Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide
Abstract
:1. Introduction
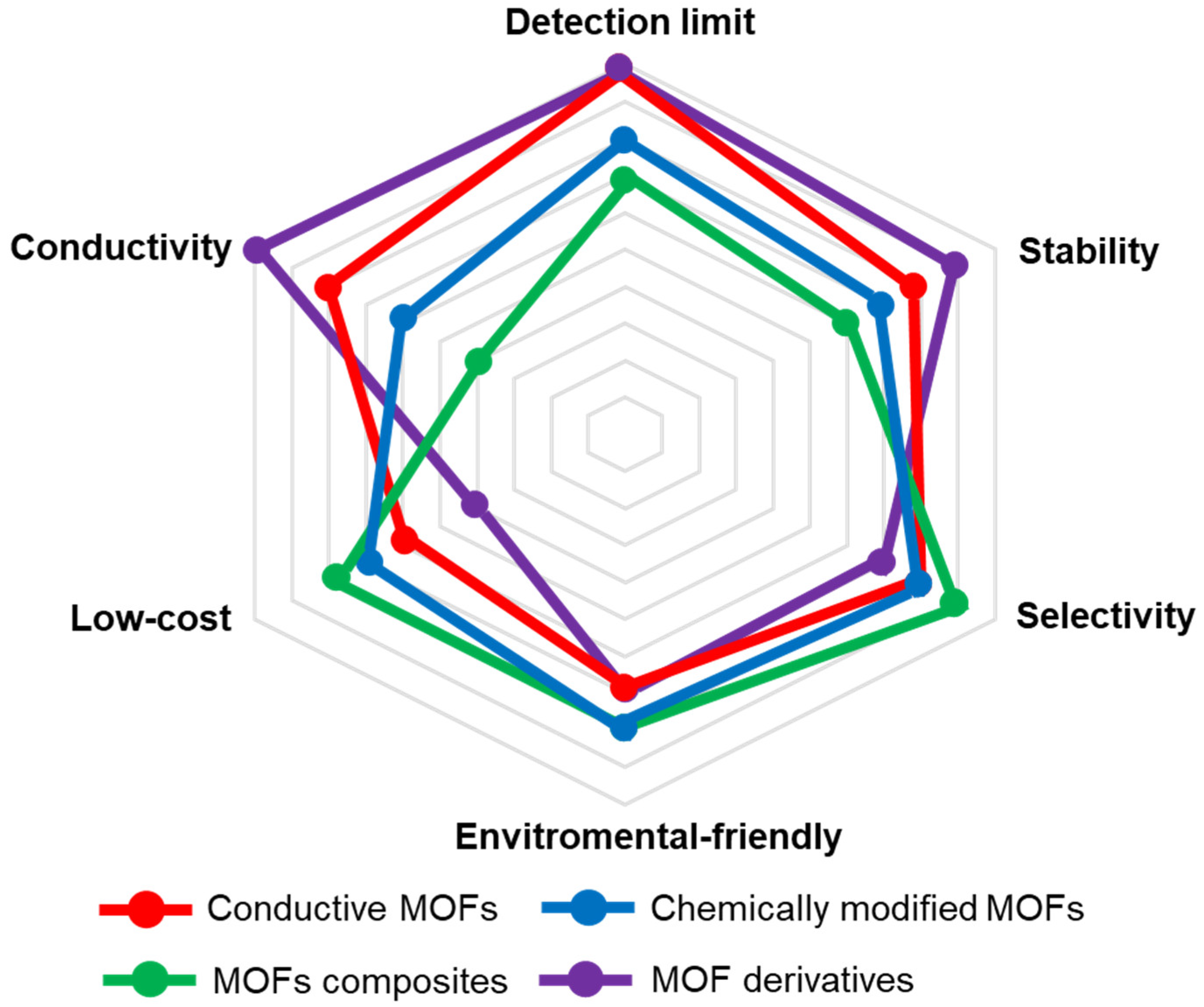
2. Research Status and Challenges
- (i)
- Conductivity
- (ii)
- Activity
- (iii)
- Selectivity
- (iv)
- Stability
- (v)
- Low-cost
- (vi)
- Environmental-friendly
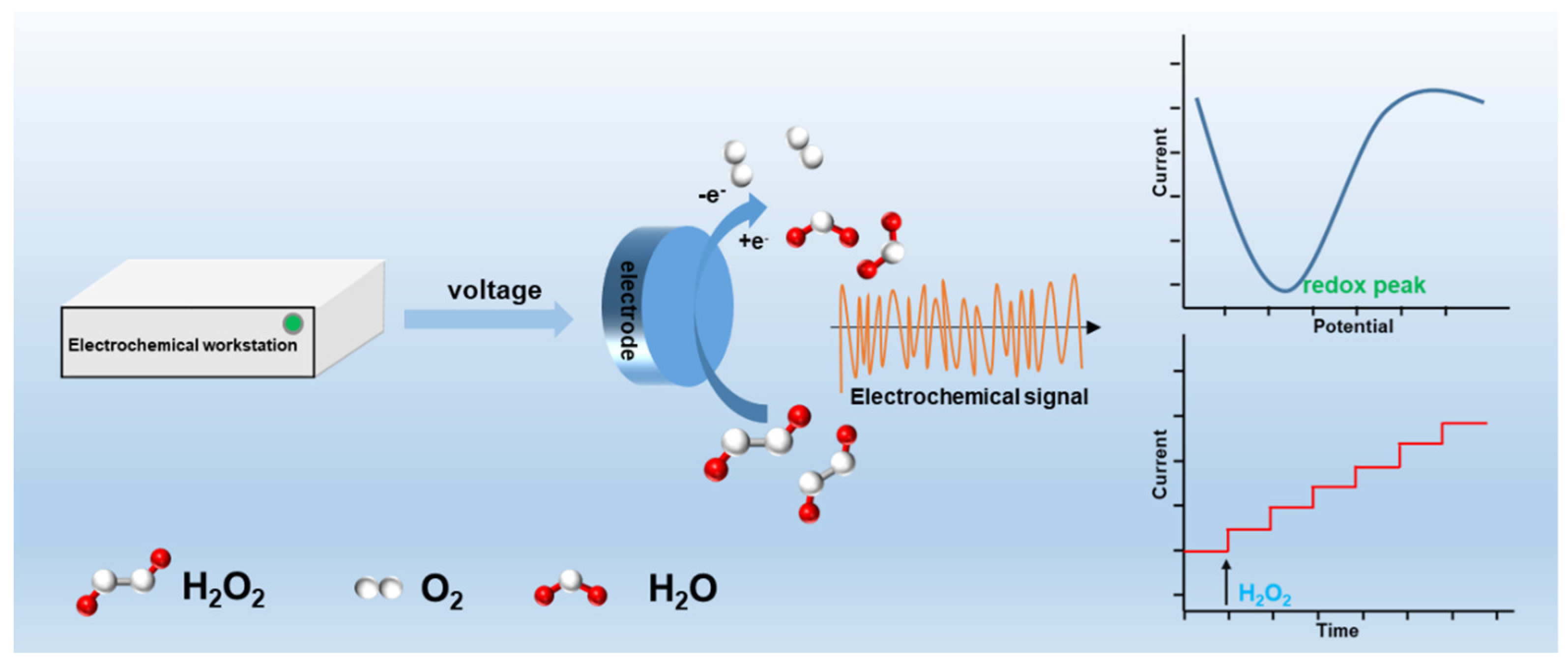
3. Sensing Mechanisms and Working Principles of MOFs-Based H2O2 Electrochemical Sensor
3.1. Electrochemical Sensor Detection Principle
3.2. Detecting under Alkaline Condition
3.3. Detecting under Neutral Condition
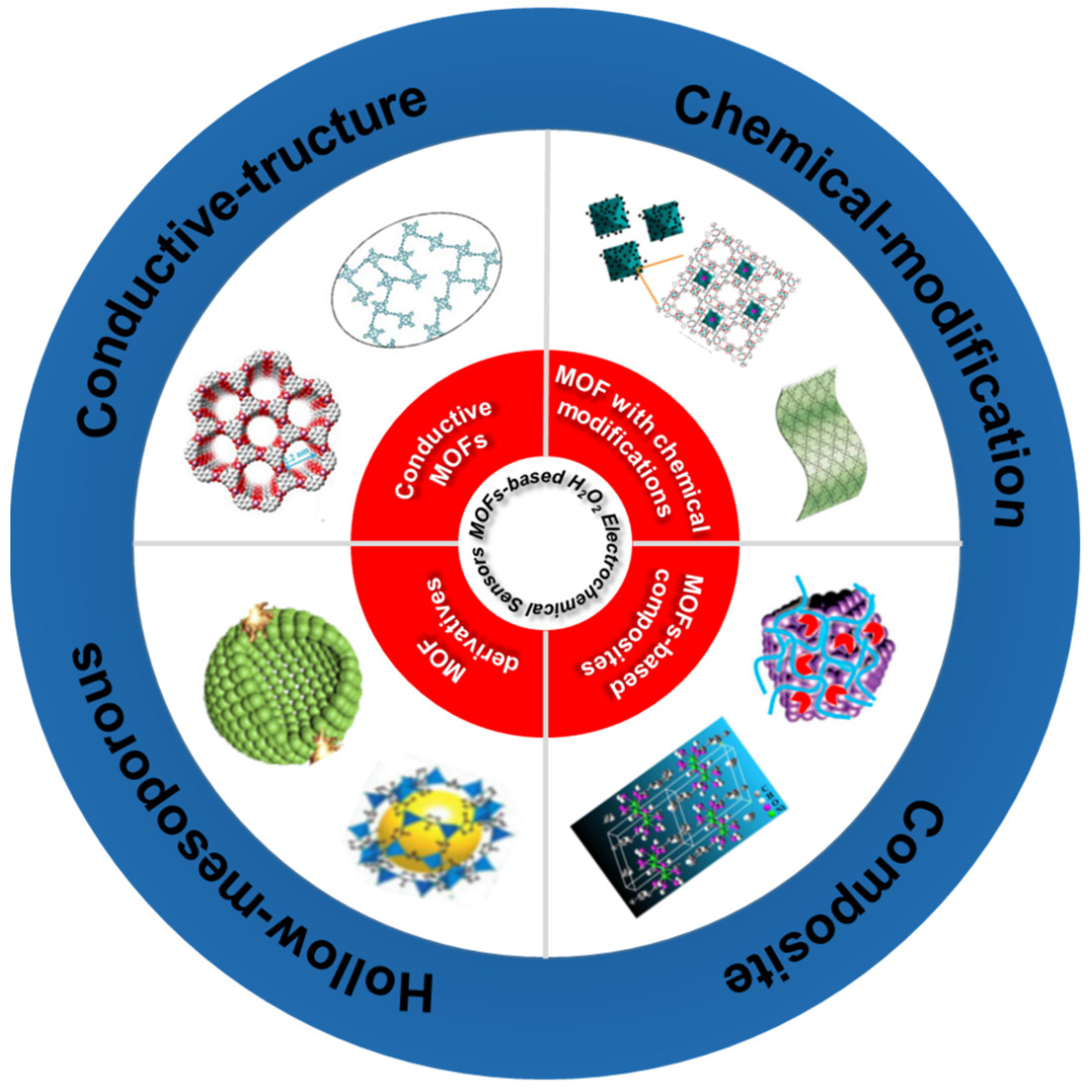
4. Design and Synthesis of MOFs Based H2O2 Sensor
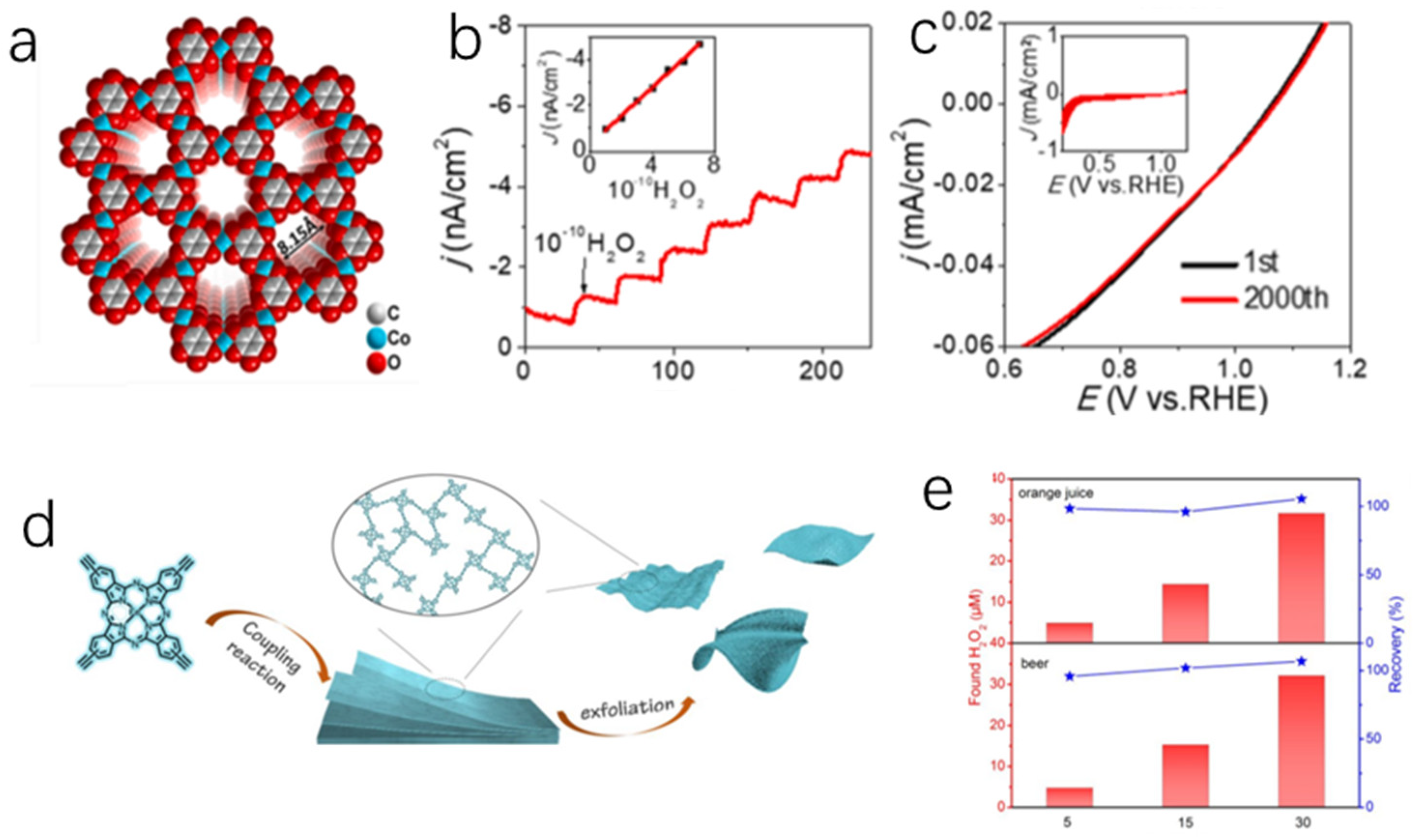
4.1. Conductive MOFs Based H2O2 Sensor
4.2. Chemically Modified MOFs Based H2O2 Sensor
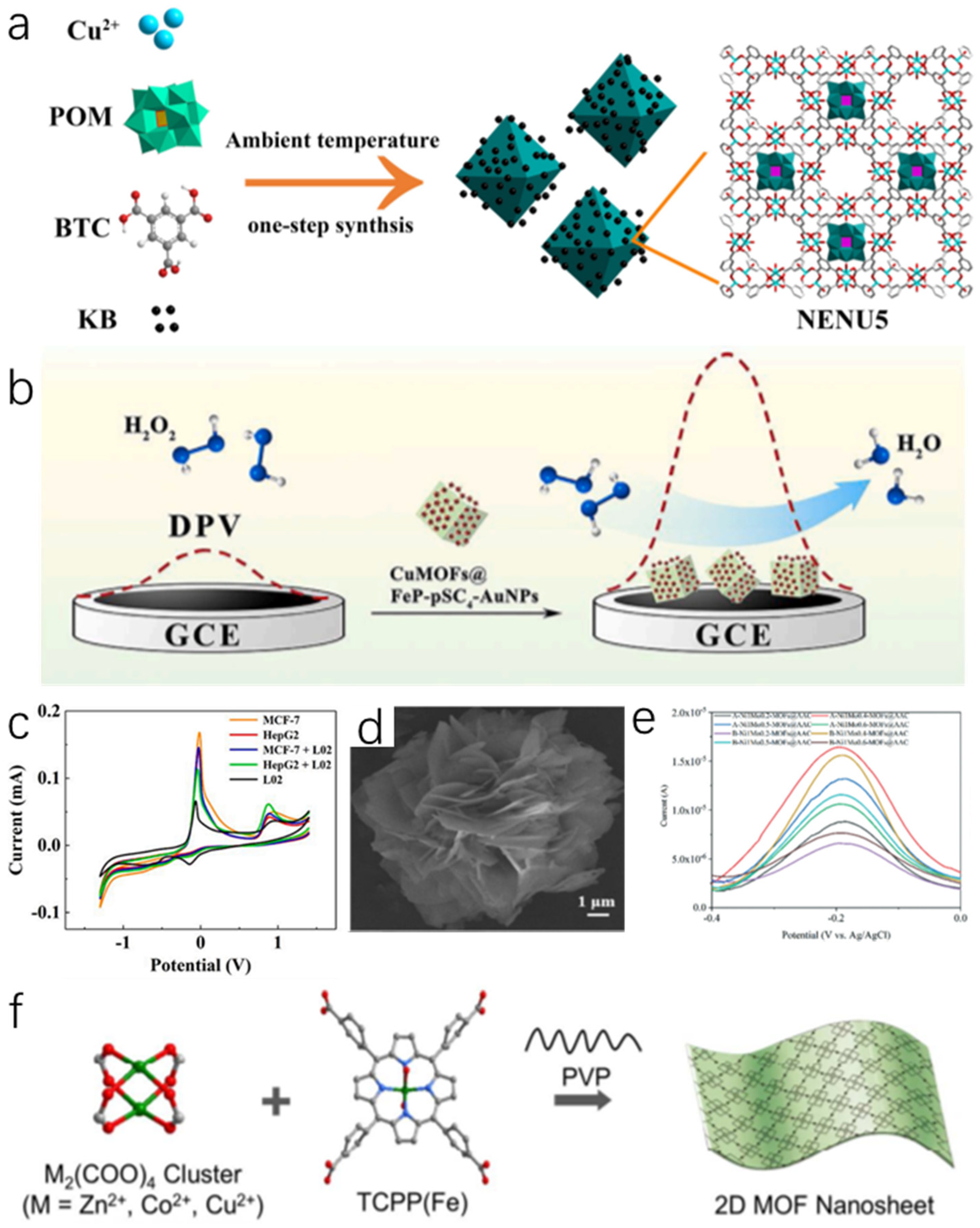
4.3. MOF Composites Based H2O2 Sensor
4.4. MOF Derivatives Based H2O2 Sensor
5. Conclusions and Outlook
- (i)
- Ultra-low detection limit
- (ii)
- Long-term stability
- (iii)
- Large-scale production
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Salimi, A.; Hallaj, R.; Soltanian, S.; Mamkhezri, H. Nanomolar Detection of Hydrogen Peroxide on Glassy Carbon Electrode Modified with Electrodeposited Cobalt Oxide Nanoparticles. Anal. Chim. Acta 2007, 594, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wang, G.; Zheng, B.; Li, L.; Lv, B.; Cao, H.; Chen, M. 3-Layer Conductive Metal-Organic Nanosheets as Electrocatalysts to Enable an Ultralow Detection Limit of H2O2. Nanoscale 2019, 11, 5058–5063. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, I.; Nemakal, M.; Sajjan, V.A.; Puttappashetty, D.B.; Sannegowda, L.K. Electropolymerized Film of Cobalt Tetrabenzimidazolephthalocyanine for the Amperometric Detection of H2O2. J. Electroanal. Chem. 2018, 826, 96–103. [Google Scholar] [CrossRef]
- Chen, W.; Cai, S.; Ren, Q.Q.; Wen, W.; Zhao, Y. Di Recent Advances in Electrochemical Sensing for Hydrogen Peroxide: A Review. Analyst 2012, 137, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Izu, K.; Yamamoto, O.; Asahi, M. Occupational Skin Injury by Hydrogen Peroxide. Dermatology 2000, 201, 61–64. [Google Scholar] [CrossRef]
- SCHRECK, A. Investigation of the Explosive Hazard of Mixtures Containing Hydrogen Peroxide and Different Alcohols. J. Hazard. Mater. 2004, 108, 1–7. [Google Scholar] [CrossRef]
- Mou, K.; Pan, W.; Han, D.A.N.; Wen, X.I.N.; Cao, F.; Miao, Y.I.; Li, P.A.N. Glycyrrhizin Protects Human Melanocytes from H2O2—Induced Oxidative Damage via the Nrf2—Dependent Induction of HO - 1. Int. J. Mol. Med. 2019, 44, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Xin, C.; Zhang, G.; Han, X.; Qin, W.; Zhang, C.W.; Yu, C.; Jing, S.; Li, L.; Huang, W. A Mitochondria-Targeted Two-Photon Fluorogenic Probe for the Dual-Imaging of Viscosity and H2O2 Levels in Parkinson’s Disease Models. J. Mater. Chem. B 2019, 7, 4243–4251. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, J.; Xu, Z.; Jin, D.; Xu, Q.; Hu, X. Nickel Metal-Organic Framework Nanosheet/Hemin Composite as Biomimetic Peroxidase for Electrocatalytic Reduction of H2O2. J. Electroanal. Chem. 2019, 845, 137–143. [Google Scholar] [CrossRef]
- Gimeno, M.P.; Mayoral, M.C.; Andrés, J.M. A Potentiometric Titration for H2O2 Determination in the Presence of Organic Compounds. Anal. Methods 2013, 5, 1510–1514. [Google Scholar] [CrossRef]
- Hu, H.-C.; Jin, H.-J.; Chai, X.-S. Rapid Determination of Hydrogen Peroxide in Pulp Bleaching Effluents by Headspace Gas Chromatography. J. Chromatogr. A 2012, 1235, 182–184. [Google Scholar] [CrossRef] [PubMed]
- Ning, D.; Liu, Q.; Wang, Q.; Du, X.-M.; Ruan, W.-J.; Li, Y. Luminescent MOF Nanosheets for Enzyme Assisted Detection of H2O2 and Glucose and Activity Assay of Glucose Oxidase. Sensors Actuators B Chem. 2019, 282, 443–448. [Google Scholar] [CrossRef]
- El-Nagar, G.A.; Sarhan, R.M.; Abouserie, A.; Maticiuc, N.; Bargheer, M.; Lauermann, I.; Roth, C. Efficient 3D-Silver Flower-like Microstructures for Non-Enzymatic Hydrogen Peroxide (H2O2) Amperometric Detection. Sci. Rep. 2017, 7, 12181. [Google Scholar] [CrossRef] [Green Version]
- Ren, H.; Long, Z.; Cui, M.; Shao, K.; Zhou, K.; Ouyang, J.; Na, N. Dual-Functional Nanoparticles for In Situ Sequential Detection and Imaging of ATP and H2O2. Small 2016, 12, 3920–3924. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Zhang, S.; Fu, Y.; Zheng, X.; Zheng, J. Shape-Controlled Synthesis of CuCo2S4 as Highly-Efficient Electrocatalyst for Nonenzymatic Detection of H2O2. Electrochim. Acta 2017, 255, 23–30. [Google Scholar] [CrossRef]
- Miah, M.R.; Ohsaka, T. Cathodic Detection of H2O2 Using Iodide-Modified Gold Electrode in Alkaline Media. Anal. Chem. 2006, 78, 1200–1205. [Google Scholar] [CrossRef] [PubMed]
- Wen, D.; Liu, Q.; Cui, Y.; Yang, H.; Kong, J. TEMPO-Functionalized Nanoporous Au Nanocomposite for the Electrochemical Detection of H2O2. Int. J. Anal. Chem. 2018, 2018, 1–11. [Google Scholar] [CrossRef]
- Manickam, P.; Vashist, A.; Madhu, S.; Sadasivam, M.; Sakthivel, A.; Kaushik, A.; Nair, M. Gold Nanocubes Embedded Biocompatible Hybrid Hydrogels for Electrochemical Detection of H2O2. Bioelectrochemistry 2020, 131, 107373. [Google Scholar] [CrossRef]
- Lin, C.Y.; Chang, C.T. Iron Oxide Nanorods Array in Electrochemical Detection of H2O2. Sensors Actuators B Chem. 2015, 220, 695–704. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, G.; Zhang, W.; Wei, Y.; Fang, B. Fixure-Reduce Method for the Synthesis of Cu2O/MWCNTs Nanocomposites and Its Application as Enzyme-Free Glucose Sensor. Biosens. Bioelectron. 2009, 24, 3395–3398. [Google Scholar] [CrossRef]
- Pang, H.; Gao, F.; Lu, Q. Glycine-Assisted Double-Solvothermal Approach for Various Cuprous Oxide Structures with Good Catalytic Activities. CrystEngComm 2010, 12, 406–412. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic Peroxidase-like Activity of Ferromagnetic Nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef] [PubMed]
- Gopalan, A.I.; Komathi, S.; Sai Anand, G.; Lee, K.P. Nanodiamond Based Sponges with Entrapped Enzyme: A Novel Electrochemical Probe for Hydrogen Peroxide. Biosens. Bioelectron. 2013, 46, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Ju, J.; Chen, W. In Situ Growth of Surfactant-Free Gold Nanoparticles on Nitrogen-Doped Graphene Quantum Dots for Electrochemical Detection of Hydrogen Peroxide in Biological Environments. Anal. Chem. 2015, 87, 1903–1910. [Google Scholar] [CrossRef]
- Peng, M.; Zhao, Y.; Chen, D.; Tan, Y. Free-Standing 3D Electrodes for Electrochemical Detection of Hydrogen Peroxide. ChemCatChem 2019, 11, 4222–4237. [Google Scholar] [CrossRef]
- Zhang, Y.; Bai, X.; Wang, X.; Shiu, K.K.; Zhu, Y.; Jiang, H. Highly Sensitive Graphene-Pt Nanocomposites Amperometric Biosensor and Its Application in Living Cell H2O2 Detection. Anal. Chem. 2014, 86, 9459–9465. [Google Scholar] [CrossRef]
- Patella, B.; Buscetta, M.; Di Vincenzo, S.; Ferraro, M.; Aiello, G.; Sunseri, C.; Pace, E.; Inguanta, R.; Cipollina, C. Electrochemical Sensor Based on RGO/Au Nanoparticles for Monitoring H2O2 Released by Human Macrophages. Sensors Actuators B Chem. 2021, 327, 128901. [Google Scholar] [CrossRef]
- Kan, M.X.; Wang, X.J.; Zhang, H.M. Detection of H2O2 at a Composite Film Modified Electrode with Highly Dispersed Ag Nanoparticles in Nafion. Chinese Chem. Lett. 2011, 22, 458–460. [Google Scholar] [CrossRef]
- Chou, T.C.; Wu, K.Y.; Hsu, F.X.; Lee, C.K. Pt-MWCNT Modified Carbon Electrode Strip for Rapid and Quantitative Detection of H2O2 in Food. J. Food Drug Anal. 2018, 26, 662–669. [Google Scholar] [CrossRef] [Green Version]
- Niamlaem, M.; Boonyuen, C.; Sangthong, W.; Limtrakul, J.; Zigah, D.; Kuhn, A.; Warakulwit, C. Highly Defective Carbon Nanotubes for Sensitive, Low-Cost and Environmentally Friendly Electrochemical H2O2 Sensors: Insight into Carbon Supports. Carbon N. Y. 2020, 170, 154–164. [Google Scholar] [CrossRef]
- Bao, J.; Yang, H.; Zhao, J.; Yang, C.; Duan, Y.; Lu, W.; Gao, M.; Chen, M.; Huo, D.; Hou, C. In SituDetection of Released H2O2 from Living Cells by Carbon Cloth-Supported Graphene/Au-Pt Nanoparticles. ACS Appl. Nano Mater. 2021, 4, 9449–9458. [Google Scholar] [CrossRef]
- Wang, H.; Chen, W.; Chen, Q.; Liu, N.; Cheng, H.; Li, T. Metal-Organic Framework (MOF)-Au@Pt Nanoflowers Composite Material for Electrochemical Sensing of H2O2 in Living Cells. J. Electroanal. Chem. 2021, 897, 115603. [Google Scholar] [CrossRef]
- Zong, C.; Li, B.; Wang, J.; Liu, X.; Zhao, W.; Zhang, Q.; Nie, X.; Yu, Y. Visual and Colorimetric Determination of H2O2 and Glucose Based on Citrate-Promoted H2O2 Sculpturing of Silver Nanoparticles. Microchim. Acta 2018, 185, 199. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Li, H.; Zhang, X.X.; Liu, N.; Zhang, X.X. NiO/Graphene Nanocomposite for Determination of H2O2 with a Low Detection Limit. Talanta 2015, 144, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Hai, X.; Chen, X.W.; Wang, J.H. In Situ Growth of Silver Nanoparticles on Graphene Quantum Dots for Ultrasensitive Colorimetric Detection of H2O2 and Glucose. Anal. Chem. 2014, 86, 6689–6694. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, B.; Li, L.; Song, J.; Song, L.; Zhang, M. Fewer-Layer Conductive Metal-Organic Langmuir-Blodgett Films as Electrocatalysts Enable an Ultralow Detection Limit of H2O2. Appl. Surf. Sci. 2021, 539, 1–6. [Google Scholar] [CrossRef]
- Jiao, L.; Wang, Y.; Jiang, H.L.; Xu, Q. Metal–Organic Frameworks as Platforms for Catalytic Applications. Adv. Mater. 2018, 30, 1–23. [Google Scholar] [CrossRef]
- Zou, C.; Wu, C. De Functional Porphyrinic Metal-Organic Frameworks: Crystal Engineering and Applications. Dalt. Trans. 2012, 41, 3879–3888. [Google Scholar] [CrossRef]
- Nath, I.; Chakraborty, J.; Verpoort, F. Metal Organic Frameworks Mimicking Natural Enzymes: A Structural and Functional Analogy. Chem. Soc. Rev. 2016, 45, 4127–4170. [Google Scholar] [CrossRef]
- Lei, J.; Qian, R.; Ling, P.; Cui, L.; Ju, H. Design and Sensing Applications of Metal-Organic Framework Composites. TrAC—Trends Anal. Chem. 2014, 58, 71–78. [Google Scholar] [CrossRef]
- Gu, Z.Y.; Park, J.; Raiff, A.; Wei, Z.; Zhou, H.C. Metal-Organic Frameworks as Biomimetic Catalysts. ChemCatChem 2014, 6, 67–75. [Google Scholar] [CrossRef]
- Gkaniatsou, E.; Sicard, C.; Ricoux, R.; Mahy, J.P.; Steunou, N.; Serre, C. Metal-Organic Frameworks: A Novel Host Platform for Enzymatic Catalysis and Detection. Mater. Horizons 2017, 4, 55–63. [Google Scholar] [CrossRef]
- Wang, H.S. Metal–Organic Frameworks for Biosensing and Bioimaging Applications. Coord. Chem. Rev. 2017, 349, 139–155. [Google Scholar] [CrossRef]
- Yi, F.Y.; Chen, D.; Wu, M.K.; Han, L.; Jiang, H.L. Chemical Sensors Based on Metal–Organic Frameworks. Chempluschem 2016, 81, 675–690. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Liu, X.; Chai, H.; Huang, Y. Recent Advances in the Construction and Analytical Applications of Metal-Organic Frameworks-Based Nanozymes. TrAC Trends Anal. Chem. 2018, 105, 391–403. [Google Scholar] [CrossRef]
- Liu, Y.L.; Zhao, X.J.; Yang, X.X.; Li, Y.F. A Nanosized Metal-Organic Framework of Fe-MIL-88NH2 as a Novel Peroxidase Mimic Used for Colorimetric Detection of Glucose. Analyst 2013, 138, 4526–4531. [Google Scholar] [CrossRef]
- Meier, J.; Hofferber, E.; Stapleton, J.A.; Iverson, N.M. Hydrogen Peroxide Sensors for Biomedical Applications. Chemosensors 2019, 7, 64. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, J.M.; Martins, P.R.; Rocha, D.P.; Matias, T.A.; Julião, M.S.S.; Munoz, R.A.A.; Angnes, L. Recent Trends and Perspectives in Electrochemical Sensors Based on MOF-Derived Materials. J. Mater. Chem. C 2021, 9, 8718–8745. [Google Scholar] [CrossRef]
- Zhong, Y.; Li, X.; Chen, J.; Wang, X.; Wei, L.; Fang, L.; Kumar, A.; Zhuang, S.Z.; Liu, J. Recent Advances in MOF-Based Nanoplatforms Generating Reactive Species for Chemodynamic Therapy. Dalt. Trans. 2020, 49, 11045–11058. [Google Scholar] [CrossRef]
- Cruz-Navarro, J.A.; Hernandez-Garcia, F.; Alvarez Romero, G.A. Novel Applications of Metal-Organic Frameworks (MOFs) as Redox-Active Materials for Elaboration of Carbon-Based Electrodes with Electroanalytical Uses. Coord. Chem. Rev. 2020, 412, 213263. [Google Scholar] [CrossRef]
- Kholdeeva, O.; Maksimchuk, N. Metal-Organic Frameworks in Oxidation Catalysis with Hydrogen Peroxide. Catalysts 2021, 11, 283. [Google Scholar] [CrossRef]
- Zhao, F.; Sun, T.; Geng, F.; Chen, P.; Gao, Y. Metal-Organic Frameworks-Based Electrochemical Sensors and Biosensors. Int. J. Electrochem. Sci. 2016, 14, 5287–5304. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Liu, L.; Yang, X.; Xu, X. Electrochemical Investigation of a New Cu-MOF and Its Electrocatalytic Activity towards H2O2 Oxidation in Alkaline Solution. Electrochem. commun. 2013, 33, 131–134. [Google Scholar] [CrossRef]
- Lopa, N.S.; Rahman, M.M.; Ahmed, F.; Chandra Sutradhar, S.; Ryu, T.; Kim, W. A Base-Stable Metal-Organic Framework for Sensitive and Non-Enzymatic Electrochemical Detection of Hydrogen Peroxide. Electrochim. Acta 2018, 274, 49–56. [Google Scholar] [CrossRef]
- Liu, W.; Pan, H.; Liu, C.; Su, C.; Liu, W.; Wang, K.; Jiang, J. Ultrathin Phthalocyanine-Conjugated Polymer Nanosheet-Based Electrochemical Platform for Accurately Detecting H2O2 in Real Time. ACS Appl. Mater. Interfaces 2019, 11, 11466–11473. [Google Scholar] [CrossRef]
- Xia, L.; Luan, X.; Qu, F.; Lu, L. Co-MOF/Titanium Nanosheet Array: An Excellent Electrocatalyst for Non-Enzymatic Detection of H2O2 Released from Living Cells. J. Electroanal. Chem. 2020, 878, 114553. [Google Scholar] [CrossRef]
- Hu, X.; Huang, Y.; Chen, J.; Zhu, X.; Mao, Z.; Wang, Y.; Hu, R.; Chen, H. MOFs Supported Nanonetworks Hybrid Flower-like Catalysts via Supramolecular-Mediated Cascade Self-Assembly for Sensitive Sensing of H2O2. Sensors Actuators B Chem. 2021, 342, 130076. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, M.; Ma, Y.; Tan, H.; Wang, Y.; Li, Y. Hybridized Polyoxometalate-Based Metal-Organic Framework with Ketjenblack for the Nonenzymatic Detection of H2O2. Chem. An Asian J. 2018, 13, 2054–2059. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, T.; Lu, N.; Xu, Z.; Song, Y.; Liu, Y.; Liu, M.; Zhao, P.; Zhang, Z.; Yan, X. Catalytic Amplification Based on Hierarchical Heterogeneity Bimetal-Organic Nanostructures with Peroxidase-like Activity. Anal. Chim. Acta 2021, 1173, 338713. [Google Scholar] [CrossRef]
- Guo, X.; Lin, C.; Zhang, M.; Duan, X.; Dong, X.; Sun, D.; Pan, J.; You, T. 2D/3D Copper-Based Metal-Organic Frameworks for Electrochemical Detection of Hydrogen Peroxide. Front. Chem. 2021, 9, 1–11. [Google Scholar] [CrossRef]
- Wang, M.Q.; Zhang, Y.; Bao, S.J.; Yu, Y.N.; Ye, C. Ni(II)-Based Metal-Organic Framework Anchored on Carbon Nanotubes for Highly Sensitive Non-Enzymatic Hydrogen Peroxide Sensing. Electrochim. Acta 2016, 190, 365–370. [Google Scholar] [CrossRef]
- Li, C.; Wu, R.; Zou, J.; Zhang, T.; Zhang, S.; Zhang, Z.; Hu, X.; Yan, Y.; Ling, X. MNPs@anionic MOFs/ERGO with the Size Selectivity for the Electrochemical Determination of H2O2 Released from Living Cells. Biosens. Bioelectron. 2018, 116, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pascal, K.; Jin, X.J. Ni-Mo Modified Metal-Organic Frameworks for High-Performance Supercapacitance and Enzymeless H2O2 detection. CrystEngComm 2020, 22, 5145–5161. [Google Scholar] [CrossRef]
- Cheng, D.; Li, P.; Zhu, X.; Liu, M.; Zhang, Y.; Liu, Y. Enzyme-Free Electrochemical Detection of Hydrogen Peroxide Based on the Three-Dimensional Flower-like Cu-Based Metal Organic Frameworks and MXene Nanosheets†. Chinese J. Chem. 2021, 39, 2181–2187. [Google Scholar] [CrossRef]
- Qiao, X.; Arsalan, M.; Ma, X.; Wang, Y.; Yang, S.; Wang, Y.; Sheng, Q.; Yue, T. A Hybrid of Ultrathin Metal-Organic Framework Sheet and Ultrasmall Copper Nanoparticles for Detection of Hydrogen Peroxide with Enhanced Activity. Anal. Bioanal. Chem. 2021, 413, 839–851. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.; Sofi, F.A.; Rashid, N.; Ingole, P.P.; Bhat, M.A. Metal-Organic Framework Functionalized Sulphur Doped Graphene: A Promising Platform for Selective and Sensitive Electrochemical Sensing of Acetaminophen, Dopamine and H2O2. New J. Chem. 2022, 46, 1588–1600. [Google Scholar] [CrossRef]
- Zheng, J.; Zhao, P.; Zhou, S.; Chen, S.; Liang, Y.; Tian, F.; Zhou, J.; Huo, D.; Hou, C. Development of Au-Pd@UiO-66-on-ZIF-L/CC as a Self-Supported Electrochemical Sensor for: In Situ Monitoring of Cellular Hydrogen Peroxide. J. Mater. Chem. B 2021, 9, 9031–9040. [Google Scholar] [CrossRef]
- Li, J.; Liu, Z.X.; Li, Y.X.; Shu, G.; Zhang, X.J.; Marks, R.S.; Shan, D. 2-Methylimidazole-Assisted Morphology Modulation of a Copper-Based Metal-Organic Framework Transducer for Enhanced Electrochemical Peroxidase-like Activity. Electroanalysis 2021, 1–8. [Google Scholar] [CrossRef]
- Liu, X.; Luo, C.; Wu, J.; He, N.; Yu, R.; Liu, X. Construction of a Non-Enzymatic Electrochemical Sensor Based on Metal-Organic-Framework-Derived Manganese Oxide Microspheres for the Detection of Hydrogen Peroxide. ChemElectroChem 2021, 8, 4141–4147. [Google Scholar] [CrossRef]
- Kim, S.E.; Muthurasu, A. Highly Oriented Nitrogen-Doped Carbon Nanotube Integrated Bimetallic Cobalt Copper Organic Framework for Non-Enzymatic Electrochemical Glucose and Hydrogen Peroxide Sensor. Electroanalysis 2021, 33, 1333–1345. [Google Scholar] [CrossRef]
- Mathew, G.; Daniel, M.; Peramaiah, K.; Ganesh, M.-R.; Neppolian, B. Real-Time Electrochemical Quantification of H2O2 in Living Cancer Cells Using Bismuth Based MOF. J. Electroanal. Chem. 2022, 914, 116255. [Google Scholar] [CrossRef]
- Zhang, T.; Xing, Y.; Song, Y.; Gu, Y.; Yan, X.; Lu, N.; Liu, H.; Xu, Z.; Xu, H.; Zhang, Z.; et al. AuPt/MOF-Graphene: A Synergistic Catalyst with Surprisingly High Peroxidase-like Activity and Its Application for H2O2 Detection. Anal. Chem. 2019, 91, 10589–10595. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, L.P.; Zhou, Z.; Li, Q.; Huo, L.H.; Zhao, H. Efficient Nonenzymatic H2O2 Biosensor Based on ZIF-67 MOF Derived Co Nanoparticles Embedded N-Doped Mesoporous Carbon Composites. Sensors Actuators B Chem. 2018, 276, 142–149. [Google Scholar] [CrossRef]
- Salman, F.; Zengin, A.; Çelik Kazici, H. Synthesis and Characterization of Fe3O4-Supported Metal–Organic Framework MIL-101(Fe) for a Highly Selective and Sensitive Hydrogen Peroxide Electrochemical Sensor. Ionics (Kiel). 2020, 26, 5221–5232. [Google Scholar] [CrossRef]
- Hu, Y.; Bai, C.; Li, M.; Hojamberdiev, M.; Geng, D.; Li, X. Tailoring Atomically Dispersed Cobalt-Nitrogen Active Sites in Wrinkled Carbon Nanosheets: Via “Fence” Isolation for Highly Sensitive Detection of Hydrogen Peroxide. J. Mater. Chem. A 2022, 10, 3190–3200. [Google Scholar] [CrossRef]
- Zhou, S.; Wang, X.; Zhao, P.; Zheng, J.; Yang, M.; Huo, D.; Hou, C. ZIF-67 MOF-Derived Co Nanoparticles Supported on N-Doped Carbon Skeletons for the Amperometric Determination of Hydrogen Peroxide. Microchim. Acta 2021, 188, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xin, Y.; Ma, Y.; Wang, P. Copper and Molybdenum Dioxide Co-Doped Octahedral Porous Carbon Framework for High Sensitivity Electrochemical Detection of Hydrogen Peroxide. Ionics (Kiel). 2022, 28, 919–925. [Google Scholar] [CrossRef]
- Mei, H.; Xie, J.; Li, Z.; Lou, C.; Lei, G.; Liu, X.; Zhang, J. Rational Design of ZnO@ZIF-8 Nanoarrays for Improved Electrochemical Detection of H2O2. CrystEngComm 2022, 24, 1645–1654. [Google Scholar] [CrossRef]
- Xiong, L.; Zhang, Y.; Wu, S.; Chen, F.; Lei, L.; Yu, L.; Li, C. Co3O4 Nanoparticles Uniformly Dispersed in Rational Porous Carbon Nano-Boxes for Significantly Enhanced Electrocatalytic Detection of H2O2 Released from Living Cells. Int. J. Mol. Sci. 2022, 23, 3799. [Google Scholar] [CrossRef]
- Park, J.; Lee, M.; Feng, D.; Huang, Z.; Hinckley, A.C.; Yakovenko, A.; Zou, X.; Cui, Y.; Bao, Z. Stabilization of Hexaaminobenzene in a 2D Conductive Metal-Organic Framework for High Power Sodium Storage. J. Am. Chem. Soc. 2018, 140, 10315–10323. [Google Scholar] [CrossRef]
- Lin, S.S.; Gurol, M.D. Catalytic Decomposition of Hydrogen Peroxide on Iron Oxide: Kinetics, Mechanism, and Implications. Environ. Sci. Technol. 1998, 32, 1417–1423. [Google Scholar] [CrossRef]
- Ensing, B.; Buda, F.; Baerends, E.J. Fenton-like Chemistry in Water: Oxidation Catalysis by Fe(III) and H2O2. J. Phys. Chem. A 2003, 107, 5722–5731. [Google Scholar] [CrossRef]
- Barb, W.G.; Baxendale, J.H.; George, P.; Hargrave, K.R. Reactions of Ferrous and Ferric Ions with Hydrogen Peroxide. Part II. - The Ferric Ion Reaction. Trans. Faraday Soc. 1951, 47, 591–616. [Google Scholar] [CrossRef]
- Wiegand, H.L.; Orths, C.T.; Kerpen, K.; Lutze, H.V.; Schmidt, T.C. Investigation of the Iron–Peroxo Complex in the Fenton Reaction: Kinetic Indication, Decay Kinetics, and Hydroxyl Radical Yields. Environ. Sci. Technol. 2017, 51, 14321–14329. [Google Scholar] [CrossRef] [PubMed]
- Thakur, B.; Karve, V.V.; Sun, D.T.; Semrau, A.L.; Weiß, L.J.K.; Grob, L.; Fischer, R.A.; Queen, W.L.; Wolfrum, B. An Investigation into the Intrinsic Peroxidase-Like Activity of Fe-MOFs and Fe-MOFs/Polymer Composites. Adv. Mater. Technol. 2021, 6, 1–9. [Google Scholar] [CrossRef]
- Li, C.; Zhang, L.; Chen, J.; Li, X.; Sun, J.; Zhu, J.; Wang, X.; Fu, Y. Recent Development and Applications of Electrical Conductive MOFs. Nanoscale 2021, 13, 485–509. [Google Scholar] [CrossRef]
- Li, P.; Wang, B. Recent Development and Application of Conductive MOFs. Isr. J. Chem. 2018, 58, 1010–1018. [Google Scholar] [CrossRef]
- Contreras-Pereda, N.; Pané, S.; Puigmartí-Luis, J.; Ruiz-Molina, D. Conductive Properties of Triphenylene MOFs and COFs. Coord. Chem. Rev. 2022, 460. [Google Scholar] [CrossRef]
- Zhu, Y.; Tang, Z. Conductive 2D MOF Coupled with Superprotonic Conduction and Interfacial Pseudo-Capacitance. Matter 2020, 2, 798–800. [Google Scholar] [CrossRef]
- Shu, Y.; Chen, J.; Xu, Q.; Wei, Z.; Liu, F.; Lu, R.; Xu, S.; Hu, X. MoS 2 Nanosheet–Au Nanorod Hybrids for Highly Sensitive Amperometric Detection of H2O2 in Living Cells. J. Mater. Chem. B 2017, 5, 1446–1453. [Google Scholar] [CrossRef]
- Sarhan, R.M.; El-Nagar, G.A.; Abouserie, A.; Roth, C. Silver–Iron Hierarchical Microflowers for Highly Efficient H2O2 Nonenzymatic Amperometric Detection. ACS Sustain. Chem. Eng. 2019, 7, 4335–4342. [Google Scholar] [CrossRef]
- Chen, D.; Zhuang, X.; Zhai, J.; Zheng, Y.; Lu, H.; Chen, L. Preparation of Highly Sensitive Pt Nanoparticles-Carbon Quantum Dots/Ionic Liquid Functionalized Graphene Oxide Nanocomposites and Application for H2O2 Detection. Sensors Actuators B Chem. 2018, 255, 1500–1506. [Google Scholar] [CrossRef]
- Li, M.; Xu, S.; Tang, M.; Liu, L.; Gao, F.; Wang, Y. Direct Electrochemistry of Horseradish Peroxidase on Graphene-Modified Electrode for Electrocatalytic Reduction towards H2O2. Electrochim. Acta 2011, 56, 1144–1149. [Google Scholar] [CrossRef]
- Bai, G.; Xu, X.; Dai, Q.; Zheng, Q.; Yao, Y.; Liu, S.; Yao, C. An Electrochemical Enzymatic Nanoreactor Based on Dendritic Mesoporous Silica Nanoparticles for Living Cell H2O2 Detection. Analyst 2019, 144, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, L.; Zhang, R.; Bai, Y.; Zhu, R.; Pang, H. Recent Advances in the Development of Electronically and Ionically Conductive Metal-Organic Frameworks. Coord. Chem. Rev. 2021, 439, 213915. [Google Scholar] [CrossRef]
- Sun, L.; Miyakai, T.; Seki, S.; Dincă, M. Mn 2 (2,5-Disulfhydrylbenzene-1,4-Dicarboxylate): A Microporous Metal–Organic Framework with Infinite (−Mn–S−) ∞ Chains and High Intrinsic Charge Mobility. J. Am. Chem. Soc. 2013, 135, 8185–8188. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Jacobs, B.; Allendorf, M.D.; Long, J.R. Conductivity, Doping, and Redox Chemistry of a Microporous Dithiolene-Based Metal−Organic Framework. Chem. Mater. 2010, 22, 4120–4122. [Google Scholar] [CrossRef]
- Guo, X.; Cao, Q.; Liu, Y.; He, T.; Liu, J.; Huang, S.; Tang, H.; Ma, M. Organic Electrochemical Transistor for in Situ Detection of H2O2 Released from Adherent Cells and Its Application in Evaluating the In Vitro Cytotoxicity of Nanomaterial. Anal. Chem. 2020, 92, 908–915. [Google Scholar] [CrossRef]
- Hu, X.-W.; Mao, C.-J.; Song, J.-M.; Niu, H.-L.; Zhang, S.-Y.; Cui, R.-J. Direct Electrochemistry and Electrocatalytic Behavior of Hemoglobin Entrapped in Ag@C Nanocables/Gold Nanoparticles Nanocomposites Film. J. Nanosci. Nanotechnol. 2012, 12, 7980–7985. [Google Scholar] [CrossRef]
- Sheberla, D.; Sun, L.; Blood-Forsythe, M.A.; Er, S.; Wade, C.R.; Brozek, C.K.; Aspuru-Guzik, A.; Dincă, M. High Electrical Conductivity in Ni 3 (2,3,6,7,10,11-Hexaiminotriphenylene) 2, a Semiconducting Metal–Organic Graphene Analogue. J. Am. Chem. Soc. 2014, 136, 8859–8862. [Google Scholar] [CrossRef]
- Tominaka, S.; Coudert, F.-X.; Dao, T.D.; Nagao, T.; Cheetham, A.K. Insulator-to-Proton-Conductor Transition in a Dense Metal–Organic Framework. J. Am. Chem. Soc. 2015, 137, 6428–6431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.Y.; Hu, X.P.; Yao, H.C.; Zang, S.Q.; Hou, H.W.; Mak, T.C.W. Alkaline Earth Metal (Mg, Sr, Ba)-Organic Frameworks Based on 2,2′,6,6′-Tetracarboxybiphenyl for Proton Conduction. Inorg. Chem. 2014, 53, 12050–12057. [Google Scholar] [CrossRef] [PubMed]
- Zou, L.; Yao, S.; Zhao, J.; Li, D.S.; Li, G.; Huo, Q.; Liu, Y. Enhancing Proton Conductivity in a 3D Metal-Organic Framework by the Cooperation of Guest [Me2NH2]+ Cations, Water Molecules, and Host Carboxylates. Cryst. Growth Des. 2017, 17, 3556–3561. [Google Scholar] [CrossRef]
- Ponomareva, V.G.; Kovalenko, K.A.; Chupakhin, A.P.; Dybtsev, D.N.; Shutova, E.S.; Fedin, V.P. Imparting High Proton Conductivity to a Metal-Organic Framework Material by Controlled Acid Impregnation. J. Am. Chem. Soc. 2012, 134, 15640–15643. [Google Scholar] [CrossRef] [PubMed]
- Phang, W.J.; Jo, H.; Lee, W.R.; Song, J.H.; Yoo, K.; Kim, B.; Hong, C.S. Superprotonic Conductivity of a UiO-66 Framework Functionalized with Sulfonic Acid Groups by Facile Postsynthetic Oxidation. Angew. Chemie Int. Ed. 2015, 54, 5142–5146. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Wei, M.-J.; Wang, H.-N.; Zang, H.-Y.; Zhou, Z.-Y. Multifunctional Luminescent Zn(ii)-Based Metal–Organic Framework for High Proton-Conductivity and Detection of Cr 3+ Ions in the Presence of Mixed Metal Ions. Dalt. Trans. 2018, 47, 1383–1387. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Wang, Y.; Lei, Y. Direct Electrochemistry and Electrocatalysis of Novel Single-Walled Carbon Nanotubes–Hemoglobin Composite Microbelts—Towards the Development of Sensitive and Mediator-Free Biosensor. Biosens. Bioelectron. 2010, 26, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.-X.; Qian, Y.; Cao, X.-X.; Xia, X.-H. Direct Electrochemistry of Cytochrome c on a Graphene/Poly (3,4-Ethylenedioxythiophene) Nanocomposite Modified Electrode. Electrochem. commun. 2012, 20, 1–3. [Google Scholar] [CrossRef]
- Sheng, M.; Gao, Y.; Sun, J.; Gao, F. Carbon Nanodots–Chitosan Composite Film: A Platform for Protein Immobilization, Direct Electrochemistry and Bioelectrocatalysis. Biosens. Bioelectron. 2014, 58, 351–358. [Google Scholar] [CrossRef]
- Lu, C.; Ben, T.; Xu, S.; Qiu, S. Electrochemical Synthesis of a Microporous Conductive Polymer Based on a Metal–Organic Framework Thin Film. Angew. Chemie Int. Ed. 2014, 53, 6454–6458. [Google Scholar] [CrossRef]
- Qin, X.; Lu, W.; Luo, Y.; Chang, G.; Sun, X. Preparation of Ag Nanoparticle-Decorated Polypyrrole Colloids and Their Application for H2O2 Detection. Electrochem. commun. 2011, 13, 785–787. [Google Scholar] [CrossRef]
- Chansi; Bhardwaj, R.; Rao, R.P.; Mukherjee, I.; Agrawal, P.K.; Basu, T.; Bharadwaj, L.M. Layered Construction of Nano Immuno-Hybrid Embedded MOF as an Electrochemical Sensor for Rapid Quantification of Total Pesticides Load in Vegetable Extract. J. Electroanal. Chem. 2020, 873, 114386. [Google Scholar] [CrossRef]
- Usov, P.M.; Ahrenholtz, S.R.; Maza, W.A.; Stratakes, B.; Epley, C.C.; Kessinger, M.C.; Zhu, J.; Morris, A.J. Cooperative Electrochemical Water Oxidation by Zr Nodes and Ni-Porphyrin Linkers of a PCN-224 MOF Thin Film. J. Mater. Chem. A 2016, 4, 16818–16823. [Google Scholar] [CrossRef] [Green Version]
- Xu, S.; Liu, R.; Shi, X.; Ma, Y.; Hong, M.; Chen, X.; Wang, T.; Li, F.; Hu, N.; Yang, Z. A Dual CoNi MOF Nanosheet/Nanotube Assembled on Carbon Cloth for High Performance Hybrid Supercapacitors. Electrochim. Acta 2020, 342, 136124. [Google Scholar] [CrossRef]
- Wu, S.; Li, C.; Shi, H.; Huang, Y.; Li, G. Design of Metal-Organic Framework-Based Nanoprobes for Multicolor Detection of DNA Targets with Improved Sensitivity. Anal. Chem. 2018, 90, 9929–9935. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.H.; Chen, C.; He, C.; Zhu, Y.Y.; Hong, D.L.; He, X.T.; An, P.J.; Wu, H.S.; Sun, B.W. Single-Layered Two-Dimensional Metal-Organic Framework Nanosheets as an in Situ Visual Test Paper for Solvents. ACS Appl. Mater. Interfaces 2018, 10, 28860–28867. [Google Scholar] [CrossRef] [Green Version]
- Yuan, K.; Song, T.; Wang, D.; Zou, Y.; Li, J.; Zhang, X.; Tang, Z.; Hu, W. Bimetal-Organic Frameworks for Functionality Optimization: MnFe-MOF-74 as a Stable and Efficient Catalyst for the Epoxidation of Alkenes with H2O2. Nanoscale 2018, 10, 1591–1597. [Google Scholar] [CrossRef]
- Li, Y.; Cheng, C.; Yang, Y.P.; Dun, X.J.; Gao, J.; Jin, X.J. A Novel Electrochemical Sensor Based on CuO/H-C3N4/RGO Nanocomposite for Efficient Electrochemical Sensing Nitrite. J. Alloys Compd. 2019, 798, 764–772. [Google Scholar] [CrossRef]
- Li, Y.; Cui, L.; Jia, M.; Xu, L.; Gao, J.; Jin, X. Hydrophilic “Bridge” Tannins for Stabilizing the Metal Selenides onto Activated Carbon for Binder-Free and Ultralong-Life Asymmetric Supercapacitors. New J. Chem. 2019, 43, 5592–5602. [Google Scholar] [CrossRef]
- Li, Y.; Xu, L.; Gao, J.; Jin, X. Hydrothermal Fabrication of Reduced Graphene Oxide/Activated Carbon/MnO2 Hybrids with Excellent Electrochemical Performance for Supercapacitors. RSC Adv. 2017, 7, 39024–39033. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Wang, J.; Teng, X.; Liu, Y.; He, X.; Chen, Z. A Novel Bimetallic Nickel–Molybdenum Carbide Nanowire Array for Efficient Hydrogen Evolution. ChemSusChem 2018, 11, 2717–2723. [Google Scholar] [CrossRef] [PubMed]
- Ai, L.; Li, L.; Zhang, C.; Fu, J.; Jiang, J. MIL-53(Fe): A Metal-Organic Framework with Intrinsic Peroxidase-like Catalytic Activity for Colorimetric Biosensing. Chem. A Eur. J. 2013, 19, 15105–15108. [Google Scholar] [CrossRef] [PubMed]
- Valekar, A.H.; Batule, B.S.; Kim, M.I.; Cho, K.H.; Hong, D.Y.; Lee, U.H.; Chang, J.S.; Park, H.G.; Hwang, Y.K. Novel Amine-Functionalized Iron Trimesates with Enhanced Peroxidase-like Activity and Their Applications for the Fluorescent Assay of Choline and Acetylcholine. Biosens. Bioelectron. 2018, 100, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Lu, Q.; Ma, Q.; Zhang, H. Two-Dimensional Metal–Organic Framework Nanosheets. Small Methods 2017, 1, 1–8. [Google Scholar] [CrossRef]
- Zhao, M.; Huang, Y.; Peng, Y.; Huang, Z.; Ma, Q.; Zhang, H. Two-Dimensional Metal-Organic Framework Nanosheets: Synthesis and Applications. Chem. Soc. Rev. 2018, 47, 6267–6295. [Google Scholar] [CrossRef]
- Liu, X.; Yan, Z.; Zhang, Y.; Liu, Z.; Sun, Y.; Ren, J.; Qu, X. Two-Dimensional Metal-Organic Framework/Enzyme Hybrid Nanocatalyst as a Benign and Self-Activated Cascade Reagent for in Vivo Wound Healing. ACS Nano 2019, 13, 5222–5230. [Google Scholar] [CrossRef]
- Cheng, H.; Liu, Y.; Hu, Y.; Ding, Y.; Lin, S.; Cao, W.; Wang, Q.; Wu, J.; Muhammad, F.; Zhao, X.; et al. Monitoring of Heparin Activity in Live Rats Using Metal-Organic Framework Nanosheets as Peroxidase Mimics. Anal. Chem. 2017, 89, 11552–11559. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Yang, Y.; Liu, B.; Wu, Y. A Facile Colorimetric Sensor for Ultrasensitive and Selective Detection of Lead(II) in Environmental and Biological Samples Based on Intrinsic Peroxidase-Mimic Activity of WS2 Nanosheets. Anal. Chim. Acta 2020, 1106, 115–125. [Google Scholar] [CrossRef]
- Wang, W.; Zou, Y.; Yan, J.; Liu, J.; Chen, H.; Li, S.; Zhang, L. Ultrasensitive Colorimetric Immunoassay for HCG Detection Based on Dual Catalysis of Au@Pt Core–Shell Nanoparticle Functionalized by Horseradish Peroxidase. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 193, 102–108. [Google Scholar] [CrossRef]
- Das, N. Recovery of Precious Metals through Biosorption - A Review. Hydrometallurgy 2010, 103, 180–189. [Google Scholar] [CrossRef]
- Xia, Y.; Yang, X. Toward Cost-Effective and Sustainable Use of Precious Metals in Heterogeneous Catalysts. Acc. Chem. Res. 2017, 50, 450–454. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Mu, C.; Yan, B.; Qin, X.; Shen, C.; Xue, H.; Pang, H. Materials Horizons Nanoparticle/MOF Composites: Preparations and Applications. Mater. Horizons 2017, 4, 557–569. [Google Scholar] [CrossRef]
- Copéret, C. Synthesis of Solid Catalysts. Edited by Krijn P. de Jong. ChemCatChem 2010, 2, 706. [Google Scholar] [CrossRef]
- Ding, K.; Cullen, D.A.; Zhang, L.; Cao, Z.; Roy, A.D.; Ivanov, I.N.; Cao, D. A General Synthesis Approach for Supported Bimetallic Nanoparticles via Surface Inorganometallic Chemistry. Science 2018, 362, 560–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, T.; Zhang, M.; Wu, P.; Tang, Y.J.; Li, S.L.; Shen, F.C.; Wang, X.L.; Zhou, X.P.; Lan, Y.Q. POM-Based Metal-Organic Framework/Reduced Graphene Oxide Nanocomposites with Hybrid Behavior of Battery-Supercapacitor for Superior Lithium Storage. Nano Energy 2017, 34, 205–214. [Google Scholar] [CrossRef]
- Xia, W.; Qu, C.; Liang, Z.; Zhao, B.; Dai, S.; Qiu, B.; Jiao, Y.; Zhang, Q.; Huang, X.; Guo, W.; et al. High-Performance Energy Storage and Conversion Materials Derived from a Single Metal-Organic Framework/Graphene Aerogel Composite. Nano Lett. 2017, 17, 2788–2795. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, H.B.; Lou, X.W. Metal-Organic-Frameworks-Derived General Formation of Hollow Structures with High Complexity. J. Am. Chem. Soc. 2013, 135, 10664–10672. [Google Scholar] [CrossRef]
- Zou, F.; Hu, X.; Li, Z.; Qie, L.; Hu, C.; Zeng, R.; Jiang, Y.; Huang, Y. MOF-Derived Porous ZnO/ZnFe2O4/C Octahedra with Hollow Interiors for High-Rate Lithium-Ion Batteries. Adv. Mater. 2014, 26, 6622–6628. [Google Scholar] [CrossRef]
- Wang, T.; Kim, H.K.; Liu, Y.; Li, W.; Griffiths, J.T.; Wu, Y.; Laha, S.; Fong, K.D.; Podjaski, F.; Yun, C.; et al. Bottom-up Formation of Carbon-Based Structures with Multilevel Hierarchy from MOF-Guest Polyhedra. J. Am. Chem. Soc. 2018, 140, 6130–6136. [Google Scholar] [CrossRef] [Green Version]
- Zhou, A.; Guo, R.M.; Zhou, J.; Dou, Y.; Chen, Y.; Li, J.R. Pd@ZIF-67 Derived Recyclable Pd-Based Catalysts with Hierarchical Pores for High-Performance Heck Reaction. ACS Sustain. Chem. Eng. 2018, 6, 2103–2111. [Google Scholar] [CrossRef]
- Zhang, C.; Li, L.; Ju, J.; Chen, W. Electrochemical Sensor Based on Graphene-Supported Tin Oxide Nanoclusters for Nonenzymatic Detection of Hydrogen Peroxide. Electrochim. Acta 2016, 210, 181–189. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Niu, X.; Pan, C.; Chen, H.L.; Chen, X. High-Performance Electrochemical Biosensor for Nonenzymatic H2O2 Sensing Based on Au@C-Co3O4 Heterostructures. Biosens. Bioelectron. 2018, 118, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhang, Y.; Song, J.; Chen, N.; Zhou, J.; Huang, Z.; Ma, Y.; Zhang, L.; Wang, L. MnO2/Graphene Nanocomposites for Nonenzymatic Electrochemical Detection of Hydrogen Peroxide. Electroanalysis 2015, 27, 353–359. [Google Scholar] [CrossRef]
- Qin, Y.; Sun, Y.; Li, Y.; Li, C.; Wang, L.; Guo, S. MOF Derived Co3O4/N-Doped Carbon Nanotubes Hybrids as Efficient Catalysts for Sensitive Detection of H2O2 and Glucose. Chinese Chem. Lett. 2020, 31, 774–778. [Google Scholar] [CrossRef]
- Cui, S.; Gu, S.; Ding, Y.; Zhang, J.; Zhang, Z.; Hu, Z. Hollow Mesoporous CuCo2O4 Microspheres Derived from Metal Organic Framework: A Novel Functional Materials for Simultaneous H2O2 Biosensing and Glucose Biofuel Cell. Talanta 2018, 178, 788–795. [Google Scholar] [CrossRef]

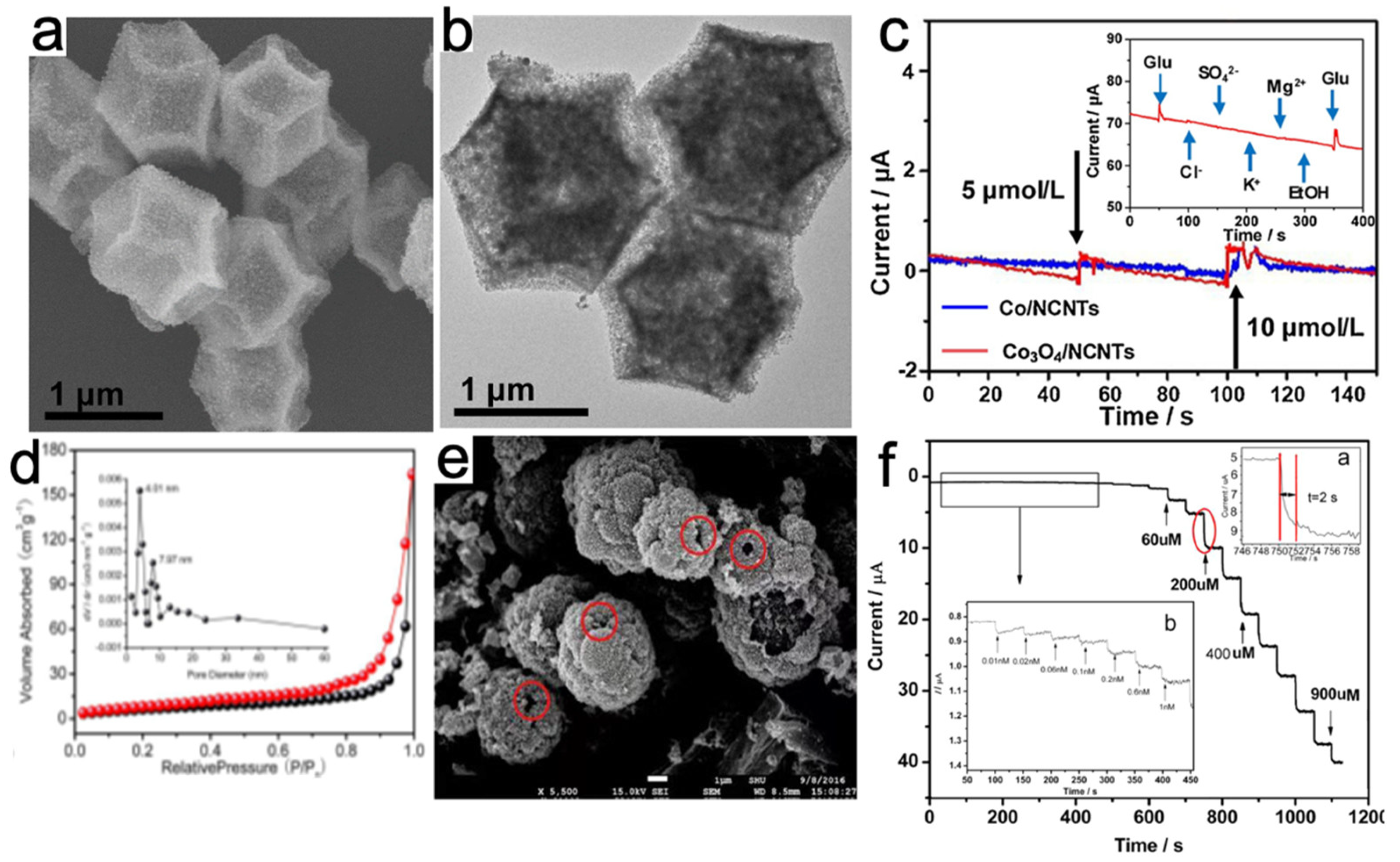
| Electrochemical Sensor | Electrolyte | Detection Limit (μM) | Linear Range | Practical Application | Reference |
|---|---|---|---|---|---|
| Conductive MOFs basedH2O2 sensors | |||||
| [Co3(HHTP)2]n | 0.1 M NaOH | 2.9 | - | - | [2] |
| [Co3(HOB)2]n | 0.1 M NaOH | 0.00308 | - | - | [36] |
| [Cu(adp)(BIB)(H2O)]n/GC | 0.1 M KOH | 0.068 | 0.1 μM–2.75 μM | [53] | |
| 2D Co-MOF | 0.1 M KOH | 0.69 | 0.5 μM–832.5 μM | [54] | |
| FePc-CP NSs | 0.1 M PBS | 0.017 | 0.1–1000 μM | A549 live cells, Orange juice and beer | [55] |
| Co-MOF/TM | 0.1 M PBS | 0.25 | 1–13,000 μM | A549 cells | [56] |
| CuMOFs@FeP-pSC4-AuNPs | 10 mM PBS | 47 | 0.5–2.5 mM | Cancer cells | [57] |
| NENU5 | 0.1 M PBS | 1.03 | 10–50,000 μM | - | [58] |
| CuCo-BDC/GO | 0.1 M PBS | 0.069 | 100 nM–3.5 mM | Human serum samples | [59] |
| HKUST-1/GCE | 0.1 M PBS | 0.68 | 2 μM–3 Mm and 3–25 mM | Milk sample | [60] |
| MOF composites based H2O2 sensors | |||||
| MIL-53-CrIIIAS/GCE | 0.1 M NaOH | 3.52 | 25–500 mM, | - | [54] |
| Ni(II)-MOF/CNTs nanocomposites | 0.1 M NaOH | 2.1 | 0.01–51.6 mM | [61] | |
| MNPs@Y-1, 4-NDC-MOF/ERGO | 0.1 M PBS | 0.18 | 4–11,000 μM | A549 cells | [62] |
| Ni–MOF nanosheets/Hemin | 0.1 M PBS | 0.2 | 1–400 | Human serum samples | [9] |
| GCE/GO/poly(CoTBIPc) | 0.1 M PBS | 0.6 | 2–200 μM | - | [3] |
| A-Ni1Mo0.5-MOFs@AAC | PSB | 0.185 | - | - | [63] |
| CuCo-BDC/GO | 0.1 M PBS | 0.069 | 100 nM -3.5 mM | Diluted human serums | [59] |
| CuMOF/MXene/GCE | 0.1 M PBS | 0.35 | 1 µM–6.12 mM | Serum | [64] |
| Cu-TCPP MOF/Cu5.4O | 0.1 M PBS | 0.13 | 0.0001- 0.59 mM and 1.59–20.59 mM | Living cells | [65] |
| Cu-MOF@S-Gr | 0.1 M PBS | 0.0113 ± 0.00004 | 0.1–3 μM | Tap water | [66] |
| Au–Pd@UiO-66-on-ZIF-L/CC | 0.01 M PBS | 0.0212 | 1 μM–19.6 mM | A549 cells | [67] |
| Cu@BDC(NH2)@2-MI | 0.1 M PBS | 0.97 | 10 μM–13.28 mM | [68] | |
| MnOx | 0.2 M PBS | 0.000232 | 0.000696–742 μM | Human serum and milk sample | [69] |
| NCNT MOF CoCu | 0.1 M PBS | 0.206 | 0.05–3.5 mM | Serum samples | [70] |
| Ag-Bi BDC (s) MOF/GCE | 0.1 M PBS | 0.020.1 | 10 μM–5 mM and 5 mM–145 mM | THP-1 and AtT-20 cancer cells | [71] |
| MOF derivatives based H2O2 sensors | |||||
| AuPt/ZIF-8−rGO | 0.1 M PBS | 0.019 | 0.1–18,000 μM | Human serum | [72] |
| MOF-Au@Pt nanoflowers | PBS | 0.086 | 0.8 μM–3 mM | Suspension of living cell | [32] |
| Co-NC RDCs | 0.1 M PBS | 0.143 | 0.001–30 mM | [73] | |
| MIL-101(Fe)@Fe3O4/NGCE | 0.1 M PBS | 0.15 | 0.001–0.01 mM | Human blood plasma | [74] |
| Co (4%)–N/CNS | 0.4 M PB | 0.00618 | 1–500 μM and 500 μM–0.1 M | Human serum | [75] |
| Co-NPs/NCs | 0.01 M PBS | 0.12 | 10–2080 μM and 2080–11,800 μM | Human serum sample | [76] |
| Cu-MoO2-C | PBS | 0.16 | 0.25–6.25 mM | [77] | |
| ZnO@ZIF-8 | 0.1 M PBS | 3 | 20–11,550 μM | [78] | |
| Co3O4@CNBs | 0.01 M PBS | 0.00232 | 10 nM–359 μM | HUVEC cells and 4T1, A549 cancer cells | [79] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, S.; Zhang, T.; Zhu, X.; Zu, S.; Xie, Z.; Lu, X.; Zhang, M.; Song, L.; Jin, Y. Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide. Molecules 2022, 27, 4571. https://doi.org/10.3390/molecules27144571
Wang S, Zhang T, Zhu X, Zu S, Xie Z, Lu X, Zhang M, Song L, Jin Y. Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide. Molecules. 2022; 27(14):4571. https://doi.org/10.3390/molecules27144571
Chicago/Turabian StyleWang, Shuhan, Tong Zhang, Xukun Zhu, Shu Zu, Zexin Xie, Xiaoxiang Lu, Mingdao Zhang, Li Song, and Yachao Jin. 2022. "Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide" Molecules 27, no. 14: 4571. https://doi.org/10.3390/molecules27144571
APA StyleWang, S., Zhang, T., Zhu, X., Zu, S., Xie, Z., Lu, X., Zhang, M., Song, L., & Jin, Y. (2022). Metal–Organic Frameworks for Electrocatalytic Sensing of Hydrogen Peroxide. Molecules, 27(14), 4571. https://doi.org/10.3390/molecules27144571







