Involvement of Resveratrol against Brain Cancer: A Combination Strategy with a Pharmaceutical Approach
Abstract
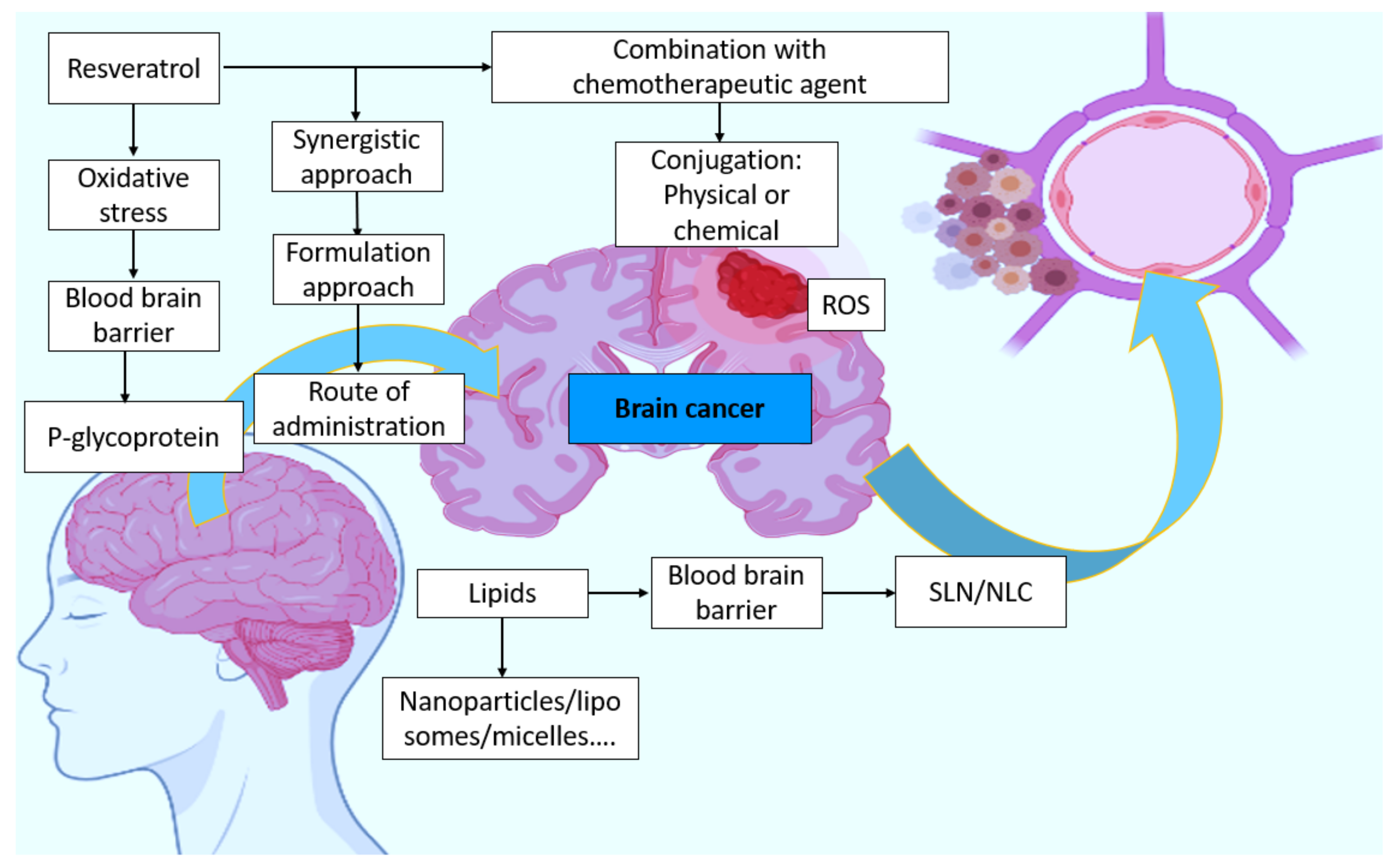
:1. A Basic Outline on Resveratrol
2. A Background Search on Brain Cancer
3. Methodology
4. Correlation between Oxidative Stress, Antioxidants, and BT
Oxidative Stress
5. RES—A General Concept and a Basic Outline
5.1. Resveratrol and Its Pharmacological Action in the General Aspect
5.1.1. Resveratrol’s Pharmacological Influence on Immunity
5.1.2. Resveratrol’s Effect on Cancer
5.1.3. Toxicity Effects of Resveratrol
6. RES Brain Delivery
7. Barriers
8. Combination, Synergistic Response, and the Response to Conventional Therapy
9. Possible Route of Administration
10. RES about Autophagy in Brain Cells
11. Control of Cellular Proliferation, Apoptosis, and Differentiation
12. Cellular and Molecular Mechanisms
13. Lipids and Their Advantages in BT
14. Formulation-Based Approaches
15. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shaito, A.; Posadino, A.M.; Younes, N.; Hasan, H.; Halabi, S.; Alhababi, D.; Al-Mohannadi, A.; Abdel-Rahman, W.M.; Eid, A.H.; Nasrallah, G.K.; et al. Potential Adverse Effects of Resveratrol: A Literature Review. Int. J. Mol. Sci. 2020, 21, 2084. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santos, A.C.; Pereira, I.; Pereira-Silva, M.; Ferreira, L.; Caldas, M.; Magalhães, M.; Figueiras, A.; Ribeiro, A.J.; Veiga, F. Nanocarriers for Resveratrol Delivery: Impact on Stability and Solubility Concerns. Trends Food Sci. Technol. 2019, 91, 483–497. [Google Scholar] [CrossRef]
- Annaji, M.; Poudel, I.; Boddu, S.H.S.; Arnold, R.D.; Tiwari, A.K.; Babu, R.J. Resveratrol-loaded Nanomedicines for Cancer Applications. Cancer Rep. 2021, 4, e1353. [Google Scholar] [CrossRef]
- Kaur, A.; Tiwari, R.; Tiwari, G.; Ramachandran, V. Resveratrol: A Vital Therapeutic Agent with Multiple Health Benefits. Drug Res. 2022, 72, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.-G.; Wang, C.-C.; Lee, Y.-S.; Sie, Y.-Y.; Chang, C.-I.; Hou, W.-C. Vitisin A, a Resveratrol Tetramer, Improves Scopolamine-Induced Impaired Learning and Memory Functions in Amnesiac ICR Mice. Biomedicines 2022, 10, 273. [Google Scholar] [CrossRef]
- Liu, Y.; Song, X.; Wu, M.; Wu, J.; Liu, J. Synergistic Effects of Resveratrol and Temozolomide against Glioblastoma Cells: Underlying Mechanism and Therapeutic Implications. Cancer Manag. Res. 2020, 12, 8341. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Islam, F.; Ahmad, S.U.; Olawale, O.A.; Alhumaydhi, F.A.; Marzouki, R.; Baig, A.A.; Emran, T. Bin Emerging Trends in the Delivery of Resveratrol by Nanostructures: Applications of Nanotechnology in Life Sciences. J. Nanomater. 2022, 2022, 3083728. [Google Scholar] [CrossRef]
- Jøraholmen, M.W.; Johannessen, M.; Gravningen, K.; Puolakkainen, M.; Acharya, G.; Basnet, P.; Škalko-Basnet, N. Liposomes-in-Hydrogel Delivery System Enhances the Potential of Resveratrol in Combating Vaginal Chlamydia Infection. Pharmaceutics 2020, 12, 1203. [Google Scholar] [CrossRef]
- Bagchi, S.; Chhibber, T.; Lahooti, B.; Verma, A.; Borse, V.; Jayant, R.D. In-Vitro Blood-Brain Barrier Models for Drug Screening and Permeation Studies: An Overview. Drug Des. Devel. Ther. 2019, 13, 3591. [Google Scholar] [CrossRef] [Green Version]
- Sevenich, L. Brain-Resident Microglia and Blood-Borne Macrophages Orchestrate Central Nervous System Inflammation in Neurodegenerative Disorders and Brain Cancer. Front. Immunol. 2018, 9, 697. [Google Scholar] [CrossRef]
- Hays, P. Trends in Precision Oncology and Precision Medicine 2.0. In Advancing Healthcare through Personalized Medicine; Springer: Berlin/Heidelberg, Germany, 2021; pp. 419–480. [Google Scholar]
- Lim, J.X.; Karlsson, B.; Pang, A.; Vellayappan, B.A.; Nga, V. Stereotactic Radiosurgery in Alveolar Soft Part Sarcoma Brain Metastases: Case Series and Literature Review. J. Clin. Neurosci. 2021, 93, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Ardern-Holmes, S.; White, C.; Bahure, S.; So, S.; McCowage, G.; Hovey, E.; Troon, S.; De Souza, P.; Simes, J.; Slancar, M. The Effect of Bevacizumab on Vestibular Schwannoma Related to Neurofibromatosis Type 2. Australas. J. Neurosci. 2021, 31, 5–14. [Google Scholar] [CrossRef]
- Uddin, M.; Mamun, A.A.; Rahman, M.; Kabir, M.; Alkahtani, S.; Alanazi, I.S.; Perveen, A.; Ashraf, G.M.; Bin-Jumah, M.N.; Abdel-Daim, M.M. Exploring the Promise of Flavonoids to Combat Neuropathic Pain: From Molecular Mechanisms to Therapeutic Implications. Front. Neurosci. 2020, 14, 478. [Google Scholar] [CrossRef] [PubMed]
- Kadry, H.; Noorani, B.; Cucullo, L. A Blood–Brain Barrier Overview on Structure, Function, Impairment, and Biomarkers of Integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef]
- Patel, D.K.; Gwathmey, K.G. Neoplastic Nerve Lesions. Neurol. Sci. 2022, 43, 3019–3038. [Google Scholar] [CrossRef]
- Zuazo-Gaztelu, I.; Casanovas, O. Unraveling the Role of Angiogenesis in Cancer Ecosystems. Front. Oncol. 2018, 8, 248. [Google Scholar] [CrossRef]
- Yang, L.; Xie, X.; Tu, Z.; Fu, J.; Xu, D.; Zhou, Y. The Signal Pathways and Treatment of Cytokine Storm in COVID-19. Signal Transduct. Target. Ther. 2021, 6, 255. [Google Scholar] [CrossRef]
- Kumar, M.; Noronha, S.; Rangaraj, N.; Moiyadi, A.; Shetty, P.; Singh, V.K. Choice of Intraoperative Ultrasound Adjuncts for Brain Tumor Surgery. medRxiv 2022. [Google Scholar] [CrossRef]
- Elfadadny, A.; El-Husseiny, H.M.; Abugomaa, A.; Ragab, R.F.; Mady, E.A.; Aboubakr, M.; Samir, H.; Mandour, A.S.; El-Mleeh, A.; El-Far, A.H.; et al. Role of Multidrug Resistance-Associated Proteins in Cancer Therapeutics: Past, Present, and Future Perspectives. Environ. Sci. Pollut. Res. 2021, 28, 49447–49466. [Google Scholar] [CrossRef]
- Quiñones, S. Sea Moss for Hair: Discover How You Can Solve Hair Loss, Hair Damage, Hair Breakage, Frizz, Split-Ends, Scalp Irritation, and Much More Using Dr. Sebi’s Guide on How to Use Sea Moss on Hair; 2021. [Google Scholar]
- Ozsahin, D.U.; Meck, K.; Halimani, S.T.; Uzun, B.; Ozsahin, I. Fuzzy PROMETHEE-Based Evaluation of Brain Cancer Treatment Techniques. In Applications of Multi-Criteria Decision-Making Theories in Healthcare and Biomedical Engineering; Elsevier: Amsterdam, The Netherlands, 2021; pp. 41–58. [Google Scholar]
- Silva dos Santos, J.; Goncalves Cirino, J.P.; de Oliveira Carvalho, P.; Ortega, M.M. The Pharmacological Action of Kaempferol in Central Nervous System Diseases: A Review. Front. Pharmacol. 2021, 11, 565700. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Moher, D. Updating Guidance for Reporting Systematic Reviews: Development of the PRISMA 2020 Statement. J. Clin. Epidemiol. 2021, 134, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Almatroodi, S.A.; A Alsahli, M.; SM Aljohani, A.; Alhumaydhi, F.A.; Babiker, A.Y.; Khan, A.A.; Rahmani, A.H. Potential Therapeutic Targets of Resveratrol, a Plant Polyphenol, and Its Role in the Therapy of Various Types of Cancer. Molecules 2022, 27, 2665. [Google Scholar] [CrossRef]
- Chai, N.; Zhang, H.; Li, L.; Yu, X.; Liu, Y.; Lin, Y.; Wang, L.; Yan, J.; Nikolaevna, S.E.; Zhao, Y. Spermidine Prevents Heart Injury in Neonatal Rats Exposed to Intrauterine Hypoxia by Inhibiting Oxidative Stress and Mitochondrial Fragmentation. Oxid. Med. Cell. Longev. 2019, 2019, 5406468. [Google Scholar] [PubMed]
- Jardim, F.R.; de Rossi, F.T.; Nascimento, M.X.; da Silva Barros, R.G.; Borges, P.A.; Prescilio, I.C.; de Oliveira, M.R. Resveratrol and Brain Mitochondria: A Review. Mol. Neurobiol. 2018, 55, 2085–2101. [Google Scholar] [CrossRef] [PubMed]
- Owoade, A.O.; Adetutu, A.; Olorunnisola, O.S. Free Radicals as Mediators of Oxidative Damage and Disease. IOSR J. Pharm. Biol. Sci. 2019, 14, 57–64. [Google Scholar]
- Vaiserman, A.; Cuttler, J.M.; Socol, Y. Low-Dose Ionizing Radiation as a Hormetin: Experimental Observations and Therapeutic Perspective for Age-Related Disorders. Biogerontology 2021, 22, 145–164. [Google Scholar] [CrossRef]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The Effects of Oxidative Stress on the Development of Atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef] [PubMed]
- Mansoor, S.; Ali Wani, O.; Lone, J.K.; Manhas, S.; Kour, N.; Alam, P.; Ahmad, A.; Ahmad, P. Reactive Oxygen Species in Plants: From Source to Sink. Antioxidants 2022, 11, 225. [Google Scholar] [CrossRef]
- Karak, P. Biological Activities of Flavonoids: An Overview. Int. J. Pharm. Sci. Res 2019, 10, 1567–1574. [Google Scholar]
- Obaidi, I.; Higgins, M.; Bahar, B.; Davis, J.L.; McMorrow, T. Identification of the Multifaceted Chemopreventive Activity of Curcumin against the Carcinogenic Potential of the Food Additive, KBrO3. Curr. Pharm. Des. 2018, 24, 595–614. [Google Scholar] [CrossRef]
- Sotler, R.; Poljšak, B.; Dahmane, R.; Jukić, T.; Pavan Jukić, D.; Rotim, C.; Trebše, P.; Starc, A. Prooxidant Activities of Antioxidants and Their Impact on Health. Acta Clin. Croat. 2019, 58, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Sivandzade, F.; Prasad, S.; Bhalerao, A.; Cucullo, L. NRF2 and NF-κB Interplay in Cerebrovascular and Neurodegenerative Disorders: Molecular Mechanisms and Possible Therapeutic Approaches. Redox Biol. 2019, 21, 101059. [Google Scholar] [CrossRef] [PubMed]
- Vicente, O.; Boscaiu, M. Flavonoids: Antioxidant Compounds for Plant Defence... and for a Healthy Human Diet. Not. Bot. Horti Agrobot. Cluj-Napoca 2018, 46, 14–21. [Google Scholar] [CrossRef] [Green Version]
- Velásquez-Jiménez, D.; Corella-Salazar, D.A.; Zuñiga-Martínez, B.S.; Domínguez-Avila, J.A.; Montiel-Herrera, M.; Salazar-López, N.J.; Rodrigo-Garcia, J.; Villegas-Ochoa, M.A.; González-Aguilar, G.A. Phenolic Compounds That Cross the Blood–Brain Barrier Exert Positive Health Effects as Central Nervous System Antioxidants. Food Funct. 2021, 12, 10356–10369. [Google Scholar] [CrossRef]
- Mahomoodally, M.F.; Sadeer, N.; Edoo, M.; Venugopala, K.N. The Potential Application of Novel Drug Delivery Systems for Phytopharmaceuticals and Natural Extracts–Current Status and Future Perspectives. Mini Rev. Med. Chem. 2021, 21, 2731–2746. [Google Scholar] [CrossRef]
- Azhari, H. Surface Modified Cubosomes for Drug Delivery across the Blood-Brain Barrier. Ph.D. Thesis, University of Otago, Dunedin, New Zealand, 2018. [Google Scholar]
- Cayero-Otero, M.D.; Espinosa-Oliva, A.M.; Herrera, A.J.; Garcia-Dominguez, I.; Fernandez-Arevalo, M.; Martin-Banderas, L.; de Pablos, R.M. Potential Use of Nanomedicine for the Anti-Inflammatory Treatment of Neurodegenerative Diseases. Curr. Pharm. Des. 2018, 24, 1589–1616. [Google Scholar] [CrossRef]
- Rahman, M.; Akter, R.; Bhattacharya, T.; Abdel-Daim, M.M.; Alkahtani, S.; Arafah, M.W.; Al-Johani, N.S.; Alhoshani, N.M.; Alkeraishan, N.; Alhenaky, A.; et al. Resveratrol and Neuroprotection: Impact and Its Therapeutic Potential in Alzheimer’s Disease. Front. Pharmacol. 2020, 11, 2272. [Google Scholar] [CrossRef]
- Kiskova, T.; Kubatka, P.; Büsselberg, D.; Kassayova, M. The Plant-Derived Compound Resveratrol in Brain Cancer: A Review. Biomolecules 2020, 10, 161. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Liu, R.H. Potential Mechanisms of Action of Dietary Phytochemicals for Cancer Prevention by Targeting Cellular Signaling Transduction Pathways. J. Agric. Food Chem. 2018, 66, 3260–3276. [Google Scholar] [CrossRef]
- Ntolkeras, G.; Barba, C.; Mavropoulos, A.; Vasileiadis, G.K.; Dardiotis, E.; Sakkas, L.I.; Hadjigeorgiou, G.; Bogdanos, D.P. On the Immunoregulatory Role of Statins in Multiple Sclerosis: The Effects on Th17 Cells. Immunol. Res. 2019, 67, 310–324. [Google Scholar] [CrossRef]
- Corogeanu, D.; Diebold, S.S. Direct and Indirect Engagement of Dendritic Cell Function by Antibodies Developed for Cancer Therapy. Clin. Exp. Immunol. 2022, uxac026. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, S.; Hussaini, R.; White, R.; Atwi, D.; Fried, A.; Sampat, S.; Piao, L.; Pan, Q.; Banerjee, P. TriCurin, a Synergistic Formulation of Curcumin, Resveratrol, and Epicatechin Gallate, Repolarizes Tumor-Associated Macrophages and Triggers an Immune Response to Cause Suppression of HPV+ Tumors. Cancer Immunol. Immunother. 2018, 67, 761–774. [Google Scholar] [CrossRef] [PubMed]
- Ascough, S.; Paterson, S.; Chiu, C. Induction and Subversion of Human Protective Immunity: Contrasting Influenza and Respiratory Syncytial Virus. Front. Immunol. 2018, 9, 323. [Google Scholar] [CrossRef] [Green Version]
- Bai, Z.; Zhao, X.; Li, C.; Sheng, C.; Li, H. EV71 Virus Reduces Nrf2 Activation to Promote Production of Reactive Oxygen Species in Infected Cells. Gut Pathog. 2020, 12, 22. [Google Scholar] [CrossRef]
- Liu, Z.; Ni, H.; Yu, L.; Xu, S.; Bo, R.; Qiu, T.; Gu, P.; Zhu, T.; He, J.; Wusiman, A.; et al. Adjuvant Activities of CTAB-Modified Polygonatum Sibiricum Polysaccharide Cubosomes on Immune Responses to Ovalbumin in Mice. Int. J. Biol. Macromol. 2020, 148, 793–801. [Google Scholar] [CrossRef] [PubMed]
- Gozin, A.; Sellak, H.; Franzini, E.; Pasquier, C. Reactive Oxygen Species Increase Neutrophil Adherence to Endothelial Cells and Activate Tyrosine Phosphorylation of Cytoskeleton Proteins. In Antioxidant Food Supplements in Human Health; Elsevier: Amsterdam, The Netherlands, 1999; pp. 371–384. [Google Scholar]
- Kapadia, G.J.; Azuine, M.A.; Tokuda, H.; Takasaki, M.; Mukainaka, T.; Konoshima, T.; Nishino, H. Chemopreventive Effect of Resveratrol, Sesamol, Sesame Oil and Sunflower Oil in the Epstein–Barr Virus Early Antigen Activation Assay and the Mouse Skin Two-Stage Carcinogenesis. Pharmacol. Res. 2002, 45, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.N.; Khan, I.; Dey, D.K.; Cho, K.-H.; Hwang, B.S.; Bae, K.B.; Kang, S.C.; Park, J.G. Decursinol Angelate Ameliorates 12-O-Tetradecanoyl Phorbol-13-Acetate (TPA)-Induced NF-ΚB Activation on Mice Ears by Inhibiting Exaggerated Inflammatory Cell Infiltration, Oxidative Stress and pro-Inflammatory Cytokine Production. Food Chem. Toxicol. 2019, 132, 110699. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, F.; Li, S.; Liu, R.; Jin, T.; Dou, Y.; Zhou, Z.; Zhang, J. A Multifunctional Nanotherapy for Targeted Treatment of Colon Cancer by Simultaneously Regulating Tumor Microenvironment. Theranostics 2019, 9, 3732. [Google Scholar] [CrossRef]
- Honari, M.; Shafabakhsh, R.; Reiter, R.J.; Mirzaei, H.; Asemi, Z. Resveratrol Is a Promising Agent for Colorectal Cancer Prevention and Treatment: Focus on Molecular Mechanisms. Cancer Cell Int. 2019, 19, 180. [Google Scholar] [CrossRef] [Green Version]
- Salem, H.F.; Kharshoum, R.M.; Abou-Taleb, H.A.; Naguib, D.M. Brain Targeting of Resveratrol through Intranasal Lipid Vesicles Labelled with Gold Nanoparticles: In Vivo Evaluation and Bioaccumulation Investigation Using Computed Tomography and Histopathological Examination. J. Drug Target. 2019, 27, 1127–1134. [Google Scholar] [CrossRef]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-Inflammatory Action and Mechanisms of Resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Karthika, C.; Appu, A.P.; Akter, R.; Rahman, M.H.; Tagde, P.; Ashraf, G.M.; Abdel-Daim, M.M.; ul Hassan, S.S.; Abid, A.; Bungau, S. Potential Innovation against Alzheimer’s Disorder: A Tricomponent Combination of Natural Antioxidants (Vitamin E, Quercetin, and Basil Oil) and the Development of Its Intranasal Delivery. Environ. Sci. Pollut. Res. 2022, 29, 10950–10965. [Google Scholar] [CrossRef] [PubMed]
- Tagde, P.; Tagde, S.; Tagde, P.; Bhattacharya, T.; Monzur, S.M.; Rahman, M.; Otrisal, P.; Behl, T.; Abdel-Daim, M.M.; Aleya, L. Nutraceuticals and Herbs in Reducing the Risk and Improving the Treatment of COVID-19 by Targeting SARS-CoV-2. Biomedicines 2021, 9, 1266. [Google Scholar] [CrossRef]
- Means, J.C.; Lopez, A.A.; Koulen, P. Resveratrol Protects Optic Nerve Head Astrocytes from Oxidative Stress-Induced Cell Death by Preventing Caspase-3 Activation, Tau Dephosphorylation at Ser422 and Formation of Misfolded Protein Aggregates. Cell. Mol. Neurobiol. 2020, 40, 911–926. [Google Scholar] [CrossRef]
- Assadpour, E.; Mahdi Jafari, S. A Systematic Review on Nanoencapsulation of Food Bioactive Ingredients and Nutraceuticals by Various Nanocarriers. Crit. Rev. Food Sci. Nutr. 2019, 59, 3129–3151. [Google Scholar] [CrossRef]
- Serov, N.; Vinogradov, V. Artificial Intelligence to Bring Nanomedicine to Life. Adv. Drug Deliv. Rev. 2022, 184, 114194. [Google Scholar] [CrossRef] [PubMed]
- Küįüktürkmen, B.; Bozkır, A. A New Approach for Drug Targeting to the Central Nervous System: Lipid Nanoparticles. Nanoarchitecton. Biomed. 2019, 335–369. [Google Scholar] [CrossRef]
- Böhmdorfer, M.; Szakmary, A.; Schiestl, R.H.; Vaquero, J.; Riha, J.; Brenner, S.; Thalhammer, T.; Szekeres, T.; Jäger, W. Involvement of UDP-Glucuronosyltransferases and Sulfotransferases in the Excretion and Tissue Distribution of Resveratrol in Mice. Nutrients 2017, 9, 1347. [Google Scholar] [CrossRef] [Green Version]
- Cao, G.; Yang, S.; Cao, J.; Tan, Z.; Wu, L.; Dong, F.; Ding, W.; Zhang, F. The Role of Oxidative Stress in Intervertebral Disc Degeneration. Oxid. Med. Cell. Longev. 2022, 2022, 2166817. [Google Scholar] [CrossRef]
- Castelli, V.; Grassi, D.; Bocale, R.; d’Angelo, M.; Antonosante, A.; Cimini, A.; Ferri, C.; Desideri, G. Diet and Brain Health: Which Role for Polyphenols? Curr. Pharm. Des. 2018, 24, 227–238. [Google Scholar] [CrossRef]
- Lin, M.; Liu, N.; Qin, Z.; Wang, Y. Mitochondrial-Derived Damage-Associated Molecular Patterns Amplify Neuroinflammation in Neurodegenerative Diseases. Acta Pharmacol. Sin. 2022, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Jászai, J.; Schmidt, M.H.H. Trends and Challenges in Tumor Anti-Angiogenic Therapies. Cells 2019, 8, 1102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haibe, Y.; Kreidieh, M.; El Hajj, H.; Khalifeh, I.; Mukherji, D.; Temraz, S.; Shamseddine, A. Resistance Mechanisms to Anti-Angiogenic Therapies in Cancer. Front. Oncol. 2020, 10, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruinsmann, F.A.; Vaz, G.R.; Alves, A.D.C.S.; Aguirre, T.; Pohlmann, A.R.; Guterres, S.S.; Sonvico, F. Nasal Drug Delivery of Anticancer Drugs for the Treatment of Glioblastoma: Preclinical and Clinical Trials. Molecules 2019, 24, 4312. [Google Scholar] [CrossRef] [Green Version]
- Wu, B.; Xu, M.-M.; Fan, C.; Feng, C.-L.; Lu, Q.-K.; Lu, H.-M.; Xiang, C.-G.; Bai, F.; Wang, H.-Y.; Wu, Y.-W.; et al. STING Inhibitor Ameliorates LPS-Induced ALI by Preventing Vascular Endothelial Cells-Mediated Immune Cells Chemotaxis and Adhesion. Acta Pharmacol. Sin. 2021, 1–12. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R. Can Curcumin along with Chemotherapeutic Drug and Lipid Provide an Effective Treatment of Metastatic Colon Cancer and Alter Multidrug Resistance? Med. Hypotheses 2019, 132, 109325. [Google Scholar] [CrossRef]
- Reddy, S.; Tatiparti, K.; Sau, S.; Iyer, A.K. Recent Advances in Nano Delivery Systems for Blood-Brain Barrier (BBB) Penetration and Targeting of Brain Tumors. Drug Discov. Today 2021, 26, 1944–1952. [Google Scholar] [CrossRef]
- Smith, L.J.; Silverman, L.; Sakai, D.; Le Maitre, C.L.; Mauck, R.L.; Malhotra, N.R.; Lotz, J.C.; Buckley, C.T. Advancing Cell Therapies for Intervertebral Disc Regeneration from the Lab to the Clinic: Recommendations of the ORS Spine Section. JOR Spine 2018, 1, e1036. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Rahman, M.H.; Akter, R.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Akhtar, M.F.; Abdel-Daim, M.M. Multiple Strategies with the Synergistic Approach for Addressing Colorectal Cancer. Biomed. Pharmacother. 2021, 140, 111704. [Google Scholar] [CrossRef]
- Karthika, C.; Sureshkumar, R. Incorporation of Natural Assumption to Deal with Cancer. Environ. Sci. Pollut. Res. 2021, 28, 4902–4917. [Google Scholar] [CrossRef]
- Zhu, M.; Wang, S. Functional Nucleic-Acid-Decorated Spherical Nanoparticles: Preparation Strategies and Current Applications in Cancer Therapy. Small Sci. 2021, 1, 2000056. [Google Scholar] [CrossRef]
- Koshani, R.; Jafari, S.M. Ultrasound-Assisted Preparation of Different Nanocarriers Loaded with Food Bioactive Ingredients. Adv. Colloid Interface Sci. 2019, 270, 123–146. [Google Scholar] [CrossRef] [PubMed]
- Saraf, S.; Jain, A.; Tiwari, A.; Verma, A.; Panda, P.K.; Jain, S.K. Advances in Liposomal Drug Delivery to Cancer: An Overview. J. Drug Deliv. Sci. Technol. 2020, 56, 101549. [Google Scholar] [CrossRef]
- Botti, G.; Dalpiaz, A.; Pavan, B. Targeting Systems to the Brain Obtained by Merging Prodrugs, Nanoparticles, and Nasal Administration. Pharmaceutics 2021, 13, 1144. [Google Scholar] [CrossRef] [PubMed]
- Moura, R.P.; Martins, C.; Pinto, S.; Sousa, F.; Sarmento, B. Blood-Brain Barrier Receptors and Transporters: An Insight on Their Function and How to Exploit Them through Nanotechnology. Expert Opin. Drug Deliv. 2019, 16, 271–285. [Google Scholar] [CrossRef]
- Rock, C.L.; Thomson, C.; Gansler, T.; Gapstur, S.M.; McCullough, M.L.; Patel, A.V.; Andrews, K.S.; Bandera, E.V.; Spees, C.K.; Robien, K. American Cancer Society Guideline for Diet and Physical Activity for Cancer Prevention. CA Cancer J. Clin. 2020, 70, 245–271. [Google Scholar] [CrossRef]
- Du, H.-Y.; Olivo, M.; Mahendran, R.; Huang, Q.; Shen, H.-M.; Ong, C.-N.; Bay, B.-H. Hypericin Photoactivation Triggers Down-Regulation of Matrix Metalloproteinase-9 Expression in Well-Differentiated Human Nasopharyngeal Cancer Cells. Cell. Mol. life Sci. 2007, 64, 979–988. [Google Scholar] [CrossRef]
- Shahcheraghi, S.H.; Salemi, F.; Peirovi, N.; Ayatollahi, J.; Alam, W.; Khan, H.; Saso, L. Nrf2 Regulation by Curcumin: Molecular Aspects for Therapeutic Prospects. Molecules 2021, 27, 167. [Google Scholar] [CrossRef]
- Abbott, N.J.; Romero, I.A. Transporting Therapeutics across the Blood-Brain Barrier. Mol. Med. Today 1996, 2, 106–113. [Google Scholar] [CrossRef]
- Blakeley, J. Drug Delivery to Brain Tumors. Curr. Neurol. Neurosci. Rep. 2008, 8, 235–241. [Google Scholar] [CrossRef] [Green Version]
- D’Amico, R.S.; Khatri, D.; Reichman, N.; Patel, N.V.; Wong, T.; Fralin, S.R.; Li, M.; Ellis, J.A.; Ortiz, R.; Langer, D.J. Super Selective Intra-Arterial Cerebral Infusion of Modern Chemotherapeutics after Blood–Brain Barrier Disruption: Where Are We Now, and Where We Are Going. J. Neurooncol. 2020, 147, 261–278. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wu, Q.; Mo, L.; Nassi, M. Intra-Arterial Chemotherapy Is Not Superior to Intravenous Chemotherapy for Malignant Gliomas: A Systematic Review and Meta-Analysis. Eur. Neurol. 2013, 70, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Ellis, J.A.; Ornstein, E.; Bruce, J.N. Intraarterial Drug Delivery for Glioblastoma Mutiforme. J. Neurooncol. 2015, 124, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Donovan, M.D. Effect of Route of Administration and Distribution on Drug Action. In Modern Pharmaceutics Volume 1 Basic Principles and Systems; CRC Press: Boca Raton, FL, USA, 2009; p. 155. [Google Scholar]
- Pająk, B. Looking for the Holy Grail—Drug Candidates for Glioblastoma Multiforme Chemotherapy. Biomedicines 2022, 10, 1001. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Yamashita, D.; Inoue, A.; Suehiro, S.; Ohue, S.; Kunieda, T. Is Interstitial Chemotherapy with Carmustine (BCNU) Wafers Effective against Local Recurrence of Glioblastoma? A Pharmacokinetic Study by Measurement of BCNU in the Tumor Resection Cavity. Brain Sci. 2022, 12, 567. [Google Scholar] [CrossRef] [PubMed]
- Parodi, A.; Rudzińska, M.; Deviatkin, A.A.; Soond, S.M.; Baldin, A.V.; Zamyatnin, A.A. Established and Emerging Strategies for Drug Delivery across the Blood-Brain Barrier in Brain Cancer. Pharmaceutics 2019, 11, 245. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.-A.; Harn, H.-J.; Chen, K.-P.; Lee, J.-H.; Lin, S.-Z.; Chiu, T.-L. Targeting the Axl and MTOR Pathway Synergizes Immunotherapy and Chemotherapy to Butylidenephthalide in a Recurrent GBM. J. Oncol. 2022, 2022, 3236058. [Google Scholar] [CrossRef]
- Stawicki, B.; Schacher, T.; Cho, H. Nanogels as a Versatile Drug Delivery System for Brain Cancer. Gels 2021, 7, 63. [Google Scholar] [CrossRef]
- Rahman, H.S.; Tan, B.L.; Othman, H.H.; Chartrand, M.S.; Pathak, Y.; Mohan, S.; Abdullah, R.; Alitheen, N.B. An Overview of In Vitro, In Vivo, and Computational Techniques for Cancer-Associated Angiogenesis Studies. Biomed Res. Int. 2020, 2020, 8857428. [Google Scholar] [CrossRef]
- Gatto, L.; Di Nunno, V.; Franceschi, E.; Tosoni, A.; Bartolini, S.; Brandes, A.A. Pharmacotherapeutic Treatment of Glioblastoma: Where Are We to Date? Drugs 2022, 82, 491–510. [Google Scholar] [CrossRef]
- Jaramillo-Botero, A.; Abrol, R.; van Duin, A.; Goddard III, W.A. Multiscale-Multiparadigm Modeling and Simulation of Nanometer Scale Systems and Processes for Nanomedical Applications. In Nanomedicine; Jenny Stanford Publishing: Dubai, United Arab Emirates, 2019; pp. 245–300. ISBN 0429065760. [Google Scholar]
- Warren, K.E. Beyond the Blood: Brain Barrier: The Importance of Central Nervous System (CNS) Pharmacokinetics for the Treatment of CNS Tumors, Including Diffuse Intrinsic Pontine Glioma. Front. Oncol. 2018, 8, 239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Novak, D.; Hüser, L.; Elton, J.J.; Umansky, V.; Altevogt, P.; Utikal, J. SOX2 in Development and Cancer Biology. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2020; Volume 67, pp. 74–82. [Google Scholar]
- Gosselet, F.; Loiola, R.A.; Roig, A.; Rosell, A.; Culot, M. Central Nervous System Delivery of Molecules across the Blood-Brain Barrier. Neurochem. Int. 2021, 144, 104952. [Google Scholar] [CrossRef] [PubMed]
- Nzou, G.; Seeds, M.C.; Wicks, R.T.; Atala, A.J. Fundamental Neurovascular Components for the Development of Complex and Dynamic in Vitro Brain Equivalent Models. J. Alzheimers Neurodegener. Dis. 2019, 5, 21. [Google Scholar] [CrossRef]
- Schiopu, S.R.I.; Habl, G.; Haefner, M.; Katayama, S.; Herfarth, K.; Debus, J.; Sterzing, F. Helical Tomotherapy in Patients with Leptomeningeal Metastases. Cancer Manag. Res. 2019, 11, 401. [Google Scholar] [CrossRef] [Green Version]
- Feldman, L.; Chen, M. Delivery of Antineoplastic Therapeutics to the Central Nervous System. In Cancer Regional Therapy; Springer: Cham, Switzerland, 2019; pp. 427–438. [Google Scholar]
- Gheorghiu, M.L.; Negreanu, F.; Fleseriu, M. Updates in the Medical Treatment of Pituitary Adenomas. Horm. Metab. Res. 2020, 52, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Hecker, A.; Schellnegger, M.; Hofmann, E.; Luze, H.; Nischwitz, S.P.; Kamolz, L.; Kotzbeck, P. The Impact of Resveratrol on Skin Wound Healing, Scarring, and Aging. Int. Wound J. 2022, 19, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Nath, R.; Roy, R.; Barai, G.; Bairagi, S.; Manna, S.; Chakraborty, R. Modern Developments of Nano Based Drug Delivery System by Combined with Phytochemicals-Presenting New Aspects. Int. J. Sci. Res. Sci. Technol. 2021, 8, 107–129. [Google Scholar]
- Bonferoni, M.C.; Rossi, S.; Sandri, G.; Ferrari, F.; Gavini, E.; Rassu, G.; Giunchedi, P. Nanoemulsions for “Nose-to-Brain” Drug Delivery. Pharmaceutics 2019, 11, 84. [Google Scholar] [CrossRef] [Green Version]
- Hirlekar, R.S.; Momin, A.M. Advances in Drug Delivery from Nose to Brain: An Overview. Curr. Drug ther. 2018, 13, 4–24. [Google Scholar] [CrossRef]
- Fan, Y.; Chen, M.; Zhang, J.; Maincent, P.; Xia, X.; Wu, W. Updated Progress of Nanocarrier-Based Intranasal Drug Delivery Systems for Treatment of Brain Diseases. Crit. Rev. Ther. Drug Carr. Syst. 2018, 35, 433–467. [Google Scholar] [CrossRef]
- Ahirrao, M.; Shrotriya, S. In Vitro and in Vivo Evaluation of Cubosomal in Situ Nasal Gel Containing Resveratrol for Brain Targeting. Drug Dev. Ind. Pharm. 2017, 43, 1686–1693. [Google Scholar] [CrossRef] [PubMed]
- Trotta, V.; Pavan, B.; Ferraro, L.; Beggiato, S.; Traini, D.; Des Reis, L.G.; Scalia, S.; Dalpiaz, A. Brain Targeting of Resveratrol by Nasal Administration of Chitosan-Coated Lipid Microparticles. Eur. J. Pharm. Biopharm. 2018, 127, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Akter, R.; Rahman, H.; Behl, T.; Chowdhury, M.A.R.; Manirujjaman, M.; Bulbul, I.J.; Elshenaw, S.E.; Tit, D.M.; Bungau, S. Prospective Role of Polyphenolic Compounds in the Treatment of Neurodegenerative Diseases. CNS Neurol. Disord. Targets Formerly Curr. Drug Targets-CNS Neurol. Disord. 2021, 20, 430–450. [Google Scholar]
- Correia, S.C.; Cardoso, S.; Santos, R.X.; Carvalho, C.; Santos, M.S.; Perry, G.; Smith, M.A.; Moreira, P.I. New Insights into the Mechanisms of Mitochondrial Preconditioning-Triggered Neuroprotection. Curr. Pharm. Des. 2011, 17, 3381–3389. [Google Scholar] [CrossRef] [PubMed]
- Zubova, S.G.; Suvorova, I.I.; Karpenko, M.N. Macrophage and Microglia Polarization: Focus on Autophagy-Dependent Reprogramming. Front. Biosci. 2022, 14, 3. [Google Scholar] [CrossRef] [PubMed]
- Forni, C.; Facchiano, F.; Bartoli, M.; Pieretti, S.; Facchiano, A.; D’Arcangelo, D.; Norelli, S.; Valle, G.; Nisini, R.; Beninati, S. Beneficial Role of Phytochemicals on Oxidative Stress and Age-Related Diseases. Biomed Res. Int. 2019, 2019, 8748253. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhen, L.; Li, Z.; Xu, W.; Leng, H.; Xu, W.; Zheng, V.; Luria, V.; Pan, J.; Tao, Y.; et al. Trans-Resveratrol Ameliorates Anxiety-like Behaviors and Neuropathic Pain in Mouse Model of Post-Traumatic Stress Disorder. J. Psychopharmacol. 2020, 34, 726–736. [Google Scholar] [CrossRef]
- Akter, R.; Rahman, M.; Kaushik, D.; Mittal, V.; Uivarosan, D.; Nechifor, A.C.; Behl, T.; Karthika, C.; Stoicescu, M.; Munteanu, M.A.; et al. Chemo-Preventive Action of Resveratrol: Suppression of P53—A Molecular Targeting Approach. Molecules 2021, 26, 5325. [Google Scholar] [CrossRef]
- Wu, H.; Chen, L.; Zhu, F.; Han, X.; Sun, L.; Chen, K. The Cytotoxicity Effect of Resveratrol: Cell Cycle Arrest and Induced Apoptosis of Breast Cancer 4T1 Cells. Toxins 2019, 11, 731. [Google Scholar] [CrossRef] [Green Version]
- Lin, J.-C.; Tsai, J.-T.; Chao, T.-Y.; Ma, H.-I.; Liu, W.-H. The STAT3/Slug Axis Enhances Radiation-Induced Tumor Invasion and Cancer Stem-like Properties in Radioresistant Glioblastoma. Cancers 2018, 10, 512. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, H.-M.; Guz-Montgomery, K.; Lowe, D.B.; Saha, D. Pathogenetic Features and Current Management of Glioblastoma. Cancers 2021, 13, 856. [Google Scholar] [CrossRef] [PubMed]
- Shoaib, A.; Tabish, M.; Ali, S.; Arafah, A.; Wahab, S.; Almarshad, F.M.; Rashid, S.; Rehman, M.U. Dietary Phytochemicals in Cancer Signalling Pathways: Role of MiRNA Targeting. Curr. Med. Chem. 2021, 28, 8036–8067. [Google Scholar] [CrossRef] [PubMed]
- Tseng, S.-H.; Lin, S.-M.; Chen, J.-C.; Su, Y.-H.; Huang, H.-Y.; Chen, C.-K.; Lin, P.-Y.; Chen, Y. Resveratrol Suppresses the Angiogenesis and Tumor Growth of Gliomas in Rats. Clin. Cancer Res. 2004, 10, 2190–2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, M.; Zhang, J.; Li, Z.; Bai, X.; Ma, J.; Li, Y. Liraglutide Protects Nucleus Pulposus Cells against High-Glucose Induced Apoptosis by Activating PI3K/Akt/MTOR/Caspase-3 and PI3K/Akt/GSK3β/Caspase-3 Signaling Pathways. Front. Med. 2021, 8, 630962. [Google Scholar] [CrossRef]
- Saeed, M.E.M.; Rahama, M.; Kuete, V.; Dawood, M.; Elbadawi, M.; Sugimoto, Y.; Efferth, T. Collateral Sensitivity of Drug-Resistant ABCB5-and Mutation-Activated EGFR Overexpressing Cells towards Resveratrol Due to Modulation of SIRT1 Expression. Phytomedicine 2019, 59, 152890. [Google Scholar] [CrossRef]
- Hussain, Y.; Luqman, S.; Meena, A. Research Progress in Flavonoids as Potential Anticancer Drug Including Synergy with Other Approaches. Curr. Top. Med. Chem. 2020, 20, 1791–1809. [Google Scholar] [CrossRef]
- Laaniste, L.; Srivastava, P.K.; Stylianou, J.; Syed, N.; Cases-Cunillera, S.; Shkura, K.; Zeng, Q.; Rackham, O.J.L.; Langley, S.R.; Delahaye-Duriez, A. Integrated Systems-genetic Analyses Reveal a Network Target for Delaying Glioma Progression. Ann. Clin. Transl. Neurol. 2019, 6, 1616–1638.z. [Google Scholar] [CrossRef] [Green Version]
- Zobeiri, M.; Parvizi, F.; Kalhori, M.R.; Majnooni, M.B.; Farzaei, M.H.; Abdollahi, M. Targeting MiRNA by Natural Products: A Novel Therapeutic Approach for Nonalcoholic Fatty Liver. Evidence-Based Complement. Altern. Med. 2021, 2021, 6641031. [Google Scholar] [CrossRef]
- Yazici, A.; Marinelli, L.; Cacciatore, I.; Emsen, B.; Eusepi, P.; Di Biase, G.; Di Stefano, A.; Mardinoğlu, A.; Türkez, H. Potential Anticancer Effect of Carvacrol Codrugs on Human Glioblastoma Cells. Curr. Drug Deliv. 2021, 18, 350–356. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic Insights and Perspectives Involved in Neuroprotective Action of Quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar]
- Van Gastel, J. The Investigation of the GIT2-RXFP3 Synergistic System and Its Potential Role in Aging and Age-Related Disorders. Ph.D. Thesis, University of Antwerp, Antwerp, Belgium, 2020. [Google Scholar]
- Tilekar, K.; Upadhyay, N.; Iancu, C.V.; Pokrovsky, V.; Choe, J.; Ramaa, C.S. Power of Two: Combination of Therapeutic Approaches Involving Glucose Transporter (GLUT) Inhibitors to Combat Cancer. Biochim. Biophys. Acta (BBA)-Reviews Cancer 2020, 1874, 188457. [Google Scholar] [CrossRef] [PubMed]
- Sartaj, A.; Baboota, S.; Ali, J. Assessment of Combination Approaches of Phytoconstituents with Chemotherapy for the Treatment of Breast Cancer: A Systematic Review. Curr. Pharm. Des. 2021, 27, 4630–4648. [Google Scholar] [CrossRef] [PubMed]
- Villota, H.; Röthlisberger, S.; Pedroza-Díaz, J. Modulation of the Canonical Wnt Signaling Pathway by Dietary Polyphenols, an Opportunity for Colorectal Cancer Chemoprevention and Treatment. Nutr. Cancer 2022, 74, 384–404. [Google Scholar] [CrossRef]
- Sagwal, S.K.; Bekeschus, S. ROS Pleiotropy in Melanoma and Local Therapy with Physical Modalities. Oxid. Med. Cell. Longev. 2021, 2021, 6816214. [Google Scholar] [CrossRef] [PubMed]
- D’Uva, G.; Baci, D.; Albini, A.; Noonan, D.M. Cancer Chemoprevention Revisited: Cytochrome P450 Family 1B1 as a Target in the Tumor and the Microenvironment. Cancer Treat. Rev. 2018, 63, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Manzanedo-Delgado, A.; Mason, S.; Pumarola, M.; Stabile, F. Diagnostic Imaging, Treatment and Outcome in a 14-month-old Dobermann with Brainstem Gemistocytic Astrocytoma. Vet. Rec. Case Reports 2021, 9, e202. [Google Scholar] [CrossRef]
- Rajagopal, R.; Raman, N.; Ong, L.C.; Foo, J.C.; Fong, C.Y. Health-Related Quality of Life among Malaysian Pediatric Survivors of Central Nervous System Tumor. Pediatr. Hematol. Oncol. 2022, 1–14. [Google Scholar] [CrossRef]
- Persano, F.; Batasheva, S.; Fakhrullina, G.; Gigli, G.; Leporatti, S.; Fakhrullin, R. Recent Advances in the Design of Inorganic and Nano-Clay Particles for the Treatment of Brain Disorders. J. Mater. Chem. B 2021, 9, 2756–2784. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Odeh, F.; Al-Adaileh, F.; Al-Taher, S.; Jaber, A.M.; Alshaer, W.; Al Bawab, A.; Mubarak, M.S. Lipid Nanostructures for Targeting Brain Cancer. Heliyon 2021, 7, e07994. [Google Scholar] [CrossRef]
- Campora, S.; Ghersi, G. Smart Nanoparticles in Biomedicine: An Overview of Recent Developments and Applications. Preprints 2021, 2021020619. [Google Scholar] [CrossRef]
- Zein, R.; Sharrouf, W.; Selting, K. Physical Properties of Nanoparticles That Result in Improved Cancer Targeting. J. Oncol. 2020, 2020, 5194780. [Google Scholar] [CrossRef] [PubMed]
- Iturrioz-Rodríguez, N.; Bertorelli, R.; Ciofani, G. Lipid-Based Nanocarriers for the Treatment of Glioblastoma. Adv. nanobiomed Res. 2021, 1, 2000054. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, W.; Ourselin, S.; Vercauteren, T. Automatic Brain Tumor Segmentation Based on Cascaded Convolutional Neural Networks with Uncertainty Estimation. Front. Comput. Neurosci. 2019, 13, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, L.A.; Satapathy, B.S.; Pattnaik, G.; Barik, B.; Patro, C.S.; Das, S. Malignant Brain Tumor: Current Progresses in Diagnosis, Treatment and Future Strategies. Ann. Rom. Soc. Cell Biol. 2021, 25, 16922–16932. [Google Scholar]
- Das, R.P.; Gandhi, V.V.; Singh, B.G.; Kunwar, A. Passive and Active Drug Targeting: Role of Nanocarriers in Rational Design of Anticancer Formulations. Curr. Pharm. Des. 2019, 25, 3034–3056. [Google Scholar] [CrossRef]
- Beringhs, A.O.; Sadabad, R.K.; Lu, X. 11.1 Current State of Tumor Imaging in the Clinic. In Biomaterials for Cancer Therapeutics; Elsevier: Amsterdam, The Netherlands, 2020; p. 291. [Google Scholar]
- Garg, J.; Pathania, K.; Sah, S.P.; Pawar, S. V Nanostructured Lipid Carriers: A Promising Drug Carrier for Targeting Brain Tumours. Futur. J. Pharm. Sci. 2022, 8, 25. [Google Scholar] [CrossRef]
- Akanda, M.; Getti, G.; Nandi, U.; Mithu, M.S.; Douroumis, D. Bioconjugated Solid Lipid Nanoparticles (SLNs) for Targeted Prostate Cancer Therapy. Int. J. Pharm. 2021, 599, 120416. [Google Scholar] [CrossRef]
- Costa, C.P.; Moreira, J.N.; Lobo, J.M.S.; Silva, A.C. Intranasal Delivery of Nanostructured Lipid Carriers, Solid Lipid Nanoparticles and Nanoemulsions: A Current Overview of in Vivo Studies. Acta Pharm. Sin. B 2021, 11, 925–940. [Google Scholar] [CrossRef]
- Falzarano, M.S.; Flesia, C.; Cavalli, R.; Guiot, C.; Ferlini, A. Nanodiagnostics and Nanodelivery Applications in Genetic Alterations. Curr. Pharm. Des. 2018, 24, 1717–1726. [Google Scholar] [CrossRef]
- Xu, L.; Wang, X.; Liu, Y.; Yang, G.; Falconer, R.J.; Zhao, C.-X. Lipid Nanoparticles for Drug Delivery. Adv. NanoBiomed Res. 2022, 2, 2100109. [Google Scholar]
- Shirazi, A.S.; Varshochian, R.; Rezaei, M.; Ardakani, Y.H.; Dinarvand, R. SN38 Loaded Nanostructured Lipid Carriers (NLCs); Preparation and in Vitro Evaluations against Glioblastoma. J. Mater. Sci. Mater. Med. 2021, 32, 78. [Google Scholar] [CrossRef] [PubMed]
- Sarecka-Hujar, B.; Banyś, A.; Ostróżka-Cieślik, A.; Balwierz, R.; Dolińska, B. Evaluation of the Potential of Nanoparticles Containing Active Substances in Selected Chronic Diseases. Adv. Clin. Exp. Med 2020, 29, 385–397. [Google Scholar] [CrossRef] [PubMed]
- Umeyor, C.E.; Uronnachi, E.M. Important Pharmaceutical Applications of Man-Made Lipid Nanocarriers for Sustained Drug Delivery and Future Outlook. In Importance & Applications of Nanotechnology; MedDocs Publishers LLC: Reno, NV, USA, 2020. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karthika, C.; Najda, A.; Klepacka, J.; Zehravi, M.; Akter, R.; Akhtar, M.F.; Saleem, A.; Al-Shaeri, M.; Mondal, B.; Ashraf, G.M.; et al. Involvement of Resveratrol against Brain Cancer: A Combination Strategy with a Pharmaceutical Approach. Molecules 2022, 27, 4663. https://doi.org/10.3390/molecules27144663
Karthika C, Najda A, Klepacka J, Zehravi M, Akter R, Akhtar MF, Saleem A, Al-Shaeri M, Mondal B, Ashraf GM, et al. Involvement of Resveratrol against Brain Cancer: A Combination Strategy with a Pharmaceutical Approach. Molecules. 2022; 27(14):4663. https://doi.org/10.3390/molecules27144663
Chicago/Turabian StyleKarthika, Chenmala, Agnieszka Najda, Joanna Klepacka, Mehrukh Zehravi, Rokeya Akter, Muhammad Furqan Akhtar, Ammara Saleem, Majed Al-Shaeri, Banani Mondal, Ghulam Md. Ashraf, and et al. 2022. "Involvement of Resveratrol against Brain Cancer: A Combination Strategy with a Pharmaceutical Approach" Molecules 27, no. 14: 4663. https://doi.org/10.3390/molecules27144663
APA StyleKarthika, C., Najda, A., Klepacka, J., Zehravi, M., Akter, R., Akhtar, M. F., Saleem, A., Al-Shaeri, M., Mondal, B., Ashraf, G. M., Tagde, P., Ramproshad, S., Ahmad, Z., Khan, F. S., & Rahman, M. H. (2022). Involvement of Resveratrol against Brain Cancer: A Combination Strategy with a Pharmaceutical Approach. Molecules, 27(14), 4663. https://doi.org/10.3390/molecules27144663









