Neocornuside A–D, Four Novel Iridoid Glycosides from Fruits of Cornus officinalis and Their Antidiabetic Activity
Abstract
1. Introduction
2. Results and Discussion
2.1. Structure Elucidation
2.2. Cell Viability of Compounds 1–10 in Insulin-Induced HepG2 Cells
2.3. Glucose Consumption of Compounds 1–10 in Insulin-Induced HepG2 Cells
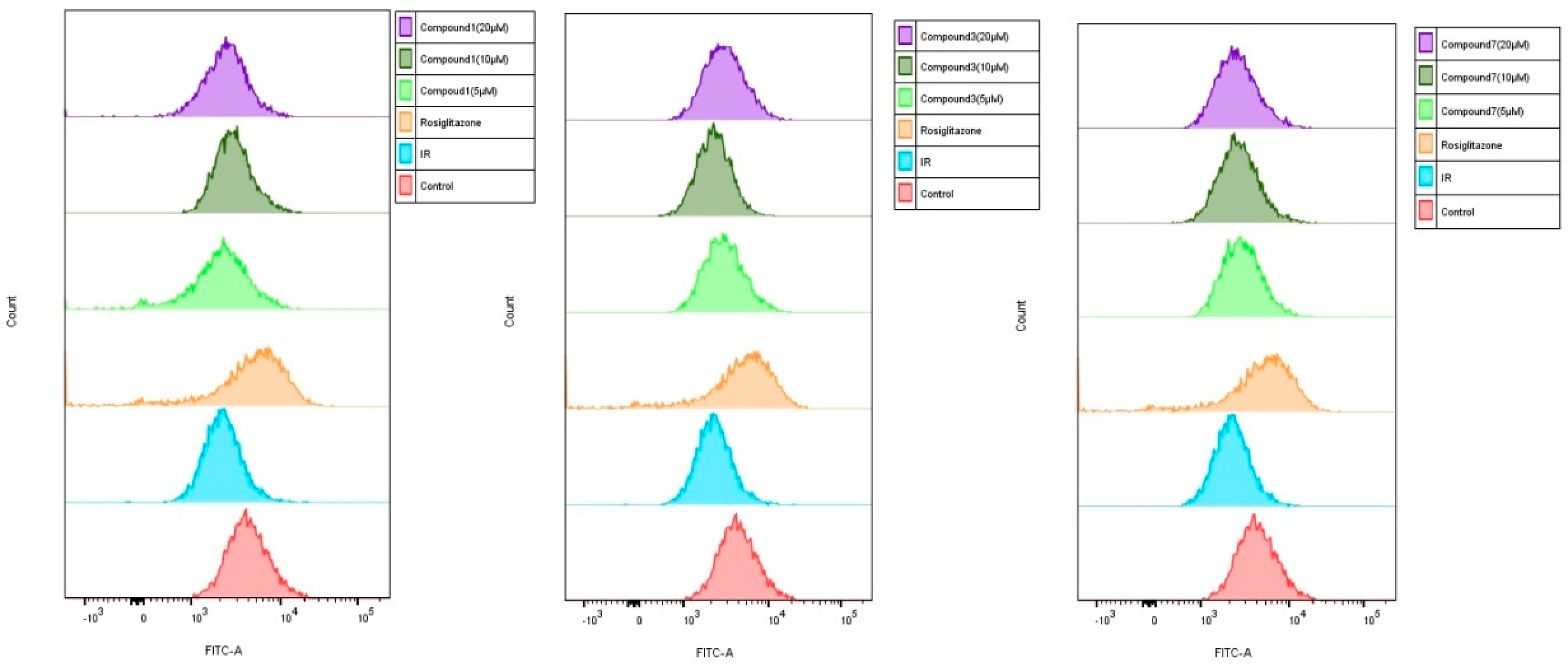
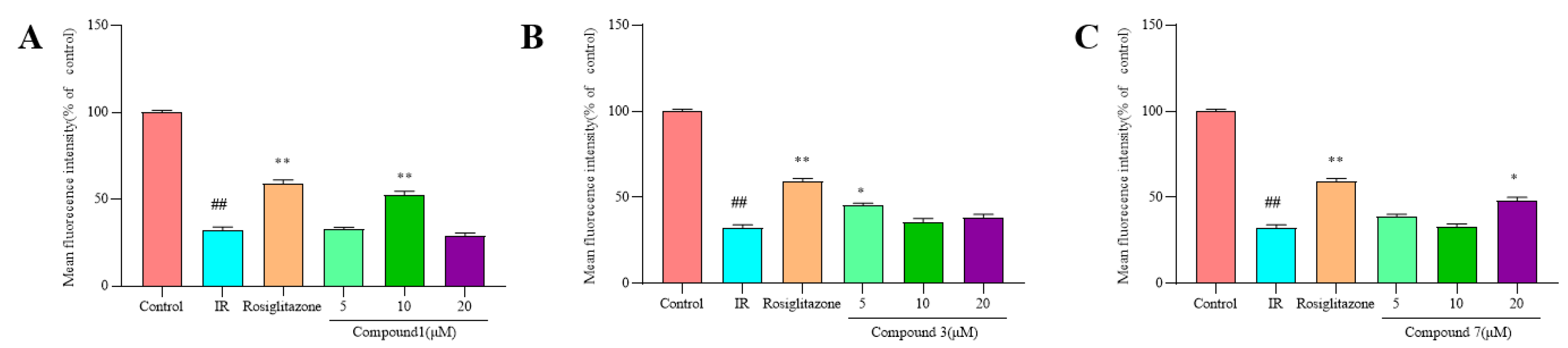
2.4. Effect of Compounds 1, 3, and 7 on Glucose Uptake in Insulin-Induced HepG2 Cells
3. Experimental
3.1. General Experimental Procedures
3.2. Plant Materials
3.3. Extraction and Isolation
3.4. Antidiabetic Evaluation
3.4.1. Cell Culture and Treatment
3.4.2. Cell Viability Assay
3.4.3. Glucose Consumption Assay
3.4.4. Glucose Uptake Assay
3.5. Acid Hydrolysis of Compounds 1–4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Ma, W.; Wang, K.J.; Cheng, C.S.; Yan, G.Q.; Lu, W.L.; Ge, J.F.; Cheng, Y.X.; Li, N. Bioactive compounds from Cornus officinalis fruits and their effects on diabetic nephropathy. J. Ethnopharmacol. 2014, 153, 840–845. [Google Scholar] [CrossRef]
- Ye, X.S.; He, J.; Zhang, J.L.; Pang, X.B.; Zhang, L.; Qiao, H.Y.; Pan, X.G.; Zhang, J.; Liu, S.N.; Zhang, W.K.; et al. Chemical constituents from ripe fruit of Cornus officinalis. Zhongguo Zhong Yao Za Zhi 2016, 41, 4605–4609. [Google Scholar] [PubMed]
- Klymenko, S.; Kucharska, A.Z.; Sokol-Letowska, A.; Piorecki, N.; Przybylska, D.; Grygorieva, O. Iridoids, flavonoids, and antioxidant capacity of Cornus mas, C. officinalis, and C. mas x C. officinalis fruits. Biomolecules 2021, 11, 776. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhong, X.J.; Zhou, N.; Ji, L.L.; Li, J.J.; Cai, N.; Wang, Q.B.; Lin, P.C.; Shang, X.Y. Secoiridoid glycosides from the fruits of Cornus officinalis. Nat. Prod. Res. 2022, 36, 2329–2335. [Google Scholar] [CrossRef]
- Lee, D.Y.; Song, M.C.; Yoo, K.H.; Bang, M.H.; Chung, I.S.; Kim, S.H.; Kim, D.K.; Kwon, B.M.; Jeong, T.S.; Park, M.H.; et al. Lignans from the fruits of Cornus kousa Burg. and their cytotoxic effects on human cancer cell lines. Arch. Pharm. Res. 2007, 30, 402–407. [Google Scholar] [CrossRef]
- He, J.; Xu, Y.; Xu, J.K.; Ye, X.S.; Zhang, X.X.; Zhang, W.K. Cornusglucosides A and B, two new iridoid glucosides from the fruit of Cornus officinalis. Chem. Biodivers. 2019, 16, e1900421. [Google Scholar] [CrossRef]
- He, K.; Song, S.; Zou, Z.; Feng, M.; Wang, D.; Wang, Y.; Li, X.; Ye, X. The hypoglycemic and synergistic effect of loganin, morroniside, and ursolic acid isolated from the fruits of Cornus officinalis. Phytother. Res. 2016, 30, 283–291. [Google Scholar] [CrossRef]
- Gao, X.; Liu, Y.; An, Z.; Ni, J. Active components and pharmacological effects of Cornus officinalis: Literature review. Front. Pharmacol. 2021, 12, 633447. [Google Scholar] [CrossRef] [PubMed]
- Qu, Y.J.; Zhen, R.R.; Zhang, L.M.; Gu, C.; Chen, L.; Peng, X.; Hu, B.; An, H.M. Uncovering the active compounds and effective mechanisms of the dried mature sarcocarp of Cornus officinalis Sieb. Et Zucc. for the treatment of Alzheimer's disease through a network pharmacology approach. BMC Complement. Med. Ther. 2020, 20, 157. [Google Scholar] [CrossRef]
- Lee, N.H.; Seo, C.S.; Lee, H.Y.; Jung, D.Y.; Lee, J.K.; Lee, J.A.; Song, K.Y.; Shin, H.K.; Lee, M.Y.; Seo, Y.B.; et al. Hepatoprotective and antioxidative activities of Cornus officinalis against acetaminophen-induced hepatotoxicity in mice. Evid. Based Complement. Alternat. Med. 2012, 2012, 804924. [Google Scholar]
- Wang, Z.X.; Lian, W.W.; He, J.; He, X.L.; Wang, Y.M.; Pan, C.H.; Li, M.; Zhang, W.K.; Liu, L.Q.; Xu, J.K. Cornuside ameliorates cognitive impairments in scopolamine induced AD mice: Involvement of neurotransmitter and oxidative stress. J. Ethnopharmacol. 2022, 293, 115252. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.L.; Wang, X.; Li, J.J.; Zhong, X.J.; Zhang, B.; Juan, J.; Shang, X.Y. New iridoid derivatives from the fruits of Cornus officinalis and their neuroprotective activities. Molecules 2019, 24, 625. [Google Scholar] [CrossRef] [PubMed]
- Akbar, M.; Ali, U.; Khalil, T.; Iqbal, M.S.; Amin, A.; Naeem, R.; Nazir, A.; Waqas, H.M.; Aslam, Z.; Jafri, F.I.; et al. Cornus macrophylla, the antibacterial activity of organic leaf extracts and the characterization of the more lipophilic components by GC/MS. Molecules 2020, 25, 2395. [Google Scholar] [CrossRef]
- Pieczykolan, A.; Pietrzak, W.; Gawlik-Dziki, U.; Nowak, R. Antioxidant, anti-inflammatory, and anti-diabetic activity of phenolic acids fractions obtained from Aerva lanata (L.) Juss. Molecules 2021, 26, 3486. [Google Scholar] [CrossRef] [PubMed]
- Spychaj, R.; Kucharska, A.Z.; Szumny, A.; Przybylska, D.; Pejcz, E.; Piorecki, N. Potential valorization of Cornelian cherry (Cornus mas L.) stones: Roasting and extraction of bioactive and volatile compounds. Food Chem. 2021, 358, 129802. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Wang, Y.H.; Ren, D.Y.; Li, J.R.; Yuan, G.X.; An, L.P.; Du, P.G.; Ma, J. Antidiabetic mechanism of Coptis chinensis polysaccharide through its antioxidant property involving the JNK pathway. Pharm. Biol. 2015, 53, 1022–1029. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Ke, Y.; Liu, Y.; Luo, X.; Li, C.; Zhang, Z.; Liu, A.; Shen, L.; Chen, H.; et al. Antidiabetic activity of polysaccharides from Suillellus luridus in streptozotocin-induced diabetic mice. Int. J. Biol. Macromol. 2018, 119, 134–140. [Google Scholar] [CrossRef]
- Hafizur, R.M.; Babiker, R.; Yagi, S.; Chishti, S.; Kabir, N.; Choudhary, M.I. The antidiabetic effect of Geigeria alata is mediated by enhanced insulin secretion, modulation of beta-cell function, and improvement of antioxidant activity in streptozotocin-induced diabetic rats. J. Endocrinol. 2012, 214, 329–335. [Google Scholar] [CrossRef]
- Dzydzan, O.; Brodyak, I.; Sokol-Letowska, A.; Kucharska, A.Z.; Sybirna, N. Loganic acid, an iridoid glycoside extracted from Cornusmas L. fruits, reduces of carbonyl/oxidative stress biomarkers in plasma and restores antioxidant balance in leukocytes of rats with streptozotocin-induced diabetes mellitus. Life 2020, 10, 349. [Google Scholar] [CrossRef]
- Sip, S.; Szymanowska, D.; Chanaj-Kaczmarek, J.; Skalicka-Wozniak, K.; Budzynska, B.; Wronikowska-Denysiuk, O.; Slowik, T.; Szulc, P.; Cielecka-Piontek, J. Potential for prebiotic stabilized Cornus mas L. lyophilized extract in the prophylaxis of diabetes mellitus in streptozotocin diabetic rats. Antioxidants 2022, 11, 380. [Google Scholar] [CrossRef]
- Ye, X.S.; He, J.; Cheng, Y.C.; Zhang, L.; Qiao, H.Y.; Pan, X.G.; Zhang, J.; Liu, S.N.; Zhang, W.K.; Xu, J.K. Cornusides A-O, bioactive iridoid glucoside dimers from the fruit of Cornus officinalis. J. Nat. Prod. 2017, 80, 3103–3111. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.H.; Fu, R.; Zhang, P.Y.; Lou, S.L.; Yang, X.C.; Chen, Y.; Ma, T.; Zhang, Y.; Xi, Z.X.; Liu, J.Q. A chromosome-level Camptotheca acuminata genome assembly provides insights into the evolutionary origin of camptothecin biosynthesis. Nat. Commun. 2021, 12, 3531. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, S.; Zhang, S. Six new triterpenoid saponins from the root and stem bark of Cephalanthus occidentalis. Planta. Med. 2005, 71, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Yin, L.; Zhang, Y.; Huang, X.; Zhao, F.; Cui, X.; Shi, L.; Xu, L. Study on anti-inflammatory efficacy and correlative ingredients with pharmacodynamics detected in acute inflammation rat model serum from Caulis Lonicerae japonicae. Phytomedicine 2016, 23, 597–610. [Google Scholar] [CrossRef]
- Liang, J.; Sun, W. Elution-extrusion countercurrent chromatography separation of six pairs of isomeric iridoids from Cornus officinalis Sieb. et Zucc. guided by ion current extraction in mass spectrometry. J. Sep. Sci. 2018, 41, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Pivovarova, O.; von Loeffelholz, C.; Ilkavets, I.; Sticht, C.; Zhuk, S.; Murahovschi, V.; Lukowski, S.; Docke, S.; Kriebel, J.; de las Heras Gala, T.; et al. Modulation of insulin degrading enzyme activity and liver cell proliferation. Cell Cycle 2015, 14, 2293–2300. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hagiwara, A.; Nishiyama, M.; Ishizaki, S. Branched-chain amino acids prevent insulin-induced hepatic tumor cell proliferation by inducing apoptosis through mTORC1 and mTORC2-dependent mechanisms. J. Cell. Physiol. 2012, 227, 2097–2105. [Google Scholar] [CrossRef]
- Popov, V.B.; Lim, J.K. Impact of insulin-sensitizing agents on risk for liver cancer and liver-related death in diabetic patients with compensated hepatitis C cirrhosis. J. Clin. Endocrinol. Metab. 2011, 96, 2398–2400. [Google Scholar] [CrossRef][Green Version]
- Gong, P.X.; Li, Q.Y.; Wu, Y.C.; Lu, W.Y.; Zeng, J.; Li, H.J. Structural elucidation and antidiabetic activity of fucosylated chondroitin sulfate from sea cucumber Stichopus japonicas. Carbohydr. Polym. 2021, 262, 117969. [Google Scholar] [CrossRef]
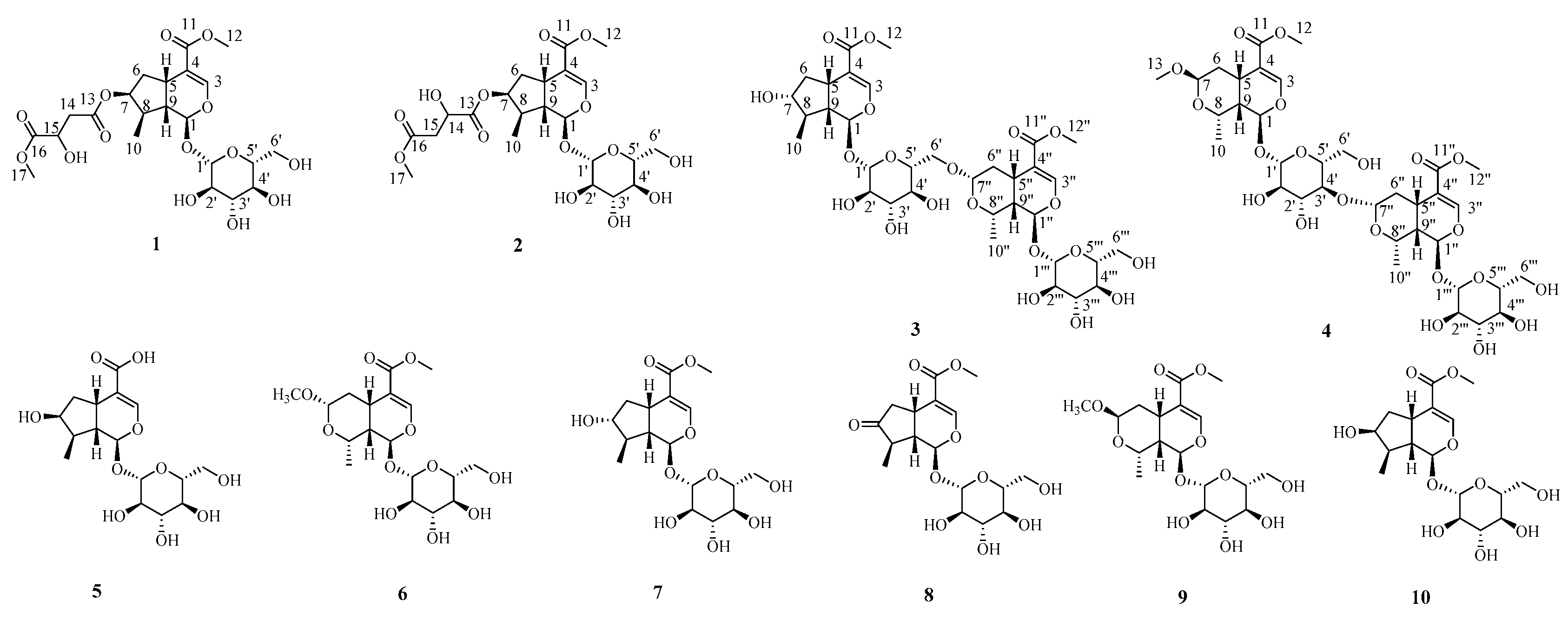
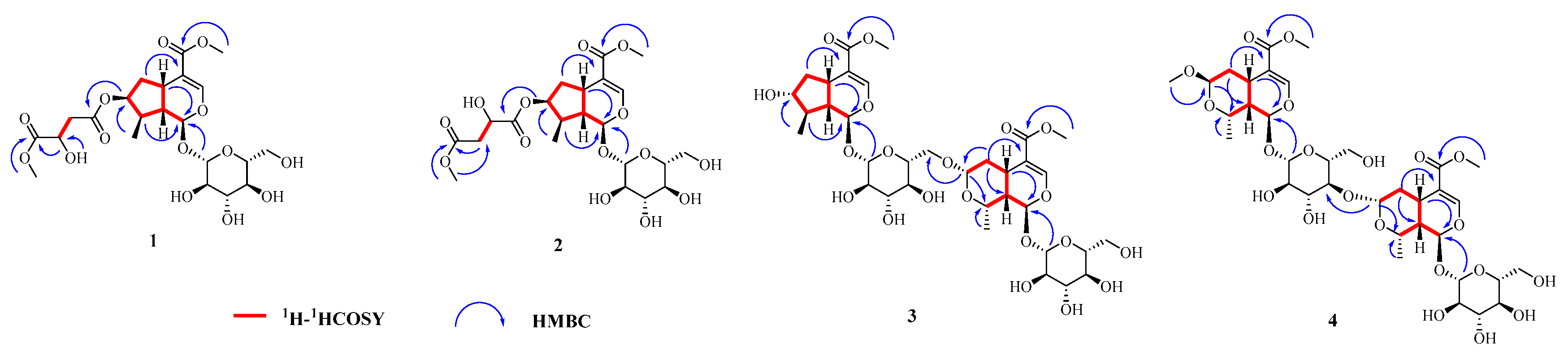
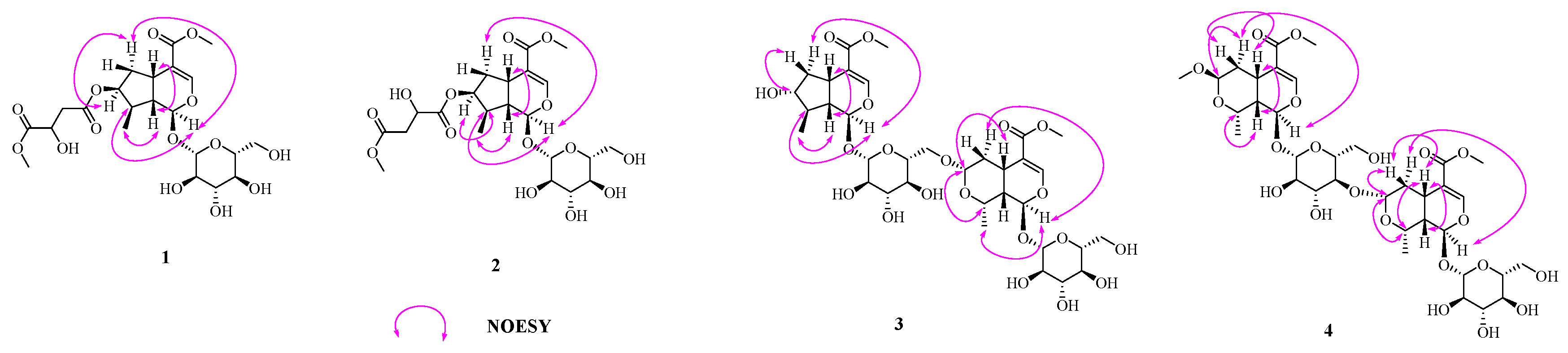


| 1 a | 2 b | |||
|---|---|---|---|---|
| Position | 13C | 1H (J in Hz) | 13C | 1H (J in Hz) |
| 1 | 95.3 | 5.20 d (4.5) | 97.5 | 5.29 d (4.9) |
| 3 | 150.9 | 7.38 d (1.0) | 152.6 | 7.43d (1.3) |
| 4 | 111.4 | 113.1 | ||
| 5 | 30.7 | 2.94 m | 32.6 | 3.10 q (8.0) |
| 6 | 38.7 | 2.13 m 1.67 m | 40.3 | 2.37 ddd (14.7, 8.0, 1.5) 1.76 ddd (14.7, 8.0, 5.0) |
| 7 | 77.0 | 5.04 m | 79.9 | 5.21 t (4.6) |
| 8 | 38.9 | 1.97 m | 40.9 | 2.14 m |
| 9 | 45.2 | 1.92 m | 46.9 | 2.07 td (8.9, 4.9) |
| 10 | 13.1 | 0.95 d (6.7) | 13.6 | 1.07 d (6.8) |
| 11 | 166.8 | 169.3 | ||
| 12 | 51.1 | 3.62 s | 52.3 | 3.69 s |
| 13 | 169.6 | 174.1 | ||
| 14 | 39.3 | 2.72 dd (15.6, 5.5) 2.63 dd (15.6, 6.9) | 68.8 | 4.52 dd (7.0, 5.2) |
| 15 | 67.0 | 4.39, dd (12.3, 5.8) | 40.0 | 2.83 dd (16.0, 5.2) 2.74 dd (16.0, 7.0) |
| 16 | 173.2 | 172.4 | ||
| 17 | 51.8 | 3.64 s | 51.7 | 3.70 s |
| 1′ | 98.7 | 4.46 d (7.9) | 100.2 | 4.66 d (7.9) |
| 2′ | 73.2 | 2.97 m | 74.7 | 3.19 m |
| 3′ | 76.8 | 3.15 m | 78.0 | 3.37 t (8.9) |
| 4′ | 70.1 | 3.03 m | 71.6 | 3.26 m |
| 5′ | 77.3 | 3.14 m | 78.4 | 3.32 m |
| 6′ | 61.2 | 3.68 m 3.44 m | 62.8 | 3.90 dd (11.9, 2.1) 3.65 dd (11.9, 6.2) |
| 3 | 4 | |||
|---|---|---|---|---|
| Position | 13C | 1H (J in Hz) | 13C | 1H (J in Hz) |
| 1 | 97.9 | 5.24 d (5.3) | 95.4 | 5.91 d (3.2) |
| 3 | 152.4 | 7.41 s | 154.5 | 7.53 s |
| 4 | 113.3 | 111.7 | ||
| 5 | 31.2 | 2.88 m | 28.0 | 3.10 dt (12.9, 4.7) |
| 6 | 41.9 | 2.51 dt (12.9, 7.4) 1.34 m | 33.8 | 1.93 dd (13.9, 4.7) 1.52 td (13.9, 3.9) |
| 7 | 79.5 | 3.68 m | 99.5 | 4.74 d (3.7) |
| 8 | 44.3 | 1.72 q (7.5) | 66.3 | 4.29 qd (6.6, 2.1) |
| 9 | 46.9 | 1.84 m | 40.4 | 1.83 m |
| 10 | 17.6 | 1.16 d (6.7) | 19.6 | 1.34 d (6.9) |
| 11 | 169.5 | 168.7 | ||
| 12 | 51.8 | 3.69 s | 51.8 | 3.70 s |
| 13 | 55.0 | 3.35 s | ||
| 1′ | 100.6 | 4.65 d (8.0) | 99.7 | 4.87 d (8.7) |
| 2′ | 74.7 | 3.22 m | 73.5 | 3.25 m |
| 3′ | 78.5 | 3.29 m | 86.8 | 3.57 t (8.9) |
| 4′ | 71.0 | 3.40 m | 70.4 | 3.38 m |
| 5′ | 78.0 | 3.38 m | 78.0 | 3.37 m |
| 6′ | 68.7 | 4.02 m 3.93 dd (11.9, 1.5) | 62.6 | 3.90 dd (12.2, 1.8) 3.87 dd (12.2, 6.5) |
| 1″ | 95.9 | 5.82 d (9.2) | 96.1 | 5.89 d (3.3) |
| 3″ | 154.5 | 7.52 s | 154.6 | 7.52 s |
| 4″ | 110.8 | 110.8 | ||
| 5″ | 31.9 | 2.85 m | 32.1 | 2.87 dt (12.9, 4.6) |
| 6″ | 35.7 | 2.07 ddd (13.2, 4.6, 2.2) 3.65 td (13.2, 9.7) | 35.4 | 2.26 ddd (13.4, 4.6, 2.4) 1.32 m |
| 7″ | 104.0 | 4.71 dd (9.7, 2.2) | 103.8 | 4.80 dd (9.7, 2.4) |
| 8″ | 74.3 | 3.98 dd (6.9, 2.3) | 74.5 | 4.05 qd (6.4, 1.7) |
| 9″ | 40.1 | 1.81 m | 40.0 | 1.81 m |
| 10″ | 19.7 | 1.41 d (6.80) | 19.7 | 1.45 d (6.8) |
| 11″ | 168.6 | 168.6 | ||
| 12″ | 51.7 | 3.70 s | 51.7 | 3.71 s |
| 1‴ | 100.0 | 4.78 d (7.8) | 100.4 | 4.79 d (7.9) |
| 2‴ | 75.1 | 3.23 m | 75.1 | 3.22 m |
| 3‴ | 77.7 | 3.39 m | 78.6 | 3.28 d (2.3) |
| 4‴ | 71.7 | 3.27 m | 71.6 | 3.27 m |
| 5‴ | 77.0 | 3.41 m | 78.4 | 3.33 m |
| 6‴ | 62.9 | 3.89 dd (12.2, 2.2) 3.66 m | 62.7 | 3.66 m |
| Compound | EC50 a (μM) |
|---|---|
| 1 | 0.582 |
| 3 | 1.275 |
| 7 | 0.742 |
| Rosiglitazone b | 1.127 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; Hao, Z.; Wang, X.; Zhou, S.; Zhu, D.; Yang, Y.; Wei, J.; Li, M.; Zheng, X.; Feng, W. Neocornuside A–D, Four Novel Iridoid Glycosides from Fruits of Cornus officinalis and Their Antidiabetic Activity. Molecules 2022, 27, 4732. https://doi.org/10.3390/molecules27154732
Yang M, Hao Z, Wang X, Zhou S, Zhu D, Yang Y, Wei J, Li M, Zheng X, Feng W. Neocornuside A–D, Four Novel Iridoid Glycosides from Fruits of Cornus officinalis and Their Antidiabetic Activity. Molecules. 2022; 27(15):4732. https://doi.org/10.3390/molecules27154732
Chicago/Turabian StyleYang, Meng, Zhiyou Hao, Xiaolan Wang, Shiqi Zhou, Denghui Zhu, Ying Yang, Junjun Wei, Meng Li, Xiaoke Zheng, and Weisheng Feng. 2022. "Neocornuside A–D, Four Novel Iridoid Glycosides from Fruits of Cornus officinalis and Their Antidiabetic Activity" Molecules 27, no. 15: 4732. https://doi.org/10.3390/molecules27154732
APA StyleYang, M., Hao, Z., Wang, X., Zhou, S., Zhu, D., Yang, Y., Wei, J., Li, M., Zheng, X., & Feng, W. (2022). Neocornuside A–D, Four Novel Iridoid Glycosides from Fruits of Cornus officinalis and Their Antidiabetic Activity. Molecules, 27(15), 4732. https://doi.org/10.3390/molecules27154732








