Engineered 3D-Printed Polyvinyl Alcohol Scaffolds Incorporating β-Tricalcium Phosphate and Icariin Induce Bone Regeneration in Rat Skull Defect Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation and Characterisation of 3D-Printed Scaffolds
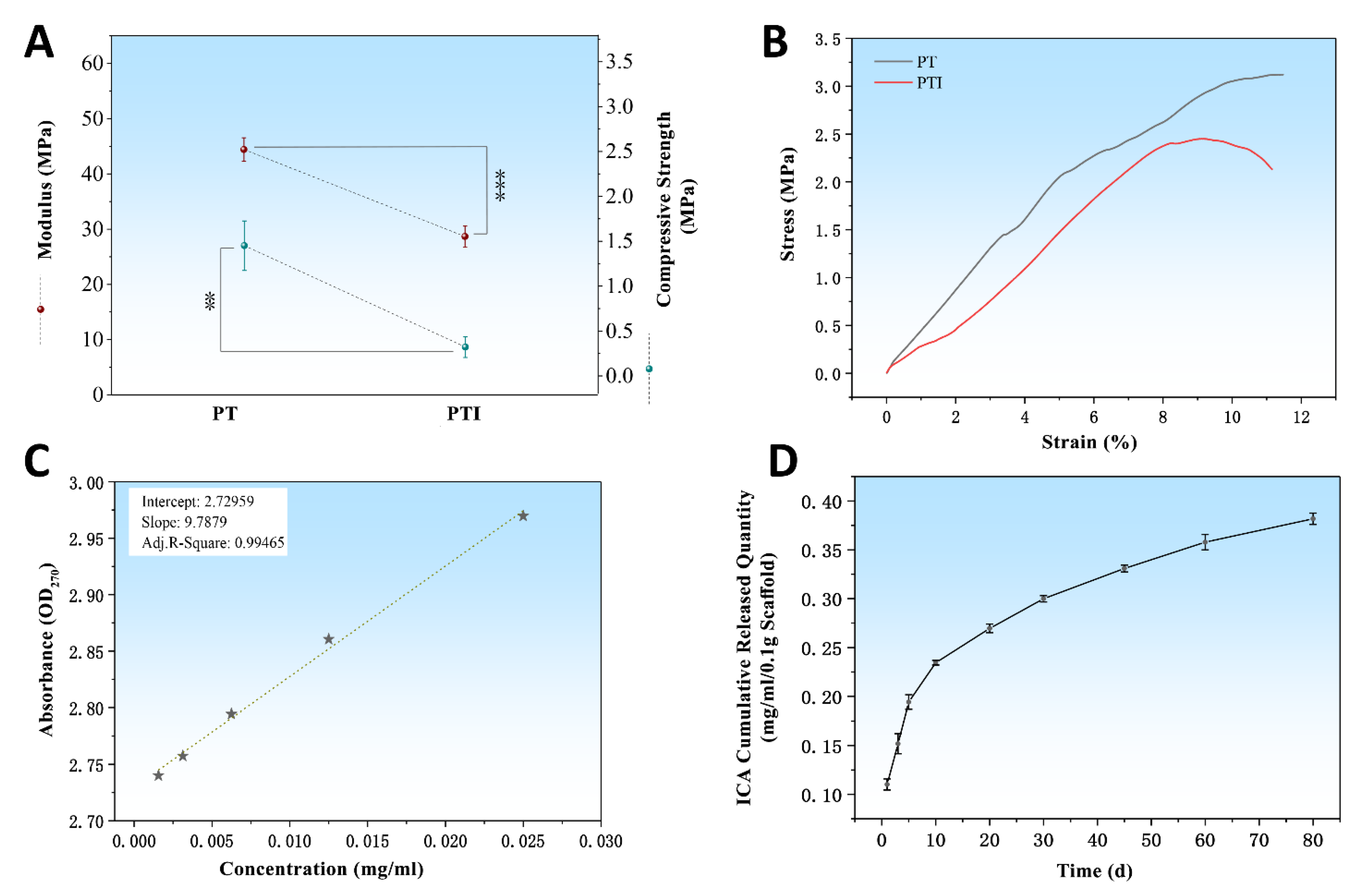
2.3. ICA Release Characteristics
2.4. In Vitro Cell Experiments
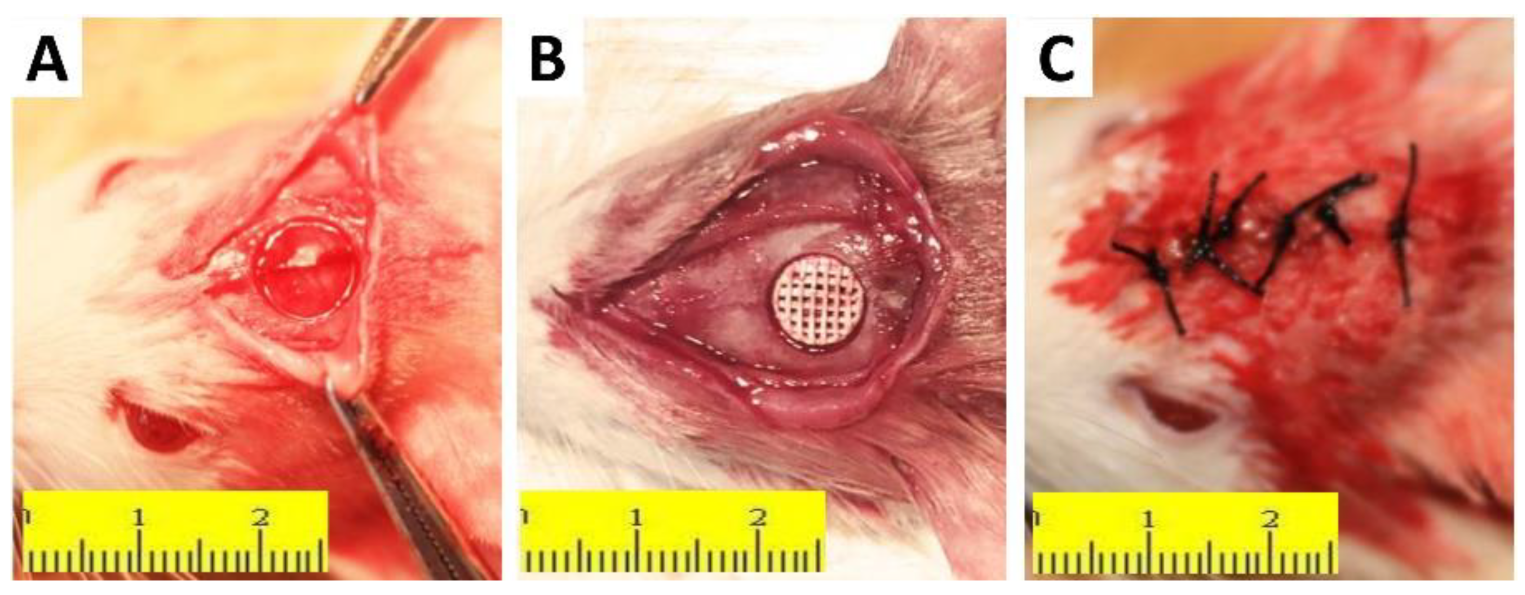
2.5. In Vivo Animal Experiments
2.6. Micro-CT and Histological Evaluation
2.7. Statistical Analysis
3. Results
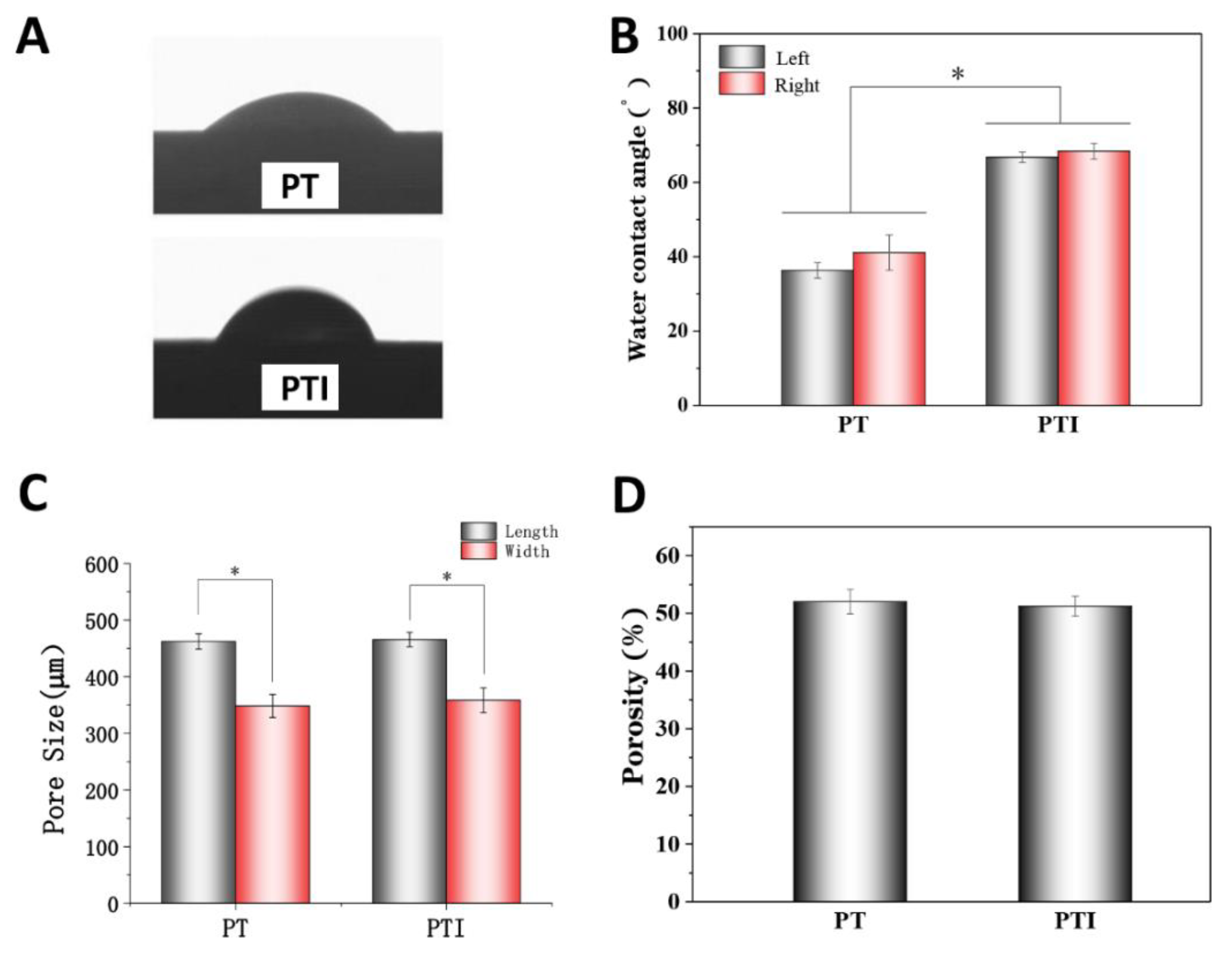
3.1. Characterisation
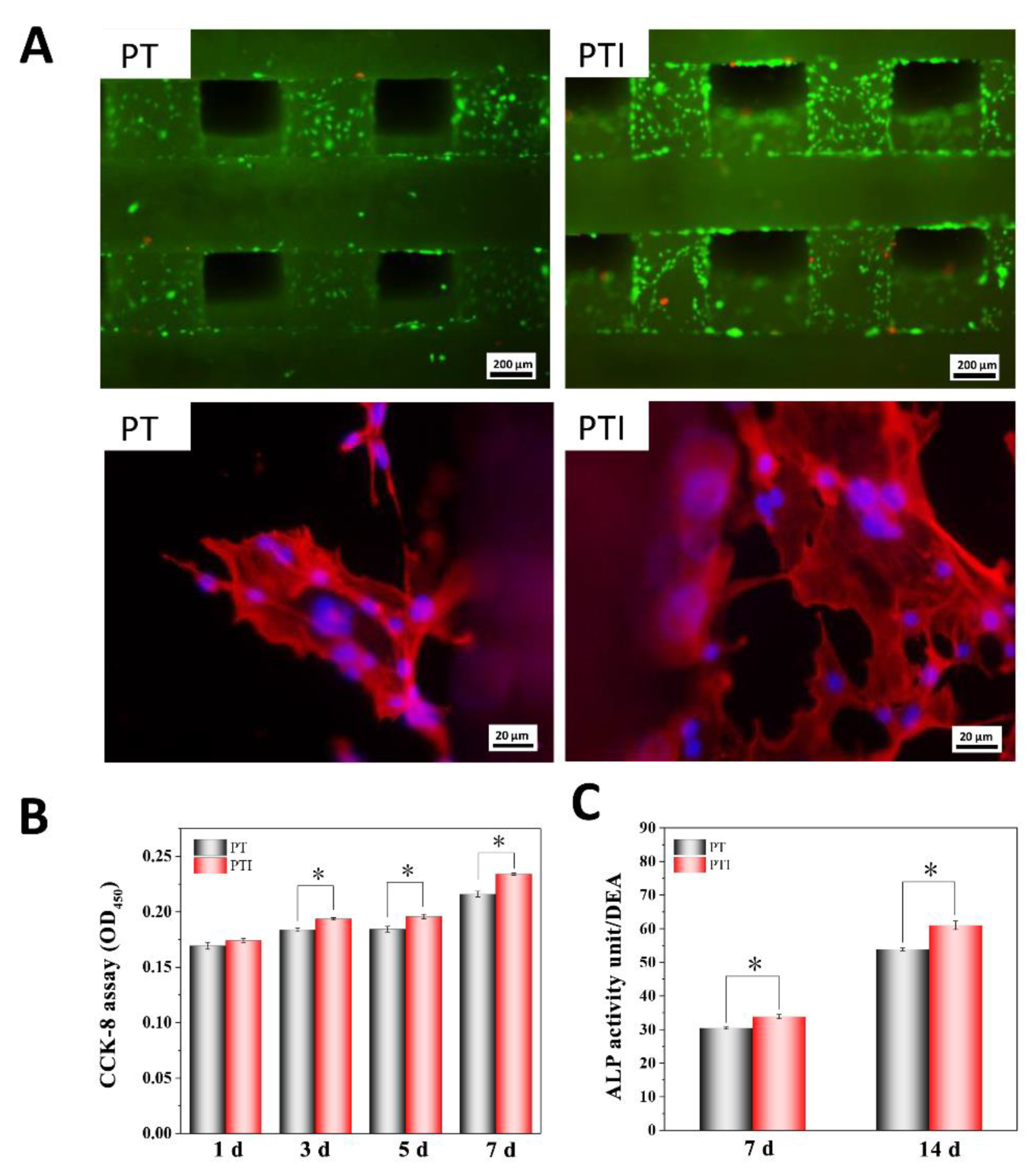
3.2. Cell Compatibility and Adhesion Ability
3.3. ALP Activity
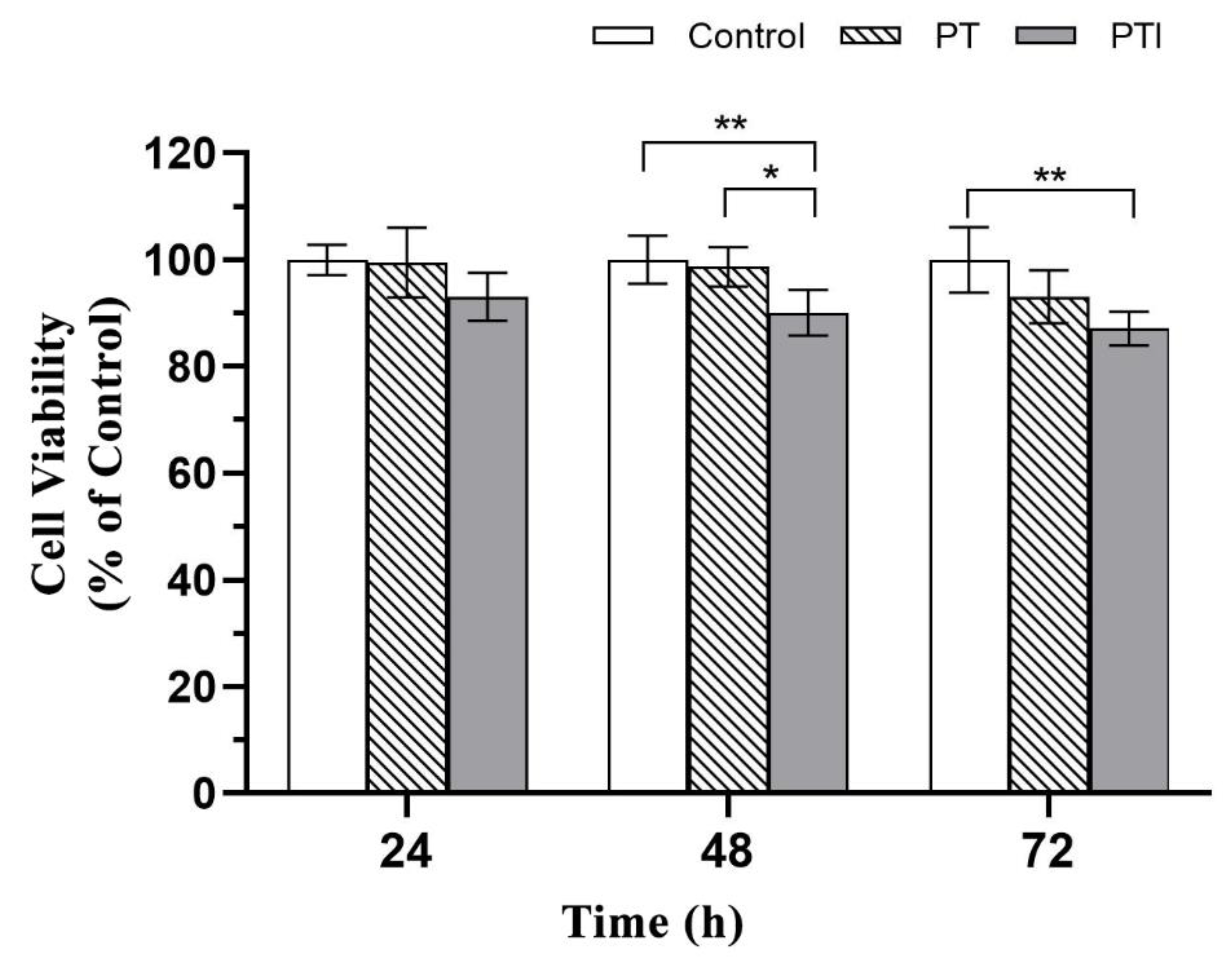
3.4. Toxicity of Scaffolds to EA.hy926 Cells
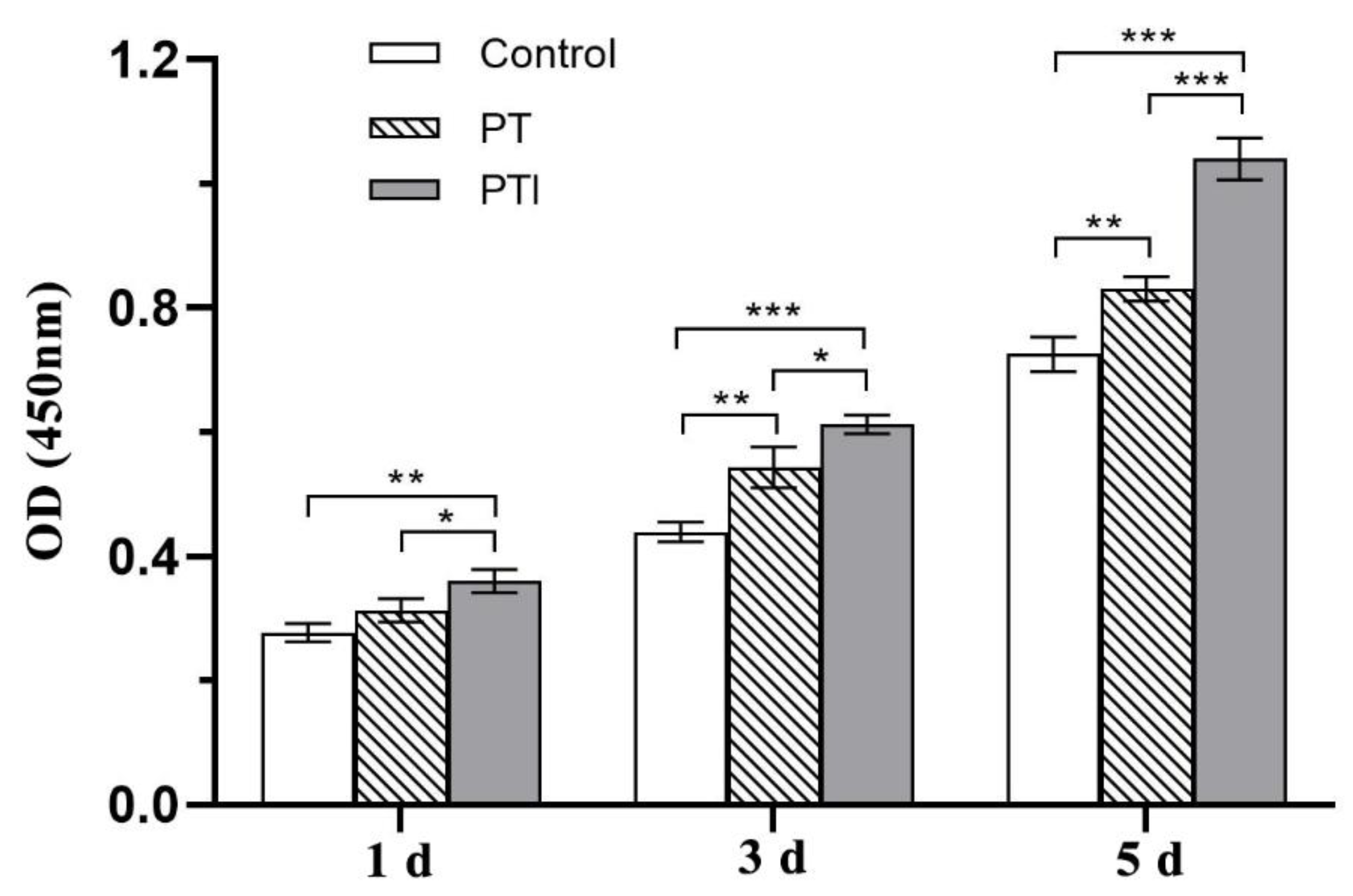
3.5. Effect of Scaffolds on the Proliferation of EA.hy926 Cells
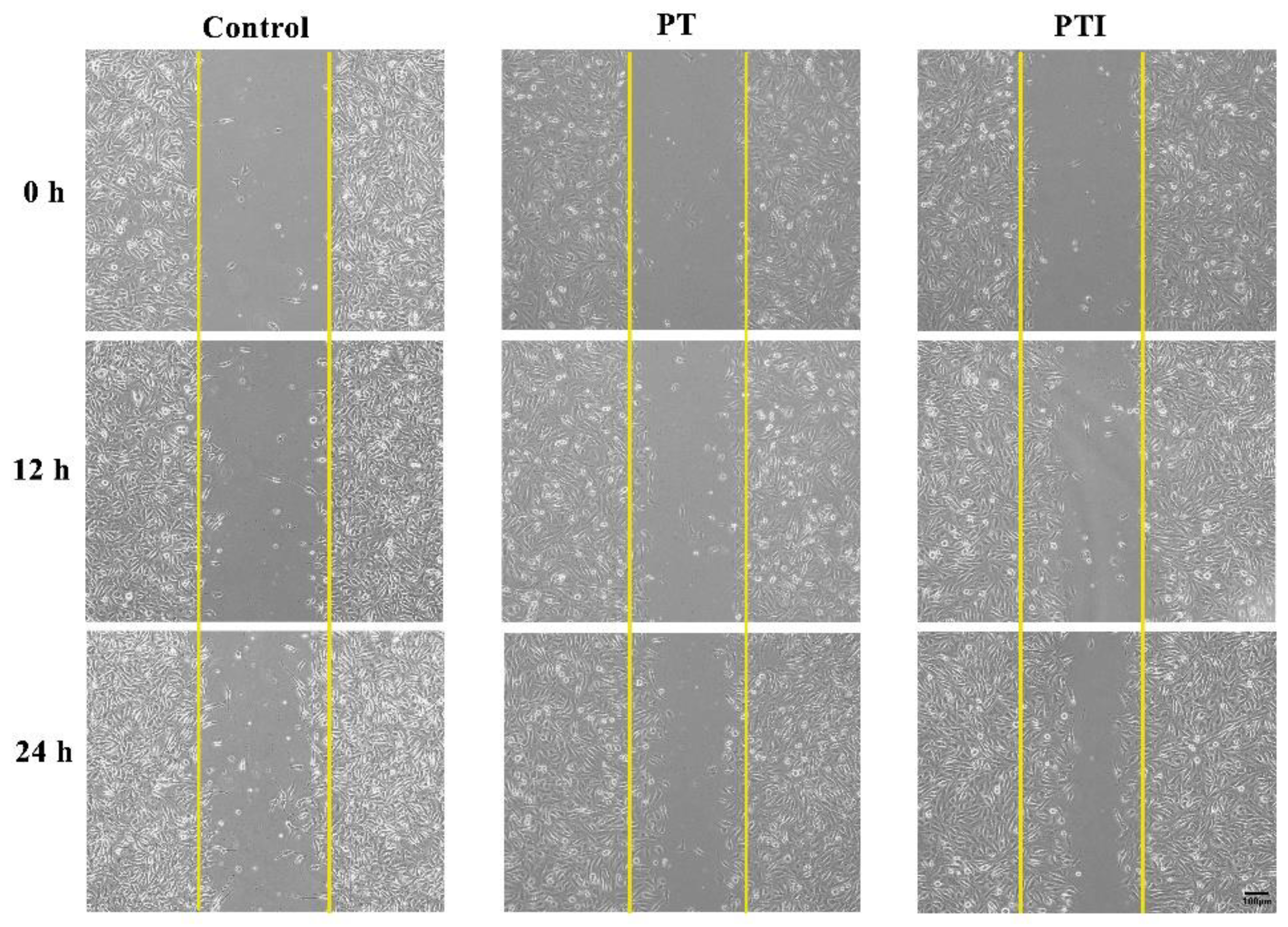
3.6. Effect of Scaffolds’ Leaching Solution on Migration of EA.hy926 Cells
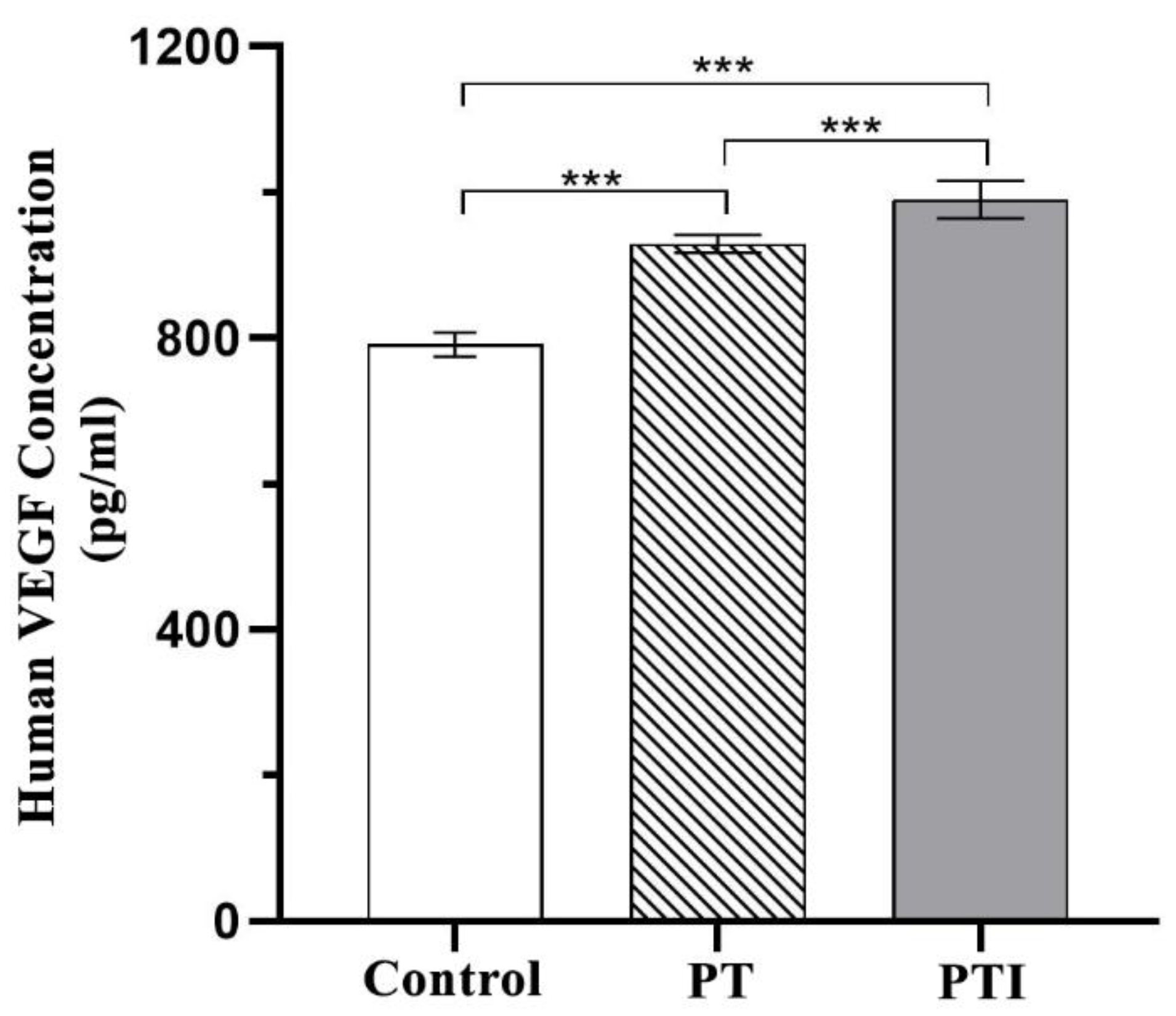
3.7. Effect of Scaffolds on the Expression of VEGF in EA.hy926 Cells
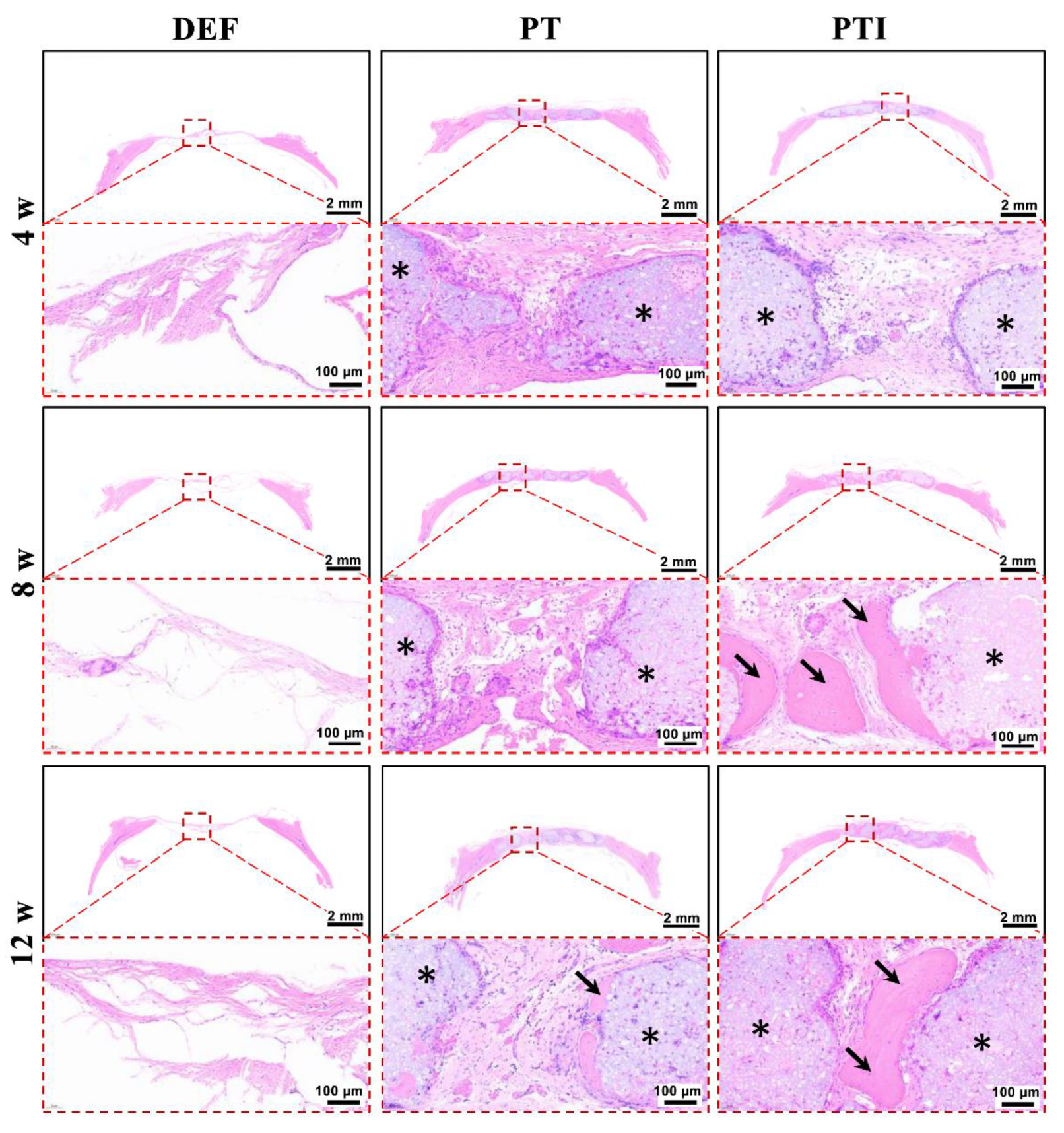
3.8. In Vivo Osteogenesis of the Scaffold
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhang, Y.; Ma, W.; Zhan, Y.; Mao, C.; Shao, X.; Xie, X.; Wei, X.; Lin, Y. Nucleic acids and analogs for bone regeneration. Bone Res. 2018, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Chen, J.; Liu, S.; Jin, Y. Stem cell-based bone and dental regeneration: A view of microenvironmental modulation. Int. J. Oral Sci. 2019, 11, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schultz, B.J.; McLaurin, T.M.; Leucht, P. Treatment of Segmental Bone Defects: Biology and Treatment Options. Bull. NYU Hosp. Jt. Dis. 2022, 80, 53–64. [Google Scholar]
- Meiser, S.; Arora, R.; Petersen, J.; Keiler, A.; Liebensteiner, M.C.; Pallua, J.D.; Wurm, A. Radiographic and clinical outcome of tibial plateau fractures treated with bone allograft. Arch. Orthop. Trauma Surg. 2022, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tokish, J.M.; Brinkman, J.C.; Hassebrock, J.D. Arthroscopic Technique for Distal Tibial Allograft Bone Augmentation with Suture Anchor Fixation for Anterior Shoulder Instability. Arthrosc. Tech. 2022, 11, e903–e909. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhou, T.; Lin, S.; Shi, S.; Lin, Y. Nanomaterials for craniofacial and dental tissue engineering. J. Dent. Res. 2017, 96, 725–732. [Google Scholar] [CrossRef] [PubMed]
- Jazayeri, H.E.; Tahriri, M.; Razavi, M.; Khoshroo, K.; Fahimipour, F.; Dashtimoghadam, E.; Almeida, L.; Tayebi, L. A current overview of materials and strategies for potential use in maxillofacial tissue regeneration. Mater. Sci. Eng. C 2017, 70, 913–929. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Wang, S.; Zhou, C.; Cheng, L.; Gao, X.; Xie, X.; Sun, J.; Wang, H.; Weir, M.D.; Reynolds, M.A. Advanced smart biomaterials and constructs for hard tissue engineering and regeneration. Bone Res. 2018, 6, 31. [Google Scholar] [CrossRef]
- Memarian, P.; Pishavar, E.; Zanotti, F.; Trentini, M.; Camponogara, F.; Soliani, E.; Gargiulo, P.; Isola, M.; Zavan, B. Active Materials for 3D Printing in Small Animals: Current Modalities and Future Directions for Orthopedic Applications. Int. J. Mol. Sci. 2022, 23, 1045. [Google Scholar] [CrossRef]
- Barreto, I.B.; Peñaranda, L.A.F.; Delgado, F.D.C.; Córdova, R.A.S.; Arana, B.A.C. 3D Printing in Bone Tissue Engineering: Bibliographic Review of Current Advances and Future Potential. In Technological Adoption and Trends in Health Sciences Teaching, Learning, and Practice; IGI Global: Hershey, PA, USA, 2022; pp. 245–264. [Google Scholar]
- Zhou, Y.; Grayson, W. Three-dimensional printing of scaffolds for facial reconstruction. MRS Bull. 2022, 47, 91–97. [Google Scholar] [CrossRef]
- Jiang, L.; Wang, Y.; Wang, X.; Ning, F.; Wen, S.; Zhou, Y.; Chen, S.; Betts, A.; Jerrams, S.; Zhou, F.-L. Electrohydrodynamic printing of a dielectric elastomer actuator and its application in tunable lenses. Compos. Part A Appl. Sci. Manuf. 2021, 147, 106461. [Google Scholar] [CrossRef]
- Ma, X.; Tao, L.; Chen, X.; Li, W.; Peng, Z.; Chen, Y.; Jin, J.; Zhang, X.; Xiong, Q.; Zhong, Z. Clinical application of three-dimensional reconstruction and rapid prototyping technology of multislice spiral computed tomography angiography for the repair of ventricular septal defect of tetralogy of Fallot. Genet. Mol. Res. 2015, 14, 1301–1309. [Google Scholar] [CrossRef]
- Withers, P.J.; Bouman, C.; Carmignato, S.; Cnudde, V.; Grimaldi, D.; Hagen, C.K.; Maire, E.; Manley, M.; Du Plessis, A.; Stock, S.R. X-ray computed tomography. Nat. Rev. Methods Primers 2021, 51, R29. [Google Scholar] [CrossRef]
- Hasegawa, K.; Okamoto, M.; Hatsushikano, S.; Caseiro, G.; Watanabe, K. Difference in whole spinal alignment between supine and standing positions in patients with adult spinal deformity using a new comparison method with slot-scanning three-dimensional X-ray imager and computed tomography through digital reconstructed radiography. BMC Musculoskelet. Disord. 2018, 19, 437. [Google Scholar]
- Brennan, M.Á.; Monahan, D.S.; Brulin, B.; Gallinetti, S.; Humbert, P.; Tringides, C.; Canal, C.; Ginebra, M.P.; Layrolle, P. Biomimetic versus sintered macroporous calcium phosphate scaffolds enhanced bone regeneration and human mesenchymal stromal cell engraftment in calvarial defects. Acta Biomater. 2021, 135, 689–704. [Google Scholar] [CrossRef]
- Ashammakhi, N.; Gonzalez, A.M.; Törmälä, P.; Jackson, I. New resorbable bone fixation. Biomaterials in craniomaxillofacial surgery: Present and future. Eur. J. Plast. Surg. 2004, 26, 383–390. [Google Scholar] [CrossRef]
- Yu, Q.; Chen, L.; Qiu, Z.; Zhang, Y.; Song, T.; Cui, F. Skull repair materials applied in cranioplasty: History and progress. Transl. Neurosci. Clin. 2017, 3, 48–57. [Google Scholar] [CrossRef] [Green Version]
- Huang, G.-y.; Shan, L.-j. Research on the digital design and manufacture of titanium alloy skull repair prosthesi. In Proceedings of the 2011 5th International Conference on Bioinformatics and Biomedical Engineering, Wuhan, China, 10–12 May 2011; pp. 1–4. [Google Scholar]
- Xue, X.; Zhang, H.; Liu, H.; Wang, S.; Li, J.; Zhou, Q.; Chen, X.; Ren, X.; Jing, Y.; Deng, Y.; et al. Rational Design of Multifunctional CuS Nanoparticle-PEG Composite Soft Hydrogel-Coated 3D Hard Polycaprolactone Scaffolds for Efficient Bone Regeneration. Adv. Funct. Mater. 2022, 2202470. [Google Scholar] [CrossRef]
- Diao, J.; OuYang, J.; Deng, T.; Liu, X.; Feng, Y.; Zhao, N.; Mao, C.; Wang, Y. 3D-Plotted Beta-Tricalcium Phosphate Scaffolds with Smaller Pore Sizes Improve In Vivo Bone Regeneration and Biomechanical Properties in a Critical-Sized Calvarial Defect Rat Model. Adv. Healthc. Mater. 2018, 7, e1800441. [Google Scholar] [CrossRef]
- Lai, Y.; Cao, H.; Wang, X.; Chen, S.; Zhang, M.; Wang, N.; Yao, Z.; Dai, Y.; Xie, X.; Zhang, P.; et al. Porous composite scaffold incorporating osteogenic phytomolecule icariin for promoting skeletal regeneration in challenging osteonecrotic bone in rabbits. Biomaterials 2018, 153, 1–13. [Google Scholar] [CrossRef]
- Wu, S.; Ma, J.; Liu, J.; Liu, C.; Ni, S.; Dai, T.; Wang, Y.; Weng, Y.; Zhao, H.; Zhou, D. Immunomodulation of Telmisartan-Loaded PCL/PVP Scaffolds on Macrophages Promotes Endogenous Bone Regeneration. ACS Appl. Mater. Interfaces 2022, 14, 15942–15955. [Google Scholar] [CrossRef] [PubMed]
- Salah, M.; Naini, F.B.; Tayebi, L. 3D Printing and Bioprinting of Biomaterials and Bioceramic Scaffolds: Clinical Outcomes and Implications in Bone Tissue Engineering and Maxillofacial Reconstructive Surgery. In Innovative Bioceramics in Translational Medicine II; Springer: Berlin/Heidelberg, Germany, 2022; pp. 15–33. [Google Scholar]
- Gnanavel, S.; Kaavya, P. Design and development of novel 3D bone scaffold for implant application. Mater. Today Proc. 2022, 59, 775–780. [Google Scholar] [CrossRef]
- Hindy, O.A.; Goker, M.; Yilgor Huri, P. Nanoscale agents within 3D-printed constructs: Intersection of nanotechnology and personalized bone tissue engineering. Emergent Mater. 2022, 5, 195–205. [Google Scholar] [CrossRef]
- Lee, J.; Lee, S.; Ahmad, T.; Madhurakkat Perikamana, S.K.; Lee, J.; Kim, E.M.; Shin, H. Human adipose-derived stem cell spheroids incorporating platelet-derived growth factor (PDGF) and bio-minerals for vascularized bone tissue engineering. Biomaterials 2020, 255, 120192. [Google Scholar] [CrossRef]
- Vallittu, P.K.; Posti, J.P.; Piitulainen, J.M.; Serlo, W.; Maatta, J.A.; Heino, T.J.; Pagliari, S.; Syrjanen, S.M.; Forte, G. Biomaterial and implant induced ossification: In vitro and in vivo findings. J. Tissue Eng. Regen. Med. 2020, 14, 1157–1168. [Google Scholar] [CrossRef]
- Yang, T.; Hu, Y.; Wang, C.; Binks, B.P. Fabrication of Hierarchical Macroporous Biocompatible Scaffolds by Combining Pickering High Internal Phase Emulsion Templates with Three-Dimensional Printing. ACS Appl. Mater. Interfaces 2017, 9, 22950–22958. [Google Scholar] [CrossRef]
- Hong, L.; Tabata, Y.; Miyamoto, S.; Yamamoto, M.; Yamada, K.; Hashimoto, N.; Ikada, Y. Bone regeneration at rabbit skull defects treated with transforming growth factor-beta 1 incorporated into hydrogels with different levels of biodegradability. J. Neurosurg. 2000, 92, 315–325. [Google Scholar] [CrossRef]
- Sasaki, T.; Niizuma, K.; Kanoke, A.; Matsui, K.; Ogita, S.; Rashad, S.; Kawai, T.; Watanabe, M.; Endo, H.; Takahashi, T.; et al. Octacalcium phosphate collagen composite (OCP/Col) enhance bone regeneration in a rat model of skull defect with dural defect. Heliyon 2020, 6, e03347. [Google Scholar] [CrossRef]
- Bi, M.; Han, H.; Dong, S.; Zhang, Y.; Xu, W.; Zhu, B.; Wang, J.; Zhou, Y.; Ding, J. Collagen-Coated Poly(lactide-co-glycolide)/Hydroxyapatite Scaffold Incorporated with DGEA Peptide for Synergistic Repair of Skull Defect. Polymers 2018, 10, 109. [Google Scholar] [CrossRef] [Green Version]
- Zhuo, Z.; Wan, Y.; Guan, D.; Ni, S.; Wang, L.; Zhang, Z.; Liu, J.; Liang, C.; Yu, Y.; Lu, A.; et al. A Loop-Based and AGO-Incorporated Virtual Screening Model Targeting AGO-Mediated miRNA–mRNA Interactions for Drug Discovery to Rescue Bone Phenotype in Genetically Modified Mice. Adv. Sci. 2020, 7, 1903451. [Google Scholar] [CrossRef]
- Alavarse, A.C.; de Oliveira Silva, F.W.; Colque, J.T.; da Silva, V.M.; Prieto, T.; Venancio, E.C.; Bonvent, J.-J. Tetracycline hydrochloride-loaded electrospun nanofibers mats based on PVA and chitosan for wound dressing. Mater. Sci. Eng. C 2017, 77, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, R.S.; Das, A.; Alzhrani, R.M.; Kang, D.; Bhaduri, S.B.; Boddu, S.H. Comparison of electrospun and solvent cast polylactic acid (PLA)/poly (vinyl alcohol)(PVA) inserts as potential ocular drug delivery vehicles. Mater. Sci. Eng. C 2017, 77, 895–903. [Google Scholar] [CrossRef] [PubMed]
- Tocchio, A.; Tamplenizza, M.; Martello, F.; Gerges, I.; Rossi, E.; Argentiere, S.; Rodighiero, S.; Zhao, W.; Milani, P.; Lenardi, C. Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials 2015, 45, 124–131. [Google Scholar] [CrossRef]
- Torres, P.M.C.; Abrantes, J.C.C.; Kaushal, A.; Pina, S.; Döbelin, N.; Bohner, M.; Ferreira, J.M.F. Influence of Mg-doping, calcium pyrophosphate impurities and cooling rate on the allotropic α ↔ β-tricalcium phosphate phase transformations. J. Eur. Ceram. Soc. 2016, 36, 817–827. [Google Scholar] [CrossRef]
- Fielding, G.; Bose, S. SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo. Acta Biomater. 2013, 9, 9137–9148. [Google Scholar] [CrossRef] [Green Version]
- Thomas, D.; Su, S.; Qiu, J.; Pantoya, M.L. Microwave synthesis of functionally graded tricalcium phosphate for osteoconduction. Mater. Today Commun. 2016, 9, 47–53. [Google Scholar] [CrossRef] [Green Version]
- Duan, M.N.; Ma, S.J.; Song, C.Y.; Li, J.; Qian, M. Three-dimensional printing of a beta-tricalcium phosphate scaffold with dual bioactivities for bone repair. Ceram. Int. 2021, 47, 4775–4782. [Google Scholar] [CrossRef]
- Batista, M.A.; Leivas, T.P.; Rodrigues, C.J.; Arenas, G.C.F.; Belitardo, D.R.; Guarniero, R. Comparison between the effects of platelet-rich plasma and bone marrow concentrate on defect consolidation in the rabbit tibia. Clinics 2011, 66, 1787–1792. [Google Scholar]
- Kinnear, C.; Burnand, D.; Clift, M.J.; Kilbinger, A.F.; Rothen-Rutishauser, B.; Petri-Fink, A. Polyvinyl alcohol as a biocompatible alternative for the passivation of gold nanorods. Angew. Chem. Int. Ed. Engl. 2014, 53, 12613–12617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, H.; Hu, C.; Zhou, C.C.; Wu, L.N.; Sun, J.X.; Zhou, X.D.; Xing, F.; Long, C.; Kong, Q.Q.; Liang, J.; et al. 3D printing of calcium phosphate scaffolds with controlled release o antibacterial functions for jaw bone repair. Mater. Des. 2020, 189, 13. [Google Scholar] [CrossRef]
- Han, L.Y.; Wu, Y.L.; Zhu, C.Y.; Wu, C.S.; Yang, C.R. Improved Pharmacokinetics of Icariin (ICA) within Formulation of PEG-PLLA/PDLA-PNIPAM Polymeric Micelles. Pharmaceutics 2019, 11, 51. [Google Scholar] [CrossRef] [Green Version]
- Qi, S.; He, J.; Zheng, H.; Chen, C.; Lan, S. Icariin Prevents Diabetes-Induced Bone Loss in Rats by Reducing Blood Glucose and Suppressing Bone Turnover. Molecules 2019, 24, 1871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, A.; Yu, C.; Lu, Q.; Li, H.; Li, Z.; He, C. Mechanism of Action of Icariin in Bone Marrow Mesenchymal Stem Cells. Stem Cells Int. 2019, 2019, 5747298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Y.M.; Cao, S.J.; Chen, J.D.; Zhao, Y.; He, F.P.; Li, Q.; Zou, L.; Shi, C. Biomimetic fabrication of icariin loaded nano hydroxyapatite reinforced bioactive porous scaffolds for bone regeneration. Chem. Eng. J. 2020, 394, 124895. [Google Scholar] [CrossRef]
- Lin, K.F.; He, S.; Song, Y.; Wang, C.M.; Gao, Y.; Li, J.Q.; Tang, P.; Wang, Z.; Bi, L.; Pei, G.X. Low-Temperature Additive Manufacturing of Biomimic Three-Dimensional Hydroxyapatite/Collagen Scaffolds for Bone Regeneration. ACS Appl. Mater. Interfaces 2016, 8, 6905–6916. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Wang, N.; Liu, P.; Sun, Y.; Wang, Y.; Fei, F.; Zhang, S.; Zheng, J.; Han, B. Poly(Dopamine) Coating on 3D-Printed Poly-Lactic-Co-Glycolic Acid/beta-Tricalcium Phosphate Scaffolds for Bone Tissue Engineering. Molecules 2019, 24, 4397. [Google Scholar] [CrossRef] [Green Version]
- Correia, T.R.; Figueira, D.R.; de Sa, K.D.; Miguel, S.P.; Fradique, R.G.; Mendonca, A.G.; Correia, I.J. 3D Printed scaffolds with bactericidal activity aimed for bone tissue regeneration. Int. J. Biol. Macromol. 2016, 93, 1432–1445. [Google Scholar] [CrossRef]
- Ye, X.; Li, L.; Lin, Z.; Yang, W.; Duan, M.; Chen, L.; Xia, Y.; Chen, Z.; Lu, Y.; Zhang, Y. Integrating 3D-printed PHBV/Calcium sulfate hemihydrate scaffold and chitosan hydrogel for enhanced osteogenic property. Carbohydr. Polym. 2018, 202, 106–114. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wen, Y.; Sheng, L.; Guo, R.; Zhang, Y.; Shao, L. Icariin activates autophagy to trigger TGFβ1 upregulation and promote angiogenesis in EA. hy926 human vascular endothelial cells. Bioengineered 2022, 13, 164–177. [Google Scholar] [CrossRef]
- Du, Y.; Taylor, C.G.; Aukema, H.M.; Zahradka, P. PD146176 affects human EA.hy926 endothelial cell function by differentially modulating oxylipin production of LOX, COX and CYP epoxygenase. Biochim. Biophys. Acta (BBA) Mol. Cell Biol. Lipids 2022, 1867, 159156. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-G.; Kim, H.-S.; An, H.; Baek, K.; Lee, J.M.; Yim, M.-J.; Ko, S.-C.; Kim, J.-Y.; Oh, G.-W.; Je, J.-G.; et al. Antihypertensive Effects of IGTGIPGIW Peptide Purified from Hippocampus abdominalis: P-eNOS and p-AKT Stimulation in EA.hy926 Cells and Lowering of Blood Pressure in SHR Model. Mar. Drugs 2022, 20, 354. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Nie, W.; Chen, L.; McCoul, D.; Liu, D.; Zhang, X.; Ji, Y.; Yu, B.; He, C. Self-Assembled Hydroxyapatite-Graphene Scaffold for Photothermal Cancer Therapy and Bone Regeneration. J. Biomed. Nanotechnol. 2018, 14, 2003–2017. [Google Scholar] [CrossRef] [PubMed]
- Lam, S.; Kuether, J.; Fong, A.; Reid, R. Cranioplasty for large-sized calvarial defects in the pediatric population: A review. Craniomaxillofacial Trauma Reconstr. 2015, 8, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.; Fan, T.T.; Chen, J.D.; Su, J.C.; Zhi, X.; Pan, P.P.; Zou, L.; Zhang, Q.Q. Magnetic bioinspired micro/nanostructured composite scaffold for bone regeneration. Colloids Surf. B Biointerfaces 2019, 174, 70–79. [Google Scholar] [CrossRef]
- Han, T.; Ji, Y.; Cui, J.; Shen, W.M. Surgical Management of Sinus Pericranii with Crouzon Syndrome. J. Craniofacial Surg. 2021, 32, 1068–1070. [Google Scholar] [CrossRef]
- Liang, H.F.; Liu, X.; Pi, Y.; Yu, Q.; Yin, Y.K.; Li, X.; Yang, Y.P.; Tian, J. 3D-Printed beta-Tricalcium Phosphate Scaffold Combined with a Pulse Electromagnetic Field Promotes the Repair of Skull Defects in Rats. Acs Biomater. Sci. Eng. 2019, 5, 5359–5367. [Google Scholar] [CrossRef]
- Isksioui, H.; Ennima, S.; Bourekkadi, S.; Abdellah, E.; Mohamed, O. Innovation and Intelligent Systems in Experimental Optimization of Fused Deposition Modeling Process Parameters: A Taguchi Process Approach for Dimension and Tolerance Control. In Education Excellence and Innovation Management: A 2025 Vision to Sustain Economic Development During Global Challenges; IBIMA: Seville, Spain, 2020; pp. 10697–10705. [Google Scholar]
- Prasad, T.S.; Sujatha, G.; Muruganandhan, J.; Patil, S.; Raj, A.T. Three-dimensional Printing in Reconstructive Oral and Maxillofacial Surgery. J. Contemp. Dent. Pract. 2018, 19, 1–2. [Google Scholar]
- Partee, B.; Hollister, S.J.; Das, S. Selective laser sintering of polycaprolactone bone tissue engineering scaffolds. In Proceedings of the Symposium on Nanoscale Materials Science in Biology and Medicine held at the 2004 MRS Fall Meeting, Boston, MA, USA, 28 November–2 December 2004; pp. 201–207. [Google Scholar]
- Gao, D.; Wang, Z.; Wu, Z.; Guo, M.; Wang, Y.; Gao, Z.; Zhang, P.; Ito, Y. 3D-printing of solvent exchange deposition modeling (SEDM) for a bilayered flexible skin substitute of poly (lactide-co-glycolide) with bioorthogonally engineered EGF. Mater. Sci. Eng. C-Mater. Biol. Appl. 2020, 112, 110942. [Google Scholar] [CrossRef]
- Lin, Z.D.; Hu, X.L.; Zhong, L.L.; Peng, D.Q.; Lu, J.L.; He, J.; Shen, X.F.; Xiong, C.D.; Xu, T.; Niu, W. Doping polyvinyl alcohol can improve the injectability of biological ceramics in 3D printing and influence the adhesion of cells to the scaffolds after sintering. Ceram. Int. 2021, 47, 25363–25372. [Google Scholar] [CrossRef]
- Montelongo, S.A.; Chiou, G.; Ong, J.L.; Bizios, R.; Guda, T. Development of bioinks for 3D printing microporous, sintered calcium phosphate scaffolds. J. Mater. Sci. Mater. Med. 2021, 32, 94. [Google Scholar] [CrossRef]
- Luo, Z.C.; Chen, X.N.; Liu, M.J.; Wang, Y.Y.; Li, D.X.; Li, X.F.; Xiao, Y.M.; Wang, Y.; Zhang, X.D. The controlled release of a novel thiolated icariin for enhanced osteoporotic bone regeneration. Mater. Des. 2021, 200, 14. [Google Scholar] [CrossRef]
- Sun, S.; Liu, H.; Hu, Y.; Wang, Y.; Zhao, M.; Yuan, Y.; Han, Y.; Jing, Y.; Cui, J.; Ren, X.; et al. Selection and identification of a novel ssDNA aptamer targeting human skeletal muscle. Bioact. Mater. 2023, 20, 166–178. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, Z.; Sun, Y.; Dai, H.; Ma, Y.; Bing, H. Engineered 3D-Printed Polyvinyl Alcohol Scaffolds Incorporating β-Tricalcium Phosphate and Icariin Induce Bone Regeneration in Rat Skull Defect Model. Molecules 2022, 27, 4535. https://doi.org/10.3390/molecules27144535
Xu Z, Sun Y, Dai H, Ma Y, Bing H. Engineered 3D-Printed Polyvinyl Alcohol Scaffolds Incorporating β-Tricalcium Phosphate and Icariin Induce Bone Regeneration in Rat Skull Defect Model. Molecules. 2022; 27(14):4535. https://doi.org/10.3390/molecules27144535
Chicago/Turabian StyleXu, Zhimin, Yidan Sun, Huanyan Dai, Yujie Ma, and Han Bing. 2022. "Engineered 3D-Printed Polyvinyl Alcohol Scaffolds Incorporating β-Tricalcium Phosphate and Icariin Induce Bone Regeneration in Rat Skull Defect Model" Molecules 27, no. 14: 4535. https://doi.org/10.3390/molecules27144535
APA StyleXu, Z., Sun, Y., Dai, H., Ma, Y., & Bing, H. (2022). Engineered 3D-Printed Polyvinyl Alcohol Scaffolds Incorporating β-Tricalcium Phosphate and Icariin Induce Bone Regeneration in Rat Skull Defect Model. Molecules, 27(14), 4535. https://doi.org/10.3390/molecules27144535





