Effect of Three Commercial Formulations Containing Effective Microorganisms (EM) on Diflufenican and Flurochloridone Degradation in Soil
Abstract
:1. Introduction
2. Results
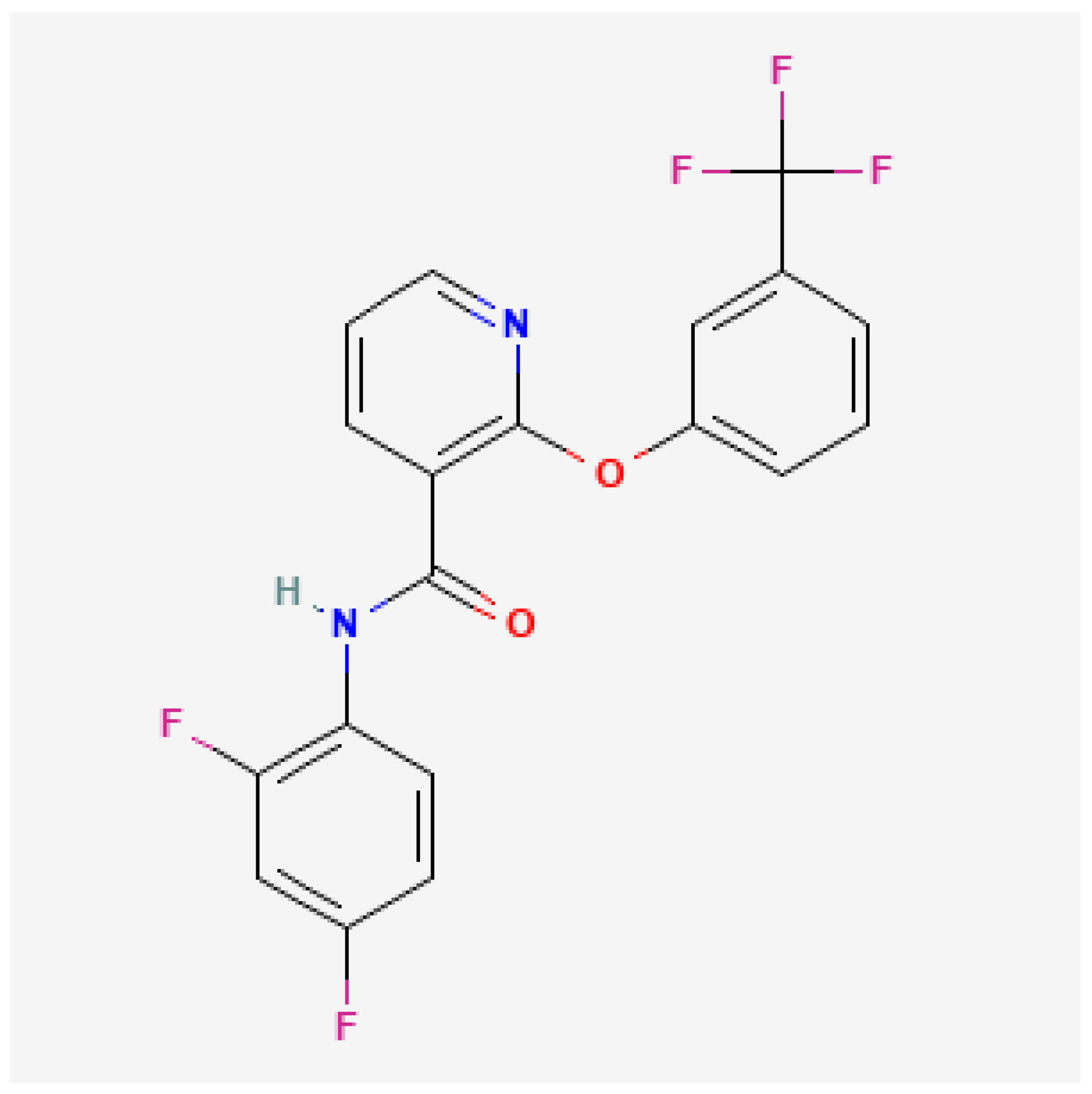
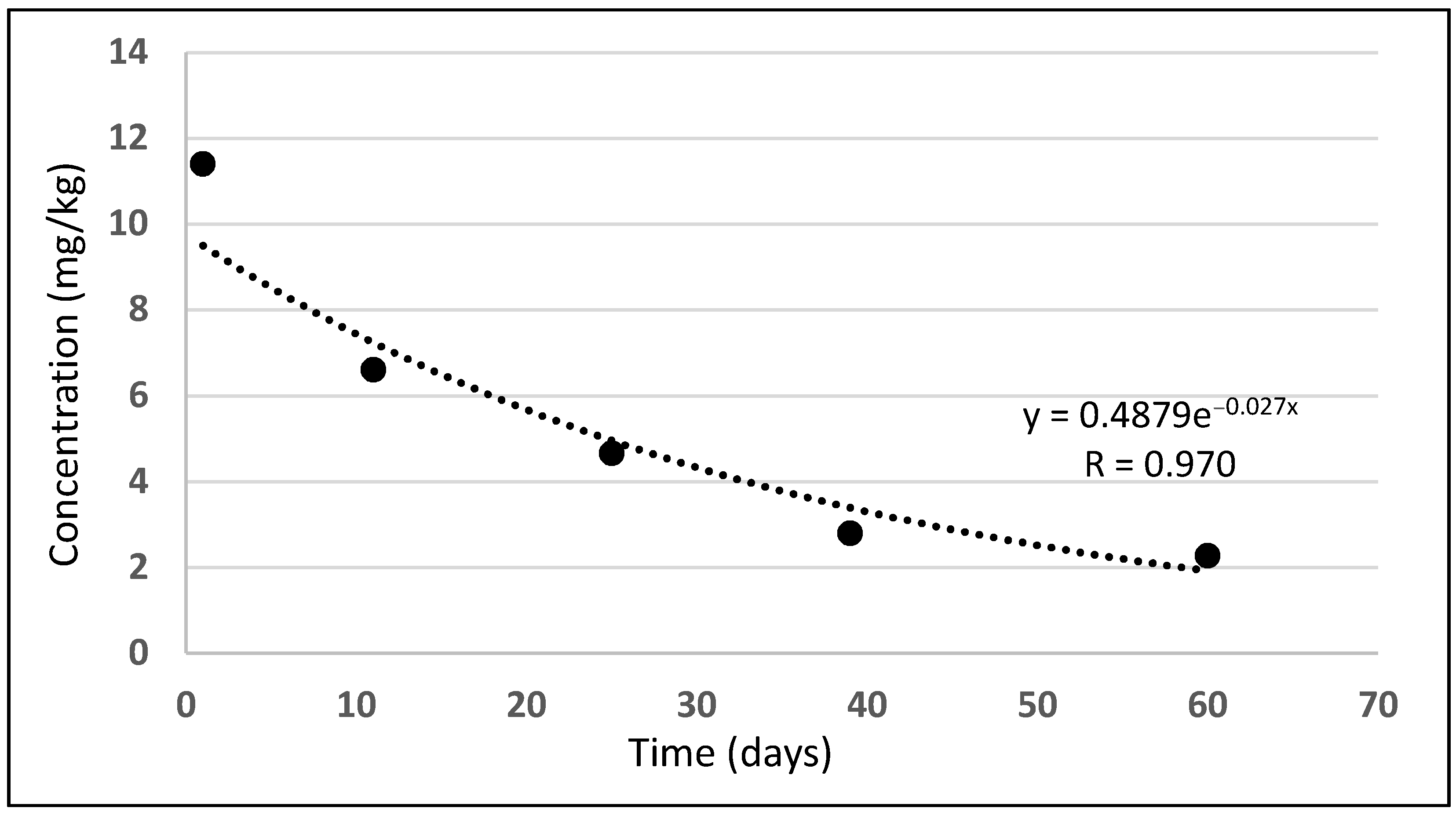
2.1. Diflufenican
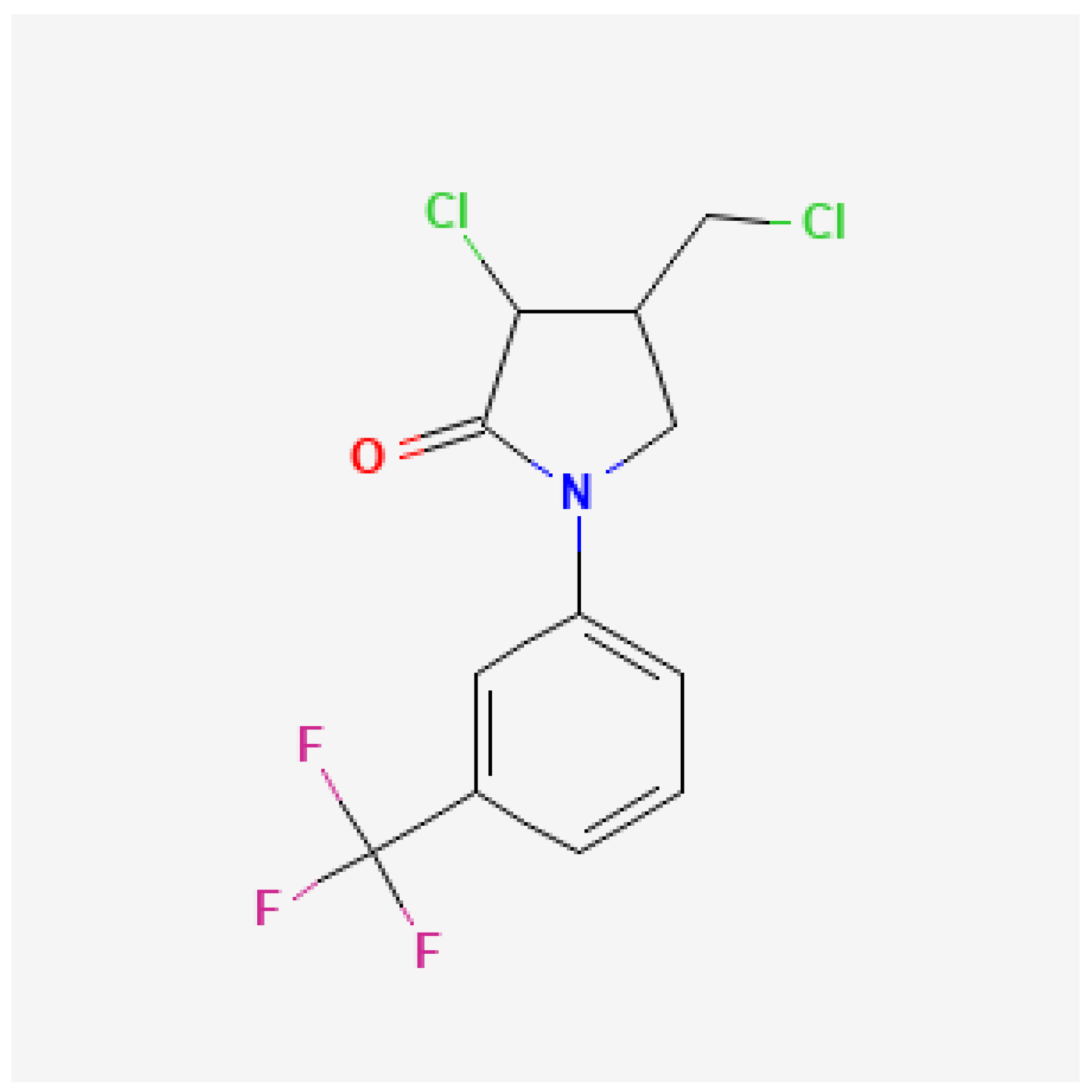
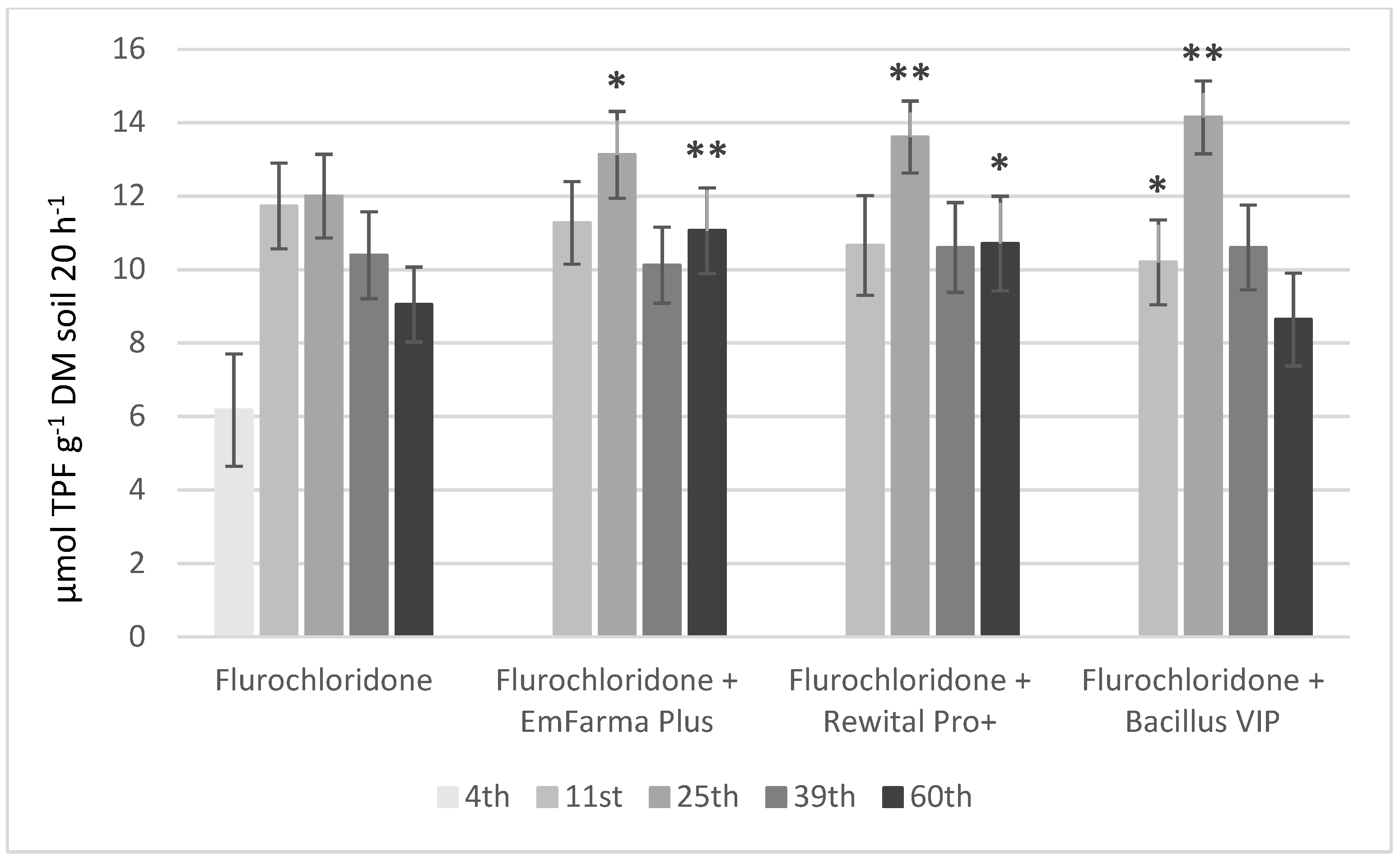
2.2. Flurochloridone
2.3. GC-MS Full Scan Analysis
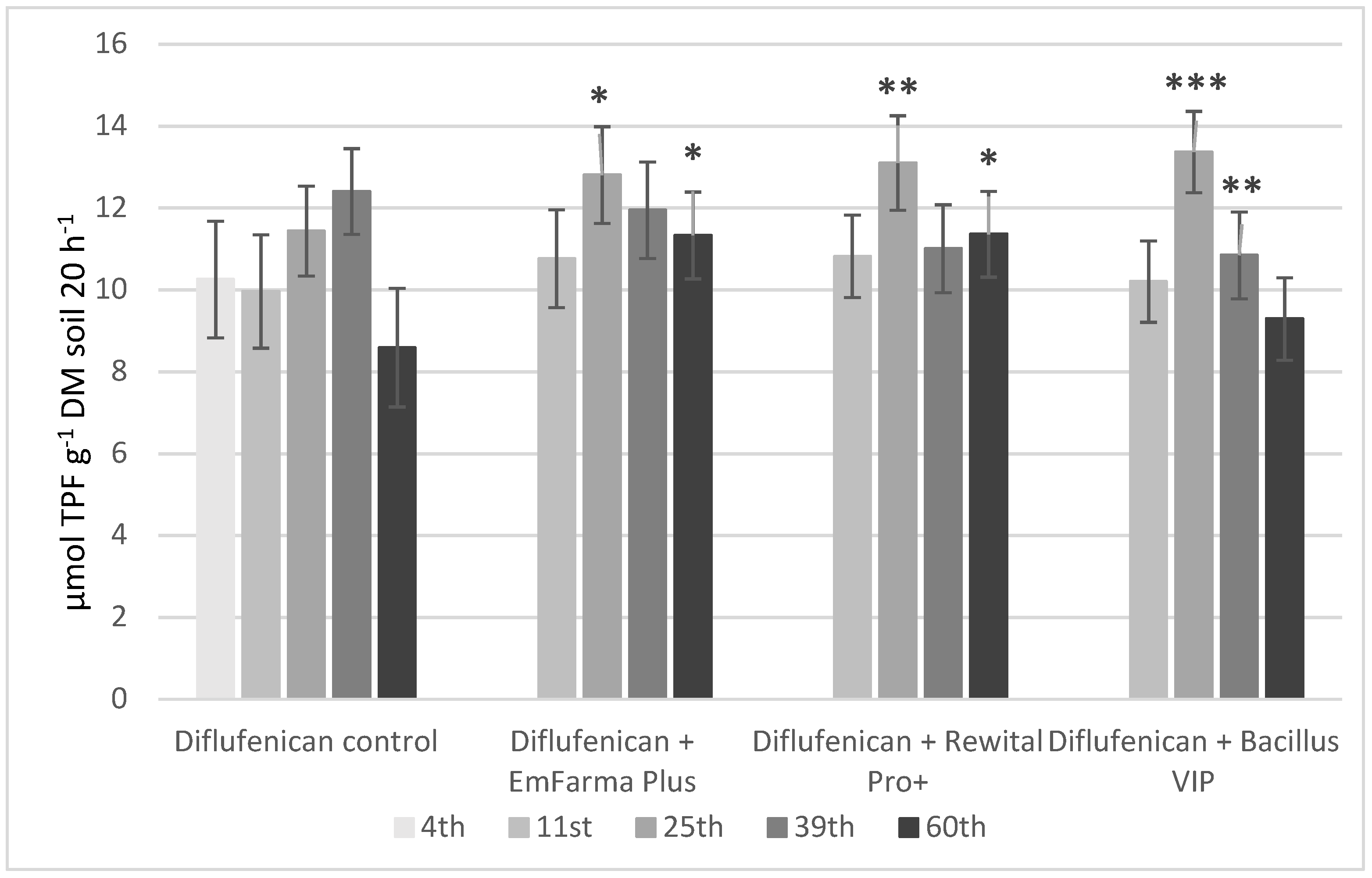
2.4. Soil Parameters: pH, Oxidoreduction Potential, and DHA
3. Discussion
3.1. Kinetics of Active Substances Diflufenican and Flurochloridone and Their Disspation and Half-Lives in Soil
3.1.1. Diflufenican
3.1.2. Flurochloridone
3.2. Influence of EM in Formulations on Herbicide Degradation in the Soil in Laboratory Conditions
3.2.1. Diflufenican
3.2.2. Flurochloridone
4. Materials and Methods
4.1. Reagents
4.2. Soil Sample Preparation
4.3. GC-MS Analysis of Pesticides Residues and Possible Metabolites
4.4. Assessment of DHA
4.5. Statistical Analysis of Results
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Jusoh, M.L.; Manaf, L.A.; Latiff, P.A. Composting of rice straw with effective microorganisms (EM) and its influence on compost quality. Iran. J. Environ. Health Sci. Eng. 2013, 10, 17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortez, J.; Billes, G.; Bouche, M.B. Effect of climate, soil type and earthworm activity on nitrogen transfer from a nitrogen-15-labelled decomposing material under field conditions. Biol. Fertil. Soils 2000, 30, 318–327. [Google Scholar] [CrossRef]
- Yamada, K.; Xu, H.-L. Properties and applications of an organic fertilizer inoculated with effective microorganisms. J. Crop Prod. 2000, 3, 255–268. [Google Scholar] [CrossRef]
- Joshi, H.; Duttand, S.; Choudhary, P.; Mundra, S. Role of Effective Microorganisms (EM) in Sustainable Agriculture. Int. J. Curr. Microbiol. App. Sci 2019, 8, 172–181. [Google Scholar] [CrossRef]
- Deiana, M.; Dessi, M.A.; Ke, B.; Liang, Y.F.; Higa, T.; Gilmour, P.S. The antioxidant cocktail effective microorganism X (EM-X) inhibits oxidant induced interleukin-8 release and the peroxidation of phospholipids in vitro. Biochem. Biophys. Res. Commun. 2002, 296, 1148–1151. [Google Scholar] [CrossRef]
- Singh, D.S.; Chand, S.; Anvar, M. Effect of organic and inorganic amendment on growth and nutrient accumulation by Isabgol (Plantago ovata) in sodic soil under greenhouse conditions. J. Med. Aromat. Plant Sci. 2003, 25, 414–419. [Google Scholar]
- Nayak, N.; Sar, K.; Sahoo, B.K.; Mahapatra, P. Beneficial effect of effective microorganisms on crop and soil—A review. J Pharm. Phytochem. 2020, 9, 3070–3074. [Google Scholar]
- Kowalska, J.; Sosnowska, D.; Remlein-Starosta, D.; Dróżdżyński, D.; Wojciechowska, R.; Łopatka, L. Efektywne Mikroorganizmy w Rolnictwie Ekologicznym; Instytut Ochrony Roślin—Państwowy Instytut Badawczy w Poznaniu: Poznań, Poland, 2011; p. 14. [Google Scholar]
- Kah, M.; Beulke, S.; Brown, C.D. Factors influencing degradation of pesticides in soil. J. Agric. Food Chem. 2007, 55, 4487–4492. [Google Scholar] [CrossRef]
- Aktar, M.W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip. Toxicol. 2009, 2, 12. [Google Scholar] [CrossRef] [Green Version]
- Wołejko, E.; Łozowicka, B.; Kaczyński, P.; Jankowska, M.; Piekut, J. The influence of effective microorganisms (EM) and yeast on the degradation of strobilurins and carboxamides in leafy vegetables monitored by LC-MS/MS and health risk assessment. Environ. Monit. Assess. 2016, 188, 64. [Google Scholar] [CrossRef] [Green Version]
- Raffa, C.M.; Chiampo, F. Bioremediation of Agricultural Soils Polluted with Pesticides: A Review. Bioengineering 2021, 8, 92. [Google Scholar] [CrossRef] [PubMed]
- Baćmaga, M.; Borowik, A.; Kucharski, J.; Tomkiel, M.; Wyszkowska, J. Microbial and enzymatic activity of soil contaminated with a mixture of diflufenican, mesoslfuron-methyl, iodosulfuron-methyl-sodium. Environ. Sci. Pollut. Res. Int. 2015, 22, 643–656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doolotkeldieva, T.; Konurbaeva, M.; Bobusheva, S. Microbial communities in pesticide-contaminated soils in Kyrgyzstan and bio-remediation possibilities. Environ. Sci. Pollut. Res. Int. 2018, 25, 31848–31862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Javaid, M.K.; Ashiq, M.; Tahir, M. Potential of Biological Agents in Decontamination of Agricultural Soil. Scientifica 2016, 2016, 1598325. [Google Scholar] [CrossRef] [Green Version]
- Szpyrka, E.; Podbielska, M.; Zwolak, A.; Piechowicz, B.; Siebielec, G.; Słowik-Borowiec, M. Influence of a Commercial Biological Fungicide containing Trichoderma harzianum Rifai T-22 on Dissipation Kinetics and Degradation of Five Herbicides in Two Types of Soil. Molecules 2020, 25, 1391. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Xiao, L.; Li, F.; Xiao, M.; Lin, D.; Long, X.; Wu, Z. Microbial degradation of pesticide residues and an emphasis on the degradation of cypermethrin and 3-phenoxy benzoic acid: A review. Molecules 2018, 23, 2313. [Google Scholar] [CrossRef] [Green Version]
- Kumari, S.; Kumar, D.; Khurana, P.S.M. Microbial degradation of pesticides: Microbial potential for degradation of pesticides. In Development in Wastewater Treatment Research and Processes; Elsevier: Amsterdam, The Netherlands, 2022; pp. 41–67. [Google Scholar] [CrossRef]
- PubChem; National Library of Medicine. National Center for Biotechnology Information. Available online: https://pubchem.ncbi.nlm.nih.gov/ (accessed on 14 February 2022).
- PPDB. Pesticide Properties Database. University of Hertfordshire: Hatfield, UK. Available online: http://sitem.herts.ac.uk/aeru/ppdb/ (accessed on 14 February 2022).
- HRAC. Herbicide Resistance Action Committee. Global Herbicide Classification Lookup. Available online: https://hracglobal.com/tools/classification-lookup (accessed on 14 February 2022).
- Wołejko, E.; Kaczyński, P.; Łozowicka, B.; Wydro, U.; Borusiewicz, A.; Hrynko, I.; Konecki, R.; Snarska, K.; Dec, D.; Malinowski, P. Dissipation of S-metolachlor in plant and soil and effect on enzymatic activities. Environ. Monit. Assess. 2017, 189, 355. [Google Scholar] [CrossRef] [Green Version]
- Rouchaud, J.; Gustin, F.; Callens, D.; Van Himme, M.; Bulcke, R. Effects of recent organic fertilizer treatment on herbicide diflufenican soil metabolism in winter wheat crops. Toxicol. Environ. Chem. 1994, 42, 191–198. [Google Scholar] [CrossRef]
- Rouchaud, J.; Neus, O.; Bulcke, R.; Cools, K.; Eelen, H.; Dekkers, T. Soil dissipation of diuron, chlortoluron, simazine, propyzamide, and diflufenican herbicides after repeated applications in fruit tree orchards. Arch. Environ. Contam. Toxicol. 2000, 39, 60–65. [Google Scholar] [CrossRef]
- Bending, G.; Lincoln, S.D.; Edmondson, R.N. Spatial variation in the degradation rate of the pesticides isoproturon, azoxystrobin and diflufenican in soil and its relationship with chemical and microbial properties. Environ. Pollut. 2006, 139, 279–287. [Google Scholar] [CrossRef]
- Conte, E.; Morali, G.; Galli, M.; Imbroglini, G.; Leake, C.R. Long-term degradation, and potential plant uptake of diflufenican under field conditions. J. Agric. Food Chem. 1998, 46, 4766–4770. [Google Scholar] [CrossRef]
- Rouchaud, J.; Neus, O.; Callens, D.; Bulcke, R. Herbicide flurochloridone soil biodegradation in potato crops. Toxicol. Environ. Chem. 1997, 61, 251–257. [Google Scholar] [CrossRef]
- Li, W.; Chen, H.; Chen, S. Study on residue and dissipation of flurochloridone by LC-MS/MS in potato and soil under open-field conditions in the China’s Qinghai Plateau. Int. J. Environ. Anal. Chem. 2021, 11. [Google Scholar] [CrossRef]
- Sharipov, U.; Kocarek, M.; Jursik, M.; Nikodem, A.; Boruvka, L. Adsorption and degradation behavior of six herbicides in different argicultural soils. Environ. Earth Sci. 2021, 80, 14. [Google Scholar] [CrossRef]
- Houot, S.; Topp, E.; Yassir, A.; Soulas, G. Dependence of accelerated degradation of atrazine on soil pH in French and Canadian soils. Soil Biol. Biochem. 2000, 32, 615–625. [Google Scholar] [CrossRef]
- Carpio, M.J.; Garcia-Delgado, C.; Marin-Benito, J.M.; Sanchez-Martin, M.J.; Rodriguez-Cruz, M.S. Soil Microbial Community Changes in a Field Treatment with Chlortoluron, Flufenacet and Diflufenican and Two Organic Amendments. Agronomy 2020, 10, 1166. [Google Scholar] [CrossRef]
- Tejada, M.; Del Toro, M.; Gomez, I.; Parrado, J. Application of diflufenican herbicide on soils amended with different organic wastes. Herbic. Environ. 2011, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Pinto, A.P.; Teixeira, D.M.; Caldeira, A.T.; Rodrigues, S.C. Biodegradation of pesticides by adapted fungi. Potential use on bio purification systems. Agrochem. Detect. Treat. Remediat. 2020, 1, 23. [Google Scholar] [CrossRef]
- EmFarma PlusTM Label. Available online: https://www.centrummikroorganizmow.pl/pl/emfarma-plus/23-emfarma-plus-10l.html (accessed on 14 February 2022).
- Rewital PRO+ Label. Available online: https://otorolny.pl/188-revital-pro-rewitalizacja-gleby-0-5l-bio-gen (accessed on 14 February 2022).
- Bacillus VIP Mikroorganizmy Probiotyczne Label. Available online: https://dobryfarmer.pl/product-pol-85-BACILLUS-VIP-Mikroorganizmy-Probiotyczne-5L-uniw.html?gclid=CjwKCAiAqIKNBhAIEiwAu_ZLDi2kMK0d0pHXIKvPoGOp1SCSDJQTl-C-C9N-cuoUfzTamtpWn1F_uxoCsDsQAvD_BwE (accessed on 14 February 2022).
- Legato 500 SC Label. Available online: https://www.adama.com/polska/pl/produkty/herbicydy/legato-500-sc (accessed on 14 February 2022).
- Racer 250 EC Label. Available online: https://www.adama.com/polska/pl/produkty/herbicydy/racer-250-ec (accessed on 14 February 2022).
- EU Pesticides Database. Directorate General for Health and Food Safety European Commission: Brussels, Belgium. Available online: https://ec.europa.eu/food/plants/pesticides/eu-pesticides-database_pl (accessed on 14 February 2022).
- PN-ISO 11465:1999; Jakość Gleby—Oznaczanie Zawartości Suche Masy Gleby i Wody w Glebie w Przeliczeniu na Suchą Masę Gleby, Metoda Wagowa. The Polish Committee for Standardization: Warsaw, Poland, 1999; p. 7.
- PN-EN 15662:2018-06; Foods of Plant Origin. Multimethod for the Determination of Pesticide Residues using GC- and LC-Based Analysis Following Acetonitrile Extraction/Partitioning and Clean-Up by Dispersive SPE. Modular QuEChERS-methodVegetable Food Multimeter for the Determination of Pesticide Residues Using GC and LC Based Analysis after Acetonitrile Extraction/Partitioning and SPE Dispersion Purification. QuEChERS Modular Method. The Polish Committee for Standardization: Warsaw, Poland, 2018; p. 82.
- Słowik-Borowiec, M.; Szpyrka, E.; Książek-Trela, P.; Podbielska, M. Simultaneous Determination of Multi-Class Pesticide Residues and PAHs in Plant Material and Soil Samples Using the Optimized QuEChERS Method and Tandem Mass Spectrometry Analysis. Molecules 2022, 27, 2140. [Google Scholar] [CrossRef]
- Casida, L.E., Jr.; Klein, D.A.; Santoro, T. Soil dehydrogenase activity. Soil Sci. 1964, 98, 371–376. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis, Part 2: Microbiological and Biochemical Properties; Weaver, R.W., Angle, J.S., Bottomley, P.S., Eds.; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–833. [Google Scholar]

| Number of Days after Formulation Application | Degradation (%) | ||
|---|---|---|---|
| EmFarma Plus™ | Rewital PRO+ | BACILLUS VIP Probiotic Microorganisms | |
| 11 | −36.5 ** | −35.5 | −32.6 |
| 25 | 7.2 | 1.4 | 7.2 |
| 39 | 3.5 | −25.0 * | −38.0 |
| 60 | 2.4 | −9.1 | −10.0 |
| Number of Days after Formulation Application | Degradation (%) | ||
|---|---|---|---|
| EmFarma Plus™ | Rewital PRO+ | BACILLUS VIP Probiotic Microorganisms | |
| 11 | 18.3 | 7.1 | 22.0 |
| 25 | 4.9 | 27.7 | 29.8 |
| 39 | 4.4 | 17.9 | 17.8 |
| 60 | 19.3 | 30.5 * | 31.2 * |
| Samples Number | Title 3 |
|---|---|
| 1–3 | soil + diflufenican |
| 4–6 | soil + diflufenican + EmFarma Plus™ |
| 7–9 | soil + diflufenican + Rewital PRO+ |
| 10–12 | soil + diflufenican + BACILLUS VIP Probiotic Microorganisms |
| 13–15 | soil + flurochloridone |
| 16–18 | soil + flurochloridone + EmFarma Plus™ |
| 19–21 | soil + flurochloridone + Rewital PRO+ |
| 22–24 | soil + flurochloridone + BACILLUS VIP Probiotic Microorganisms |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Książek-Trela, P.; Bielak, E.; Węzka, D.; Szpyrka, E. Effect of Three Commercial Formulations Containing Effective Microorganisms (EM) on Diflufenican and Flurochloridone Degradation in Soil. Molecules 2022, 27, 4541. https://doi.org/10.3390/molecules27144541
Książek-Trela P, Bielak E, Węzka D, Szpyrka E. Effect of Three Commercial Formulations Containing Effective Microorganisms (EM) on Diflufenican and Flurochloridone Degradation in Soil. Molecules. 2022; 27(14):4541. https://doi.org/10.3390/molecules27144541
Chicago/Turabian StyleKsiążek-Trela, Paulina, Ewelina Bielak, Dominika Węzka, and Ewa Szpyrka. 2022. "Effect of Three Commercial Formulations Containing Effective Microorganisms (EM) on Diflufenican and Flurochloridone Degradation in Soil" Molecules 27, no. 14: 4541. https://doi.org/10.3390/molecules27144541






