Effect of Ester Moiety on Structural Properties of Binary Mixed Monolayers of Alpha-Tocopherol Derivatives with DPPC
Abstract
:1. Introduction
2. Results
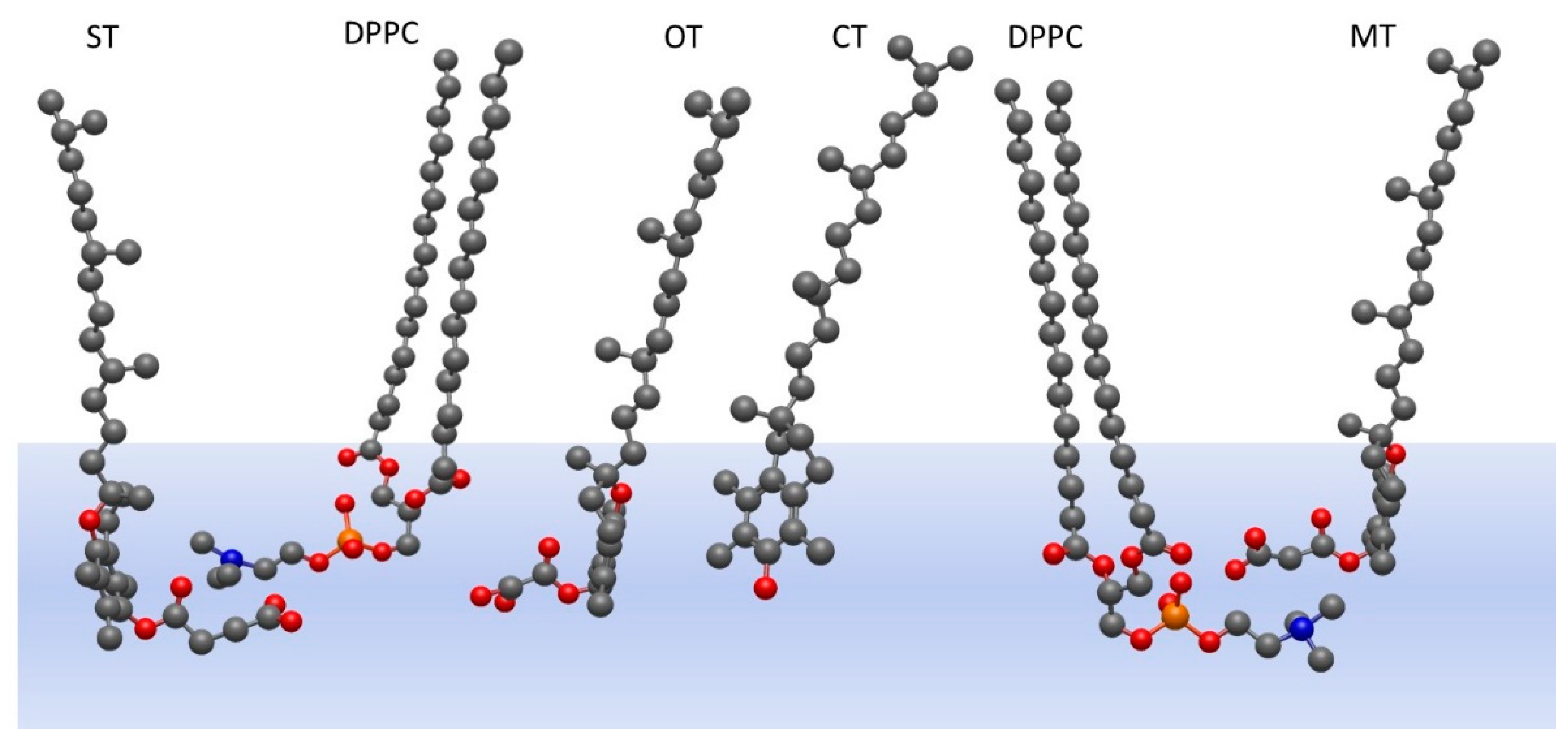
2.1. Monolayers of Pure Tocopherol and Its Derivatives
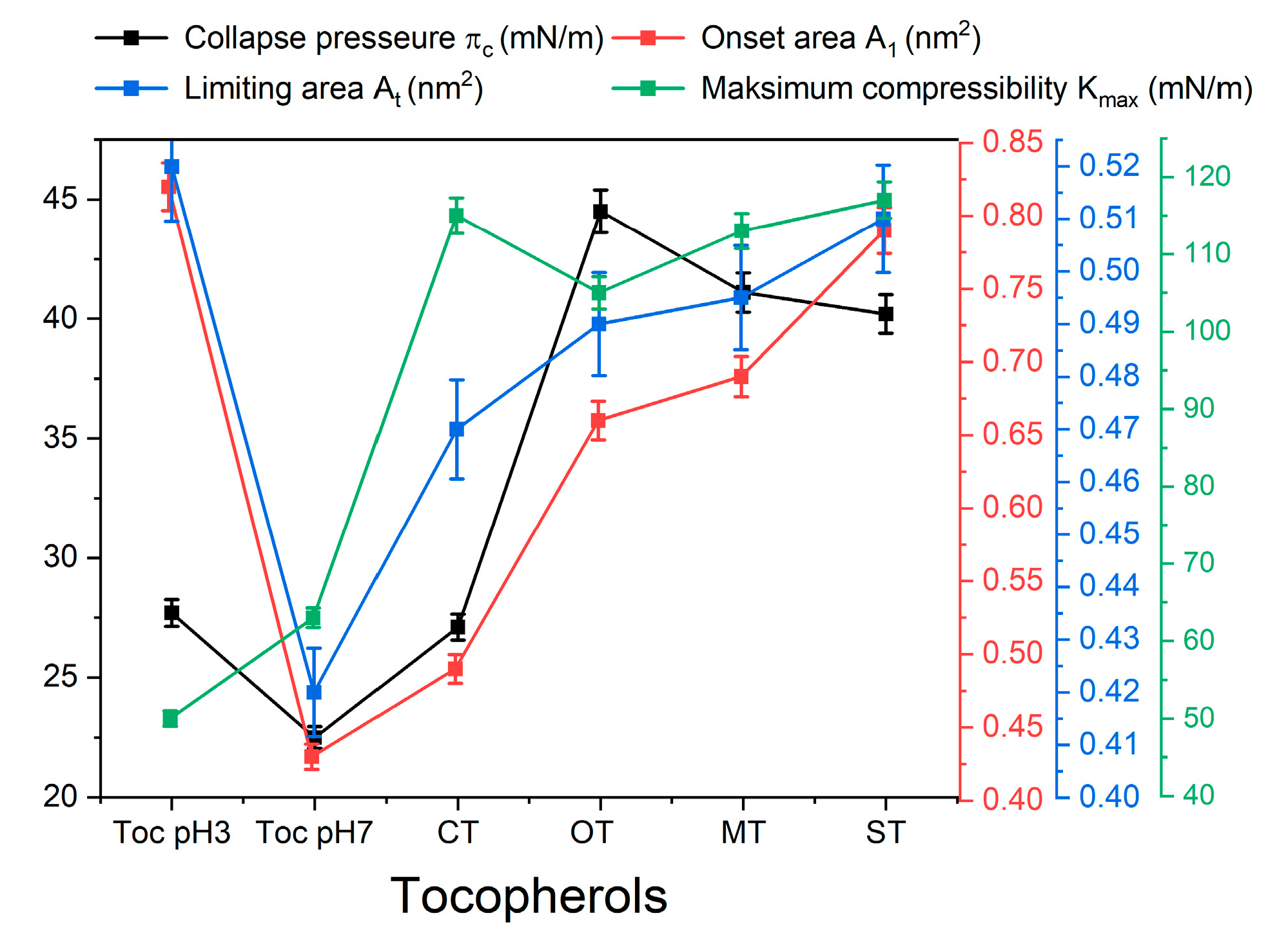
2.1.1. π-A Isotherms
2.1.2. Compressibility Analysis
2.2. Mixed Monolayers of DPPC with Tocopherol and Its Derivatives
2.2.1. π-A Isotherms of Mixed Monolayers
2.2.2. Compressibility Analysis
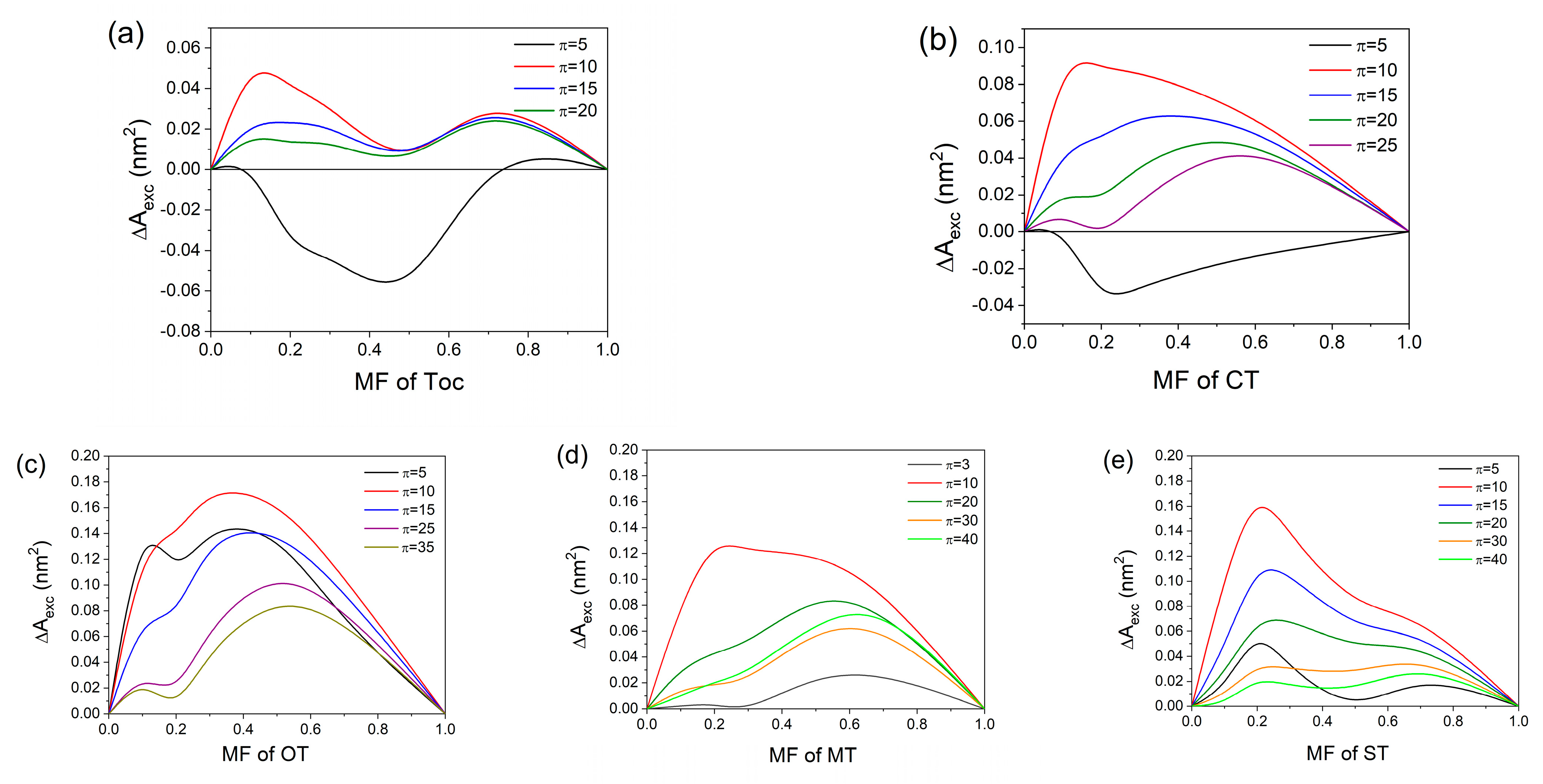
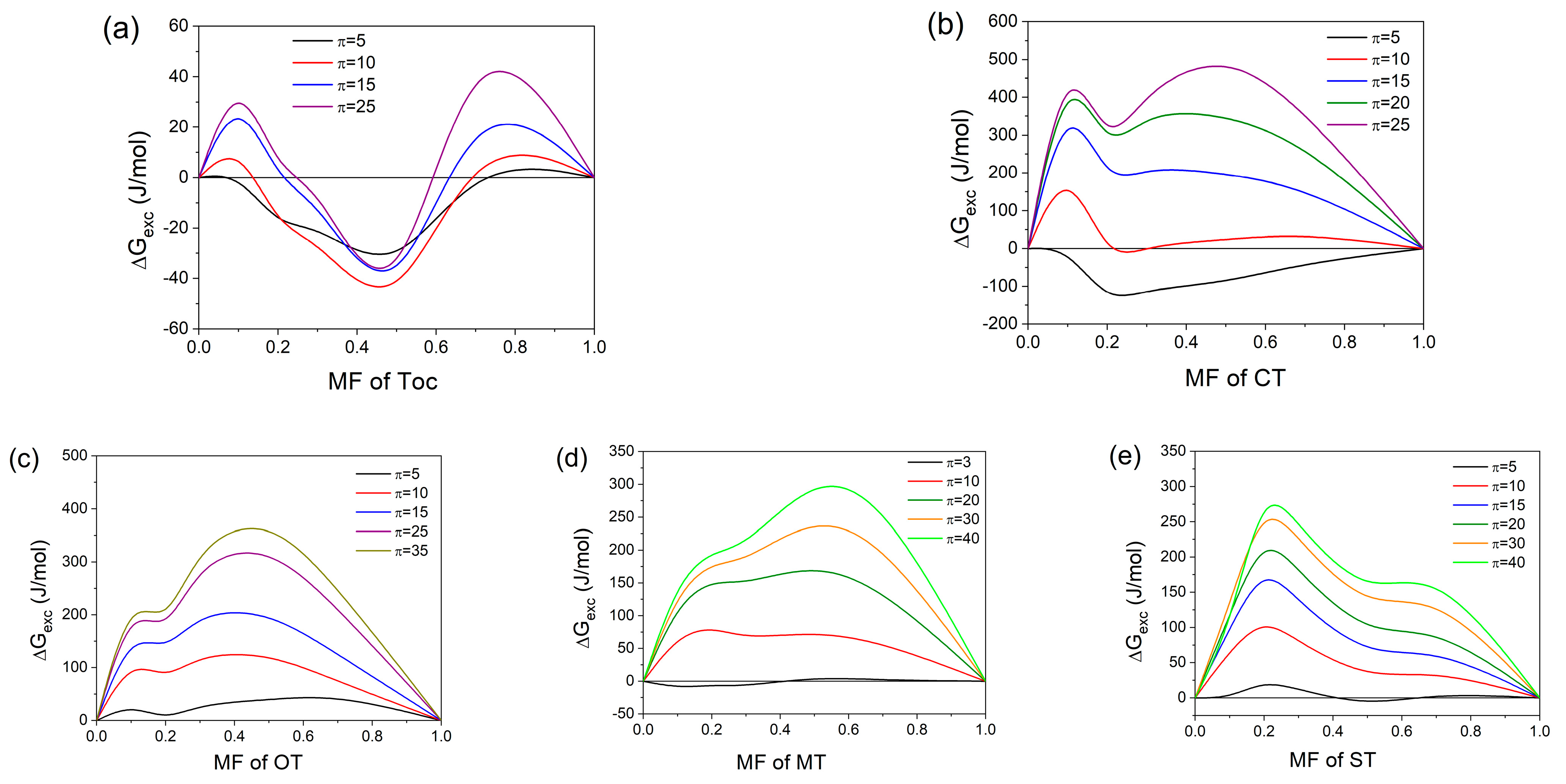
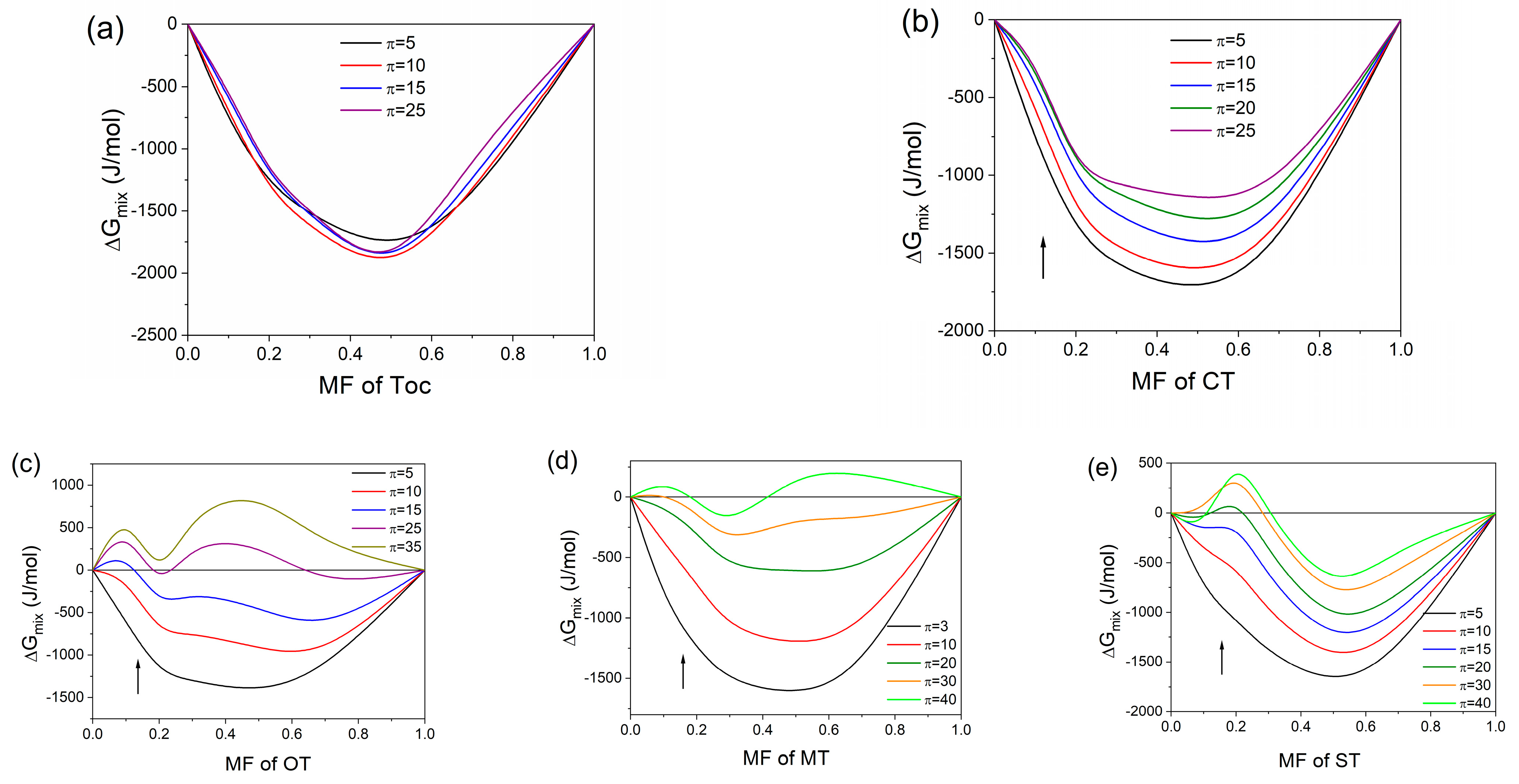
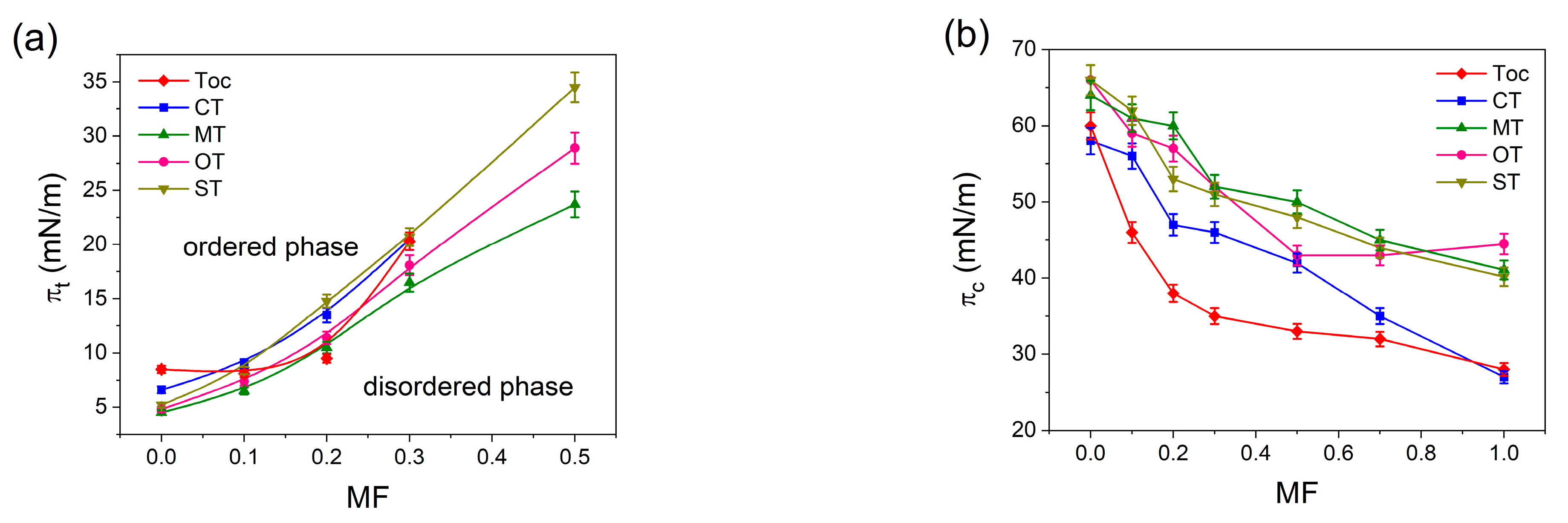
2.2.3. Miscibility Analysis and Thermodynamics of Mixed Monolayers
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Preparation of Langmuir Monolayers
4.3. Data Analysis and Fitting Procedures
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Massey, J.B.; She, H.S.; Pownall, H.J. Interaction of vitamin E with saturated phospholipid bilayers. Biochem. Biophys. Res. Commun. 1982, 106, 842–847. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. The location and function of vitamin E in membranes (Review). Mol. Membr. Biol. 2000, 17, 143–156. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Characterisation of clusters of alpha-tocopherol in gel and fluid phases of dipalmitoylglycerophosphocholine. Eur. J. Biochem. 1995, 233, 916–925. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. The effect of tocopherol on the structure and permeability of phosphatidylcholine liposomes. J. Control Release 2012, 160, 158–163. [Google Scholar] [CrossRef]
- Atkinson, J.; Harroun, T.; Wassall, S.R.; Stillwell, W.; Katsaras, J. The location and behavior of α-Tocopherol in membranes. Mol. Nutr. Food Res. 2010, 54, 641–651. [Google Scholar] [CrossRef] [Green Version]
- Jurak, M.; Conde, J.M. Characterization of the binary mixed monolayers of α-tocopherol with phospholipids at the air-water interface. Biochim. Biophys. Acta Biomembr. 2013, 1828, 2410–2418. [Google Scholar] [CrossRef] [Green Version]
- Feng-Kui, M.; Jing, W.; Uematsu, S.; Akahori, Y. Study on Monolayer of Vitamin E and Phosphatidylcholines. Acta Physico-Chim. Sin. 1995, 11, 1077–1083. [Google Scholar] [CrossRef]
- Patil, G.S.; Cornwell, D.G. Interfacial oxidation of alpha-tocopherol and the surface properties of its oxidation products. J. Lipid Res. 1978, 19, 416–422. [Google Scholar] [CrossRef]
- Maggio, B.; Diplock, A.T.; Lucy, J.A. Interactions of tocopherols and ubiquinones with monolayers of phospholipids. Biochem. J. 1977, 161, 111–121. [Google Scholar] [CrossRef] [Green Version]
- Neunert, G.; Tomaszewska-Gras, J.; Siejak, P.; Pietralik, Z.; Kozak, M.; Polewski, K. Disruptive effect of tocopherol oxalate on DPPC liposome structure: DSC, SAXS, and fluorescence anisotropy studies. Chem. Phys. Lipids 2018, 216, 104–113. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Witkowski, S.; Polewski, K. Tocopheryl succinate-induced structural changes in DPPC liposomes: DSC and ANS fluorescence studies. Molecules 2020, 25, 2780. [Google Scholar] [CrossRef] [PubMed]
- Neunert, G.; Makowiecki, J.; Piosik, E.; Hertmanowski, R.; Polewski, K.; Martynski, T. Miscibility of dl-α-tocopherol β-glucoside in DPPC monolayer at air/water and air/solid interfaces. Mater. Sci. Eng. C 2016, 67, 362–368. [Google Scholar] [CrossRef] [PubMed]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Phase transitions and structural changes in DPPC liposomes induced by a 1-carba-alpha-tocopherol analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef]
- Toimil, P.; Prieto, G.; Miñones, J.; Sarmiento, F. A comparative study of F-DPPC/DPPC mixed monolayers. Influence of subphase temperature on F-DPPC and DPPC monolayers. Phys. Chem. Chem. Phys. 2010, 12, 13323–13332. [Google Scholar] [CrossRef] [PubMed]
- Nakahara, H.; Nakamura, S.; Kawasaki, H.; Shibata, O. Properties of two-component Langmuir monolayer of single chain perfluorinated carboxylic acids with dipalmitoylphosphatidylcholine (DPPC). Colloids Surfaces B Biointerfaces 2005, 41, 285–298. [Google Scholar] [CrossRef]
- Miyoshi, T.; Kato, S. Detailed Analysis of the Surface Area and Elasticity in the Saturated 1,2-Diacylphosphatidylcholine/Cholesterol Binary Monolayer System. Langmuir 2015, 31, 9086–9096. [Google Scholar] [CrossRef]
- Dynarowicz-Łątka, P.; Kita, K. Molecular interaction in mixed monolayers at the air/water interface. Adv. Colloid Interface Sci. 1999, 79, 1–17. [Google Scholar] [CrossRef]
- Eeman, M.; Deleu, M. From biological membranes to biomimetic model membranes. Biotechnol. Agron. Soc. Environ. 2010, 14, 691–708. [Google Scholar]
- Ruckenstein, E. On the Nature of The Liquid Expanded/Liquid Condensed Phase Transition in Monolayers of Polar Molecules. J. Colloid Interface Sci. 1997, 196, 313–315. [Google Scholar] [CrossRef]
- Davies, J.T.; Rideal, E.K. The kinetic salt effect in monolayer reactions. Proc. R. Soc. A 1948, 194, 417–428. [Google Scholar]
- Lee, Y.L.; Lin, J.Y.; Chang, C.H. Thermodynamic characteristics and Langmuir-Blodgett deposition behavior of mixed DPPA/DPPC monolayers at air/liquid interfaces. J. Colloid Interface Sci. 2006, 296, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.M.; Xiao, D.; Xiao, S.J.; Wei, Y. Domain structures of phospholipid monolayer Langmuir-Blodgett films determined by atomic force microscopy. Appl. Phys. A 1994, 59, 139–143. [Google Scholar] [CrossRef]
- Yang, Q.; Xing, H.; Su, B.; Bao, Z.; Wang, J.; Yang, Y.; Ren, Q. The essential role of hydrogen-bonding interaction in the extractive separation of phenolic compounds by ionic liquid. AIChE J. 2013, 59, 1657–1667. [Google Scholar] [CrossRef]
- Baoukina, S.; Monticelli, L.; Risselada, H.J.; Marrink, S.J.; Tieleman, D.P. The molecular mechanism of lipid monolayer collapse. Proc. Natl. Acad. Sci. USA 2008, 105, 10803–10808. [Google Scholar] [CrossRef] [Green Version]
- Kavousi, S.; Novak, B.R.; Tong, X.; Moldovan, D. Molecular dynamics simulation study of the positioning and dynamics of α-tocopherol in phospholipid bilayers. Eur. Biophys. J. 2021, 50, 889–903. [Google Scholar] [CrossRef]
- Hauser, H.; Pascher, I.; Pearson, R.H.; Sundell, S. Preferred conformation and molecular packing of phosphatidylethanolamine and phosphatidylcholine. Biochim. Biophys. Acta Rev. Biomembr. 1981, 650, 21–51. [Google Scholar] [CrossRef]
- Gennis, R.B. Biomembranes: Molecular Structure and Function; Springer Advanced Texts in Chemistry; Springer: New York, NY, USA, 1989; ISBN 9780387967608. [Google Scholar]
- Först, G.; Cwiklik, L.; Jurkiewicz, P.; Schubert, R.; Hof, M. Interactions of beta-blockers with model lipid membranes: Molecular view of the interaction of acebutolol, oxprenolol, and propranolol with phosphatidylcholine vesicles by time-dependent fluorescence shift and molecular dynamics simulations. Eur. J. Pharm. Biopharm. 2014, 87, 559–569. [Google Scholar] [CrossRef]
- Mizogami, M.; Takakura, K.; Tsuchiya, H. The interactivities with lipid membranes differentially characterize selective and nonselective β1-blockers. Eur. J. Anaesthesiol. 2010, 27, 829–834. [Google Scholar] [CrossRef]
- Ausili, A.; Torrecillas, A.; De Godos, A.M.; Corbalán-García, S.; Gómez-Fernández, J.C. Phenolic Group of α-Tocopherol Anchors at the Lipid-Water Interface of Fully Saturated Membranes. Langmuir 2018, 34, 3336–3348. [Google Scholar] [CrossRef]
- Mashaghi, A.; Partovi-Azar, P.; Jadidi, T.; Nafari, N.; Maass, P.; Tabar, M.R.R.; Bonn, M.; Bakker, H.J. Hydration strongly affects the molecular and electronic structure of membrane phospholipids. J. Chem. Phys. 2012, 136, 114709. [Google Scholar] [CrossRef]
- Marsh, D. Lateral pressure in membranes. Biochim. Biophys. Acta Rev. Biomembr. 1996, 1286, 183–223. [Google Scholar] [CrossRef]
- Kanai, K.; Kikuchi, E.; Mikami, S.; Suzuki, E.; Uchida, Y.; Kodaira, K.; Miyajima, A.; Ohigashi, T.; Nakashima, J.; Oya, M. Vitamin E succinate induced apoptosis and enhanced chemosensitivity to paclitaxel in human bladder cancer cells in vitro and in vivo. Cancer Sci. 2010, 101, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Kogure, K.; Manabe, S.; Suzuki, I.; Tokumura, A.; Fukuzawa, K. Cytotoxicity of alpha-tocopheryl succinate, malonate and oxalate in normal and cancer cells in vitro and their anti-cancer effects on mouse melanoma in vivo. J. Nutr. Sci. Vitaminol. 2005, 51, 392–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prasad, K.N.; Kumar, B.; Yan, X.D.; Hanson, A.J.; Cole, W.C. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: A review. J. Am. Coll. Nutr. 2003, 22, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Baj, A.; Cedrowski, J.; Olchowik-Grabarek, E.; Ratkiewicz, A.; Witkowski, S. Synthesis, DFT Calculations, and In Vitro Antioxidant Study on Novel Carba-Analogs of Vitamin E. Antioxidants 2019, 8, 589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Witkowski, S.; Maciejewska, D.; Wawer, I. A solution and solid state conformations of chromanol esters, 13C MAS NMR and d-NMR study. J. Chem. Soc. Perkin Trans. 2000, 2, 1471–1476. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neunert, G.; Hertmanowski, R.; Witkowski, S.; Polewski, K. Effect of Ester Moiety on Structural Properties of Binary Mixed Monolayers of Alpha-Tocopherol Derivatives with DPPC. Molecules 2022, 27, 4670. https://doi.org/10.3390/molecules27154670
Neunert G, Hertmanowski R, Witkowski S, Polewski K. Effect of Ester Moiety on Structural Properties of Binary Mixed Monolayers of Alpha-Tocopherol Derivatives with DPPC. Molecules. 2022; 27(15):4670. https://doi.org/10.3390/molecules27154670
Chicago/Turabian StyleNeunert, Grażyna, Robert Hertmanowski, Stanislaw Witkowski, and Krzysztof Polewski. 2022. "Effect of Ester Moiety on Structural Properties of Binary Mixed Monolayers of Alpha-Tocopherol Derivatives with DPPC" Molecules 27, no. 15: 4670. https://doi.org/10.3390/molecules27154670
APA StyleNeunert, G., Hertmanowski, R., Witkowski, S., & Polewski, K. (2022). Effect of Ester Moiety on Structural Properties of Binary Mixed Monolayers of Alpha-Tocopherol Derivatives with DPPC. Molecules, 27(15), 4670. https://doi.org/10.3390/molecules27154670






