Effect of Poly(methacrylic acid) on the Cytokine Level in an In Vivo Tumor Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Poly(methacrylic acid) (PMAA) Synthesis
2.2. Polymerization
2.3. Molecular-Weight Characteristics of Polymers
2.4. Experimental Animals
2.5. In Vivo Subcutaneous Cancer Model
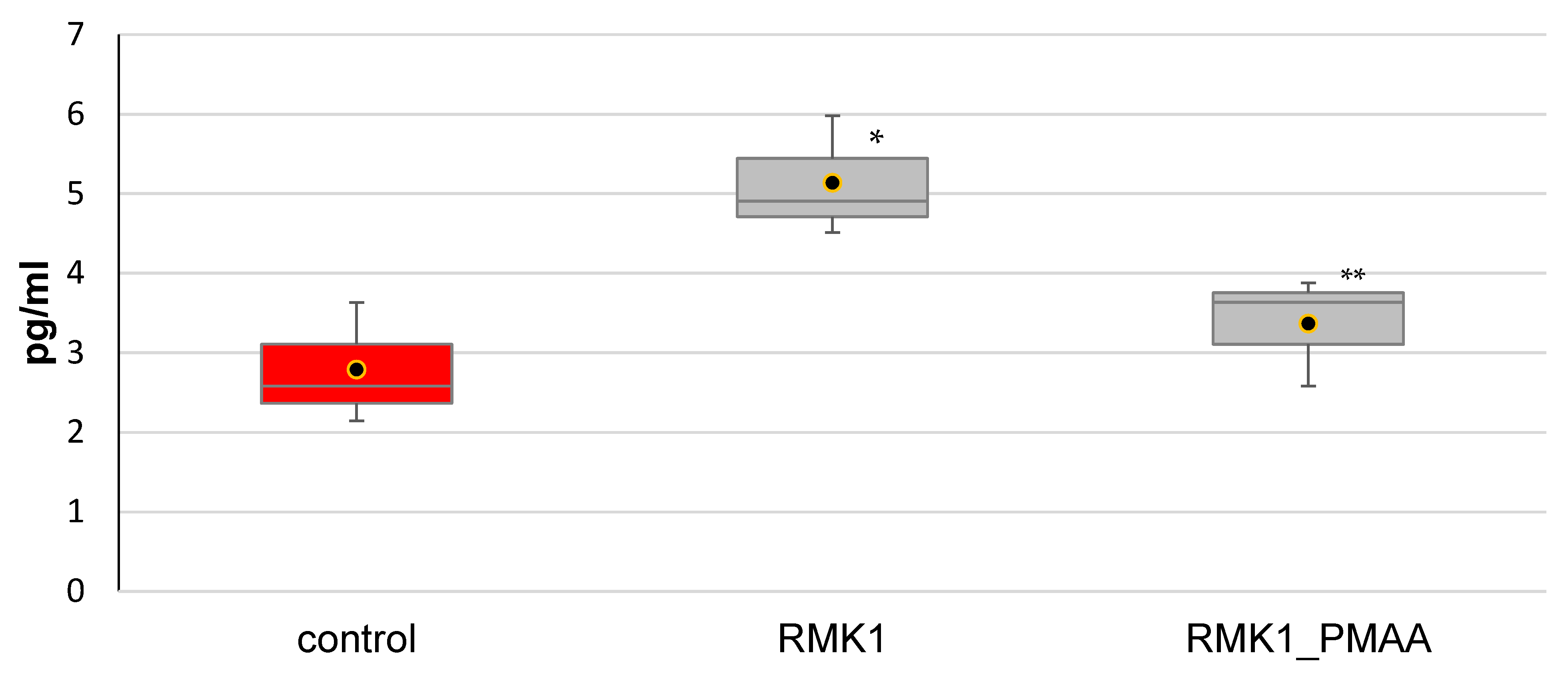
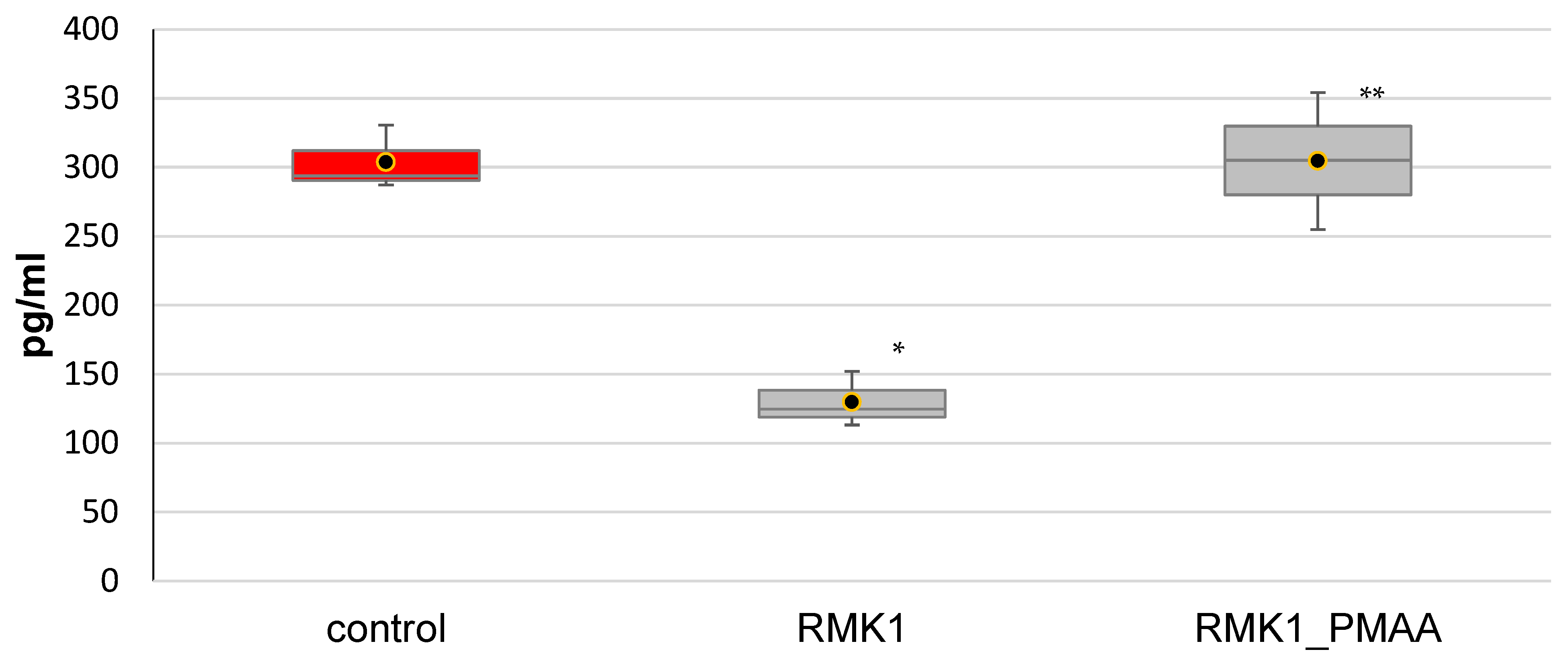
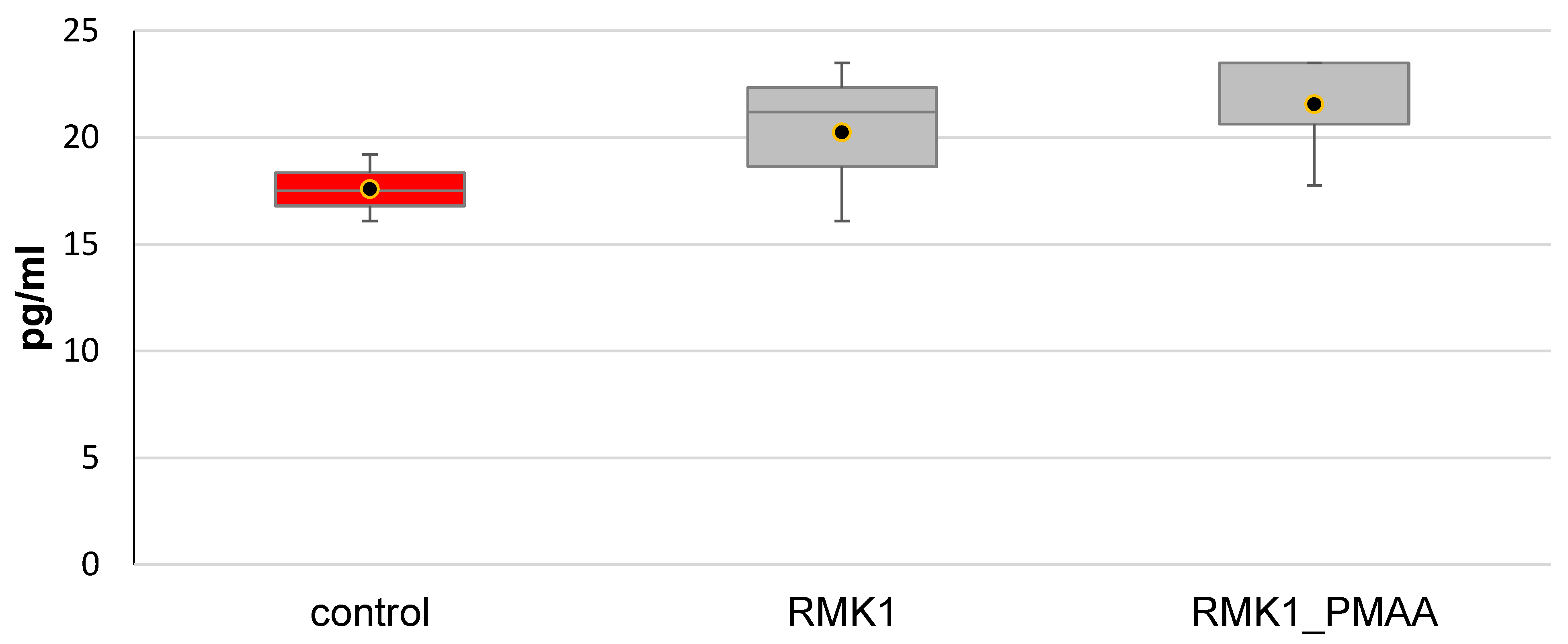
2.6. Serum Cytokine Measurements in Tumor-Bearing Rats by ELISA
2.7. Statistical Analysis
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Briukhovetska, D.; Dörr, J.; Endres, S.; Libby, P.; Dinarello, C.A.; Kobold, S. Interleukins in cancer: From biology to therapy. Nat. Cancer 2021, 21, 481–499. [Google Scholar] [CrossRef] [PubMed]
- Esquivel-Velázquez, M.; Ostoa-Saloma, P.; Palacios-Arreola, M.I.; Nava-Castro, K.E.; Castro, J.I.; Morales-Montor, J. The role of cytokines in breast cancer development and progression. J. Interferon. Cytokine Res. 2015, 35, 1–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuzhalin, A.E.; Kutikhin, A.G. Interleukins in Cancer Biology: Their Heterogeneous Role; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Holen, I.; Lefley, D.V.; Francis, S.E.; Rennicks, S.; Bradbury, S.; Coleman, R.E.; Ottewell, P. IL-1 drives breast cancer growth and bone metastasis in vivo. Oncotarget 2016, 7, 75571–75584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kitamura, T.; Qian, B.-Z.; Soong, D.; Cassetta, L.; Noy, R.; Sugano, G.; Kato, Y.; Li, J.; Pollard, J.W. CCL2-induced chemokine cascade promotes breast cancer metastasis by enhancing retention of metastasis-associated macrophages. J. Exp. Med. 2015, 212, 1043–1059. [Google Scholar] [CrossRef]
- Naimi, A.; Mohammed, R.N.; Raji, A.; Chupradit, S.; Yumashev, A.V.; Suksatan, W.; Shalaby, M.N.; Thangavelu, L.; Kamrava, S.; Shomali, N.; et al. Tumor immunotherapies by immune checkpoint inhibitors (ICIs); the pros and cons. Cell Commun. Signal. 2022, 20, 44. [Google Scholar] [CrossRef]
- Ito, F.; Chang, A.E. Cancer immunotherapy. Current status and future directions. Surg. Oncol. Clin. North Am. 2013, 22, 765–783. [Google Scholar] [CrossRef]
- Lou, P.-J.; Cheng, W.-F.; Chung, Y.-C.; Cheng, C.-Y.; Chiu, L.-H.; Young, T.-H. PMMA particle-mediated DNA vaccine for cervical cancer. J. Biomed. Mater. Res. Part A 2009, 88, 849–857. [Google Scholar] [CrossRef]
- Armarego Wilfred, L.E.; Chai Christina, L.L. Purification of Laboratory Chemicals, 7th ed.; Elsevier: Amsterdam, The Netherlands, 2012; p. 1024. [Google Scholar]
- Konoplev, V.P.; Lagova, N.D. Characteristics of the rat breast cancer to be transferred. Bull. Exp. Biol. Med. 1960, 7, 79–81. [Google Scholar]
- Heinrich, P.C.; Behrmann, I.; Haan, S.; Hermanns, H.M.; Müller-Newen, G.; Schaper, F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. Biochem. J. 2003, 374, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Masjedi, A.; Hashemi, V.; Hojjat-Farsangi, M.; Ghalamfarsa, G.; Azizi, G.; Yousefi, M.; Jadidi-Niaragh, F. The significant role of interleukin-6 and its signaling pathway in the immunopathogenesis and treatment of breast cancer. Biomed. Pharmacother. 2018, 108, 1415–1424. [Google Scholar] [CrossRef]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.-M.; Kramer, V.; Waldner, M.J.; Büttner, C.; et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 2020, 69, 1269–1282. [Google Scholar] [CrossRef]
- Ke, W.; Zhang, L.; Dai, Y. The role of IL-6 in immunotherapy of non-small cell lung cancer (NSCLC) with immune-related adverse events (irAEs). Thorac. Cancer 2020, 11, 835–839. [Google Scholar] [CrossRef]
- Sun, X.; Qu, Q.; Lao, Y.; Zhang, M.; Yin, X.; Zhu, H.; Wang, Y.; Yang, J.; Yi, J.; Hao, M. Tumor suppressor HIC1 is synergistically compromised by cancer-associated fibroblasts and tumor cells through the IL-6/pSTAT3 axis in breast cancer. BMC Cancer 2019, 19, 1180. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100. [Google Scholar] [CrossRef] [PubMed]
- Dethlefsen, C.; Højfeldt, G.; Hojman, P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res. Treat. 2013, 138, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Knüpfer, H.; Preiss, R. Significance of interleukin-6 (IL-6) in breast cancer (review). Breast Cancer Res. Treat. 2007, 102, 129–135. [Google Scholar] [CrossRef]
- Kumari, N.; Dwarakanath, B.S.; Das, A.; Bhatt, A.N. Role of interleukin-6 in cancer progression and therapeutic resistance. Tumor Biol. 2016, 37, 11553–11572. [Google Scholar] [CrossRef]
- Nemunaitis, J.; Fong, T.; Shabe, P.; Martineau, D.; Ando, D. Comparison of Serum Interleukin-10 (IL-10) Levels Between Normal Volunteers and Patients with Advanced Melanoma. Cancer Investig. 2001, 19, 239–247. [Google Scholar] [CrossRef]
- McKay, K.; Moore, P.C.; Smoller, B.R.; Hiatt, K.M. Association between natural killer cells and regression in melanocytic lesions. Hum. Pathol. 2011, 42, 1960–1964. [Google Scholar] [CrossRef]
- Petersson, M.; Charo, J.; Salazar-Onfray, F.; Noffz, G.; Mohaupt, M.; Qin, Z.; Klein, G.; Blankenstein, T.; Kiessling, R. Constitutive IL-10 production accounts for the high NK sensitivity, low MHC class I expression, and poor transporter associated with antigen processing (TAP)-1/2 function in the prototype NK target YAC-1. J. Immunol. 1998, 161, 2099–2105. [Google Scholar]
- Song, Y.; Yang, J.M. Role of interleukin (IL)-17 and T-helper (Th)17 cells in cancer. Biochem. Biophys. Res. Commun. 2017, 493, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Kuen, D.-S.; Kim, B.-S.; Chung, Y. IL-17-Producing Cells in Tumor Immunity: Friends or Foes? Immune Netw. 2020, 20, e6. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Qian, Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine 2013, 62, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Palomeras, S.; Diaz-Lagares, Á.; Viñas, G.; Setien, F.; Ferreira, H.J.; Oliveras, G.; Crujeiras, A.B.; Hernández, A.; Lum, D.H.; Welm, A.L.; et al. Epigenetic silencing of TGFBI confers resistance to trastuzumab in human breast cancer. Breast Cancer Res. 2019, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Vitiello, G.A.F.; Guembarovski, R.L.; Hirata, B.K.B.; Amarante, M.K.; de Oliveira, C.E.C.; de Oliveira, K.B.; Cebinelli, G.C.M.; Guembarovski, A.L.; Campos, C.Z.; Watanabe, M.A.E. Transforming growth factor beta 1 (TGFβ1) polymorphisms and haplotype structures have dual roles in breast cancer pathogenesis. J. Cancer Res. Clin. Oncol. 2018, 144, 645–655. [Google Scholar] [CrossRef]
- Band, A.M.; Laiho, M. Crosstalk of TGF ß and estrogen receptor signaling in breast cancer. J. Mammary Gland Biol. Neoplasia. 2011, 16, 109–115. [Google Scholar] [CrossRef]
- Joshi, A.; Cao, D. TGF-ßeta signaling, tumor microenvironment and tumor progression: The butterfly effect. Front Biosci. 2010, 15, 180–194. [Google Scholar] [CrossRef] [Green Version]
- Ciftci, R.; Tas, F.; Yasasever, C.T.; Aksit, E.; Karabulut, S.; Sen, F.; Keskin, S.; Kilic, L.; Yildiz, I.; Bozbey, H.U.; et al. High serum transforming growth factor beta 1 (TGFB1) level predicts better survival in breast cancer. Tumor Biol. 2014, 35, 6941–6948. [Google Scholar] [CrossRef]
- Syed, V. TGF-β Signaling in Cancer. J. Cell. Biochem. 2016, 117, 1279–1287. [Google Scholar] [CrossRef]
- Zhang, M.; Zhang, Y.Y.; Chen, Y.; Wang, J.; Wang, Q.; Lu, H. TGF-β Signaling and Resistance to Cancer Therapy. Front. Cell Dev. Biol. 2021, 9, 786728. [Google Scholar] [CrossRef]
- Kim, B.-G.; Malek, E.; Choi, S.H.; Ignatz-Hoover, J.J.; Driscoll, J.J. Novel therapies emerging in oncology to target the TGF-β pathway. J. Hematol. Oncol. 2021, 14, 55. [Google Scholar] [CrossRef] [PubMed]
- Guo, B.; Wu, S.; Zhu, X.; Zhang, L.; Deng, J.; Li, F.; Wang, Y.; Zhang, S.; Wu, R.; Lu, J.; et al. Micropeptide CIP 2A- BP encoded by LINC 00665 inhibits triple-negative breast cancer progression. EMBO J. 2019, 39, e102190. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Wang, J. Correlations of MRP1 gene with serum TGF-β1 and IL-8 in breast cancer patients during chemotherapy. J. BUON 2018, 23, 1302–1308. [Google Scholar] [PubMed]
- Idriss, H.T.; Naismith, J.H. TNF alpha and the TNF receptor superfamily: Structure-function relationship(s). Microsc. Res. Tech. 2000, 50, 184–195. [Google Scholar] [CrossRef]
- Chu, C.-Q. Blocking tumor necrosis factor paved the way for targeted therapeutics in inflammatory diseases. Chin. Med. J. 2021, 134, 2525–2528. [Google Scholar] [CrossRef]
- Atzeni, F.; Sarzi-Puttini, P. Tumor Necrosis Factor. In Brenner’s Encyclopedia of Genetics, 2nd ed.; Maloy, S., Hughes, K., Eds.; Academic Press: San Diego, MA, USA, 2013; pp. 229–231. [Google Scholar]
- Salomon, B.L.; Leclerc, M.; Tosello, J.; Ronin, E.; Piaggio, E.; Cohen, J.L. Tumor necrosis factor α and regulatory T cells in oncoimmunology. Front Immunol. 2018, 9, 444. [Google Scholar] [CrossRef]
- Cruceriu, D.; Baldasici, O.; Balacescu, O.; Berindan-Neagoe, I. The dual role of tumor necrosis factor-alpha (TNF-α) in breast cancer: Molecular insights and therapeutic approaches. Cell. Oncol. 2020, 43, 1–18. [Google Scholar] [CrossRef]
- Van Horssen, R.; Hagen, T.L.M.T.; Eggermont, A.M.M. TNF-α in Cancer Treatment: Molecular Insights, Antitumor Effects, and Clinical Utility. Oncol. 2006, 11, 397–408. [Google Scholar] [CrossRef]
- Ruiz, E.P.; Minute, L.; Otano, I.; Alvarez, M.; Ochoa, M.C.; Belsue, V.; De Andrea, C.; Rodriguez-Ruiz, M.E.; Perez-Gracia, J.L.; Marquez-Rodas, I.; et al. Prophylactic TNF blockade uncouples efficacy and toxicity in dual CTLA-4 and PD-1 immunotherapy. Nature 2019, 569, 428–432. [Google Scholar] [CrossRef]
- Balkwill, F. Tumour necrosis factor and cancer. Nat. Cancer 2009, 9, 361–371. [Google Scholar] [CrossRef]
- Balkwill, F. TNF-α in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Grant, R.; Mishra, P.; Nilubol, N. The Role of Tumor Necrosis Factor in Manipulating the Immunological Response of Tumor Microenvironment. Front. Immunol. 2021, 12, 656908. [Google Scholar] [CrossRef] [PubMed]
- Kokolakis, G.; Sabat, R.; Krüger-Krasagakis, S.; Eberle, J. Ambivalent Effects of Tumor Necrosis Factor Alpha on Apoptosis of Malignant and Normal Human Keratinocytes. Ski. Pharmacol. Physiol. 2021, 34, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Y.; Wolchok, J.D.; Bass, A.R. TNF in the era of immune checkpoint inhibitors: Friend or foe? Nat. Rev. Rheumatol. 2021, 17, 213–223. [Google Scholar] [CrossRef] [PubMed]
- Ottenbrite, R.M.; Regelson, W.; Kaplan, A.; Carchman, R.; Morahan, P.; Munson, A. Polymer Drugs; Donaruma, L.G., Vogel, O., Eds.; John Wiley & Sons, Inc.: New York, NY, USA, 1978; p. 263. [Google Scholar]
- Yilmaz, G.; Demir, B.; Timur, S.; Becer, C.R. Poly(methacrylic acid)-Coated Gold Nanoparticles: Functional Platforms for Theranostic Applications. Biomacromolecules 2016, 17, 2901–2911. [Google Scholar] [CrossRef]
- Tang, H.; Guo, J.; Sun, Y.; Chang, B.; Ren, Q.; Yang, W. Facile synthesis of pH sensitive polymer-coated mesoporous silica nanoparticles and their application in drug delivery. Int. J. Pharm. 2011, 421, 388–396. [Google Scholar] [CrossRef]
- Fang, W.; Wang, Z.; Zong, S.; Chen, H.; Zhu, D.; Zhong, Y.; Cui, Y. pH-controllable drug carrier with SERS activity for targeting cancer cells. BiosensBioelectron 2014, 57, 10–15. [Google Scholar] [CrossRef]
- Zhao, X.; Yang, L.; Li, X.; Jia, X.; Liu, L.; Zeng, J.; Guo, J.; Liu, P. Functionalized Graphene Oxide Nanoparticles for Cancer Cell-Specific Delivery of Antitumor Drug. Bioconjugate Chem. 2015, 26, 128–136. [Google Scholar] [CrossRef]
- Pan, Y.-J.; Chen, Y.-Y.; Wang, D.-R.; Wei, C.; Guo, J.; Lu, D.-R.; Chu, C.-C.; Wang, C.-C. Redox/pH dual stimuli-responsive biodegradable nanohydrogels with varying responses to dithiothreitol and glutathione for controlled drug release. Biomaterials 2012, 33, 6570–6579. [Google Scholar] [CrossRef]
- Shalviri, A.; Raval, G.; Prasad, P.; Chan, C.; Liu, Q.; Heerklotz, H.; Rauth, A.M.; Wu, X.Y. pH-Dependent doxorubicin release from terpolymer of starch, polymethacrylic acid and polysorbate 80 nanoparticles for overcoming multi-drug resistance in human breast cancer cells. Eur. J. Pharm. Biopharm. 2012, 82, 587–597. [Google Scholar] [CrossRef]
- Shalviri, A.; Chan, H.K.; Raval, G.; Abdekhodaie, M.J.; Liu, Q.; Heerklotz, H.; Wu, X.Y. Design of pH-responsive nanoparticles of terpolymer of poly(methacrylic acid), polysorbate 80 and starch for delivery of doxorubicin. Colloids Surf. B Biointerfaces 2013, 101, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Donaruma, L.G.; Ottenbrite, R.W.; Vogl, O. (Eds.) Polymers as Biomaterials; Wiley-Interscience: Hoboken, NJ, USA, 1980; p. 358. [Google Scholar]
- Zhukova, O.V.; Arkhipova, E.V.; Kovaleva, T.F.; Ryabov, S.A.; Ivanova, I.P.; Golovacheva, A.A.; Zykova, D.A.; Zaitsev, S.D. Immunopharmacological Properties of Methacrylic Acid Polymers as Potential Polymeric Carrier Constituents of Anticancer Drugs. Molecules 2021, 26, 4855. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Wilke, C.M.; Kryczek, I.; Chen, G.Y.; Kao, J.; Núñez, G.; Zou, W. Interleukin-10 Ablation Promotes Tumor Development, Growth, and Metastasis. Cancer Res. 2012, 72, 420–429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frances, R. Balkwill, Melania Capasso and Thorsten Hagemann. The tumor microenvironment at a glance. J. Cell Sci. 2012, 125, 5591–5596. [Google Scholar]
- Mostafiz, M.M.; Jhan, P.K.; Shim, J.-K.; Lee, K.Y. Modulation of Cytokine Secretion Allows CD4 T Cells Secreting IL-10 and IL-17 to Simultaneously Participatein Maintaining Toleranceand Immunity. PLoS ONE 2018, 13, e0208552. [Google Scholar] [CrossRef]


| [RCT Agent], mol/L | Characteristics | ||
|---|---|---|---|
| Mn∙103 | Mw∙103 | Mw/Mn | |
| 0.01 | 99.2 | 160.4 | 1.57 |
| 0.04 | 31.5 | 40.0 | 1.27 |
| 0.08 | 19.5 | 23.6 | 1.19 |
| 0.10 | 14.6 | 16.6 | 1.13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhukova, O.V.; Arkhipova, E.V.; Kovaleva, T.F.; Zykova, D.A.; Dubovskaya, N.A. Effect of Poly(methacrylic acid) on the Cytokine Level in an In Vivo Tumor Model. Molecules 2022, 27, 4572. https://doi.org/10.3390/molecules27144572
Zhukova OV, Arkhipova EV, Kovaleva TF, Zykova DA, Dubovskaya NA. Effect of Poly(methacrylic acid) on the Cytokine Level in an In Vivo Tumor Model. Molecules. 2022; 27(14):4572. https://doi.org/10.3390/molecules27144572
Chicago/Turabian StyleZhukova, Olga V., Evgenia V. Arkhipova, Tatiana F. Kovaleva, Daria A. Zykova, and Natalya A. Dubovskaya. 2022. "Effect of Poly(methacrylic acid) on the Cytokine Level in an In Vivo Tumor Model" Molecules 27, no. 14: 4572. https://doi.org/10.3390/molecules27144572






