetic Hollow Porous Carbon-Based Nanodrug Delivery System for Magnetic Resonance Imaging-Guided Synergistic Photothermal/Chemotherapy of Cancer. ACS Appl. Mater. Interfaces 2018, 10, 21939–21949. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Liang, Y.; Yin, Y.; Zhang, J.; Su, W.; White, A.M.; Jiang, B.; Xu, J.; Zhang, Y.; Stewart, S.; et al. Carbon nano-onion-mediated dual targeting of P-selectin and P-glycoprotein to overcome cancer drug resistance. Nat. Commun. 2021, 12, 212. [Google Scholar] [CrossRef] [PubMed]
- Hifni, B.; Khan, M.; Devereux, S.J.; Byrne, M.H.; Quinn, S.J.; Simpson, J.C. Investigation of the Cellular Destination of Fluorescently Labeled Carbon Nanohorns in Cultured Cells. ACS Appl. Bio Mater. 2020, 3, 6790–6801. [Google Scholar] [CrossRef]
- Cailotto, S.; Amadio, E.; Facchin, M.; Selva, M.; Pontoglio, E.; Rizzolio, F.; Riello, P.; Toffoli, G.; Benedetti, A.; Perosa, A. Carbon Dots from Sugars and Ascorbic Acid: Role of the Precursors on Morphology, Properties, Toxicity, and Drug Uptake. ACS Med. Chem. Lett. 2018, 9, 832–837. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Zhang, K.; Zhao, L.; Li, C.; Bu, W.; Shen, Y.; Gu, Z.; Chang, B.; Zheng, C.; Lin, C.; et al. Aspirin-Based Carbon Dots, a Good Biocompatibility of Material Applied for Bioimaging and Anti-Inflammation. ACS Appl. Mater. Interfaces 2016, 8, 32706–32716. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Xu, M.; Li, S.; Shen, X.; Li, T.; Yan, J.; Zhang, C.; Wu, C.; Zeng, H.; Liu, Y. Chitosan hybrid nanoparticles as a theranostic platform for targeted doxorubicin/VEGF shRNA co-delivery and dual-modality fluorescence imaging. RSC Adv. 2016, 6, 29685–29696. [Google Scholar] [CrossRef]
- Wen, Y.; Xu, M.; Liu, X.; Jin, X.; Kang, J.; Xu, D.; Sang, H.; Gao, P.; Chen, X.; Zhao, L. Magnetofluorescent nanohybrid com-prising polyglycerol grafted carbon dots and iron oxides: Colloidal synthesis and applications in cellular imaging and mag-netically enhanced drug delivery. Colloids Surf. B 2019, 173, 842. [Google Scholar] [CrossRef]
- Gurunathan, S.; Han, J.W.; Park, J.H.; Kim, E.; Choi, Y.-J.; Kwon, D.-N.; Kim, J.-H. Reduced graphene oxide-silver nano-particle nanocomposite: A potential anticancer nanotherapy. Int. J. Nanomed. 2015, 10, 6257. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.F.; Huang, F.H.; Zhang, G.L.; Bai, D.P.; Massimo, D.F.; Huang, Y.F.; Gurunathan, S. Novel biomolecule lyco-pene-reduced graphene oxide-silver nanoparticle enhances apoptotic potential of trichostatin A in human ovarian cancer cells (SKOV3). Int. J. Nanomed. 2017, 12, 7551. [Google Scholar] [CrossRef] [Green Version]
- Saranya, J.; Sreeja, B.S.; Padmalaya, G.; Radha, S.; Senthil Kumar, P. CdO nanoparticles, c-MWCNT nanoparticles and CdO nanoparticles/c-MWCNT nanocomposite fibres: In vitro assessment of anti-proliferative and apoptotic studies in HeLa cancer cell line. IET Nanobiotechnol. 2020, 14, 695. [Google Scholar] [CrossRef]
- Kim, T.H.; Sirdaarta, J.P.; Zhang, Q.; Eftekhari, E.; St. John, J.; Kennedy, D.; Cock, I.E.; Li, Q. Selective toxicity of hydrox-yl-rich carbon nanodots for cancer research. Nano Res. 2018, 11, 2204. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.; Liu, L.; Wang, H.; Zhao, W.; Li, Z.; Qu, Z.; Li, J.; Sun, T.; Wang, T.; Sui, G. Synthesis of dual functional gallic-acid-based carbon dots for bioimaging and antitumor therapy. Biomater. Sci. 2019, 7, 3258–3265. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Sui, X.; Lu, S.; Qu, Y.; Liu, T.; Wang, T. Preparation of carbon dots-based nanoparticles and their research of bi-oimaging and targeted antitumor therapy. J. Biomed. Mater. Res. B 2022, 110, 220. [Google Scholar] [CrossRef]
- Liu, Y.; Bhattarai, P.; Dai, Z.; Chen, X. Photothermal therapy and photoacoustic imaging via nanotheranostics in fighting cancer. Chem. Soc. Rev. 2018, 48, 2053–2108. [Google Scholar] [CrossRef]
- Li, Y.; Lu, J.; Zhang, J.; Zhu, X.; Liu, J.; Zhang, Y. Phase-Change Nanotherapeutic Agents Based on Mesoporous Carbon for Multimodal Imaging and Tumor Therapy. ACS Appl. Bio Mater. 2020, 3, 8705–8713. [Google Scholar] [CrossRef]
- Fang, J.; Liu, Y.; Chen, Y.; Ouyang, D.; Yang, G.; Yu, T. Graphene quantum dots-gated hollow mesoporous carbon nano-platform for targeting drug delivery and synergistic chemo-photothermal therapy. Int. J. Nanomed. 2018, 13, 5991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadhasivam, S.; Savitha, S.; Wu, C.-J.; Lin, F.-H.; Stobiński, L. Carbon encapsulated iron oxide nanoparticles surface engi-neered with polyethylene glycol-folic acid to induce selective hyperthermia in folate over expressed cancer cells. Int. J. Pharm. 2015, 480, 8. [Google Scholar] [CrossRef]
- Kim, T.E.; Jang, H.J.; Park, S.W.; Wei, J.; Cho, S.; Park, W.I.; Lee, B.R.; Yang, C.D.; Jung, Y.K. Folic Acid Functionalized Carbon Dot/Polypyrrole Nanoparticles for Specific Bioimaging and Photothermal Therapy. ACS Appl. Bio Mater. 2021, 4, 3453–3461. [Google Scholar] [CrossRef]
- Shu, Q.; Liu, J.; Chang, Q.; Liu, C.; Wang, H.; Xie, Y.; Deng, X. Enhanced Photothermal Performance by Carbon Dot-Chelated Polydopamine Nanoparticles. ACS Biomater. Sci. Eng. 2021, 7, 5497–5505. [Google Scholar] [CrossRef]
- Singh, R.K.; Kurian, A.G.; Patel, K.D.; Mandakhbayar, N.; Lee, N.-H.; Knowles, J.C.; Lee, J.-H.; Kim, H.-W. Label-Free Fluorescent Mesoporous Bioglass for Drug Delivery, Optical Triple-Mode Imaging, and Photothermal/Photodynamic Synergistic Cancer Therapy. ACS Appl. Bio Mater. 2020, 3, 2218–2229. [Google Scholar] [CrossRef]
- Rhazouani, A.; Gamrani, H.; El Achaby, M.; Aziz, K.; Gebrati, L.; Uddin, M.S.; Aziz, F. Synthesis and Toxicity of Graphene Oxide Nanoparticles: A Literature Review of In Vitro and In Vivo Studies. BioMed Res. Int. 2021, 2021, 5518999. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhou, Y.; Cheng, G.; Dong, M.; Liu, S.; Huang, C. Carbon dots as a luminescence sensor for ultrasensitive detection of phosphate and their bioimaging properties. Luminescence 2014, 30, 411–415. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.; Zhang, L.; Chen, M.; Chen, X.; Wang, J. The production of pH-sensitive photoluminescent carbon nanoparticles by the carbonization of polyethylenimine and their use for bioimaging. Carbon 2013, 55, 343. [Google Scholar] [CrossRef]
- Shi, W.; Li, X.; Ma, H. A Tunable Ratiometric pH Sensor Based on Carbon Nanodots for the Quantitative Measurement of the Intracellular pH of Whole Cells. Angew. Chem. Int. Ed. 2012, 51, 6432–6435. [Google Scholar] [CrossRef]
- Li, L.; Meng, L.; Zhang, X.; Fu, C.; Lu, Q. The ionic liquid-associated synthesis of a cellulose/SWCNT complex and its re-markable biocompatibility. J. Mater. Chem. 2009, 19, 3612. [Google Scholar] [CrossRef]
- Mahmood, M.; Casciano, D.A.; Mocan, T.; Iancu, C.; Xu, Y.; Mocan, L.; Iancu, D.T.; Dervishi, E.; Li, Z.; Abdalmuhsen, M.; et al. Cytotoxicity and biological effects of functional nanomaterials delivered to various cell lines. J. Appl. Toxicol. 2009, 30, 74–83. [Google Scholar] [CrossRef]
- Mishra, V.; Kesharwani, P.; Jain, N.K. Biomedical Applications and Toxicological Aspects of Functionalized Carbon Nano-tubes. Crit. Rev. Ther. Drug 2018, 35, 293. [Google Scholar] [CrossRef] [PubMed]
- Meng, L.; Zhang, X.; Lu, Q.; Fei, Z.; Dyson, P.J. Single walled carbon nanotubes as drug delivery vehicles: Targeting doxo-rubicin to tumors. Biomaterials 2012, 33, 1689. [Google Scholar] [CrossRef]
- Foldvari, M.; Bagonluri, M. Carbon nanotubes as functional excipients for nanomedicines: II. Drug delivery and biocompatibility issues. Nanomed. Nanotechnol. Biol. Med. 2008, 4, 183–200. [Google Scholar] [CrossRef]
- Kong, H.; Wang, L.; Zhu, Y.; Huang, Q.; Fan, C. Culture Medium-Associated Physicochemical Insights on the Cytotoxicity of Carbon Nanomaterials. Chem. Res. Toxicol. 2015, 28, 290–295. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, X.; Sun, L.; Wei, Y.; Wei, X. Cellular Toxicity and Immunological Effects of Carbon-based Nanomaterials. Part. Fibre Toxicol. 2019, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Petibone, D.; Xu, Y.; Mahmood, M.; Karmakar, A.; Casciano, D.; Ali, S.; Biris, A.S. Toxicity and efficacy of carbon nanotubes and graphene: The utility of carbon-based nanoparticles in nanomedicine. Drug Metab. Rev. 2014, 46, 232–246. [Google Scholar] [CrossRef] [PubMed]
- Uo, M.; Akasaka, T.; Watari, F.; Sato, Y.; Tohji, K. Toxicity evaluations of various carbon nanomaterials. Dent. Mater. J. 2011, 30, 245–263. [Google Scholar] [CrossRef] [Green Version]
- Liu, N.; Tang, M. Toxic effects and involved molecular pathways of nanoparticles on cells and subcellular organelles. J. Appl. Toxicol. 2019, 40, 16–36. [Google Scholar] [CrossRef] [PubMed]

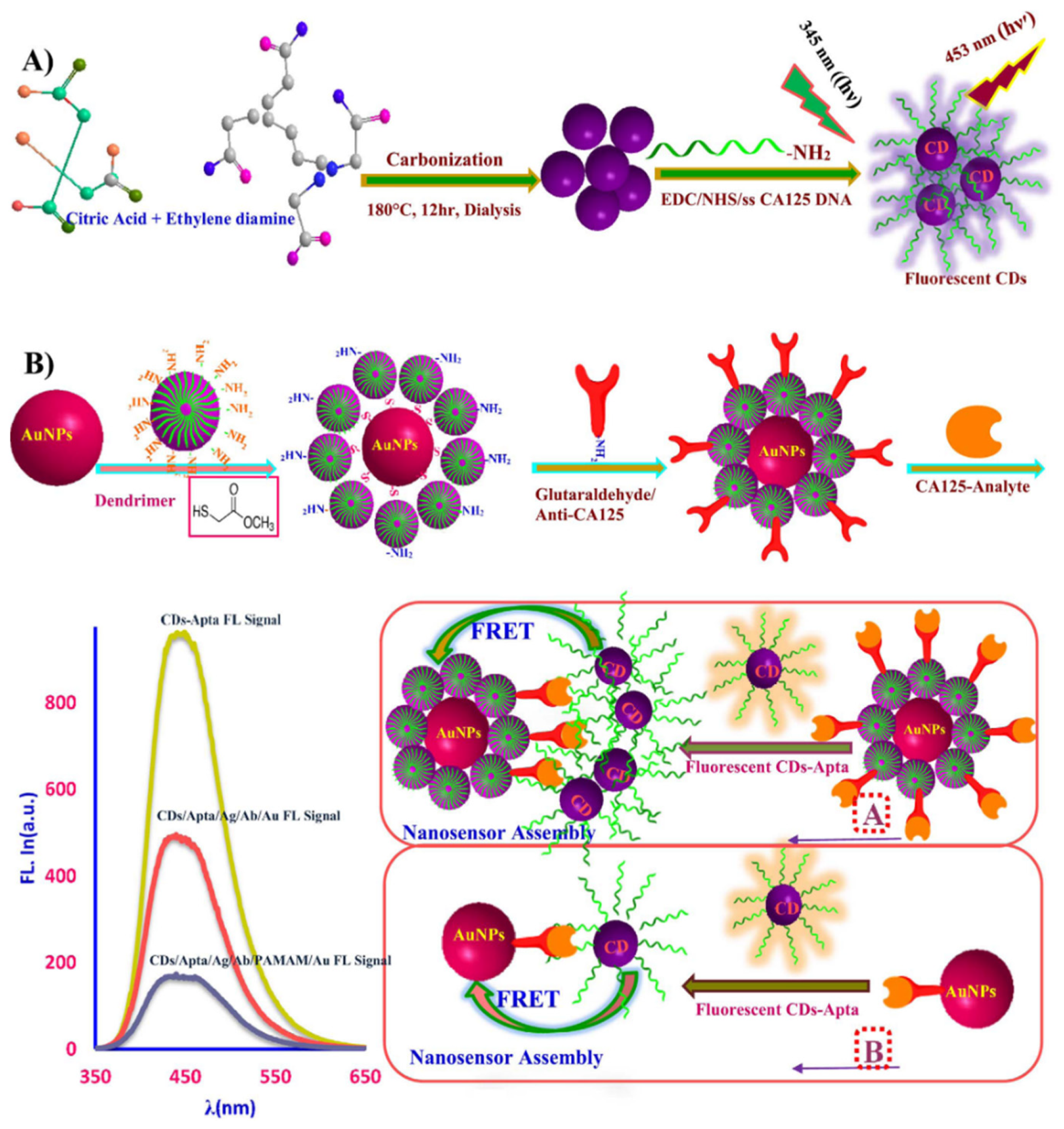
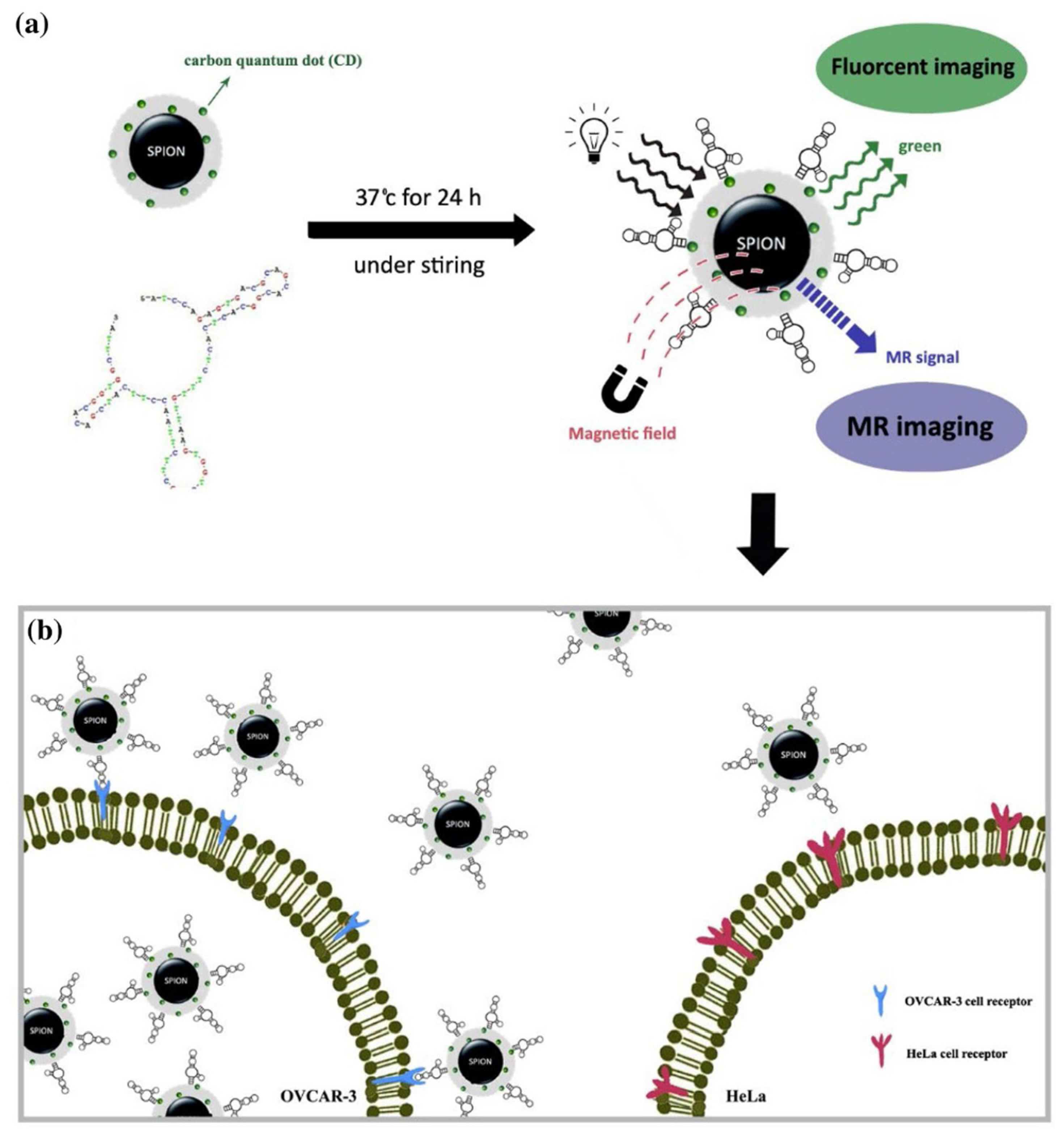
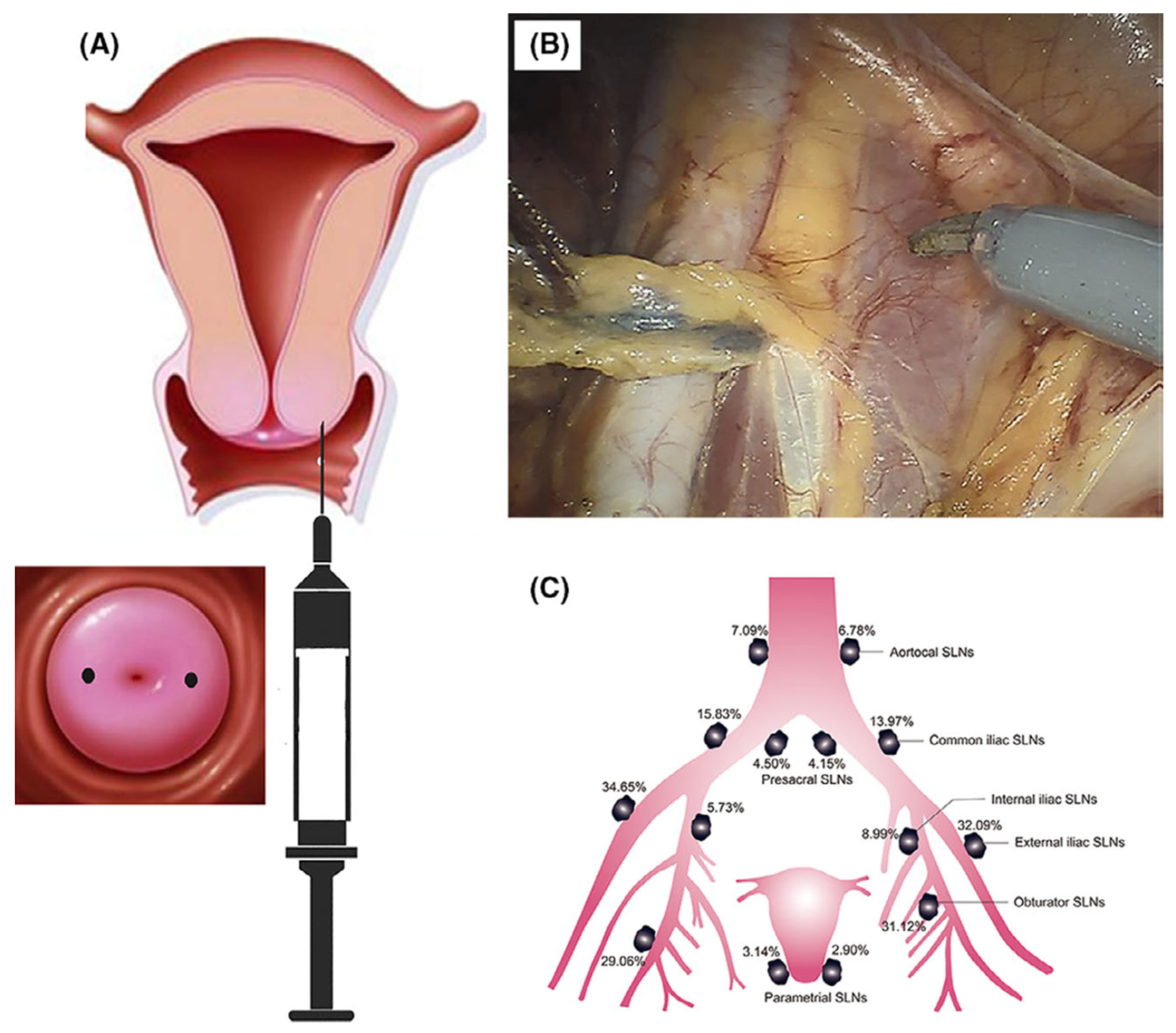

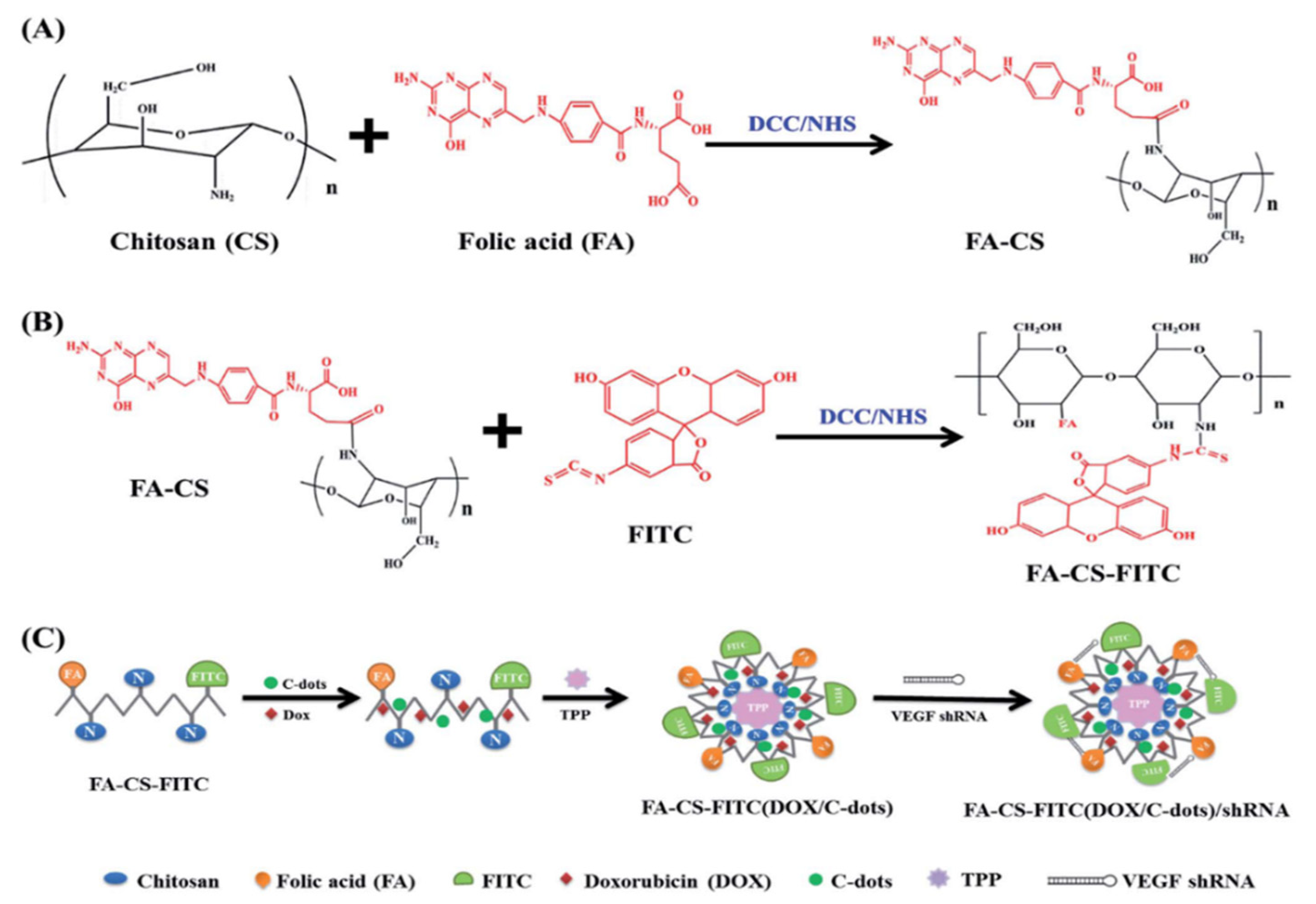
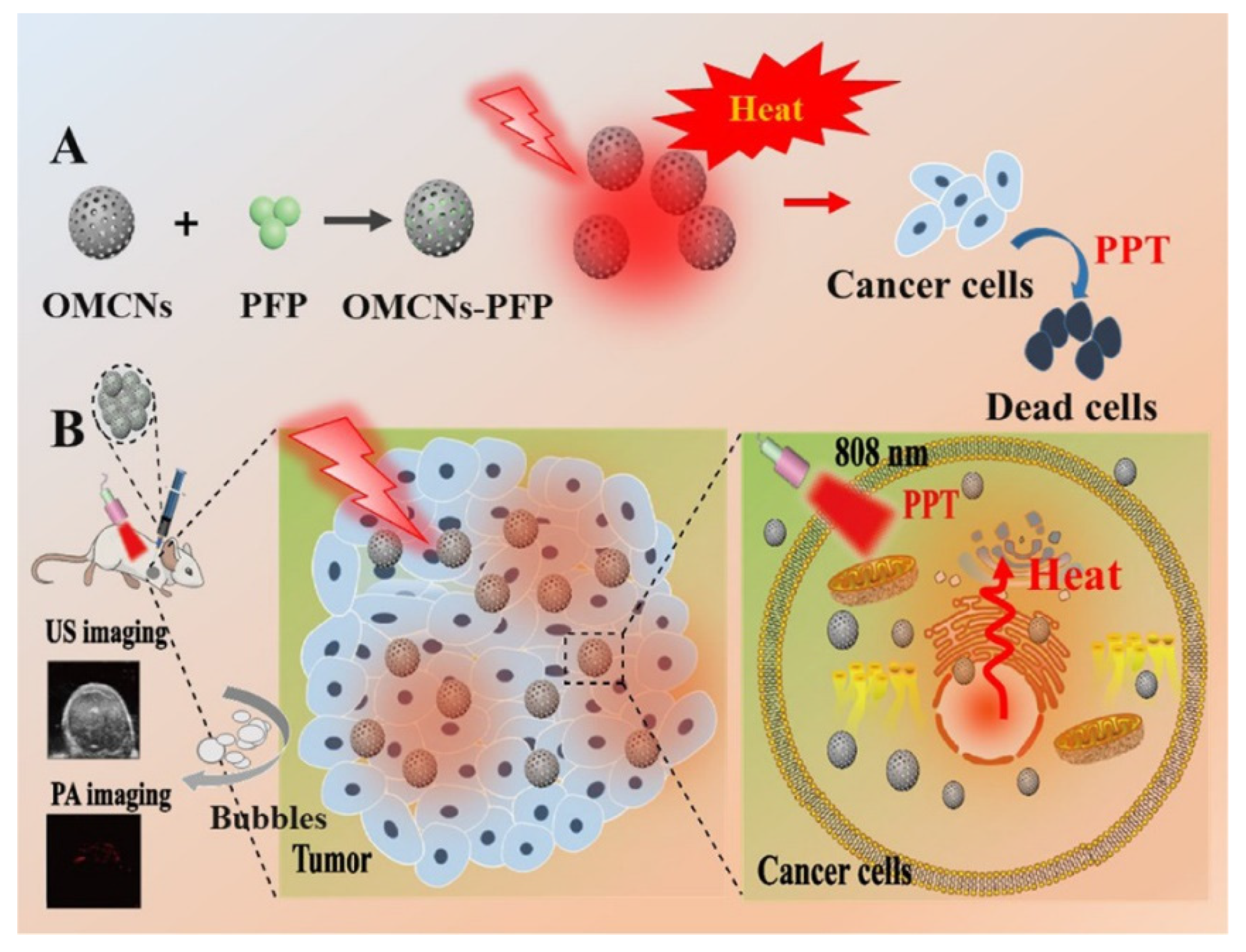
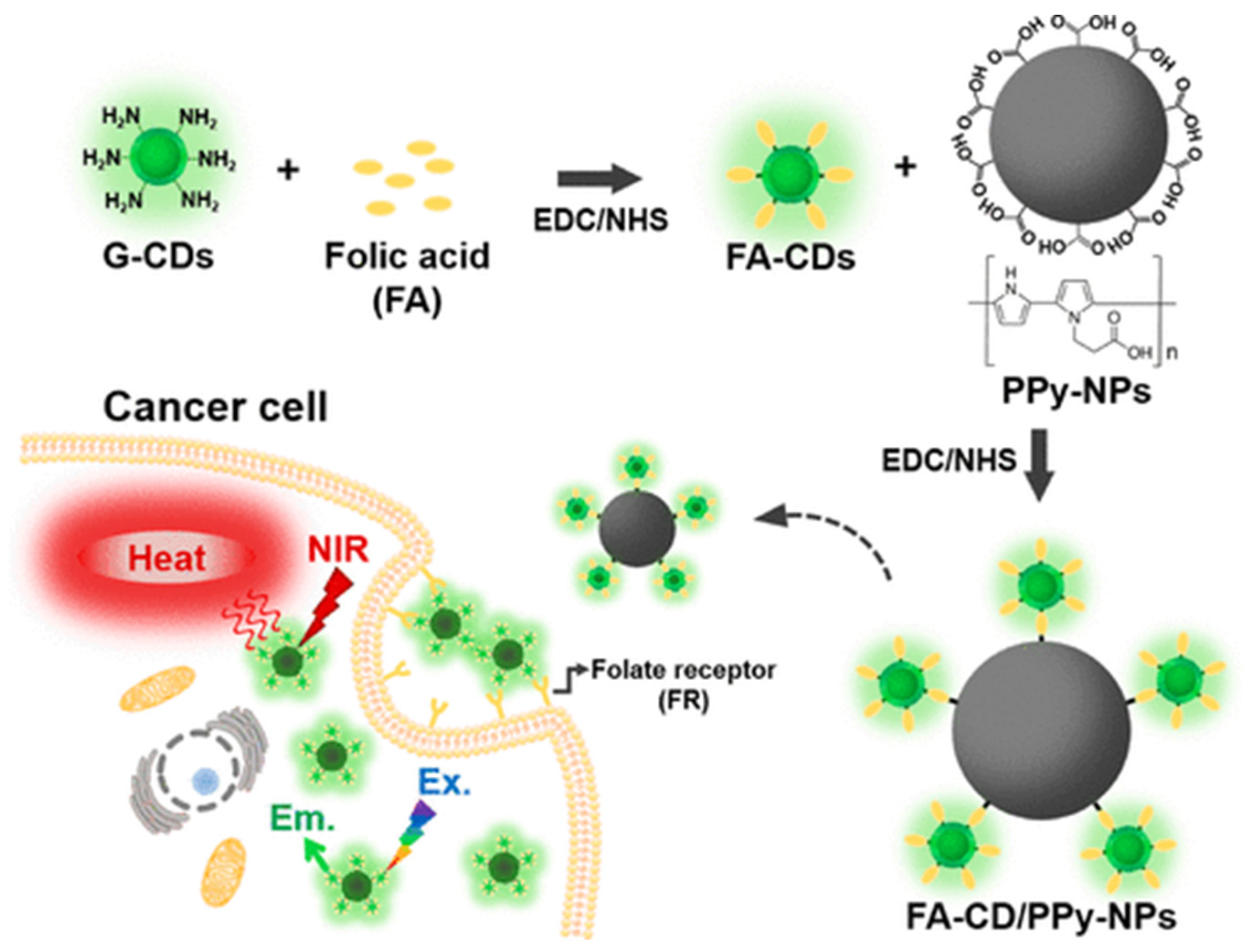
| Carbon Platform Types | Tumor Types | Targeted BIOsignals | Detection Range | Detection Limit | Ref. |
|---|---|---|---|---|---|
| Graphene | Ovarian cancer | CA 125 | 0.025 to 250 U mL−1 | 1.9 U μU mL−1 | [47] |
| Graphene | Ovarian cancer | CA 125 | 0.0001 to 300 U mL−1 | 0.042 μU mL−1 | [48] |
| Graphene | Cervical cancer | H2O2 | 0.05 to 14.2 mM | 2 μM | [49] |
| Graphene | Ovarian cancer | BRCA1 gene | 10 pM to 1 mM | 0.8 pM | [40] |
| Carbon nanotube | Ovarian cancer | CA 125 | 3.125 to 150 U mL−1 | 0.49 U mL−1 | [46] |
| Carbon nanotube | Ovarian cancer | CA 125 | 1.3 to 260 U mL−1 | 2 U mL−1 | [44] |
| Carbon nanotube | Ovarian cancer | CA 125 | 0.01–0.5 U mL−1 or 0.5–100 U mL−1 | 2 μU mL−1 | [45] |
| Carbon nanotube | Ovarian cancer | CA 125 | 0.0005 to 75 U mL−1 | 6 μU mL−1 | [33] |
| Carbon dots | Ovarian cancer | CA 125 | 0.01 to 100 U mL−1 | 9 μU mL mL−1 | [52] |
| Carbon dots | Cervical cancer | H2O2 | 20 to 800 nM | 2.4 nM | [54] |
| Carbon dots | Cervical cancer | pH | 5.0 to 7.4 | --- | [55] |
| Carbon dots | Ovarian cancer | HE4 | 0.01 to 200 nM | 2.3 pM | [34] |
| Carbon dots | Ovarian cancer | Cancer cell | 2.5 × 103 to 2 × 104 cells mL−1 | 400 cells mL−1 | [53] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, C.; Li, C.; Hu, J.; Zhu, L. The Application of Carbon Nanomaterials in Sensing, Imaging, Drug Delivery and Therapy for Gynecologic Cancers: An Overview. Molecules 2022, 27, 4465. https://doi.org/10.3390/molecules27144465
Xiao C, Li C, Hu J, Zhu L. The Application of Carbon Nanomaterials in Sensing, Imaging, Drug Delivery and Therapy for Gynecologic Cancers: An Overview. Molecules. 2022; 27(14):4465. https://doi.org/10.3390/molecules27144465
Chicago/Turabian StyleXiao, Changji, Changming Li, Jun Hu, and Lirong Zhu. 2022. "The Application of Carbon Nanomaterials in Sensing, Imaging, Drug Delivery and Therapy for Gynecologic Cancers: An Overview" Molecules 27, no. 14: 4465. https://doi.org/10.3390/molecules27144465
APA StyleXiao, C., Li, C., Hu, J., & Zhu, L. (2022). The Application of Carbon Nanomaterials in Sensing, Imaging, Drug Delivery and Therapy for Gynecologic Cancers: An Overview. Molecules, 27(14), 4465. https://doi.org/10.3390/molecules27144465





