Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly
Abstract
:1. Introduction
2. Results and Discussion
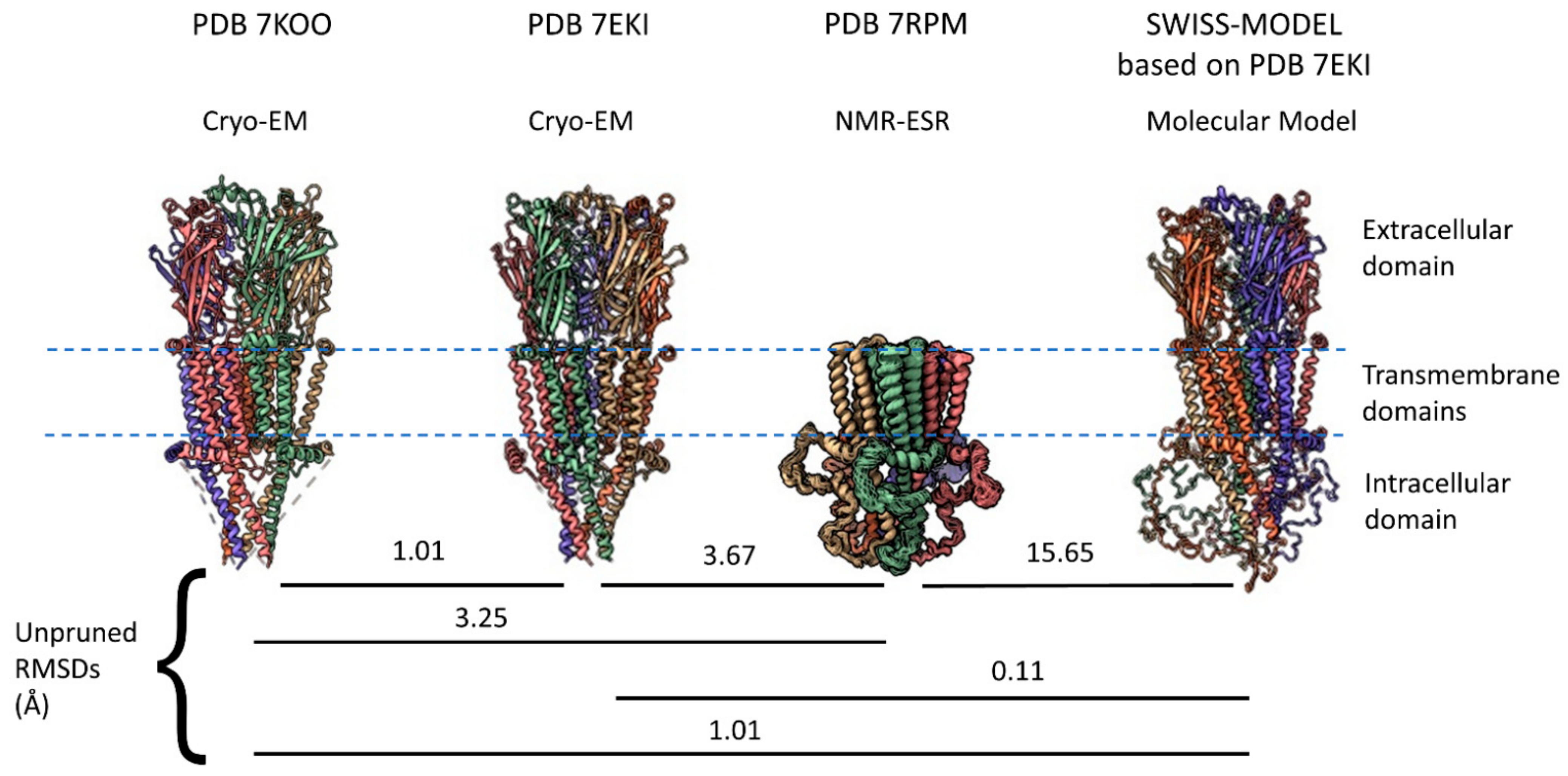
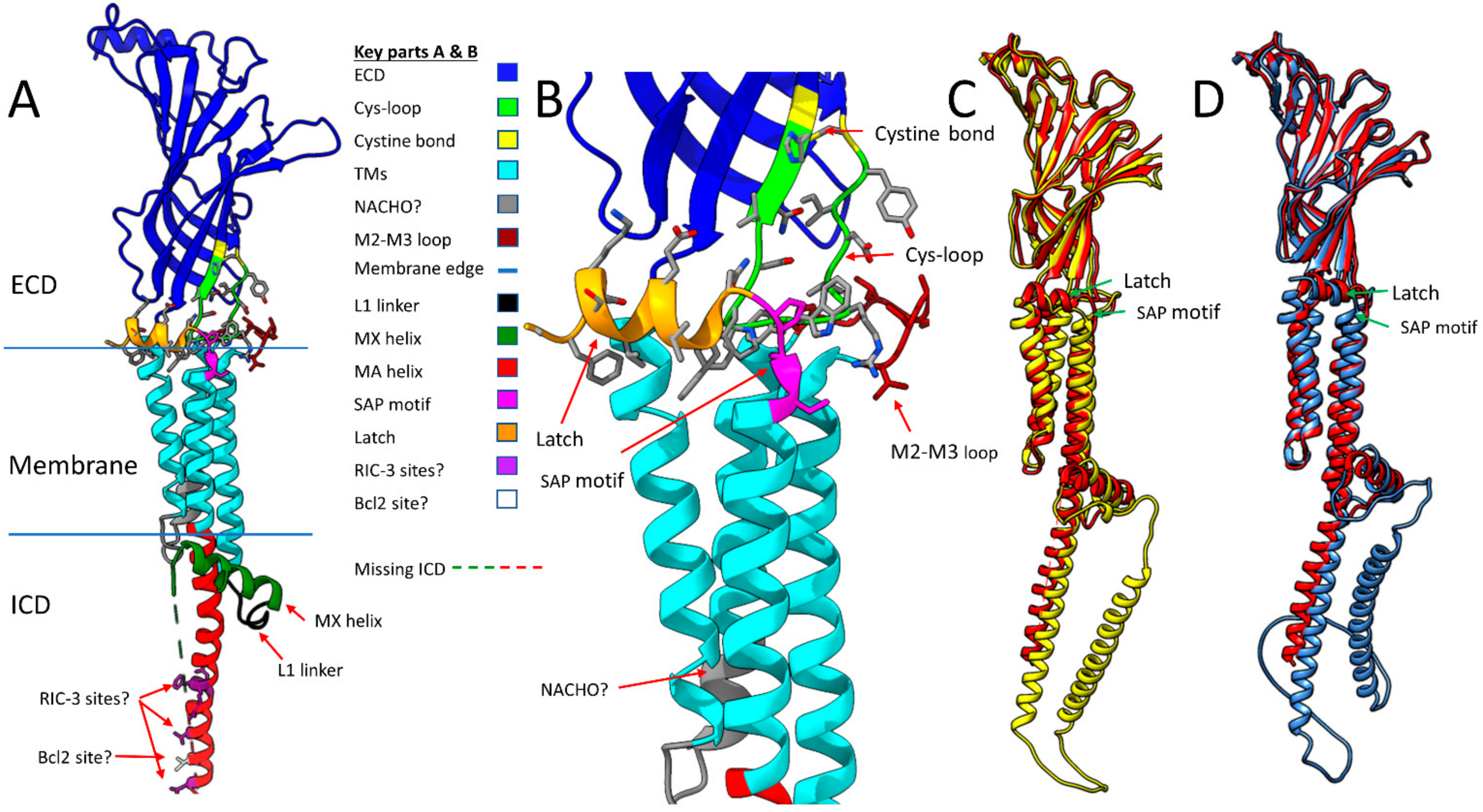
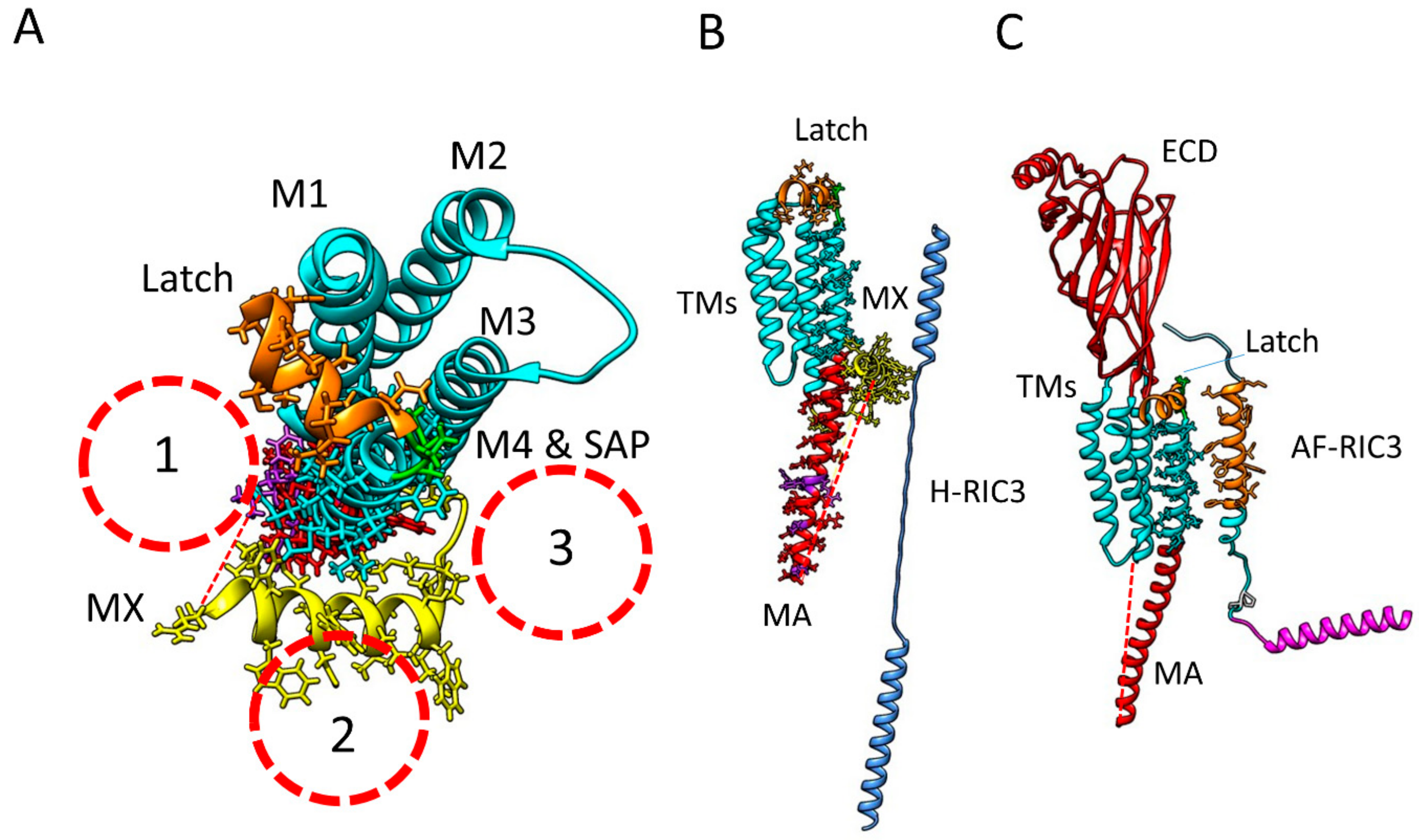
2.1. α7 Receptor Structure
2.2. How What We Know about Muscle Nicotinic Receptor Assembly Informs How We Think α7 Receptors Assemble
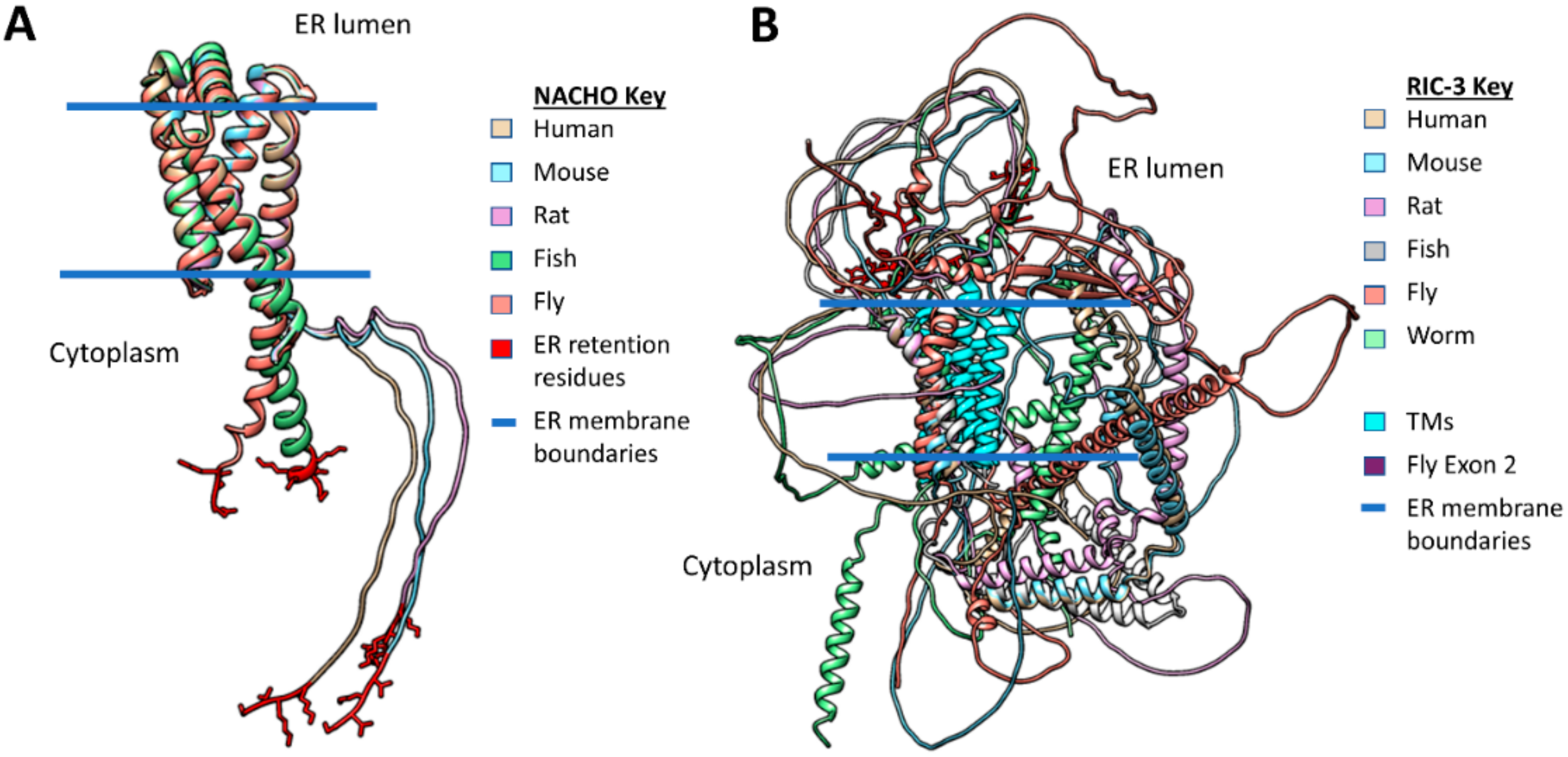
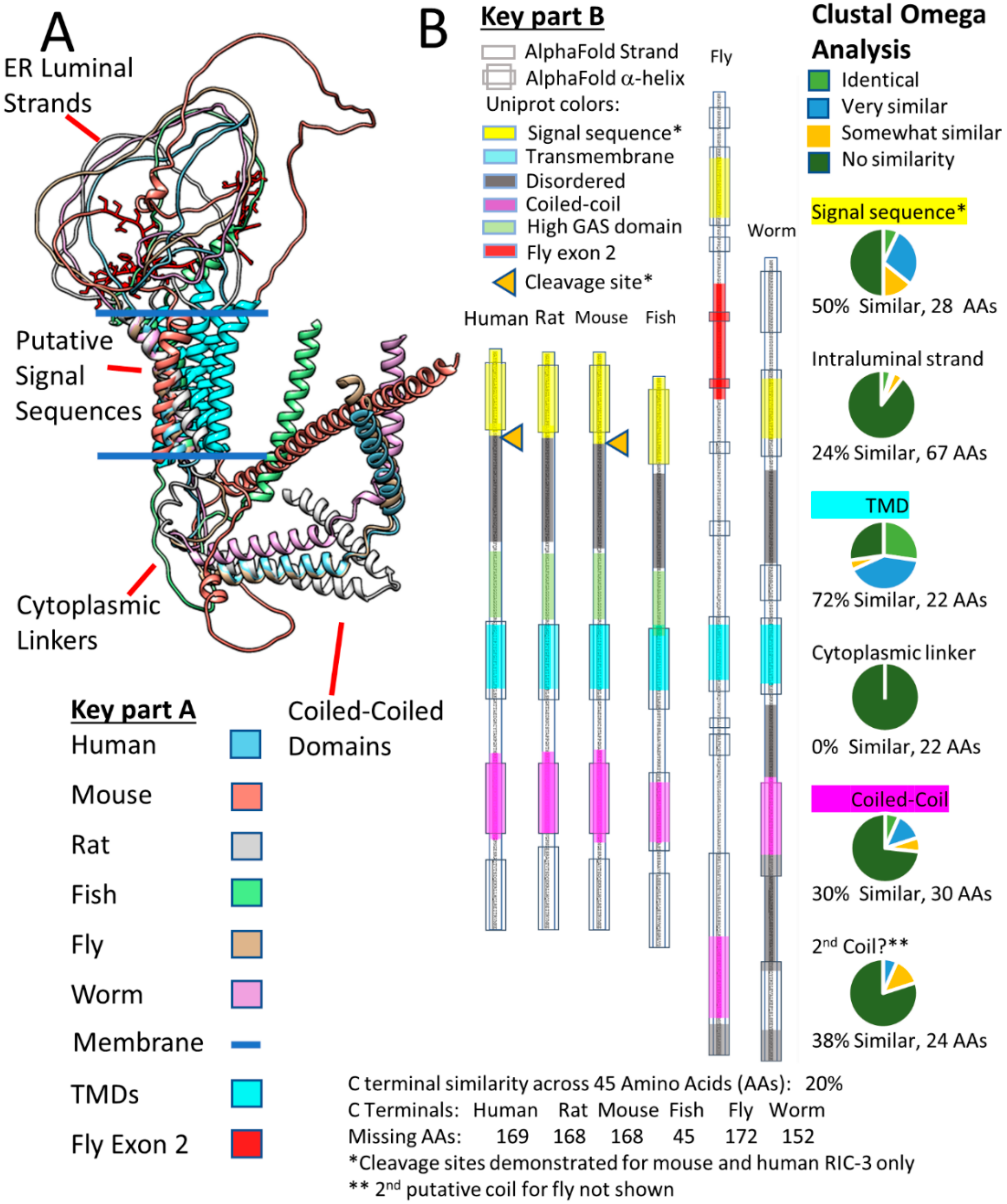
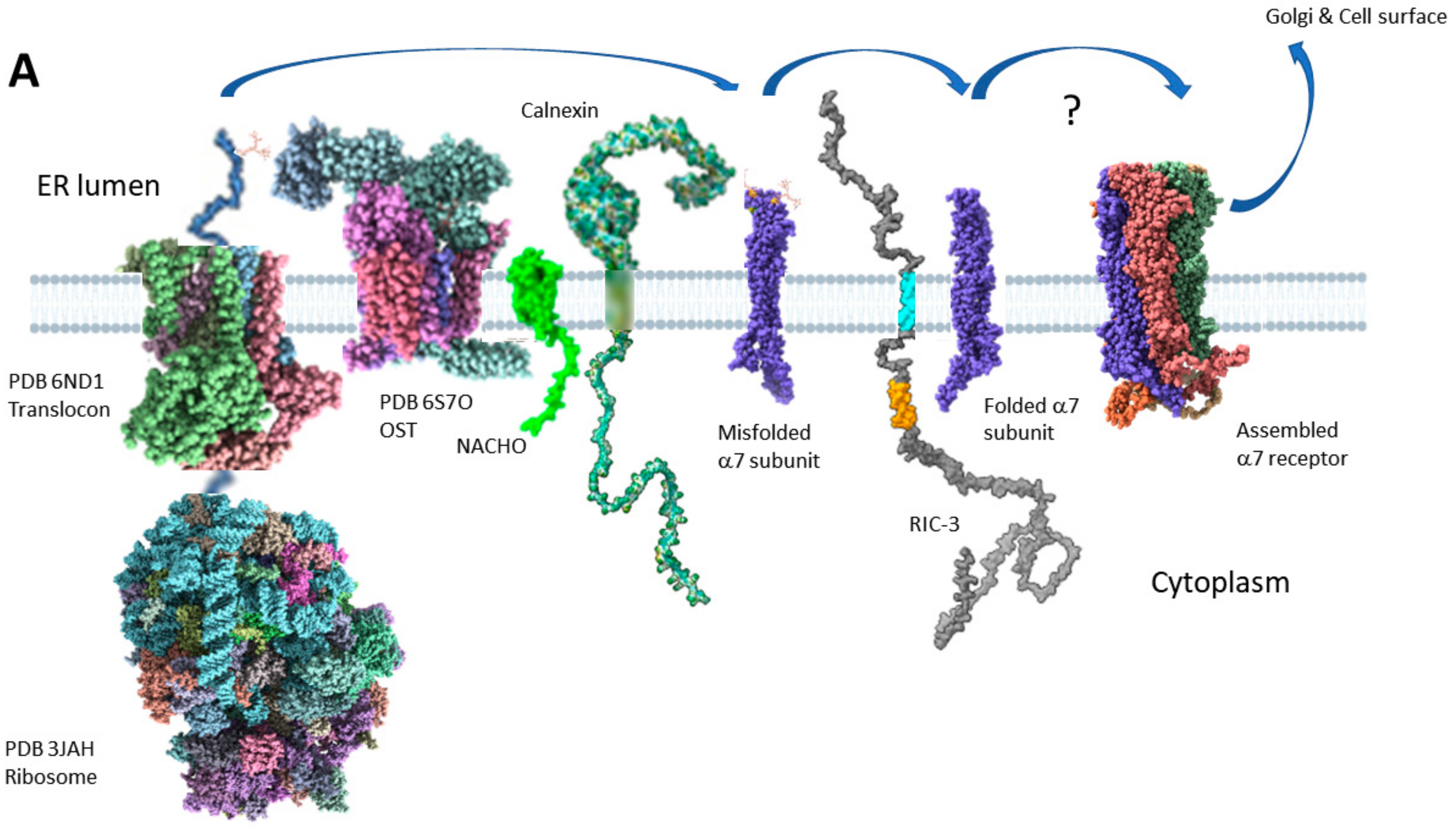
2.3. RIC-3 & NACHO Chaperone Effects and Structures (or Lack Thereof)
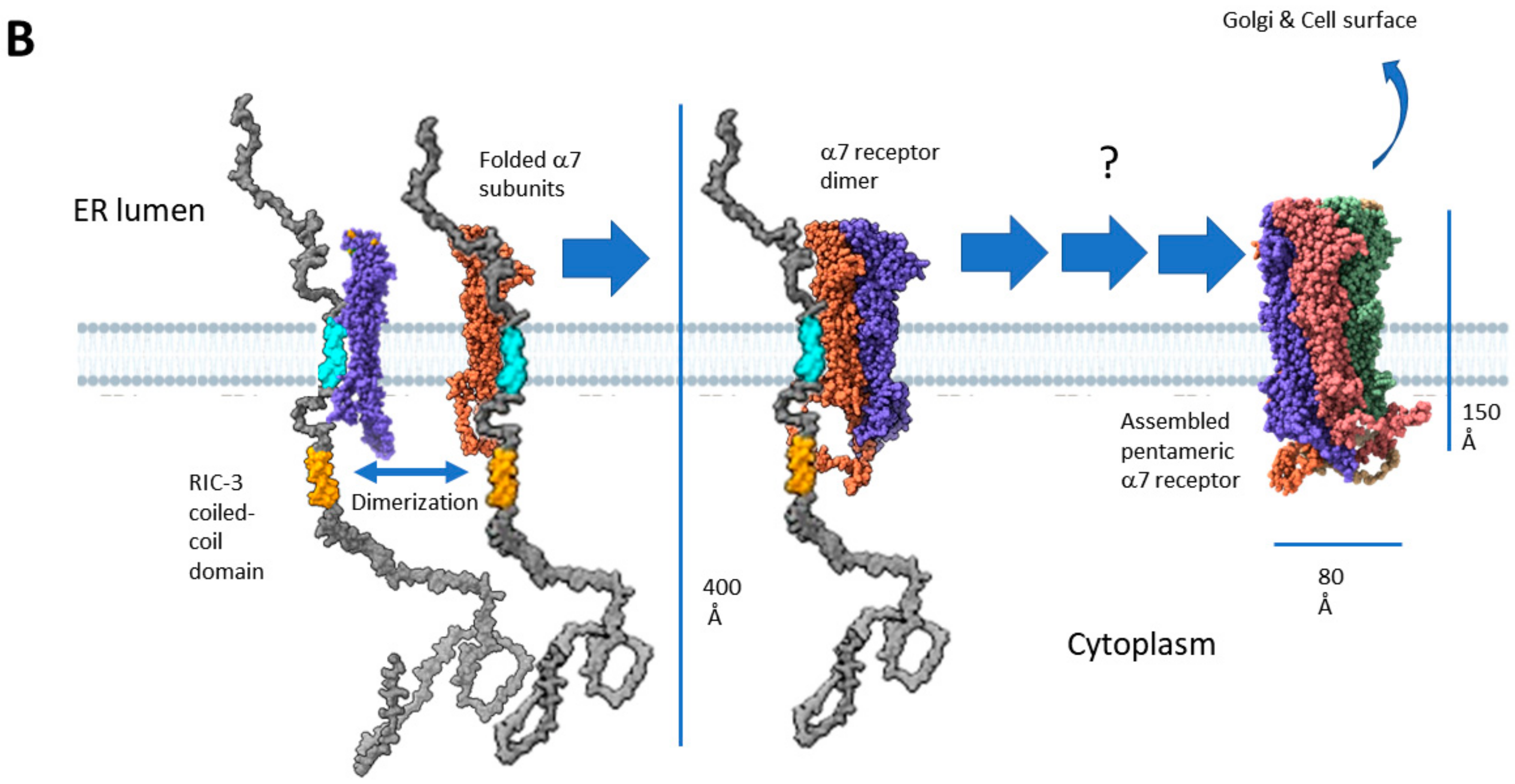
2.4. Two Models for How RIC-3 Helps Assemble α7 Receptors
2.5. Does RIC-3 Bind to α7 Receptors, and If So, Where?
2.5.1. RIC-3 Interactions with the α7 Receptor ECD
2.5.2. RIC-3 Interactions with α7 Receptor Transmembrane Domains
2.5.3. RIC-3 Interactions with the α7 Receptor Intracellular Domain
2.6. RIC-3 Integration with Other Chaperones and Regulators
3. Summary and Conclusions
4. Methods
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Overington, J.P.; Al-Lazikani, B.; Hopkins, A.L. How many drug targets are there? Nat. Rev. Drug Discov. 2006, 5, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Millar, N.S.; Harkness, P.C. Assembly and trafficking of nicotinic acetylcholine receptors (Review). Mol. Membr. Biol. 2008, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Zoli, M.; Pucci, S.; Vilella, A.; Gotti, C. Neuronal and Extraneuronal Nicotinic Acetylcholine Receptors. Curr. Neuropharmacol. 2018, 16, 338–349. [Google Scholar] [CrossRef] [PubMed]
- Corringer, P.J.; Poitevin, F.; Prevost, M.S.; Sauguet, L.; Delarue, M.; Changeux, J.P. Structure and pharmacology of pentameric receptor channels: From bacteria to brain. Structure 2012, 20, 941–956. [Google Scholar] [CrossRef] [Green Version]
- Williams, M.E.; Burton, B.; Urrutia, A.; Shcherbatko, A.; Chavez-Noriega, L.E.; Cohen, C.J.; Aiyar, J. Ric-3 promotes functional expression of the nicotinic acetylcholine receptor alpha7 subunit in mammalian cells. J. Biol. Chem. 2005, 280, 1257–1263. [Google Scholar] [CrossRef] [Green Version]
- Gu, S.; Matta, J.A.; Lord, B.; Harrington, A.W.; Sutton, S.W.; Davini, W.B.; Bredt, D.S. Brain α7 Nicotinic Acetylcholine Receptor Assembly Requires NACHO. Neuron 2016, 89, 948–955. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, D.; Lee, C.H.; Flood, D.; Marger, F.; Donnelly-Roberts, D. Therapeutic Potential of α7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2015, 67, 1025–1073. [Google Scholar] [CrossRef] [Green Version]
- Corradi, J.; Bouzat, C. Understanding the Bases of Function and Modulation of α7 Nicotinic Receptors: Implications for Drug Discovery. Mol. Pharmacol. 2016, 90, 288–299. [Google Scholar] [CrossRef] [Green Version]
- Papke, R.L.; Horenstein, N.A. Therapeutic Targeting of alpha7 Nicotinic Acetylcholine Receptors. Pharmacol. Rev. 2021, 73, 1118–1149. [Google Scholar] [CrossRef]
- Basak, S.; Gicheru, Y.; Rao, S.; Sansom, M.S.P.; Chakrapani, S. Cryo-EM reveals two distinct serotonin-bound conformations of full-length 5-HT. Nature 2018, 563, 270–274. [Google Scholar] [CrossRef]
- Morales-Perez, C.L.; Noviello, C.M.; Hibbs, R.E. X-ray structure of the human α4β2 nicotinic receptor. Nature 2016, 538, 411–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bondarenko, V.; Mowrey, D.D.; Tillman, T.S.; Seyoum, E.; Xu, Y.; Tang, P. NMR structures of the human α7 nAChR transmembrane domain and associated anesthetic binding sites. Biochim. Biophys. Acta 2014, 1838, 1389–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brejc, K.; van Dijk, W.J.; Klaassen, R.V.; Schuurmans, M.; van Der Oost, J.; Smit, A.B.; Sixma, T.K. Crystal structure of an ACh-binding protein reveals the ligand-binding doma.ain of nicotinic receptors. Nature 2001, 411, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Smit, A.B.; Syed, N.I.; Schaap, D.; van Minnen, J.; Klumperman, J.; Kits, K.S.; Lodder, H.; van der Schors, R.C.; van Elk, R.; Sorgedrager, B.; et al. A glia-derived acetylcholine-binding protein that modulates synaptic transmission. Nature 2001, 411, 261–268. [Google Scholar] [CrossRef]
- Ulens, C.; Akdemir, A.; Jongejan, A.; van Elk, R.; Bertrand, S.; Perrakis, A.; Leurs, R.; Smit, A.B.; Sixma, T.K.; Bertrand, D.; et al. Use of acetylcholine binding protein in the search for novel alpha7 nicotinic receptor ligands. In silico docking, pharmacological screening, and X-ray analysis. J. Med. Chem. 2009, 52, 2372–2383. [Google Scholar] [CrossRef] [PubMed]
- Noviello, C.M.; Gharpure, A.; Mukhtasimova, N.; Cabuco, R.; Baxter, L.; Borek, D.; Sine, S.M.; Hibbs, R.E. Structure and gating mechanism of the α7 nicotinic acetylcholine receptor. Cell 2021, 184, 2121–2134.e2113. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, S.; Zhou, Y.; Zhang, M.; Chen, H.; Eric Xu, H.; Sun, D.; Liu, L.; Tian, C. Structural basis of human α7 nicotinic acetylcholine receptor activation. Cell Res. 2021, 31, 713–716. [Google Scholar] [CrossRef]
- Bondarenko, V.; Wells, M.M.; Chen, Q.; Tillman, T.S.; Singewald, K.; Lawless, M.J.; Caporoso, J.; Brandon, N.; Coleman, J.A.; Saxena, S.; et al. Structures of highly flexible intracellular domain of human α7 nicotinic acetylcholine receptor. Nat. Commun. 2022, 13, 793. [Google Scholar] [CrossRef]
- Jumper, J.; Evans, R.; Pritzel, A.; Green, T.; Figurnov, M.; Ronneberger, O.; Tunyasuvunakool, K.; Bates, R.; Žídek, A.; Potapenko, A.; et al. Highly accurate protein structure prediction with AlphaFold. Nature 2021, 596, 583–589. [Google Scholar] [CrossRef]
- Tunyasuvunakool, K.; Adler, J.; Wu, Z.; Green, T.; Zielinski, M.; Žídek, A.; Bridgland, A.; Cowie, A.; Meyer, C.; Laydon, A.; et al. Highly accurate protein structure prediction for the human proteome. Nature 2021, 596, 590–596. [Google Scholar] [CrossRef]
- Bennett, H.M.; Lees, K.; Harper, K.M.; Jones, A.K.; Sattelle, D.B.; Wonnacott, S.; Wolstenholme, A.J. Xenopus laevis RIC-3 enhances the functional expression of the C. elegans homomeric nicotinic receptor, ACR-16, in Xenopus oocytes. J. Neurochem. 2012, 123, 911–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Yao, Y.; Tang, X.Q.; Wang, Z.Z. Mouse RIC-3, an endoplasmic reticulum chaperone, promotes assembly of the alpha7 acetylcholine receptor through a cytoplasmic coiled-coil domain. J. Neurosci. 2009, 29, 12625–12635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kweon, H.J.; Gu, S.; Witham, E.; Dhara, M.; Yu, H.; Mandon, E.D.; Jawhari, A.; Bredt, D.S. NACHO Engages N-Glycosylation ER Chaperone Pathways for α7 Nicotinic Receptor Assembly. Cell Rep. 2020, 32, 108025. [Google Scholar] [CrossRef] [PubMed]
- Crespi, A.; Colombo, S.F.; Gotti, C. Proteins and chemical chaperones involved in neuronal nicotinic receptor expression and function: An update. Br. J. Pharmacol 2018, 175, 1869–1879. [Google Scholar] [CrossRef]
- Miwa, J.M.; Anderson, K.R.; Hoffman, K.M. Lynx Prototoxins: Roles of Endogenous Mammalian Neurotoxin-Like Proteins in Modulating Nicotinic Acetylcholine Receptor Function to Influence Complex Biological Processes. Front. Pharmacol. 2019, 10, 343. [Google Scholar] [CrossRef]
- Matta, J.A.; Gu, S.; Davini, W.B.; Bredt, D.S. Nicotinic acetylcholine receptor redux: Discovery of accessories opens therapeutic vistas. Science 2021, 373, eabg6539. [Google Scholar] [CrossRef]
- Vallés, A.S.; Barrantes, F.J. Chaperoning α7 neuronal nicotinic acetylcholine receptors. Biochim. Biophys. Acta 2012, 1818, 718–729. [Google Scholar] [CrossRef] [Green Version]
- Bocquet, N.; Prado de Carvalho, L.; Cartaud, J.; Neyton, J.; Le Poupon, C.; Taly, A.; Grutter, T.; Changeux, J.P.; Corringer, P.J. A prokaryotic proton-gated ion channel from the nicotinic acetylcholine receptor family. Nature 2007, 445, 116–119. [Google Scholar] [CrossRef]
- Corringer, P.J.; Baaden, M.; Bocquet, N.; Delarue, M.; Dufresne, V.; Nury, H.; Prevost, M.; Van Renterghem, C. Atomic structure and dynamics of pentameric ligand-gated ion channels: New insight from bacterial homologues. J. Physiol. 2010, 588, 565–572. [Google Scholar] [CrossRef]
- Hilf, R.J.; Dutzler, R. X-ray structure of a prokaryotic pentameric ligand-gated ion channel. Nature 2008, 452, 375–379. [Google Scholar] [CrossRef]
- Hilf, R.J.; Dutzler, R. Structure of a potentially open state of a proton-activated pentameric ligand-gated ion channel. Nature 2009, 457, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chen, Q.; Willenbring, D.; Yoshida, K.; Tillman, T.; Kashlan, O.B.; Cohen, A.; Kong, X.P.; Xu, Y.; Tang, P. Structure of the pentameric ligand-gated ion channel ELIC cocrystallized with its competitive antagonist acetylcholine. Nat. Commun. 2012, 3, 714. [Google Scholar] [CrossRef] [PubMed]
- Tasneem, A.; Iyer, L.M.; Jakobsson, E.; Aravind, L. Identification of the prokaryotic ligand-gated ion channels and their implications for the mechanisms and origins of animal Cys-loop ion channels. Genome Biol. 2005, 6, R4. [Google Scholar] [CrossRef] [Green Version]
- Althoff, T.; Hibbs, R.E.; Banerjee, S.; Gouaux, E. X-ray structures of GluCl in apo states reveal a gating mechanism of Cys-loop receptors. Nature 2014, 512, 333–337. [Google Scholar] [CrossRef] [Green Version]
- Hibbs, R.E.; Gouaux, E. Principles of activation and permeation in an anion-selective Cys-loop receptor. Nature 2011, 474, 54–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, B.M.; Temburni, M.K.; Levey, M.S.; Bertrand, S.; Bertrand, D.; Jacob, M.H. The long internal loop of the alpha 3 subunit targets nAChRs to subdomains within individual synapses on neurons in vivo. Nat. Neurosci. 1998, 1, 557–562. [Google Scholar] [CrossRef] [Green Version]
- Borges, L.S.; Yechikhov, S.; Lee, Y.I.; Rudell, J.B.; Friese, M.B.; Burden, S.J.; Ferns, M.J. Identification of a motif in the acetylcholine receptor beta subunit whose phosphorylation regulates rapsyn association and postsynaptic receptor localization. J. Neurosci. 2008, 28, 11468–11476. [Google Scholar] [CrossRef] [Green Version]
- Temburni, M.K.; Blitzblau, R.C.; Jacob, M.H. Receptor targeting and heterogeneity at interneuronal nicotinic cholinergic synapses in vivo. J. Physiol. 2000, 525 Pt 1, 21–29. [Google Scholar] [CrossRef]
- Tsetlin, V.; Kuzmin, D.; Kasheverov, I. Assembly of nicotinic and other Cys-loop receptors. J. Neurochem. 2011, 116, 734–741. [Google Scholar] [CrossRef]
- Stokes, C.; Treinin, M.; Papke, R.L. Looking below the surface of nicotinic acetylcholine receptors. Trends Pharmacol. Sci. 2015, 36, 514–523. [Google Scholar] [CrossRef] [Green Version]
- King, J.R.; Nordman, J.C.; Bridges, S.P.; Lin, M.K.; Kabbani, N. Identification and Characterization of a G Protein-binding Cluster in α7 Nicotinic Acetylcholine Receptors. J. Biol. Chem. 2015, 290, 20060–20070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hucho, F.; Tsetlin, V.I.; Machold, J. The emerging three-dimensional structure of a receptor. The nicotinic acetylcholine receptor. Eur. J. Biochem. 1996, 239, 539–557. [Google Scholar] [CrossRef] [PubMed]
- Langlhofer, G.; Villmann, C. The Intracellular Loop of the Glycine Receptor: It’s not all about the Size. Front. Mol. Neurosci. 2016, 9, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferns, M. An Inside Job: Molecular Determinants for Postsynaptic Localization of Nicotinic Acetylcholine Receptors. Molecules 2021, 26, 3065. [Google Scholar] [CrossRef] [PubMed]
- Valor, L.M.; Mulet, J.; Sala, F.; Sala, S.; Ballesta, J.J.; Criado, M. Role of the large cytoplasmic loop of the alpha 7 neuronal nicotinic acetylcholine receptor subunit in receptor expression and function. Biochemistry 2002, 41, 7931–7938. [Google Scholar] [CrossRef]
- Dau, A.; Komal, P.; Truong, M.; Morris, G.; Evans, G.; Nashmi, R. RIC-3 differentially modulates α4β2 and α7 nicotinic receptor assembly, expression, and nicotine-induced receptor upregulation. BMC Neurosci. 2013, 14, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, M.; Bali, M.; Akabas, M.H. Modular design of Cys-loop ligand-gated ion channels: Functional 5-HT3 and GABA rho1 receptors lacking the large cytoplasmic M3M4 loop. J. Gen. Physiol. 2008, 131, 137–146. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, N.K.; Bali, M.; Akabas, M.H. Length and amino acid sequence of peptides substituted for the 5-HT3A receptor M3M4 loop may affect channel expression and desensitization. PLoS ONE 2012, 7, e35563. [Google Scholar] [CrossRef] [Green Version]
- Murray, T.A.; Liu, Q.; Whiteaker, P.; Wu, J.; Lukas, R.J. Nicotinic acetylcholine receptor alpha7 subunits with a C2 cytoplasmic loop yellow fluorescent protein insertion form functional receptors. Acta Pharmacol. Sin. 2009, 30, 828–841. [Google Scholar] [CrossRef] [Green Version]
- Pirayesh, E.; Stuebler, A.G.; Pandhare, A.; Jansen, M. Delineating the Site of Interaction of the 5-HT3a Receptor with the Chaperone Protein RIC-3. Biophys. J. 2020, 118, 934–943. [Google Scholar] [CrossRef]
- Gharpure, A.; Noviello, C.M.; Hibbs, R.E. Progress in nicotinic receptor structural biology. Neuropharmacology 2020, 171, 108086. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Lester, H.A.; Lummis, S.C. The structural basis of function in Cys-loop receptors. Q. Rev. Biophys. 2010, 43, 449–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krause, R.M.; Buisson, B.; Bertrand, S.; Corringer, P.J.; Galzi, J.L.; Changeux, J.P.; Bertrand, D. Ivermectin: A positive allosteric effector of the alpha7 neuronal nicotinic acetylcholine receptor. Mol. Pharmacol. 1998, 53, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawe, G.B.; Yu, H.; Gu, S.; Blackler, A.N.; Matta, J.A.; Siuda, E.R.; Rex, E.B.; Bredt, D.S. α7 nicotinic acetylcholine receptor upregulation by anti-apoptotic Bcl-2 proteins. Nat. Commun. 2019, 10, 2746. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.; Mulet, J.; Gutiérrez, L.M.; Ortiz, J.A.; Castelán, F.; Gerber, S.; Sala, S.; Sala, F.; Criado, M. Dual role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J. Biol. Chem. 2005, 280, 27062–27068. [Google Scholar] [CrossRef] [Green Version]
- Castillo, M.; Mulet, J.; Gutierrez, L.M.; Ortiz, J.A.; Castelan, F.; Gerber, S.; Sala, S.; Sala, F.; Criado, M. Role of the RIC-3 protein in trafficking of serotonin and nicotinic acetylcholine receptors. J. Mol. Neurosci. 2006, 30, 153–156. [Google Scholar] [CrossRef]
- Kaji, M.D.; Geary, T.G.; Beech, R.N. A Functional Comparison of Homopentameric Nicotinic Acetylcholine Receptors (ACR-16) Receptors from Necator americanus and Ancylostoma ceylanicum. Front. Mol. Neurosci. 2020, 13, 601102. [Google Scholar] [CrossRef]
- Hansen, T.V.A.; Grencis, R.K.; Issouf, M.; Neveu, C.; Charvet, C.L. Functional Characterization of the Oxantel-Sensitive Acetylcholine Receptor from. Pharmaceuticals 2021, 14, 698. [Google Scholar] [CrossRef]
- Hansen, T.V.A.; Cirera, S.; Neveu, C.; Courtot, E.; Charvet, C.L.; Calloe, K.; Klaerke, D.A.; Martin, R.J. The narrow-spectrum anthelmintic oxantel is a potent agonist of a novel acetylcholine receptor subtype in whipworms. PLoS Pathog. 2021, 17, e1008982. [Google Scholar] [CrossRef]
- Jones, A.K.; Raymond-Delpech, V.; Thany, S.H.; Gauthier, M.; Sattelle, D.B. The nicotinic acetylcholine receptor gene family of the honey bee, Apis mellifera. Genome Res. 2006, 16, 1422–1430. [Google Scholar] [CrossRef] [Green Version]
- Mongan, N.P.; Jones, A.K.; Smith, G.R.; Sansom, M.S.; Sattelle, D.B. Novel alpha7-like nicotinic acetylcholine receptor subunits in the nematode Caenorhabditis elegans. Protein Sci. 2002, 11, 1162–1171. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, S.; Kashyap, S.S.; Martin, R.J.; Robertson, A.P. Advances in our understanding of nematode ion channels as potential anthelmintic targets. Int. J. Parasitol. Drugs Drug Resist. 2022, 18, 52–86. [Google Scholar] [CrossRef] [PubMed]
- Touroutine, D.; Fox, R.M.; Von Stetina, S.E.; Burdina, A.; Miller, D.M.; Richmond, J.E. acr-16 encodes an essential subunit of the levamisole-resistant nicotinic receptor at the Caenorhabditis elegans neuromuscular junction. J. Biol. Chem. 2005, 280, 27013–27021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sattelle, D.B.; Buckingham, S.D.; Akamatsu, M.; Matsuda, K.; Pienaar, I.S.; Jones, A.K.; Sattelle, B.M.; Almond, A.; Blundell, C.D. Comparative pharmacology and computational modelling yield insights into allosteric modulation of human alpha7 nicotinic acetylcholine receptors. Biochem. Pharmacol. 2009, 78, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Ballivet, M.; Alliod, C.; Bertrand, S.; Bertrand, D. Nicotinic acetylcholine receptors in the nematode Caenorhabditis elegans. J. Mol. Biol. 1996, 258, 261–269. [Google Scholar] [CrossRef]
- Abongwa, M.; Buxton, S.K.; Courtot, E.; Charvet, C.L.; Neveu, C.; McCoy, C.J.; Verma, S.; Robertson, A.P.; Martin, R.J. Pharmacological profile of Ascaris suum ACR-16, a new homomeric nicotinic acetylcholine receptor widely distributed in Ascaris tissues. Br. J. Pharmacol. 2016, 173, 2463–2477. [Google Scholar] [CrossRef] [Green Version]
- Bentley, G.N.; Jones, A.K.; Oliveros Parra, W.G.; Agnew, A. ShAR1alpha and ShAR1beta: Novel putative nicotinic acetylcholine receptor subunits from the platyhelminth blood fluke Schistosoma. Gene 2004, 329, 27–38. [Google Scholar] [CrossRef]
- Jones, A.K.; Davis, P.; Hodgkin, J.; Sattelle, D.B. The nicotinic acetylcholine receptor gene family of the nematode Caenorhabditis elegans: An update on nomenclature. Invertebr. Neurosci. 2007, 7, 129–131. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, H.C.; Yassin, L.; Farah, H.; Michaeli, A.; Eshel, M.; Treinin, M. RIC-3 affects properties and quantity of nicotinic acetylcholine receptors via a mechanism that does not require the coiled-coil domains. J. Biol. Chem. 2005, 280, 28053–28060. [Google Scholar] [CrossRef] [Green Version]
- Ben-Ami, H.C.; Biala, Y.; Farah, H.; Elishevitz, E.; Battat, E.; Treinin, M. Receptor and Subunit Specific Interactions of RIC-3 with Nicotinic Acetylcholine Receptors. Biochemistry 2009, 48, 12329–12336. [Google Scholar] [CrossRef]
- Blanton, M.P.; Cohen, J.B. Mapping the lipid-exposed regions in the Torpedo californica nicotinic acetylcholine receptor. Biochemistry 1992, 31, 3738–3750. [Google Scholar] [CrossRef] [PubMed]
- Baenziger, J.E.; Hénault, C.M.; Therien, J.P.; Sun, J. Nicotinic acetylcholine receptor-lipid interactions: Mechanistic insight and biological function. Biochim. Biophys. Acta 2015, 1848, 1806–1817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hénault, C.M.; Sun, J.; Therien, J.P.; daCosta, C.J.; Carswell, C.L.; Labriola, J.M.; Juranka, P.F.; Baenziger, J.E. The role of the M4 lipid-sensor in the folding, trafficking, and allosteric modulation of nicotinic acetylcholine receptors. Neuropharmacology 2015, 96, 157–168. [Google Scholar] [CrossRef] [PubMed]
- Thompson, M.J.; Domville, J.A.; Edrington, C.H.; Venes, A.; Giguère, P.M.; Baenziger, J.E. Distinct functional roles for the M4 α-helix from each homologous subunit in the hetero-pentameric ligand-gated ion channel nAChR. J. Biol. Chem. 2022, 298, 102104. [Google Scholar] [CrossRef] [PubMed]
- Mesoy, S.; Jeffreys, J.; Lummis, S.C.R. Characterization of Residues in the 5HT3 Receptor M4 Region That Contribute to Function. ACS Chem. Neurosci. 2019, 10, 3167–3172. [Google Scholar] [CrossRef]
- Mesoy, S.M.; Lummis, S.C.R. M4, the Outermost Helix, is Extensively Involved in Opening of the α4β2 nACh Receptor. ACS Chem. Neurosci. 2021, 12, 133–139. [Google Scholar] [CrossRef]
- da Costa Couto, A.R.G.M.; Price, K.L.; Mesoy, S.; Capes, E.; Lummis, S.C.R. The M4 Helix Is Involved in α7 nACh Receptor Function. ACS Chem. Neurosci. 2020, 11, 1406–1412. [Google Scholar] [CrossRef]
- Unwin, N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J. Mol. Biol. 2005, 346, 967–989. [Google Scholar] [CrossRef]
- Unwin, N. Nicotinic acetylcholine receptor and the structural basis of neuromuscular transmission: Insights from Torpedo postsynaptic membranes. Q. Rev. Biophys. 2013, 46, 283–322. [Google Scholar] [CrossRef] [Green Version]
- Rahman, M.M.; Teng, J.; Worrell, B.T.; Noviello, C.M.; Lee, M.; Karlin, A.; Stowell, M.H.B.; Hibbs, R.E. Structure of the Native Muscle-type Nicotinic Receptor and Inhibition by Snake Venom Toxins. Neuron 2020, 106, 952–962.e5. [Google Scholar] [CrossRef]
- Anderson, D.J.; Blobel, G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc. Natl. Acad. Sci. USA 1981, 78, 5598–5602. [Google Scholar] [CrossRef] [Green Version]
- Anderson, D.J.; Walter, P.; Blobel, G. Signal recognition protein is required for the integration of acetylcholine receptor delta subunit, a transmembrane glycoprotein, into the endoplasmic reticulum membrane. J. Cell. Biol. 1982, 93, 501–506. [Google Scholar] [CrossRef] [Green Version]
- Green, W.N.; Claudio, T. Acetylcholine receptor assembly: Subunit folding and oligomerization occur sequentially. Cell 1993, 74, 57–69. [Google Scholar] [CrossRef]
- Green, W.N.; Millar, N.S. Ion-channel assembly. Trends Neurosci. 1995, 18, 280–287. [Google Scholar] [CrossRef]
- Green, W.N. Ion channel assembly: Creating structures that function. J. Gen. Physiol. 1999, 113, 163–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, M.M.; Lindstrom, J.; Merlie, J.P. Formation of the alpha-bungarotoxin binding site and assembly of the nicotinic acetylcholine receptor subunits occur in the endoplasmic reticulum. J. Biol. Chem. 1987, 262, 4367–4376. [Google Scholar] [CrossRef]
- Karlin, A.; Holtzman, E.; Yodh, N.; Lobel, P.; Wall, J.; Hainfeld, J. The arrangement of the subunits of the acetylcholine receptor of Torpedo californica. J. Biol. Chem. 1983, 258, 6678–6681. [Google Scholar] [CrossRef]
- Blount, P.; Merlie, J.P. Mutational analysis of mu.u.u.u.uscle nicotinic acetylcholine receptor subunit assembly. J. Cell. Biol. 1990, 111, 2613–2622. [Google Scholar] [CrossRef] [Green Version]
- Green, W.N.; Wanamaker, C.P. The role of the cystine loop in acetylcholine receptor assembly. J. Biol. Chem. 1997, 272, 20945–20953. [Google Scholar] [CrossRef] [Green Version]
- Rickert, K.W.; Imperiali, B. Analysis of the conserved glycosylation site in the nicotinic acetylcholine receptor: Potential roles in complex assembly. Chem. Biol. 1995, 2, 751–759. [Google Scholar] [CrossRef] [Green Version]
- Dellisanti, C.D.; Yao, Y.; Stroud, J.C.; Wang, Z.Z.; Chen, L. Crystal structure of the extracellular domain of nAChR alpha1 bound to alpha-bungarotoxin at 1.94 A resolution. Nat. Neurosci. 2007, 10, 953–962. [Google Scholar] [CrossRef] [PubMed]
- Kao, P.N.; Karlin, A. Acetylcholine receptor binding site contains a disulfide cross-link between adjacent half-cystinyl residues. J. Biol. Chem. 1986, 261, 8085–8088. [Google Scholar] [CrossRef]
- Olson, E.N.; Glaser, L.; Merlie, J.P. Alpha and beta subunits of the nicotinic acetylcholine receptor contain covalently bound lipid. J. Biol. Chem. 1984, 259, 5364–5367. [Google Scholar] [CrossRef]
- Alexander, J.K.; Govind, A.P.; Drisdel, R.C.; Blanton, M.P.; Vallejo, Y.; Lam, T.T.; Green, W.N. Palmitoylation of nicotinic acetylcholine receptors. J. Mol. Neurosci. 2010, 40, 12–20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blount, P.; Merlie, J.P. BIP associates with newly synthesized subunits of the mouse muscle nicotinic receptor. J. Cell. Biol. 1991, 113, 1125–1132. [Google Scholar] [CrossRef] [Green Version]
- Paulson, H.L.; Ross, A.F.; Green, W.N.; Claudio, T. Analysis of early events in acetylcholine receptor assembly. J. Cell. Biol. 1991, 113, 1371–1384. [Google Scholar] [CrossRef] [Green Version]
- Gelman, M.S.; Chang, W.; Thomas, D.Y.; Bergeron, J.J.; Prives, J.M. Role of the endoplasmic reticulum chaperone calnexin in subunit folding and assembly of nicotinic acetylcholine receptors. J. Biol. Chem. 1995, 270, 15085–15092. [Google Scholar] [CrossRef] [Green Version]
- Keller, S.H.; Lindstrom, J.; Taylor, P. Involvement of the chaperone protein calnexin and the acetylcholine receptor beta-subunit in the assembly and cell surface expression of the receptor. J. Biol. Chem. 1996, 271, 22871–22877. [Google Scholar] [CrossRef] [Green Version]
- Wanamaker, C.P.; Green, W.N. N-linked glycosylation is required for nicotinic receptor assembly but not for subunit associations with calnexin. J. Biol. Chem. 2005, 280, 33800–33810. [Google Scholar] [CrossRef] [Green Version]
- Merlie, J.P.; Smith, M.M. Synthesis and assembly of acetylcholine receptor, a multisubunit membrane glycoprotein. J. Membr. Biol. 1986, 91, 1–10. [Google Scholar] [CrossRef]
- Smith, M.M.; Schlesinger, S.; Lindstrom, J.; Merlie, J.P. The effects of inhibiting oligosaccharide trimming by 1-deoxynojirimycin on the nicotinic acetylcholine receptor. J. Biol. Chem. 1986, 261, 14825–14832. [Google Scholar] [CrossRef]
- Moretti, M.; Zoli, M.; George, A.A.; Lukas, R.J.; Pistillo, F.; Maskos, U.; Whiteaker, P.; Gotti, C. The novel α7β2-nicotinic acetylcholine receptor subtype is expressed in mouse and human basal forebrain: Biochemical and pharmacological characterization. Mol. Pharmacol. 2014, 86, 306–317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borroni, V.; Barrantes, F.J. Homomeric and Heteromeric α7 Nicotinic Acetylcholine Receptors in Health and Some Central Nervous System Diseases. Membranes 2021, 11, 664. [Google Scholar] [CrossRef]
- Rex, E.B.; Shukla, N.; Gu, S.; Bredt, D.; DiSepio, D. A Genome-Wide Arrayed cDNA Screen to Identify Functional Modulators of α7 Nicotinic Acetylcholine Receptors. SLAS Discov. 2017, 22, 155–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hurst, R.S.; Hajós, M.; Raggenbass, M.; Wall, T.M.; Higdon, N.R.; Lawson, J.A.; Rutherford-Root, K.L.; Berkenpas, M.B.; Hoffmann, W.E.; Piotrowski, D.W.; et al. A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: In vitro and in vivo characterization. J. Neurosci. 2005, 25, 4396–4405. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, J.G.; Nemecz, Á.; Nguyen, Q.T.; Muller, A.; Schroeder, L.F.; Talley, T.T.; Lindstrom, J.; Kleinfeld, D.; Taylor, P. Characterizing ligand-gated ion channel receptors with genetically encoded Ca2++ sensors. PLoS ONE 2011, 6, e16519. [Google Scholar] [CrossRef]
- Roncarati, R.; Seredenina, T.; Jow, B.; Jow, F.; Papini, S.; Kramer, A.; Bothmann, H.; Dunlop, J.; Terstappen, G.C. Functional properties of alpha7 nicotinic acetylcholine receptors co-expressed with RIC-3 in a stable recombinant CHO-K1 cell line. Assay Drug Dev. Technol. 2008, 6, 181–193. [Google Scholar] [CrossRef]
- Andersen, N.; Corradi, J.; Sine, S.M.; Bouzat, C. Stoichiometry for activation of neuronal α7 nicotinic receptors. Proc. Natl. Acad. Sci. USA 2013, 110, 20819–20824. [Google Scholar] [CrossRef] [Green Version]
- Eisele, J.L.; Bertrand, S.; Galzi, J.L.; Devillers-Thiery, A.; Changeux, J.P.; Bertrand, D. Chimaeric nicotinic-serotonergic receptor combines distinct ligand binding and channel specificities. Nature 1993, 366, 479–483. [Google Scholar] [CrossRef]
- Gee, V.J.; Kracun, S.; Cooper, S.T.; Gibb, A.J.; Millar, N.S. Identification of domains influencing assembly and ion channel properties in alpha 7 nicotinic receptor and 5-HT3 receptor subunit chimaeras. Br. J. Pharmacol. 2007, 152, 501–512. [Google Scholar] [CrossRef]
- Kracun, S.; Harkness, P.C.; Gibb, A.J.; Millar, N.S. Influence of the M3-M4 intracellular domain upon nicotinic acetylcholine receptor assembly, targeting and function. Br. J. Pharmacol. 2008, 153, 1474–1484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Craig, P.J.; Bose, S.; Zwart, R.; Beattie, R.E.; Folly, E.A.; Johnson, L.R.; Bell, E.; Evans, N.M.; Benedetti, G.; Pearson, K.H.; et al. Stable expression and characterisation of a human alpha 7 nicotinic subunit chimera: A tool for functional high-throughput screening. Eur. J. Pharmacol. 2004, 502, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Campos-Caro, A.; Sala, S.; Ballesta, J.J.; Vicente-Agulló, F.; Criado, M.; Sala, F. A single residue in the M2-M3 loop is a major determinant of coupling between binding and gating in neuronal nicotinic receptors. Proc. Natl. Acad. Sci. USA 1996, 93, 6118–6123. [Google Scholar] [CrossRef] [Green Version]
- Quiram, P.A.; Sine, S.M. Identification of residues in the neuronal alpha7 acetylcholine receptor that confer selectivity for conotoxin ImI. J. Biol. Chem. 1998, 273, 11001–11006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Guzmán, M.; Sala, F.; Sala, S.; Campos-Caro, A.; Criado, M. Role of two acetylcholine receptor subunit domains in homomer formation and intersubunit recognition, as revealed by alpha 3 and alpha 7 subunit chimeras. Biochemistry 1994, 33, 15198–15203. [Google Scholar] [CrossRef] [PubMed]
- Garg, B.K.; Loring, R.H. Evaluating Commercially Available Antibodies for Rat α7 Nicotinic Acetylcholine Receptors. J. Histochem. Cytochem. 2017, 65, 499–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Couturier, S.; Bertrand, D.; Matter, J.M.; Hernandez, M.C.; Bertrand, S.; Millar, N.; Valera, S.; Barkas, T.; Ballivet, M. A neuronal nicotinic acetylcholine receptor subunit (alpha 7) is developmentally regulated and forms a homo-oligomeric channel blocked by alpha-BTX. Neuron 1990, 5, 847–856. [Google Scholar] [CrossRef] [Green Version]
- Kassner, P.D.; Berg, D.K. Differences in the fate of neuronal acetylcholine receptor protein expressed in neurons and stably transfected cells. J. Neurobiol. 1997, 33, 968–982. [Google Scholar] [CrossRef]
- Cooper, S.T.; Millar, N.S. Host cell-specific folding and assembly of the neuronal nicotinic acetylcholine receptor alpha7 subunit. J. Neurochem. 1997, 68, 2140–2151. [Google Scholar] [CrossRef]
- Rangwala, F.; Drisdel, R.C.; Rakhilin, S.; Ko, E.; Atluri, P.; Harkins, A.B.; Fox, A.P.; Salman, S.S.; Green, W.N. Neuronal alpha-bungarotoxin receptors differ structurally from other nicotinic acetylcholine receptors. J. Neurosci. 1997, 17, 8201–8212. [Google Scholar] [CrossRef] [Green Version]
- Sweileh, W.; Wenberg, K.; Xu, J.; Forsayeth, J.; Hardy, S.; Loring, R.H. Multistep expression and assembly of neuronal nicotinic receptors is both host-cell- and receptor-subtype-dependent. Brain Res. Mol. Brain Res. 2000, 75, 293–302. [Google Scholar] [CrossRef]
- Nguyen, M.; Alfonso, A.; Johnson, C.D.; Rand, J.B. Caenorhabditis elegans mutants resistant to inhibitors of acetylcholinesterase. Genetics 1995, 140, 527–535. [Google Scholar] [CrossRef] [PubMed]
- Halevi, S.; McKay, J.; Palfreyman, M.; Yassin, L.; Eshel, M.; Jorgensen, E.; Treinin, M. The C. elegans ric-3 gene is required for maturation of nicotinic acetylcholine receptors. EMBO J. 2002, 21, 1012–1020. [Google Scholar] [CrossRef] [Green Version]
- Cheng, A.; Bollan, K.A.; Greenwood, S.M.; Irving, A.J.; Connolly, C.N. Differential subcellular localization of RIC-3 isoforms and their role in determining 5-HT3 receptor composition. J. Biol. Chem. 2007, 282, 26158–26166. [Google Scholar] [CrossRef] [Green Version]
- Millar, N.S. RIC-3: A nicotinic acetylcholine receptor chaperone. Br. J. Pharmacol. 2008, 153 (Suppl. 1), S177–S183. [Google Scholar] [CrossRef] [Green Version]
- Halevi, S.; Yassin, L.; Eshel, M.; Sala, F.; Sala, S.; Criado, M.; Treinin, M. Conservation within the RIC-3 gene family. Effectors of mammalian nicotinic acetylcholine receptor expression. J. Biol. Chem. 2003, 278, 34411–34417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Treinin, M.; Jin, Y. Cholinergic transmission in C. elegans: Functions, diversity, and maturation of ACh-activated ion channels. J. Neurochem. 2021, 158, 1274–1291. [Google Scholar] [CrossRef]
- Quik, M.; Choremis, J.; Komourian, J.; Lukas, R.J.; Puchacz, E. Similarity between rat brain nicotinic alpha-bungarotoxin receptors and stably expressed alpha-bungarotoxin binding sites. J. Neurochem. 1996, 67, 145–154. [Google Scholar] [CrossRef]
- Koperniak, T.M.; Garg, B.K.; Boltax, J.; Loring, R.H. Cell-specific effects on surface α7 nicotinic receptor expression revealed by over-expression and knockdown of rat RIC3 protein. J. Neurochem. 2013, 124, 300–309. [Google Scholar] [CrossRef]
- Matta, J.A.; Gu, S.; Davini, W.B.; Lord, B.; Siuda, E.R.; Harrington, A.W.; Bredt, D.S. NACHO Mediates Nicotinic Acetylcholine Receptor Function throughout the Brain. Cell Rep. 2017, 19, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Castelán, F.; Castillo, M.; Mulet, J.; Sala, S.; Sala, F.; Domínguez Del Toro, E.; Criado, M. Molecular characterization and localization of the RIC-3 protein, an effector of nicotinic acetylcholine receptor expression. J. Neurochem. 2008, 105, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, A.; Vinayakamoorthy, R.M.; Garg, B.K.; Thummapudi, J.P.; Oza, G.; Adhikari, K.; Agarwal, A.; Dalvi, P.; Iyer, S.; Thulasi Raman, S.; et al. Why Does Knocking out NACHO, but not RIC3, Completely Block Expression of α7 Nicotinic Receptors in Mouse Brain? Biomolecules 2020, 10, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol. 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Chetkovich, D.M.; Petralia, R.S.; Sweeney, N.T.; Kawasaki, Y.; Wenthold, R.J.; Bredt, D.S.; Nicoll, R.A. Stargazin regulates synaptic targeting of AMPA receptors by two distinct mechanisms. Nature 2000, 408, 936–943. [Google Scholar] [CrossRef]
- Vandenberghe, W.; Nicoll, R.A.; Bredt, D.S. Interaction with the unfolded protein response reveals a role for stargazin in biosynthetic AMPA receptor transport. J. Neurosci. 2005, 25, 1095–1102. [Google Scholar] [CrossRef]
- Biala, Y.; Liewald, J.F.; Ben-Ami, H.C.; Gottschalk, A.; Treinin, M. The conserved RIC-3 coiled-coil domain mediates receptor-specific interactions with nicotinic acetylcholine receptors. Mol. Biol. Cell 2009, 20, 1419–1427. [Google Scholar] [CrossRef] [Green Version]
- Lansdell, S.J.; Collins, T.; Yabe, A.; Gee, V.J.; Gibb, A.J.; Millar, N.S. Host-cell specific effects of the nicotinic acetylcholine receptor chaperone RIC-3 revealed by a comparison of human and Drosophila RIC-3 homologues. J. Neurochem. 2008, 105, 1573–1581. [Google Scholar] [CrossRef]
- Lansdell, S.J.; Gee, V.J.; Harkness, P.C.; Doward, A.I.; Baker, E.R.; Gibb, A.J.; Millar, N.S. RIC-3 enhances functional expression of multiple nicotinic acetylcholine receptor subtypes in mammalian cells. Mol. Pharmacol. 2005, 68, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Akopian, D.; Shen, K.; Zhang, X.; Shan, S.O. Sign.nal recognition particle: An essential protein-targeting machine. Annu. Rev. Biochem. 2013, 82, 693–721. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Cabanos, C.; Rapoport, T.A. Structure of the post-translational protein translocation machinery of the ER membrane. Nature 2019, 566, 136–139. [Google Scholar] [CrossRef]
- Voss, M.; Schröder, B.; Fluhrer, R. Mechanism, specificity, and physiology of signal peptide peptidase (SPP) and SPP-like proteases. Biochim. Biophys. Acta 2013, 1828, 2828–2839. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanty, S.; Chaudhary, B.P.; Zoetewey, D. Structural Insight into the Mechanism of N-Linked Glycosylation by Oligosaccharyltransferase. Biomolecules 2020, 10, 624. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, D.; Dang, H.; Patrick, J.W. Contributions of N-linked glycosylation to the expression of a functional alpha7-nicotinic receptor in Xenopus oocytes. J. Neurochem. 1998, 70, 349–357. [Google Scholar] [CrossRef]
- Bañó-Polo, M.; Baeza-Delgado, C.; Tamborero, S.; Hazel, A.; Grau, B.; Nilsson, I.; Whitley, P.; Gumbart, J.C.; von Heijne, G.; Mingarro, I. Transmembrane but not soluble helices fold inside the ribosome tunnel. Nat. Commun. 2018, 9, 5246. [Google Scholar] [CrossRef]
- Egea, P.F.; Stroud, R.M. Lateral opening of a translocon upon entry of protein suggests the mechanism of insertion into membranes. Proc. Natl. Acad. Sci. USA 2010, 107, 17182–17187. [Google Scholar] [CrossRef] [Green Version]
- Adams, B.M.; Canniff, N.P.; Guay, K.P.; Hebert, D.N. The Role of Endoplasmic Reticulum Chaperones in Protein Folding and Quality Control. Prog. Mol. Subcell. Biol. 2021, 59, 27–50. [Google Scholar] [CrossRef]
- Schrag, J.D.; Bergeron, J.J.; Li, Y.; Borisova, S.; Hahn, M.; Thomas, D.Y.; Cygler, M. The Structure of calnexin, an ER chaperone involved in quality control of protein folding. Mol. Cell 2001, 8, 633–644. [Google Scholar] [CrossRef]
- Kozlov, G.; Gehring, K. Calnexin cycle—Structural features of the ER chaperone system. FEBS J. 2020, 287, 4322–4340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozlov, G.; Pocanschi, C.L.; Rosenauer, A.; Bastos-Aristizabal, S.; Gorelik, A.; Williams, D.B.; Gehring, K. Structural basis of carbohydrate recognition by calreticulin. J. Biol. Chem. 2010, 285, 38612–38620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dunckley, T.; Wu, J.; Zhao, L.; Lukas, R.J. Mutational analysis of roles for extracellular cysteine residues in the assembly and function of human alpha 7-nicotinic acetylcholine receptors. Biochemistry 2003, 42, 870–876. [Google Scholar] [CrossRef]
- Drisdel, R.C.; Manzana, E.; Green, W.N. The role of palmitoylation in functional expression of nicotinic alpha7 receptors. J. Neurosci. 2004, 24, 10502–10510. [Google Scholar] [CrossRef] [PubMed]
- Dhara, M.; Matta, J.A.; Lei, M.; Knowland, D.; Yu, H.; Gu, S.; Bredt, D.S. Polyamine regulation of ion channel assembly and implications for nicotinic acetylcholine receptor pharmacology. Nat. Commun. 2020, 11, 2799. [Google Scholar] [CrossRef] [PubMed]
- Alexander, J.K.; Sagher, D.; Krivoshein, A.V.; Criado, M.; Jefford, G.; Green, W.N. Ric-3 promotes alpha7 nicotinic receptor assembly and trafficking through the ER subcompartment of dendrites. J. Neurosci. 2010, 30, 10112–10126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lansdell, S.J.; Millar, N.S. Molecular characterization of Dalpha6 and Dalpha7 nicotinic acetylcholine receptor subunits from Drosophila: Formation of a high-affinity alpha-bungarotoxin binding site revealed by expression of subunit chimeras. J. Neurochem. 2004, 90, 479–489. [Google Scholar] [CrossRef] [PubMed]
- Seredenina, T.; Ferraro, T.; Terstappen, G.C.; Caricasole, A.; Roncarati, R. Molecular cloning and characterization of a novel human variant of RIC-3, a putative chaperone of nicotinic acetylcholine receptors. Biosci. Rep. 2008, 28, 299–306. [Google Scholar] [CrossRef]
- Ben-David, Y.; Mizrachi, T.; Kagan, S.; Krisher, T.; Cohen, E.; Brenner, T.; Treinin, M. RIC-3 expression and splicing regulate nAChR functional expression. Mol. Brain 2016, 9, 47. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, M.J.; Paulo, J.A.; Hawrot, E. Proteomic Investigation of Murine Neuronal α7-Nicotinic Acetylcholine Receptor Interacting Proteins. J. Proteome Res. 2018, 17, 3959–3975. [Google Scholar] [CrossRef] [Green Version]
- Paulo, J.A.; Brucker, W.J.; Hawrot, E. Proteomic analysis of an alpha7 nicotinic acetylcholine receptor interactome. J. Proteome Res. 2009, 8, 1849–1858. [Google Scholar] [CrossRef] [Green Version]
- Mulcahy, M.J.; Blattman, S.B.; Barrantes, F.J.; Lukas, R.J.; Hawrot, E. Resistance to Inhibitors of Cholinesterase 3 (Ric-3) Expression Promotes Selective Protein Associations with the Human α7-Nicotinic Acetylcholine Receptor Interactome. PLoS ONE 2015, 10, e0134409. [Google Scholar] [CrossRef] [Green Version]
- Rudell, J.C.; Borges, L.S.; Yarov-Yarovoy, V.; Ferns, M. The MX-Helix of Muscle nAChR Subunits Regulates Receptor Assembly and Surface Trafficking. Front. Mol. Neurosci. 2020, 13, 48. [Google Scholar] [CrossRef]
- Margeta-Mitrovic, M.; Jan, Y.N.; Jan, L.Y. A trafficking checkpoint controls GABA(B) receptor heterodimerization. Neuron 2000, 27, 97–106. [Google Scholar] [CrossRef] [Green Version]
- Walstab, J.; Hammer, C.; Lasitschka, F.; Möller, D.; Connolly, C.N.; Rappold, G.; Brüss, M.; Bönisch, H.; Niesler, B. RIC-3 exclusively enhances the surface expression of human homomeric 5-hydroxytryptamine type 3A (5-HT3A) receptors despite direct interactions with 5-HT3A, -C, -D, and -E subunits. J. Biol. Chem. 2010, 285, 26956–26965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goyal, R.; Salahudeen, A.A.; Jansen, M. Engineering a prokaryotic Cys-loop receptor with a third functional domain. J. Biol. Chem. 2011, 286, 34635–34642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishtala, S.N.; Mnatsakanyan, N.; Pandhare, A.; Leung, C.; Jansen, M. Direct interaction of the resistance to inhibitors of cholinesterase type 3 protein with the serotonin receptor type 3A intracellular domain. J. Neurochem. 2016, 137, 528–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, H.; Jeffery, C.J. Moonlighting Proteins in the Fuzzy Logic of Cellular Metabolism. Molecules 2020, 25, 3440. [Google Scholar] [CrossRef]
- Shteingauz, A.; Cohen, E.; Biala, Y.; Treinin, M. The BTB-MATH protein BATH-42 interacts with RIC-3 to regulate maturation of nicotinic acetylcholine receptors. J. Cell Sci. 2009, 122, 807–812. [Google Scholar] [CrossRef] [Green Version]
- Safdie, G.; Liewald, J.F.; Kagan, S.; Battat, E.; Gottschalk, A.; Treinin, M. RIC-3 phosphorylation enables dual regulation of excitation and inhibition of Caenorhabditis elegans muscle. Mol. Biol. Cell 2016, 27, 2994–3003. [Google Scholar] [CrossRef]
- Séguéla, P.; Wadiche, J.; Dineley-Miller, K.; Dani, J.A.; Patrick, J.W. Molecular cloning, functional properties, and distribution of rat brain alpha 7: A nicotinic cation channel highly permeable to calcium. J. Neurosci. 1993, 13, 596–604. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, D.; Galzi, J.L.; Devillers-Thiéry, A.; Bertrand, S.; Changeux, J.P. Mutations at two distinct sites within the channel domain M2 alter calcium permeability of neuronal alpha 7 nicotinic receptor. Proc. Natl. Acad. Sci. USA 1993, 90, 6971–6975. [Google Scholar] [CrossRef] [Green Version]
- Fucile, S.; Palma, E.; Mileo, A.M.; Miledi, R.; Eusebi, F. Human neuronal threonine-for-leucine-248 alpha 7 mutant nicotinic acetylcholine receptors are highly Ca2+ permeable. Proc. Natl. Acad. Sci. USA 2000, 97, 3643–3648. [Google Scholar] [CrossRef]
- Trump, B.F.; Berezesky, I.K. Calcium-mediated cell injury and cell death. FASEB J. 1995, 9, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, C.Z.; Barrall, E.A.; Rissman, R.A.; Joiner, W.J. Unbalanced Regulation of α7 nAChRs by Ly6h and NACHO Contributes to Neurotoxicity in Alzheimer’s Disease. J. Neurosci. 2021, 41, 8461–8474. [Google Scholar] [CrossRef] [PubMed]
- Loughner, C.L.; Bruford, E.A.; McAndrews, M.S.; Delp, E.E.; Swamynathan, S.; Swamynathan, S.K. Organization, evolution and functions of the human and mouse Ly6/uPAR family genes. Hum. Genom. 2016, 10, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puddifoot, C.A.; Wu, M.; Sung, R.J.; Joiner, W.J. Ly6h regulates trafficking of alpha7 nicotinic acetylcholine receptors and nicotine-induced potentiation of glutamatergic signaling. J. Neurosci. 2015, 35, 3420–3430. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera--a visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [Green Version]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Meng, E.C.; Couch, G.S.; Croll, T.I.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Structure visualization for researchers, educators, and developers. Protein Sci. 2021, 30, 70–82. [Google Scholar] [CrossRef]
- Meng, E.C.; Pettersen, E.F.; Couch, G.S.; Huang, C.C.; Ferrin, T.E. Tools for integrated sequence-structure analysis with UCSF Chimera. BMC Bioinform. 2006, 7, 339. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Bienert, S.; Waterhouse, A.; de Beer, T.A.; Tauriello, G.; Studer, G.; Bordoli, L.; Schwede, T. The SWISS-MODEL Repository-new features and functionality. Nucleic Acids Res. 2017, 45, D313–D319. [Google Scholar] [CrossRef] [Green Version]
- Studer, G.; Rempfer, C.; Waterhouse, A.M.; Gumienny, R.; Haas, J.; Schwede, T. QMEANDisCo-distance constraints applied on model quality estimation. Bioinformatics 2020, 36, 1765–1771. [Google Scholar] [CrossRef] [PubMed]
- Waterhouse, A.; Bertoni, M.; Bienert, S.; Studer, G.; Tauriello, G.; Gumienny, R.; Heer, F.T.; de Beer, T.A.P.; Rempfer, C.; Bordoli, L.; et al. SWISS-MODEL: Homology modelling of protein structures and complexes. Nucleic Acids Res. 2018, 46, W296–W303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emanuelsson, O.; Brunak, S.; von Heijne, G.; Nielsen, H. Locating proteins in the cell using TargetP, SignalP and related tools. Nat. Protoc 2007, 2, 953–971. [Google Scholar] [CrossRef] [PubMed]
- Lupas, A.; Van Dyke, M.; Stock, J. Predicting coiled coils from protein sequences. Science 1991, 252, 1162–1164. [Google Scholar] [CrossRef]
- Jones, D.T.; Taylor, W.R.; Thornton, J.M. A model recognition approach to the prediction of all-helical membrane protein structure and topology. Biochemistry 1994, 33, 3038–3049. [Google Scholar] [CrossRef]
- Käll, L.; Krogh, A.; Sonnhammer, E.L. Advantages of combined transmembrane topology and signal peptide prediction--the Phobius web server. Nucleic Acids Res. 2007, 35, W429–W432. [Google Scholar] [CrossRef] [Green Version]
- Krogh, A.; Larsson, B.; von Heijne, G.; Sonnhammer, E.L. Predicting transmembrane protein topology with a hidden Markov model: Application to complete genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Erdős, G.; Dosztányi, Z. Analyzing Protein Disorder with IUPred2A. Curr. Protoc. Bioinform. 2020, 70, e99. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loring, R.H. Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly. Molecules 2022, 27, 4527. https://doi.org/10.3390/molecules27144527
Loring RH. Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly. Molecules. 2022; 27(14):4527. https://doi.org/10.3390/molecules27144527
Chicago/Turabian StyleLoring, Ralph H. 2022. "Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly" Molecules 27, no. 14: 4527. https://doi.org/10.3390/molecules27144527
APA StyleLoring, R. H. (2022). Speculation on How RIC-3 and Other Chaperones Facilitate α7 Nicotinic Receptor Folding and Assembly. Molecules, 27(14), 4527. https://doi.org/10.3390/molecules27144527





