Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation
Abstract
:1. Introduction
2. Results
2.1. Cell Viability of Xanthatin
2.2. Effects of Xanthatin on LPS-Stimulated on NO Production
2.3. Effects of Xanthatin on LPS-Induced Pro-Inflammatory Mediator Expression
2.4. Effects of Xanthatin on LPS-Induced iNOS, COX-2, TNF-α, IL-1β, and IL-6 mRNA Levels
2.5. Effects of Xanthatin on LPS-Stimulated on the Reactive Oxygen Species (ROS) Content
2.6. Effects of Xanthatin on LPS-Induced on the iNOS and COX-2 Expression
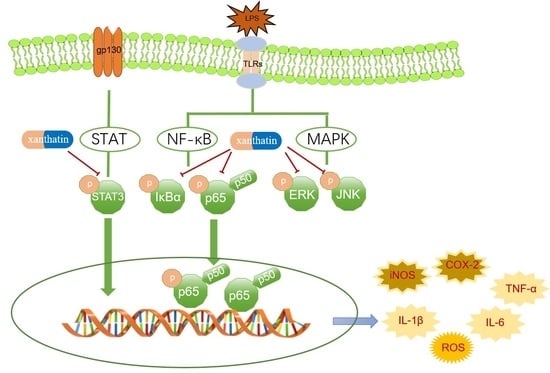
2.7. Effects of Xanthatin on LPS-Induced on the NF-κB, MAPK and STATs Signaling Pathways
2.8. Effects of Xanthatin on LPS-Induced on the NF-κB p65 Nuclear Translocation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Cell Viability Assay
4.4. Measurement of Nitric Oxide (NO)
4.5. ELISA
4.6. Quantitative Reverse Transcriptase-Polymerase Chain Reaction (qRT-PCR)
4.7. Reactive Oxygen Species (ROS) Measurement
4.8. Western Blot Analysis
4.9. The NF-κB p65 Nuclear Translocation Immunofluorescence Staining
4.10. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Libby, P. Inflammatory mechanisms: The molecular basis of inflammation and disease. Nutr. Rev. 2007, 65, S140–S146. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef] [PubMed]
- Medzhitov, R. Origin and physiological roles of inflammation. Nature 2008, 454, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Koh, W.; Shin, J.S.; Lee, J.; Lee, I.H.; Lee, S.K.; Ha, I.H.; Chung, H.J. Anti-inflammatory effect of Cortex Eucommiae via modulation of the toll-like receptor 4 pathway in lipopolysaccharide-stimulated RAW 264.7 macrophages. J. Ethnopharmacol. 2017, 209, 255–263. [Google Scholar] [CrossRef]
- Donath, M.Y.; Shoelson, S.E. Type 2 diabetes as an inflammatory disease. Nat. Rev. Immunol. 2011, 11, 98–107. [Google Scholar] [CrossRef]
- Chawla, A.; Nguyen, K.D.; Goh, Y.P. Macrophage-mediated inflammation in metabolic disease. Nat. Rev. Immunol. 2011, 11, 738–749. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, K.; Karin, M. NF-kappaB, inflammation, immunity and cancer: Coming of age. Nat. Rev. Immunol. 2018, 18, 309–324. [Google Scholar] [CrossRef]
- Pountos, I.; Theodora, G.; Howard, B.; Giannoudis, P. Nonsteroidal anti-inflammatory drugs: Prostaglandins, indications, and side effects. Int. J. Interferon Cytokine Mediat. Res. 2011, 3, 19. [Google Scholar] [CrossRef] [Green Version]
- He, Y.; Yue, Y.; Zheng, X.; Zhang, K.; Chen, S.; Du, Z. Curcumin, inflammation, and chronic diseases: How are they linked? Molecules 2015, 20, 9183–9213. [Google Scholar] [CrossRef]
- Wongrakpanich, S.; Wongrakpanich, A.; Melhado, K.; Rangaswami, J. A Comprehensive Review of Non-Steroidal Anti-Inflammatory Drug Use in The Elderly. Aging Dis. 2018, 9, 143–150. [Google Scholar] [CrossRef] [Green Version]
- Diakos, C.I.; Charles, K.A.; McMillan, D.C.; Clarke, S.J. Cancer-related inflammation and treatment effectiveness. Lancet Oncol. 2014, 15, e493–e503. [Google Scholar] [CrossRef]
- Tabas, I.; Glass, C.K. Anti-inflammatory therapy in chronic disease: Challenges and opportunities. Science 2013, 339, 166–172. [Google Scholar] [CrossRef] [Green Version]
- Yates, C.M.; Calder, P.C.; Ed Rainger, G. Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharm. Ther. 2014, 141, 272–282. [Google Scholar] [CrossRef]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage biology in development, homeostasis and disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef] [PubMed]
- Krausgruber, T.; Blazek, K.; Smallie, T.; Alzabin, S.; Lockstone, H.; Sahgal, N.; Hussell, T.; Feldmann, M.; Udalova, I.A. IRF5 promotes inflammatory macrophage polarization and TH1-TH17 responses. Nat. Immunol. 2011, 12, 231–238. [Google Scholar] [CrossRef] [PubMed]
- Koh, T.J.; DiPietro, L.A. Inflammation and wound healing: The role of the macrophage. Expert Rev. Mol. Med. 2011, 13, e23. [Google Scholar] [CrossRef] [Green Version]
- Hua, Y.; Bergers, G. Tumors vs. Chronic Wounds: An Immune Cell’s Perspective. Front. Immunol. 2019, 10, 2178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, M.S.; Lee, J.O. Structures of the toll-like receptor family and its ligand complexes. Immunity 2008, 29, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. LPS/TLR4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef]
- Guha, M.; Mackman, N. LPS induction of gene expression in human monocytes. Cell Signal 2001, 13, 85–94. [Google Scholar] [CrossRef]
- Lai, J.L.; Liu, Y.H.; Liu, C.; Qi, M.P.; Liu, R.N.; Zhu, X.F.; Zhou, Q.G.; Chen, Y.Y.; Guo, A.Z.; Hu, C.M. Indirubin Inhibits LPS-Induced Inflammation via TLR4 Abrogation Mediated by the NF-kB and MAPK Signaling Pathways. Inflammation 2017, 40, 1–12. [Google Scholar] [CrossRef]
- Ju, A.; Cho, Y.C.; Cho, S. Methanol extracts of Xanthium sibiricum roots inhibit inflammatory responses via the inhibition of nuclear factor-kappaB (NF-kappaB) and signal transducer and activator of transcription 3 (STAT3) in murine macrophages. J. Ethnopharmacol. 2015, 174, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Wang, C. Traditional uses, phytochemistry, pharmacology, pharmacokinetics and toxicology of the fruit of Tetradium ruticarpum: A review. J. Ethnopharmacol. 2020, 263, 113231. [Google Scholar] [CrossRef] [PubMed]
- Yen, P.H.; Hoang, N.H.; Trang, D.T.; Huong, P.T.T.; Tai, B.H.; Nhiem, N.X.; Kiem, P.V. A New Thiazinedione Glycoside From the Fruits of Xanthium strumarium L. Nat. Prod. Commun. 2021, 16, 1934578X211032082. [Google Scholar] [CrossRef]
- Liu, M.; Xiao, C.Q.; Sun, M.W.; Tan, M.J.; Hu, L.H.; Yu, Q. Xanthatin inhibits STAT3 and NF-kappaB signalling by covalently binding to JAK and IKK kinases. J. Cell. Mol. Med. 2019, 23, 4301–4312. [Google Scholar] [CrossRef] [PubMed]
- Kuang, H.-X.; Wang, Q.-H.; Jiang, H.; Wang, X.-j.; Yang, L.; Zhang, J.-X.; Hou, A.J.; Man, W.-J.; Wang, S.; Yang, B.-Y.; et al. The fruits of Xanthium sibiricum Patr: A review on phytochemistry, pharmacological activities, and toxicity. World J. Tradit. Chin. Med. 2020, 6, 408–442. [Google Scholar] [CrossRef]
- Kamoun, E.A.; Kenawy, E.S.; Chen, X. A review on polymeric hydrogel membranes for wound dressing applications: PVA-based hydrogel dressings. J. Adv. Res. 2017, 8, 217–233. [Google Scholar] [CrossRef]
- Olivaro, C.; Rostan, V.; Bandera, D.; Moyna, G.; Vazquez, A. Xanthane sesquiterpenoids from the roots and flowers of Xanthium cavanillesii. Nat. Prod. Res. 2016, 30, 2238–2242. [Google Scholar] [CrossRef]
- Nibret, E.; Youns, M.; Krauth-Siegel, R.L.; Wink, M. Biological activities of xanthatin from Xanthium strumarium leaves. Phytother. Res. 2011, 25, 1883–1890. [Google Scholar] [CrossRef]
- Tao, L.; Cao, Y.; Wei, Z.; Jia, Q.; Yu, S.; Zhong, J.; Wang, A.; Woodgett, J.R.; Lu, Y. Xanthatin triggers Chk1-mediated DNA damage response and destabilizes Cdc25C via lysosomal degradation in lung cancer cells. Toxicol. Appl. Pharmacol. 2017, 337, 85–94. [Google Scholar] [CrossRef]
- Shi, T.L.; Zhang, L.; Cheng, Q.Y.; Yu, J.S.; Liu, J.; Shen, Y.J.; Feng, X.J.; Shen, Y.X. Xanthatin induces apoptosis by activating endoplasmic reticulum stress in hepatoma cells. Eur. J. Pharmacol. 2019, 843, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Geng, Y.D.; Zhang, L.; Wang, G.Y.; Feng, X.J.; Chen, Z.L.; Jiang, L.; Shen, A.Z. Xanthatin mediates G2/M cell cycle arrest, autophagy and apoptosis via ROS/XIAP signaling in human colon cancer cells. Nat. Prod. Res. 2020, 34, 2616–2620. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Sheng, X.; Zhang, L.; Li, W.; Wei, Z.; Zhu, P.; Zhang, F.; Wang, A.; Woodgett, J.R.; Lu, Y. Xanthatin anti-tumor cytotoxicity is mediated via glycogen synthase kinase-3beta and beta-catenin. Biochem. Pharmacol. 2016, 115, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Fan, L.; Peng, C.; Zhang, Q.; Wang, L.; Li, L.; Wang, J.; Zhang, D.; Peng, W.; Wu, C. Traditional uses, botany, phytochemistry, pharmacology, pharmacokinetics and toxicology of Xanthium strumarium L.: A review. Molecules 2019, 24, 359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamboj, A.; Saluja, A.K. Phytopharmacological review of Xanthium strumarium L.(Cocklebur). Int. J. Green Pharm. 2010, 4, 129–139. [Google Scholar] [CrossRef]
- Shen, M.; Zhou, X.Z.; Ye, L.; Yuan, Q.; Shi, C.; Zhu, P.W.; Jiang, N.; Ma, M.Y.; Yang, Q.C.; Shao, Y. Xanthatin inhibits corneal neovascularization by inhibiting the VEGFR2mediated STAT3/PI3K/Akt signaling pathway. Int. J. Mol. Med. 2018, 42, 769–778. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.S.; Shin, J.S.; Jang, D.S.; Lee, K.T. Cnidilide, an alkylphthalide isolated from the roots of Cnidium officinale, suppresses LPS-induced NO, PGE2, IL-1beta, IL-6 and TNF-alpha production by AP-1 and NF-kappaB inactivation in RAW 264.7 macrophages. Int. Immunopharmacol. 2016, 40, 146–155. [Google Scholar] [CrossRef]
- Kearney, C.J.; Cullen, S.P.; Tynan, G.A.; Henry, C.M.; Clancy, D.; Lavelle, E.C.; Martin, S.J. Necroptosis suppresses inflammation via termination of TNF-or LPS-induced cytokine and chemokine production. Cell Death Differ. 2015, 22, 1313–1327. [Google Scholar] [CrossRef]
- Blaser, H.; Dostert, C.; Mak, T.W.; Brenner, D. TNF and ROS Crosstalk in Inflammation. Trends Cell Biol. 2016, 26, 249–261. [Google Scholar] [CrossRef]
- Chien, S.Y.; Huang, C.Y.; Tsai, C.H.; Wang, S.W.; Lin, Y.M.; Tang, C.H. Interleukin-1β induces fibroblast growth factor 2 expression and subsequently promotes endothelial progenitor cell angiogenesis in chondrocytes. Clin. Sci. 2016, 130, 667–681. [Google Scholar] [CrossRef] [Green Version]
- Kany, S.; Vollrath, J.T.; Relja, B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019, 20, 6008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, C.A.; Jones, S.A. IL-6 as a keystone cytokine in health and disease. Nat. Immunol. 2015, 16, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Diehl, S.; Rincón, M. The two faces of IL-6 on Th1/Th2 differentiation. Mol. Immunol. 2002, 39, 531–536. [Google Scholar] [CrossRef]
- Hurst, S.M.; Wilkinson, T.S.; McLoughlin, R.M.; Jones, S.; Horiuchi, S.; Yamamoto, N.; Rose-John, S.; Fuller, G.M.; Topley, N.; Jones, S.A. IL-6 and its soluble receptor orchestrate a temporal switch in the pattern of leukocyte recruitment seen during acute inflammation. Immunity 2001, 14, 705–714. [Google Scholar] [CrossRef] [Green Version]
- Park, H.H.; Kim, M.J.; Li, Y.; Park, Y.N.; Lee, J.; Lee, Y.J.; Kim, S.G.; Park, H.J.; Son, J.K.; Chang, H.W.; et al. Britanin suppresses LPS-induced nitric oxide, PGE2 and cytokine production via NF-kappaB and MAPK inactivation in RAW 264.7 cells. Int. Immunopharmacol. 2013, 15, 296–302. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Shin, E.M.; Guo, L.Y.; Youn, U.J.; Bae, K.; Kang, S.S.; Zou, L.B.; Kim, Y.S. Anti-inflammatory activity of 4-methoxyhonokiol is a function of the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via NF-κB, JNK and p38 MAPK inactivation. Eur. J. Pharmacol. 2008, 586, 340–349. [Google Scholar] [CrossRef]
- Hickey, M. Role of inducible nitric oxide synthase in the regulation of leucocyte recruitment. Clin. Sci. 2001, 100, 1–12. [Google Scholar] [CrossRef]
- Rath, M.; Müller, I.; Kropf, P.; Closs, E.I.; Munder, M. Metabolism via arginase or nitric oxide synthase: Two competing arginine pathways in macrophages. Front. Immunol. 2014, 5, 532. [Google Scholar] [CrossRef] [Green Version]
- Tsatsanis, C.; Androulidaki, A.; Dermitzaki, E.; Gravanis, A.; Margioris, A.N. Corticotropin releasing factor receptor 1 (CRF1) and CRF2 agonists exert an anti-inflammatory effect during the early phase of inflammation suppressing LPS-induced TNF-α release from macrophages via induction of COX-2 and PGE. J. Cell. Physiol. 2007, 210, 774–783. [Google Scholar] [CrossRef]
- Kundu, J.K.; Surh, Y.J. Inflammation: Gearing the journey to cancer. Mutat. Res. 2008, 659, 15–30. [Google Scholar] [CrossRef]
- Hillmer, E.J.; Zhang, H.; Li, H.S.; Watowich, S.S. STAT3 signaling in immunity. Cytokine Growth Factor Rev. 2016, 31, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhu, R.; Huang, Z.; Li, H.; Zhu, H. Lipopolysaccharide-induced toll-like receptor 4 signaling in cancer cells promotes cell survival and proliferation in hepatocellular carcinoma. Dig. Dis. Sci. 2013, 58, 2223–2236. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Qi, S.; Dai, W.; Luo, L.; Yin, Z. Arctigenin inhibits lipopolysaccharide-induced iNOS expression in RAW264.7 cells through suppressing JAK-STAT signal pathway. Int. Immunopharmacol. 2011, 11, 1095–1102. [Google Scholar] [CrossRef]
- Qi, Z.; Yin, F.; Lu, L.; Shen, L.; Qi, S.; Lan, L.; Yin, Z. Baicalein reduces lipopolysaccharide-induced inflammation via suppressing JAK/STATs activation and ROS production. Inflamm. Res. 2013, 62, 845–855. [Google Scholar] [CrossRef] [PubMed]
- Ganster, R.T.; Bradley, S.; Shao, L.; Geller, D. Complex regulation of human inducible nitric oxide synthase gene transcription by Stat 1 and NF-κB. Proc. Natl. Acad. Sci. USA 2001, 98, 8638–8643. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.; Pu, D.; Di, Q.; Zhao, X.; Ji, F.; Li, H.; Chen, W. Cirsitakaoside isolated from Premna szemaoensis reduces LPS-induced inflammatory responses in vitro and in vivo. Int. Immunopharmacol. 2018, 59, 384–390. [Google Scholar] [CrossRef]
- Hoffmann, A.; Baltimore, D. Circuitry of nuclear factor κB signaling. Immunol. Rev. 2006, 210, 171–186. [Google Scholar] [CrossRef]
- Chen, Y.E.; Fischbach, M.A.; Belkaid, Y. Skin microbiota-host interactions. Nature 2018, 553, 427–436. [Google Scholar] [CrossRef]
- Lebre, M.C.; van der Aar, A.M.; van Baarsen, L.; van Capel, T.M.; Schuitemaker, J.H.; Kapsenberg, M.L.; de Jong, E.C. Human keratinocytes express functional Toll-like receptor 3, 4, 5, and 9. J. Investig. Dermatol. 2007, 127, 331–341. [Google Scholar] [CrossRef] [Green Version]
- Miller, L.S.; Modlin, R.L. Toll-like receptors in the skin. Semin. Immunopathol. 2007, 29, 15–26. [Google Scholar] [CrossRef]
- Lawrence, T. The nuclear factor NF-kappaB pathway in inflammation. Cold Spring Harb. Perspect Biol. 2009, 1, a001651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, Y.J.; Jeong, M.; Lee, K.T.; Jang, D.S.; Choi, J.H. Isocyperol, isolated from the rhizomes of Cyperus rotundus, inhibits LPS-induced inflammatory responses via suppression of the NF-κB and STAT3 pathways and ROS stress in LPS-stimulated RAW 264.7 cells. Int. Immunopharmacol. 2016, 38, 61–69. [Google Scholar] [CrossRef]
- Park, H.Y.; Kim, G.Y.; Choi, Y.H. Naringenin attenuates the release of pro-inflammatory mediators from lipopolysaccharide-stimulated BV2 microglia by inactivating nuclear factor-κB and inhibiting mitogen-activated protein kinases. Int. J. Mol. Med. 2012, 30, 204–210. [Google Scholar] [CrossRef] [Green Version]
- Fu, Y.; Liu, B.; Zhang, N.; Liu, Z.; Liang, D.; Li, F.; Cao, Y.; Feng, X.; Zhang, X.; Yang, Z. Magnolol inhibits lipopolysaccharide-induced inflammatory response by interfering with TLR4 mediated NF-kappaB and MAPKs signaling pathways. J. Ethnopharmacol. 2013, 145, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Ahn, C.B.; Je, J.Y. Anti-inflammatory action of high molecular weight Mytilus edulis hydrolysates fraction in LPS-induced RAW264. 7 macrophage via NF-κB and MAPK pathways. Food Chem. 2016, 202, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.G.; Yoon, D.H.; Lee, W.H.; Han, S.K.; Shrestha, B.; Kim, C.H.; & Kim, T.W. Phellinus linteus inhibits inflammatory mediators by suppressing redox-based NF-κB and MAPKs activation in lipopolysaccharide-induced RAW 264.7 macrophage. J. Ethnopharmacol. 2007, 114, 307–315. [Google Scholar] [CrossRef]
- Lee, J.; Choi, J.W.; Sohng, J.K.; Pandey, R.P.; Park, Y.I. The immunostimulating activity of quercetin 3-O-xyloside in murine macrophages via activation of the ASK1/MAPK/NF-κB signaling pathway. Int. Immunopharmacol. 2016, 31, 88–97. [Google Scholar] [CrossRef]
- Guo, C.; Yang, L.; Luo, J.; Zhang, C.; Xia, Y.; Ma, T.; & Kong, L. Sophoraflavanone G from Sophora alopecuroides inhibits lipopolysaccharide-induced inflammation in RAW264. 7 cells by targeting PI3K/Akt, JAK/STAT and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2016, 38, 349–356. [Google Scholar] [CrossRef]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T.J.B.; Bulletin, P. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by poncirin through the NF-kappaB inactivation in RAW 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [Green Version]
- Yun, K.J.; Kim, J.Y.; Kim, J.B.; Lee, K.W.; Jeong, S.Y.; Park, H.J.; Lee, K.T. Inhibition of LPS-induced NO and PGE2 production by asiatic acid via NF-κB inactivation in RAW 264.7 macrophages: Possible involvement of the IKK and MAPK pathways. Int. Immunopharmacol. 2008, 8, 431–441. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, D.H.; Baek, S.H.; Lee, H.J.; Kim, M.R.; Kwon, H.J.; Lee, C.H. Rengyolone inhibits inducible nitric oxide synthase expression and nitric oxide production by down-regulation of NF-κB and p38 MAP kinase activity in LPS-stimulated RAW 264.7 cells. Biochem. Pharmacol. 2006, 71, 1198–1205. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.D.; Riches, D.W. IFN-γ+ LPS induction of iNOS is modulated by ERK, JNK/SAPK, and p38 mapk in a mouse macrophage cell line. Am. J. Physiol. Cell Physiol. 2001, 280, C441–C450. [Google Scholar] [CrossRef] [PubMed]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Choi, Y.J.; Shin, S.Y.; Li, J.; Kang, S.W.; Bae, J.Y.; Kang, Y.H. Dietary flavonoids differentially reduce oxidized LDL-induced apoptosis in human endothelial cells: Role of MAPK-and JAK/STAT-signaling. J. Nutr. 2008, 138, 983–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Levy, O.; Granot, Y. Arginine-vasopressin activates the JAK-STAT pathway in vascular smooth muscle cells. J. Biol. Chem. 2006, 281, 15597–15604. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.; Zhang, W.; Kone, B.C. Signal transducers and activators of transcription 3 (STAT3) inhibits transcription of the inducible nitric oxide synthase gene by interacting with nuclear factor κB. Biochem. J. 2002, 367, 97–105. [Google Scholar] [CrossRef]
- Kong, F.; Lee, B.H.; Wei, K. 5-hydroxymethylfurfural mitigates lipopolysaccharide-stimulated inflammation via suppression of MAPK, NF-κB and mTOR activation in RAW 264.7 cells. Molecules 2019, 24, 275. [Google Scholar] [CrossRef] [Green Version]









| Name | Primer Sequence (5′-3′) | Product (bp) |
|---|---|---|
| iNOS | F: GCTATGGCCGCTTTGATGTGR: ACCTCCAGTAGCATGTTGGC | 184 |
| COX-2 | F: TGAGTACCGCAAACGCTTCTR: ACGAGGTTTTTCCACCAGCA | 148 |
| TNF-α | F: GACGTGGAACTGGCAGAAGAR: ACTGATGAGAGGGAGGCCAT | 192 |
| IL-1β | F: AACCTTTGACCTGGGCTGTCR: AAGGTCCACGGGAAAGACAC | 144 |
| IL-6 | F: ATCCAGTTGCCTTCTTGGGAR: GGTCTGTTGGGAGTGGTATCC | 103 |
| β-actin | F: AGGGAAATCGTGCGTGACATR: AACCGCTCGTTGCCAATAGT | 149 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Chen, W.; Zheng, F.; Yu, H.; Wei, K. Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation. Molecules 2022, 27, 4603. https://doi.org/10.3390/molecules27144603
Liu Y, Chen W, Zheng F, Yu H, Wei K. Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation. Molecules. 2022; 27(14):4603. https://doi.org/10.3390/molecules27144603
Chicago/Turabian StyleLiu, Yuanqi, Wenyu Chen, Fang Zheng, Huanan Yu, and Kun Wei. 2022. "Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation" Molecules 27, no. 14: 4603. https://doi.org/10.3390/molecules27144603
APA StyleLiu, Y., Chen, W., Zheng, F., Yu, H., & Wei, K. (2022). Xanthatin Alleviates LPS-Induced Inflammatory Response in RAW264.7 Macrophages by Inhibiting NF-κB, MAPK and STATs Activation. Molecules, 27(14), 4603. https://doi.org/10.3390/molecules27144603