Electrochemical Immunoassay for Tumor Marker CA19-9 Detection Based on Self-Assembled Monolayer
Abstract
:1. Introduction
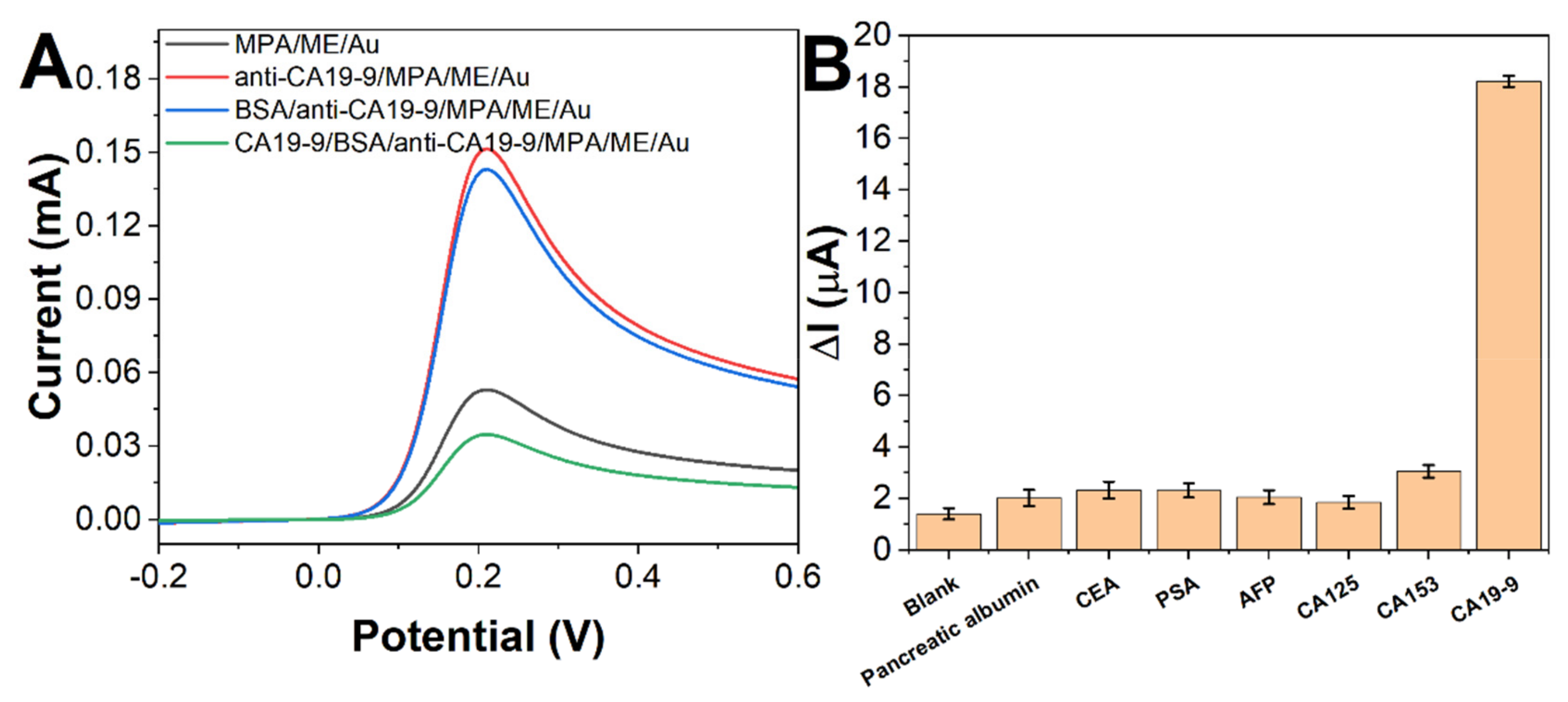
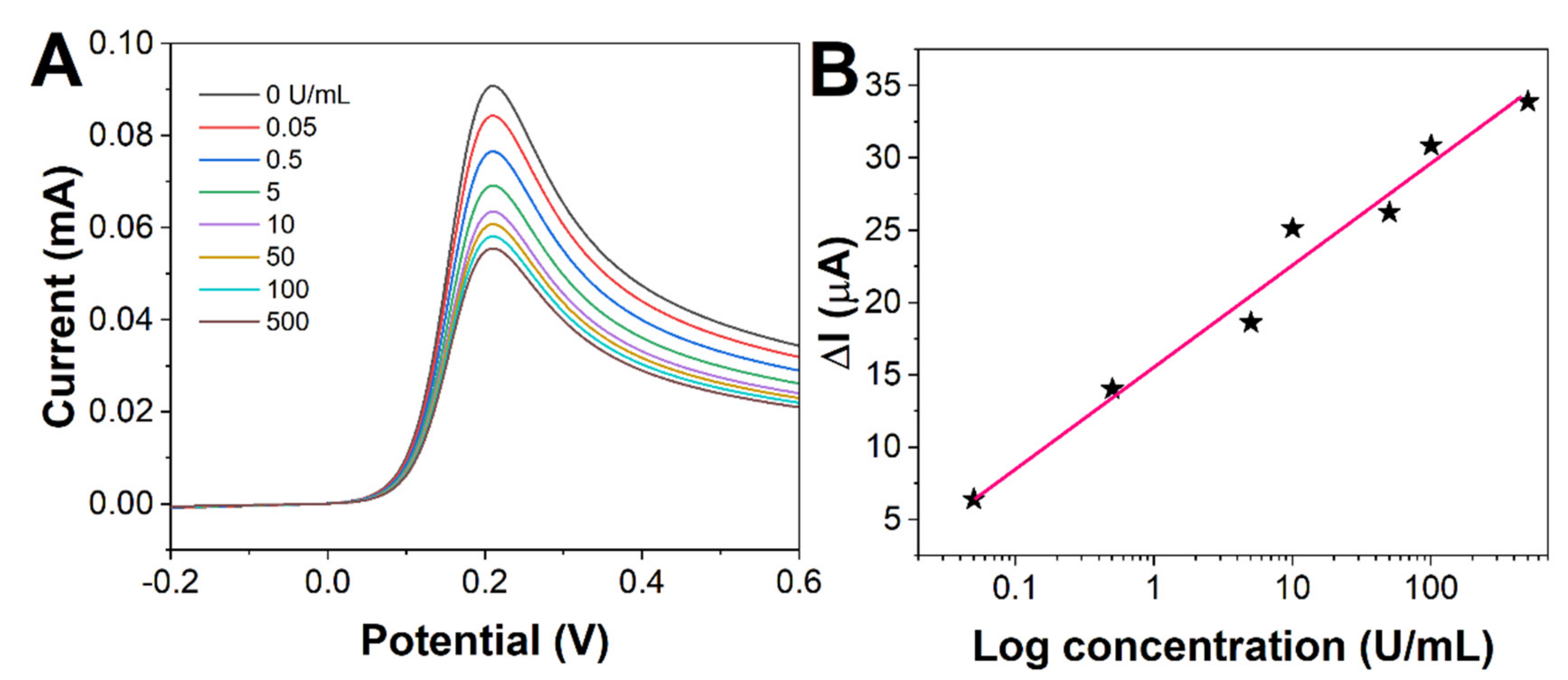
2. Results and Discussion
| Sensor | Linear Range (U/mL) | Limit of Detection (U/mL) | Reference |
|---|---|---|---|
| Anti-CA19-9/3D-ordered macroporous magnetic Au film | 0.05–15.65 | 0.01 | [31] |
| Microfluidic chip | 10.75–172 | 10.75 | [32] |
| Anti-CA19-9/AuNPs/poly(thionine)-SDS Nanocomposites | 6.5–520 | 0.26 | [33] |
| Antibody–AuNP–G-quadruplex/hemin | 0.025–1 | 0.016 | [34] |
| AuPt nanocalliandras | 0.05–50 | 0.03 | [35] |
| BSA/anti-CA19-9/MPA/ME/Au | 0.05–500 | 0.01 | This work |
3. Experimental
3.1. Reagents and Instruments
3.2. Preparation of Electrochemical Immunoassay
3.3. Electrochemical Detection of CA19-9
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Hu, J.; Li, Q.; Chen, J.; Chen, S.; Cai, Y.; Zhao, C. A Label-Free Electrochemical Immunosensor Based on Polythionine-nanogold Nanocomposite for Detection of Trypsin Using Screen-Printed Electrode. Int. J. Electrochem. Sci. 2022, 17, 220617. [Google Scholar] [CrossRef]
- Wu, M.; Liu, S.; Qi, F.; Qiu, R.; Feng, J.; Ren, X.; Rong, S.; Ma, H.; Chang, D.; Pan, H. A label-free electrochemical immunosensor for CA125 detection based on CMK-3(Au/Fc@MgAl-LDH)n multilayer nanocomposites modification. Talanta 2022, 241, 123254. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Zheng, X.; Zhang, Z.; Wu, X.; Sun, L.; Zhou, J.; Liu, M. A Label-Free Electrochemical Immunosensor for Detection of the Tumor Marker CA242 Based on Reduced Graphene Oxide-Gold-Palladium Nanocomposite. Nanomaterials 2019, 9, 1335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahari, D.; Babamiri, B.; Salimi, A.; Hallaj, R.; Amininasab, S.M. A self-enhanced ECL-RET immunosensor for the detection of CA19-9 antigen based on Ru(bpy)2(phen-NH2)2+-Amine-rich nitrogen-doped carbon nanodots as probe and graphene oxide grafted hyperbranched aromatic polyamide as platform. Anal. Chim. Acta 2020, 1132, 55–65. [Google Scholar] [CrossRef]
- Bahari, D.; Babamiri, B.; Salimi, A. An eco-friendly MIP-solid surface fluorescence immunosensor for detection of CA 19-9 tumor marker using Ni nanocluster as an emitter labels. J. Iran. Chem. Soc. 2020, 17, 2283–2291. [Google Scholar] [CrossRef]
- Jing, A.; Xu, Q.; Feng, W.; Liang, G. An Electrochemical Immunosensor for Sensitive Detection of the Tumor Marker Carcinoembryonic Antigen (CEA) Based on Three-Dimensional Porous Nanoplatinum/Graphene. Micromachines 2020, 11, 660. [Google Scholar] [CrossRef]
- Jin, M.; Liu, J.; Wu, W.; Zhou, Q.; Fu, L.; Zare, N.; Karimi, F.; Yu, J.; Lin, C.-T. Relationship between Graphene and Pedosphere: A Scientometric Analysis. Chemosphere 2022, 300, 134599. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Carbohydrate antigen 19-9 electrochemical immunosensor based on 1D-MoS2 nanorods/LiNb3O8 and polyoxometalate-incorporated gold nanoparticles. Microchem. J. 2021, 170, 106643. [Google Scholar] [CrossRef]
- Su, C.-W.; Tian, J.-H.; Ye, J.-J.; Chang, H.-W.; Tsai, Y.-C. Construction of a Label-Free Electrochemical Immunosensor Based on Zn-Co-S/Graphene Nanocomposites for Carbohydrate Antigen 19-9 Detection. Nanomaterials 2021, 11, 1475. [Google Scholar] [CrossRef]
- Kalyani, T.; Nanda, A.; Jana, S.K. Detection of a novel glycodelin biomarker using electrochemical immunosensor for endometriosis. Anal. Chim. Acta 2021, 1146, 146–154. [Google Scholar] [CrossRef]
- Rebelo, T.S.; Ribeiro, J.A.; Sales, M.G.F.; Pereira, C.M. Electrochemical immunosensor for detection of CA 15-3 biomarker in point-of-care. Sens. Bio-Sens. Res. 2021, 33, 100445. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Y.; Zhang, X.; Wang, H.; Xia, T.; Bian, C.; Liang, S.; Tang, X.; Wang, X. Electrochemical immunosensor for HBe antigen detection based on a signal amplification strategy: The co-catalysis of horseradish peroxidase and nanoporous gold. Sens. Actuators B Chem. 2019, 284, 296–304. [Google Scholar] [CrossRef]
- Zheng, Y.; Karimi-Maleh, H.; Fu, L. Advances in Electrochemical Techniques for the Detection and Analysis of Genetically Modified Organisms: An Analysis Based on Bibliometrics. Chemosensors 2022, 10, 194. [Google Scholar] [CrossRef]
- Feng, J.; Chu, C.; Ma, Z. Electrochemical Signal Substance for Multiplexed Immunosensing Interface Construction: A Mini Review. Molecules 2022, 27, 267. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Chu, C.; Ma, Z. Fenton and Fenton-like catalysts for electrochemical immunoassay: A mini review. Electrochem. Commun. 2021, 125, 106970. [Google Scholar] [CrossRef]
- Meng, X.; Xu, Y.; Zhang, N.; Ma, B.; Ma, Z.; Han, H. Ferric hydroxide nanocage triggered Fenton-like reaction to improve amperometric immunosensor. Sens. Actuators B Chem. 2021, 338, 129840. [Google Scholar] [CrossRef]
- Sangili, A.; Kalyani, T.; Chen, S.-M.; Nanda, A.; Jana, S.K. Label-Free Electrochemical Immunosensor Based on One-Step Electrochemical Deposition of AuNP-RGO Nanocomposites for Detection of Endometriosis Marker CA 125. ACS Appl. Bio Mater. 2020, 3, 7620–7630. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Xie, Y.; Sun, X.; Wang, Y. Label-Free Electrochemical Immunosensor for Ultrasensitive Detection of Carbohydrate Antigen 125 Based on Antibody-Immobilized Biocompatible MOF-808/CNT. ACS Appl. Mater. Interfaces 2021, 13, 3295–3302. [Google Scholar] [CrossRef]
- Hassanpour, S.; Hasanzadeh, M. Label-free electrochemical-immunoassay of cancer biomarkers: Recent progress and challenges in the efficient diagnosis of cancer employing electroanalysis and based on point of care (POC). Microchem. J. 2021, 168, 106424. [Google Scholar] [CrossRef]
- Biswas, S.; Lan, Q.; Li, C.; Xia, X.-H. Morphologically Flex Sm-MOF Based Electrochemical Immunosensor for Ultrasensitive Detection of a Colon Cancer Biomarker. Anal. Chem. 2022, 94, 3013–3019. [Google Scholar] [CrossRef]
- Zumpano, R.; Polli, F.; D’Agostino, C.; Antiochia, R.; Favero, G.; Mazzei, F. Nanostructure-Based Electrochemical Immunosensors as Diagnostic Tools. Electrochem 2021, 2, 10–28. [Google Scholar] [CrossRef]
- Filik, H.; Avan, A.A. Nanostructures for nonlabeled and labeled electrochemical immunosensors: Simultaneous electrochemical detection of cancer markers: A review. Talanta 2019, 205, 120153. [Google Scholar] [CrossRef] [PubMed]
- Alarfaj, N.A.; El-Tohamy, M.F.; Oraby, H. New label-free ultrasensitive electrochemical immunosensor-based Au/MoS2/rGO nanocomposites for CA 27–29 breast cancer antigen detection. New J. Chem. 2018, 42, 11046–11053. [Google Scholar] [CrossRef]
- Liao, X.; Wang, X.; Sun, C.; Chen, S.; Zhang, M.; Mei, L.; Qi, Y.; Hong, C. Ratiometric electrochemical immunosensor triggered by an advanced oxidation process for the ultrasensitive detection of carcinoembryonic antigen. Sens. Actuators B Chem. 2022, 362, 131804. [Google Scholar] [CrossRef]
- Arya, S.K.; Estrela, P. Recent Advances in Enhancement Strategies for Electrochemical ELISA-Based Immunoassays for Cancer Biomarker Detection. Sensors 2018, 18, 2010. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Mao, S.; Zhu, J.; Fu, L.; Zare, N.; Karimi, F. Current Status of Electrochemical Detection of Sunset Yellow Based on Bibliometrics. Food Chem. Toxicol. 2022, 164, 113019. [Google Scholar] [CrossRef]
- Kim, J.; Park, M. Recent Progress in Electrochemical Immunosensors. Biosensors 2021, 11, 360. [Google Scholar] [CrossRef]
- Ibáñez-Redín, G.; Furuta, R.H.; Wilson, D.; Shimizu, F.M.; Materon, E.M.; Arantes, L.M.R.B.; Melendez, M.E.; Carvalho, A.L.; Reis, R.M.; Chaur, M.N. Screen-printed interdigitated electrodes modified with nanostructured carbon nano-onion films for detecting the cancer biomarker CA19-9. Mater. Sci. Eng. C 2019, 99, 1502–1508. [Google Scholar] [CrossRef]
- Mic, M.; Varodi, C.; Pogacean, F.; Socaci, C.; Coros, M.; Stefan-van Staden, R.-I.; Pruneanu, S. Sensing and Interaction of His-Tagged CA19-9 Antigen with Graphene-Modified Electrodes. Chemosensors 2020, 8, 112. [Google Scholar] [CrossRef]
- Mo, G.; He, X.; Qin, D.; Meng, S.; Wu, Y.; Deng, B. Spatially-resolved dual-potential sandwich electrochemiluminescence immunosensor for the simultaneous determination of carbohydrate antigen 19–9 and carbohydrate antigen 24-2. Biosens. Bioelectron. 2021, 178, 113024. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, X.; Tang, Y.; Ge, L.; Guo, B.; Yao, C. Amperometric carbohydrate antigen 19-9 immunosensor based on three dimensional ordered macroporous magnetic Au film coupling direct electrochemistry of horseradish peroxidase. Anal. Chim. Acta 2014, 815, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Karimi-Maleh, H.; Fu, L. Evaluation of Antioxidants Using Electrochemical Sensors: A Bibliometric Analysis. Sensors 2022, 22, 3238. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Jiang, Z.; Zhao, C.; Han, W.; Lin, L.; Liu, A.; Weng, S.; Lin, X. Simple and effective label-free electrochemical immunoassay for carbohydrate antigen 19-9 based on polythionine-Au composites as enhanced sensing signals for detecting different clinical samples. Int. J. Nanomed. 2017, 12, 3049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, M.; Zhao, S.; Huang, Y.; Zhao, L.; Liu, Y.-M. Signal amplification in capillary electrophoresis based chemiluminescent immunoassays by using an antibody–gold nanoparticle–DNAzyme assembly. Talanta 2014, 124, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.; Liu, Y.; Xue, Y.; Wang, A.-J.; Wu, L.; Feng, J.-J. L-Proline bio-inspired synthesis of AuPt nanocalliandras as sensing platform for label-free electrochemical immunoassay of carbohydrate antigen 19-9. Sens. Actuators B Chem. 2017, 250, 61–68. [Google Scholar] [CrossRef]
- Mollarasouli, F.; Kurbanoglu, S.; Ozkan, S.A. The Role of Electrochemical Immunosensors in Clinical Analysis. Biosensors 2019, 9, 86. [Google Scholar] [CrossRef] [PubMed] [Green Version]

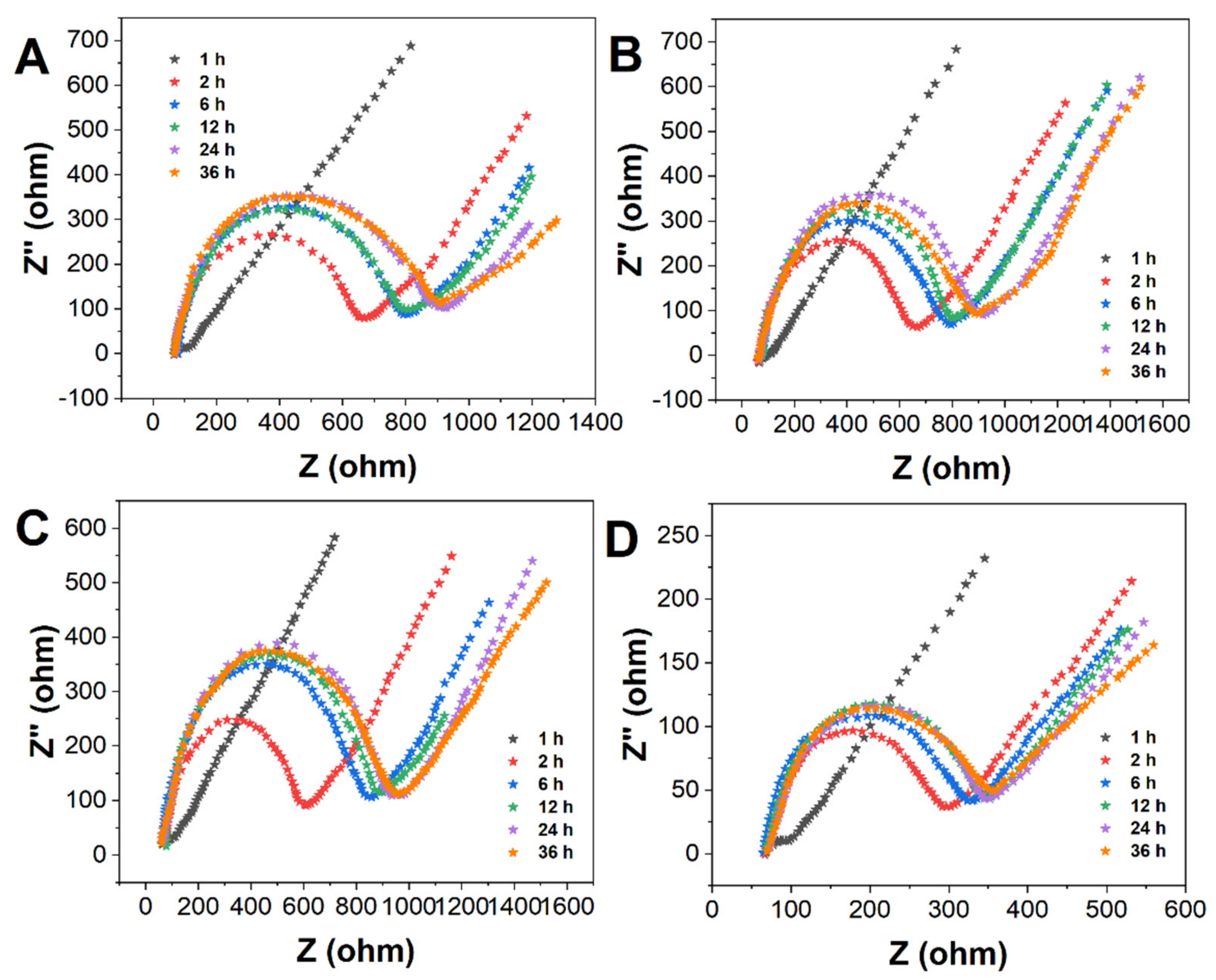

| MPA (%) | 1 h | 2 h | 6 h | 12 h | 24 h |
|---|---|---|---|---|---|
| 100 | 73.31 | 150.23 | 221.64 | 338.20 | 584.30 |
| 50 | 261.10 | 635.21 | 740.22 | 766.20 | 878.30 |
| 25 | 242.40 | 488.50 | 719.30 | 805.22 | 1120.30 |
| 10 | 204.14 | 228.51 | 244.63 | 251.20 | 262.28 |
| Sample | Add (U/mL) | Found (U/mL) | RSD (%) | Recovery (%) |
|---|---|---|---|---|
| 1 | 1.00 | 0.97, 1.03, 1.07, 1.15, 0.95 | 8.05 | 115.0 |
| 2 | 3.00 | 3.05, 2.98, 2.91, 3.09, 3.03 | 6.94 | 103.0 |
| 3 | 5.00 | 5.08, 5.01, 4.89, 4.93, 5.12 | 9.71 | 102.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, Z.; Cai, X.; Cui, W.; Zhang, J. Electrochemical Immunoassay for Tumor Marker CA19-9 Detection Based on Self-Assembled Monolayer. Molecules 2022, 27, 4578. https://doi.org/10.3390/molecules27144578
Wei Z, Cai X, Cui W, Zhang J. Electrochemical Immunoassay for Tumor Marker CA19-9 Detection Based on Self-Assembled Monolayer. Molecules. 2022; 27(14):4578. https://doi.org/10.3390/molecules27144578
Chicago/Turabian StyleWei, Zheng, Xiaoping Cai, Weifeng Cui, and Junping Zhang. 2022. "Electrochemical Immunoassay for Tumor Marker CA19-9 Detection Based on Self-Assembled Monolayer" Molecules 27, no. 14: 4578. https://doi.org/10.3390/molecules27144578
APA StyleWei, Z., Cai, X., Cui, W., & Zhang, J. (2022). Electrochemical Immunoassay for Tumor Marker CA19-9 Detection Based on Self-Assembled Monolayer. Molecules, 27(14), 4578. https://doi.org/10.3390/molecules27144578






