Biological Risk Assessment of Three Dental Composite Materials following Gas Plasma Exposure
Abstract
:1. Introduction
2. Results
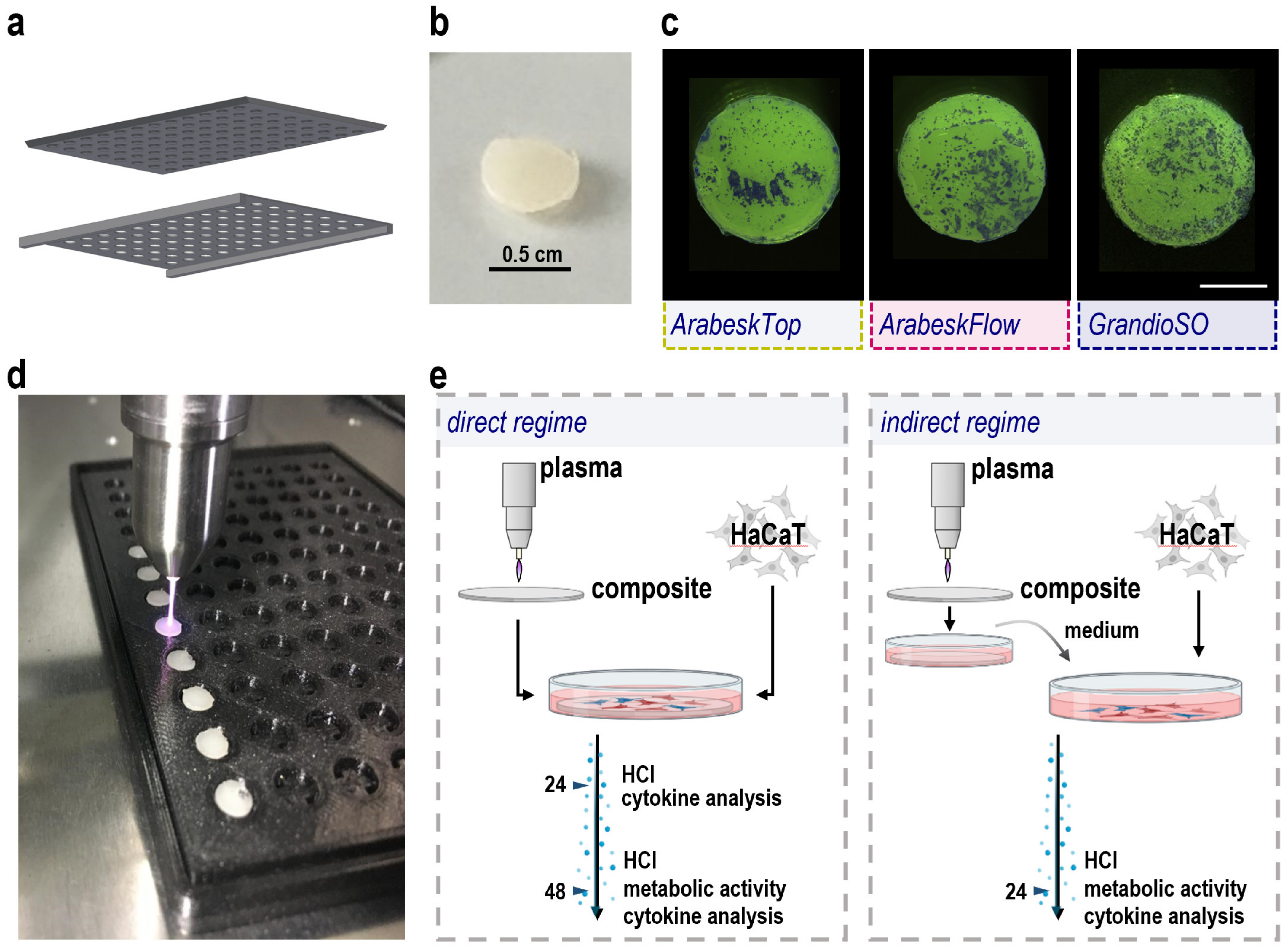
2.1. Composite Manufacturing, Gas Plasma Treatment, and Cell Culture Workflow Setup
2.2. Cytotoxicity Analysis of Three Types of Gas Plasma-Treated Dental Composites
2.3. Secretion Profiling of Three Types of Gas Plasma-Treated Dental Composites
2.4. Composite Particle Release Analysis
3. Discussion
4. Materials and Methods
4.1. Resin-Based Composite Chip Manufacturing
4.2. Cell Culture
4.3. Gas Plasma Setup and Exposure
4.4. Stereo Microscopy
4.5. Metabolic Activity
4.6. High Content Imaging
4.7. Flow Cytometry
4.8. Chemokine and Cytokine Analysis
4.9. Statistical Analysis
4.10. Photon Correlation Spectroscopy
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laroussi, M.; Lu, X.; Keidar, M. Perspective: The physics, diagnostics, and applications of atmospheric pressure low temperature plasma sources used in plasma medicine. J. Appl. Phys. 2017, 122, 020901. [Google Scholar] [CrossRef]
- Setsuhara, Y. Low-temperature atmospheric-pressure plasma sources for plasma medicine. Arch. Biochem. Biophys. 2016, 605, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Laroussi, M. Nonthermal decontamination of biological media by atmospheric-pressure plasmas: Review, analysis, and prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Purevdorj, D.; Igura, N.; Ariyada, O.; Hayakawa, I. Effect of feed gas composition of gas discharge plasmas on bacillus pumilus spore mortality. Lett. Appl. Microbiol. 2003, 37, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Busco, G.; Robert, E.; Chettouh-Hammas, N.; Pouvesle, J.M.; Grillon, C. The emerging potential of cold atmospheric plasma in skin biology. Free Radic. Biol. Med. 2020, 161, 290–304. [Google Scholar] [CrossRef]
- Bekeschus, S.; von Woedtke, T.; Emmert, S.; Schmidt, A. Medical gas plasma-stimulated wound healing: Evidence and mechanisms. Redox Biol. 2021, 46, 102116. [Google Scholar] [CrossRef] [PubMed]
- Hui, W.L.; Perrotti, V.; Iaculli, F.; Piattelli, A.; Quaranta, A. The emerging role of cold atmospheric plasma in implantology: A review of the literature. Nanomaterials 2020, 10, 1505. [Google Scholar] [CrossRef]
- Preissner, S.; Kastner, I.; Schutte, E.; Hartwig, S.; Schmidt-Westhausen, A.M.; Paris, S.; Preissner, R.; Hertel, M. Adjuvant antifungal therapy using tissue tolerable plasma on oral mucosa and removable dentures in oral candidiasis patients: A randomised double-blinded split-mouth pilot study. Mycoses 2016, 59, 467–475. [Google Scholar] [CrossRef]
- Jablonowski, L.; Fricke, K.; Matthes, R.; Holtfreter, B.; Schluter, R.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Removal of naturally grown human biofilm with an atmospheric pressure plasma jet: An in-vitro study. J. Biophotonics 2017, 10, 718–726. [Google Scholar] [CrossRef] [Green Version]
- Kucuk, D.; Savran, L.; Ercan, U.K.; Yarali, Z.B.; Karaman, O.; Kantarci, A.; Saglam, M.; Koseoglu, S. Evaluation of efficacy of non-thermal atmospheric pressure plasma in treatment of periodontitis: A randomized controlled clinical trial. Clin. Oral Investig. 2020, 24, 3133–3145. [Google Scholar] [CrossRef]
- Choi, B.B.; Choi, J.H.; Kang, T.H.; Lee, S.J.; Kim, G.C. Enhancement of osteoblast differentiation using no-ozone cold plasma on human periodontal ligament cells. Biomedicines 2021, 9, 1542. [Google Scholar] [CrossRef] [PubMed]
- Moharamzadeh, K.; Van Noort, R.; Brook, I.M.; Scutt, A.M. Cytotoxicity of resin monomers on human gingival fibroblasts and hacat keratinocytes. Dent. Mater. 2007, 23, 40–44. [Google Scholar] [CrossRef]
- Wende, K.; Bekeschus, S.; Schmidt, A.; Jatsch, L.; Hasse, S.; Weltmann, K.D.; Masur, K.; von Woedtke, T. Risk assessment of a cold argon plasma jet in respect to its mutagenicity. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2016, 798–799, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Miebach, L.; Freund, E.; Clemen, R.; Weltmann, K.D.; Metelmann, H.R.; von Woedtke, T.; Gerling, T.; Wende, K.; Bekeschus, S. Conductivity augments ros and rns delivery and tumor toxicity of an argon plasma jet. Free Radic. Biol. Med. 2022, 180, 210–219. [Google Scholar] [CrossRef] [PubMed]
- Farr, N.; Thanarak, J.; Schafer, J.; Quade, A.; Claeyssens, F.; Green, N.; Rodenburg, C. Understanding surface modifications induced via argon plasma treatment through secondary electron hyperspectral imaging. Adv. Sci 2021, 8, 2003762. [Google Scholar] [CrossRef]
- Reghu, V.R.; Shankar, V.; Ramaswamy, P. Challenges in plasma spraying of 8%y2o3-zro2 thermal barrier coatings on al alloy automotive piston and influence of vibration and thermal fatigue on coating characteristics. Mater. Today Proc. 2018, 5, 23927–23936. [Google Scholar] [CrossRef]
- Aleman, C.; Fabregat, G.; Armelin, E.; Buendia, J.J.; Llorca, J. Plasma surface modification of polymers for sensor applications. J. Mater. Chem B 2018, 6, 6515–6533. [Google Scholar] [CrossRef] [Green Version]
- Stancu, E.C.; Quade, A.; Weltmann, K.D. Polystyrene surface modification for serum-free cell culture using an atmospheric pressure dielectric barrier discharge. Rom. Rep. Phys. 2019, 71, 409. [Google Scholar]
- Choi, J.H.; Song, Y.S.; Song, K.; Lee, H.J.; Hong, J.W.; Kim, G.C. Skin renewal activity of non-thermal plasma through the activation of β-catenin in keratinocytes. Sci. Rep. 2017, 7, 6146. [Google Scholar] [CrossRef] [Green Version]
- Blackert, S.; Haertel, B.; Wende, K.; von Woedtke, T.; Lindequist, U. Influence of non-thermal atmospheric pressure plasma on cellular structures and processes in human keratinocytes (hacat). J. Dermatol. Sci. 2013, 70, 173–181. [Google Scholar] [CrossRef]
- Adachi, T.; Matsuda, Y.; Ishii, R.; Kamiya, T.; Hara, H. Ability of plasma-activated acetated ringer’s solution to induce a549 cell injury is enhanced by a pre-treatment with histone deacetylase inhibitors. J. Clin. Biochem. Nutr. 2020, 67, 232–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatia, R.; Strudwick, X.; Cowin, A.J.; Szili, E.J. Investigation of helium plasma jet-treated serum and cell media on the viability of skin cells. J. Biomater. Tissue Eng. 2018, 8, 892–899. [Google Scholar] [CrossRef]
- Choi, B.B.R.; Choi, J.H.; Hong, J.W.; Song, K.W.; Lee, H.J.; Kim, U.K.; Kim, G.C. Selective killing of melanoma cells with non-thermal atmospheric pressure plasma and p-fak antibody conjugated gold nanoparticles. Int. J. Med. Sci. 2017, 14, 1101–1109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clemen, R.; Heirman, P.; Lin, A.; Bogaerts, A.; Bekeschus, S. Physical plasma-treated skin cancer cells amplify tumor cytotoxicity of human natural killer (nk) cells. Cancers 2020, 12, 3575. [Google Scholar] [CrossRef]
- Schmidt, A.; Bekeschus, S.; Jablonowski, H.; Barton, A.; Weltmann, K.D.; Wende, K. Role of ambient gas composition on cold physical plasma-elicited cell signaling in keratinocytes. Biophys. J. 2017, 112, 2397–2407. [Google Scholar] [CrossRef] [Green Version]
- Colombo, I.; Sangiovanni, E.; Maggio, R.; Mattozzi, C.; Zava, S.; Corbett, Y.; Fumagalli, M.; Carlino, C.; Corsetto, P.A.; Scaccabarozzi, D.; et al. Hacat cells as a reliable in vitro differentiation model to dissect the inflammatory/repair response of human keratinocytes. Mediat. Inflamm. 2017, 2017, 7435621. [Google Scholar] [CrossRef]
- Casas, C.; Paul, C.; Lahfa, M.; Livideanu, B.; Lejeune, O.; Alvarez-Georges, S.; Saint-Martory, C.; Degouy, A.; Mengeaud, V.; Ginisty, H.; et al. Quantification of demodex folliculorum by pcr in rosacea and its relationship to skin innate immune activation. Exp. Dermatol. 2012, 21, 906–910. [Google Scholar] [CrossRef]
- Di Cioccio, V.; Strippoli, R.; Bizzarri, C.; Troiani, G.; Cervellera, M.N.; Gloaguen, I.; Colagrande, A.; Cattozzo, E.M.; Pagliei, S.; Santoni, A.; et al. Key role of proline-rich tyrosine kinase 2 in interleukin-8 (cxcl8/il-8)-mediated human neutrophil chemotaxis. Immunology 2004, 111, 407–415. [Google Scholar] [CrossRef]
- de Oliveira, S.; Reyes-Aldasoro, C.C.; Candel, S.; Renshaw, S.A.; Mulero, V.; Calado, A. Cxcl8 (il-8) mediates neutrophil recruitment and behavior in the zebrafish inflammatory response. J. Immunol. 2013, 190, 4349–4359. [Google Scholar] [CrossRef]
- Deshmane, S.L.; Kremlev, S.; Amini, S.; Sawaya, B.E. Monocyte chemoattractant protein-1 (mcp-1): An overview. J. Interferon Cytokine Res. 2009, 29, 313–326. [Google Scholar] [CrossRef]
- Mann, A.; Breuhahn, K.; Schirmacher, P.; Blessing, M. Keratinocyte-derived granulocyte-macrophage colony stimulating factor accelerates wound healing: Stimulation of keratinocyte proliferation, granulation tissue formation, and vascularization. J. Investig. Dermatol. 2001, 117, 1382–1390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khajah, M.; Millen, B.; Cara, D.C.; Waterhouse, C.; McCafferty, D.M. Granulocyte-macrophage colony-stimulating factor (gm-csf): A chemoattractive agent for murine leukocytes in vivo. J. Leukoc. Biol. 2011, 89, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Zhou, F. Inflammasomes in common immune-related skin diseases. Front. Immunol. 2020, 11, 882. [Google Scholar] [CrossRef] [PubMed]
- Pazos-Castro, D.; Gonzalez-Klein, Z.; Montalvo, A.Y.; Hernandez-Ramirez, G.; Romero-Sahagun, A.; Esteban, V.; Garrido-Arandia, M.; Tome-Amat, J.; Diaz-Perales, A. Nlrp3 priming due to skin damage precedes ltp allergic sensitization in a mouse model. Sci. Rep. 2022, 12, 3329. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Shim, J.H.; Kim, K.H.; Cho, Y.S.; Choi, H.S.; Song, E.Y.; Myung, P.K.; Kang, J.S.; Suh, S.K.; Park, S.N.; Yoon, D.Y. Protective effect of oxidative stress in hacat keratinocytes expressing e7 oncogene. Amino Acids 2008, 34, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Sasabe, E.; Tomomura, A.; Kitamura, N.; Yamamoto, T. Metal nanoparticles-induced activation of nlrp3 inflammasome in human oral keratinocytes is a possible mechanism of oral lichenoid lesions. Toxicol. Vitro 2020, 62, 104663. [Google Scholar] [CrossRef]
- Rutkowski, R.; Daeschlein, G.; von Woedtke, T.; Smeets, R.; Gosau, M.; Metelmann, H.R. Long-term risk assessment for medical application of cold atmospheric pressure plasma. Diagnostics 2020, 10, 210. [Google Scholar] [CrossRef] [Green Version]
- Bekeschus, S.; Schmidt, A.; Weltmann, K.-D.; von Woedtke, T. The plasma jet kinpen—A powerful tool for wound healing. Clin. Plasma Med. 2016, 4, 19–28. [Google Scholar] [CrossRef]
- Stratmann, B.; Costea, T.C.; Nolte, C.; Hiller, J.; Schmidt, J.; Reindel, J.; Masur, K.; Motz, W.; Timm, J.; Kerner, W.; et al. Effect of cold atmospheric plasma therapy vs standard therapy placebo on wound healing in patients with diabetic foot ulcers: A randomized clinical trial. JAMA Netw. Open 2020, 3, e2010411. [Google Scholar] [CrossRef]
- Evert, K.; Kocher, T.; Schindler, A.; Müller, M.; Müller, K.; Pink, C.; Holtfreter, B.; Schmidt, A.; Dombrowski, F.; Schubert, A.; et al. Repeated exposure of the oral mucosa over 12 months with cold plasma is not carcinogenic in mice. Sci. Rep. 2021, 11, 20672. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.; Woedtke, T.V.; Stenzel, J.; Lindner, T.; Polei, S.; Vollmar, B.; Bekeschus, S. One year follow-up risk assessment in skh-1 mice and wounds treated with an argon plasma jet. Int. J. Mol. Sci. 2017, 18, 868. [Google Scholar] [CrossRef] [PubMed]
- Bekeschus, S.; Schmidt, A.; Kramer, A.; Metelmann, H.R.; Adler, F.; von Woedtke, T.; Niessner, F.; Weltmann, K.D.; Wende, K. High throughput image cytometry micronucleus assay to investigate the presence or absence of mutagenic effects of cold physical plasma. Environ. Mol. Mutagen. 2018, 59, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Boxhammer, V.; Li, Y.F.; Koritzer, J.; Shimizu, T.; Maisch, T.; Thomas, H.M.; Schlegel, J.; Morfill, G.E.; Zimmermann, J.L. Investigation of the mutagenic potential of cold atmospheric plasma at bactericidal dosages. Mutat. Res. 2013, 753, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Maisch, T.; Bosserhoff, A.K.; Unger, P.; Heider, J.; Shimizu, T.; Zimmermann, J.L.; Morfill, G.E.; Landthaler, M.; Karrer, S. Investigation of toxicity and mutagenicity of cold atmospheric argon plasma. Environ. Mol. Mutagen. 2017, 58, 172–177. [Google Scholar] [CrossRef]
- Hasse, S.; Hahn, O.; Kindler, S.; Woedtke, T.v.; Metelmann, H.-R.; Masur, K. Atmospheric pressure plasma jet application on human oral mucosa modulates tissue regeneration. Plasma Med. 2014, 4, 117–129. [Google Scholar] [CrossRef]
- Gornig, D.C.; Maletz, R.; Ottl, P.; Warkentin, M. Influence of artificial aging: Mechanical and physicochemical properties of dental composites under static and dynamic compression. Clin. Oral Investig. 2022, 26, 1491–1504. [Google Scholar] [CrossRef]
- Reuter, S.; von Woedtke, T.; Weltmann, K.D. The kinpen-a review on physics and chemistry of the atmospheric pressure plasma jet and its applications. J. Phys. D Appl. Phys. 2018, 51, 233001. [Google Scholar] [CrossRef] [Green Version]





| Name (Abbrev.) | Type | Resin Matrix/Filler Size | Commercial Provider |
|---|---|---|---|
| ArabeskTop (AT) | flowable composite (low filled, <70%) | BisGMA, TEGDMA, UDMA/0.05–1.00 µm | Voco, Germany |
| ArabeskFlow (AF) | flowable composite (medium filled, 71–79%) | BisGMA, TEGDMA, UDMA/~1.00 µm | Voco, Germany |
| GrandioSO (GS) | flowable composite (high filled, >80%) | BisGMA, TEGDMA, BisEMA/20–40 µm | Voco, Germany |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekeschus, S.; Miebach, L.; Pommerening, J.; Clemen, R.; Witzke, K. Biological Risk Assessment of Three Dental Composite Materials following Gas Plasma Exposure. Molecules 2022, 27, 4519. https://doi.org/10.3390/molecules27144519
Bekeschus S, Miebach L, Pommerening J, Clemen R, Witzke K. Biological Risk Assessment of Three Dental Composite Materials following Gas Plasma Exposure. Molecules. 2022; 27(14):4519. https://doi.org/10.3390/molecules27144519
Chicago/Turabian StyleBekeschus, Sander, Lea Miebach, Jonas Pommerening, Ramona Clemen, and Katharina Witzke. 2022. "Biological Risk Assessment of Three Dental Composite Materials following Gas Plasma Exposure" Molecules 27, no. 14: 4519. https://doi.org/10.3390/molecules27144519






