Biodiesel Production Using Wild Apricot (Prunus aitchisonii) Seed Oil via Heterogeneous Catalysts
Abstract
:1. Introduction
2. Methodology
2.1. Equipment and Chemicals Used in Seed Oil Extraction
2.2. Extraction of Oil
2.2.1. Chemical Extraction of Oil
2.2.2. Mechanical Extraction of Oils
2.3. Calculation of Free-Fatty Acid (FFA) Contents of Prunus aitchisonii Seeds Oil
2.4. Production of Heterogeneous Nano-Catalysts (HNC)
2.4.1. Calcined Prunus aitchisonii Cake (CPC)
2.4.2. KOH-Activated P. aitchisonii Cake (KPC)
2.4.3. Titanium Dioxide (TiO2) Nanoparticles through Precipitation Methods
2.5. Illustration of Heterogeneous Nanocatalysts (KPC, KPC, and TiO2)
2.5.1. XRD (X-ray Diffraction)
2.5.2. Scanning Electron Microscopic Technique (SEM)
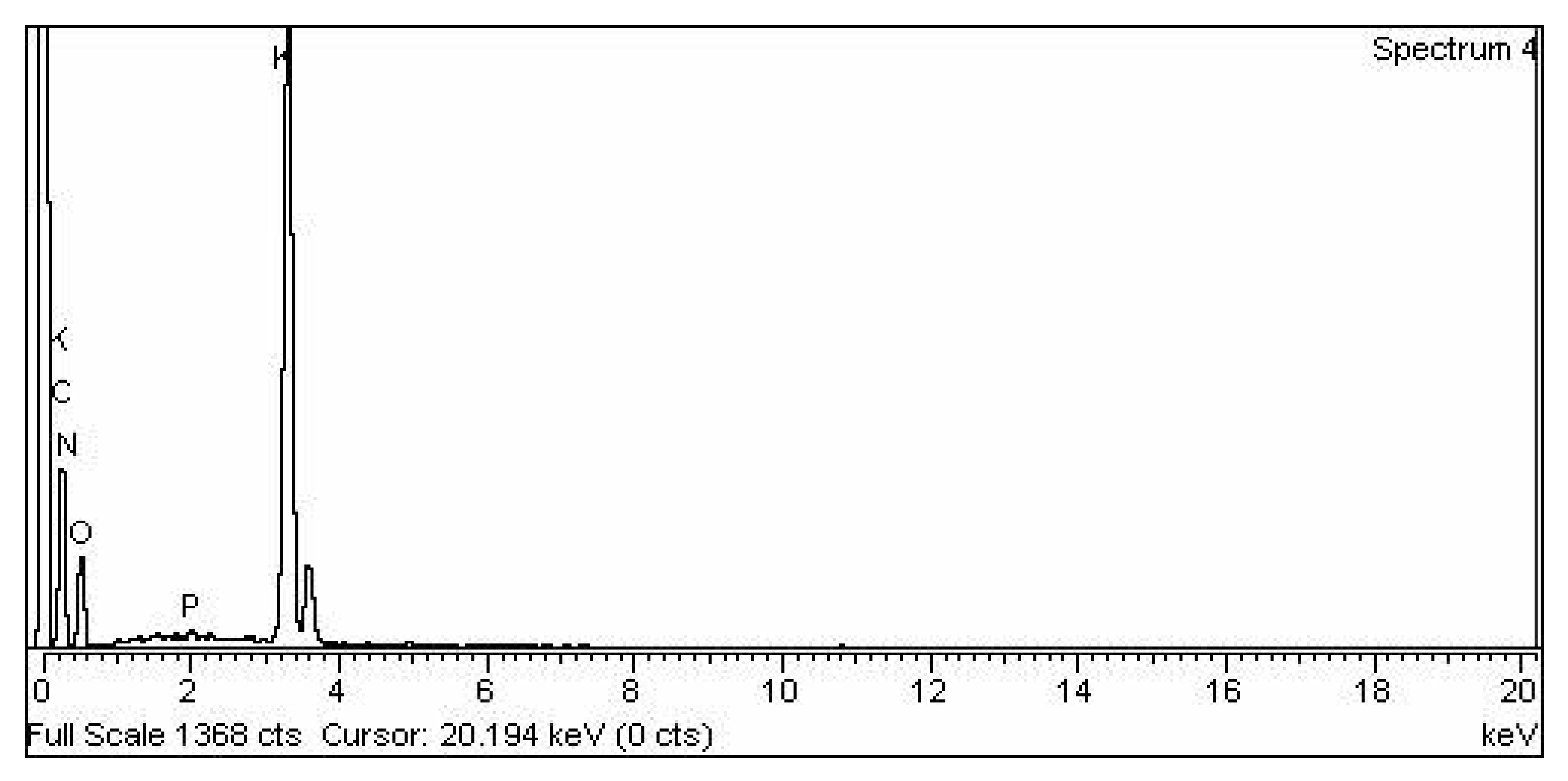
2.5.3. (EDS or EDX) Energy-Dispersive X-ray Composition Analysis
2.6. Biofuel/Diesel Production through Reflux (Trans-) Esterification Reaction
2.7. Characterization of Manufactured Fatty Acids-Methyl Esters
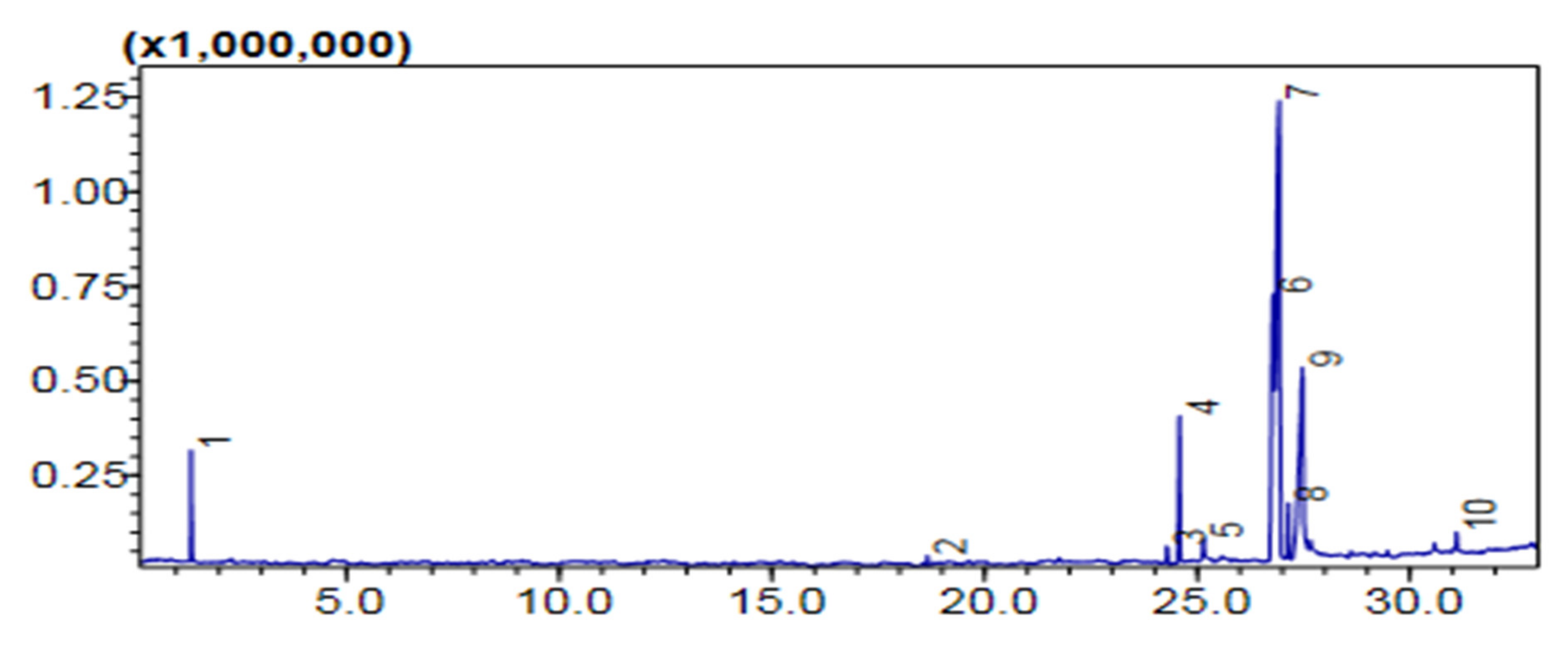
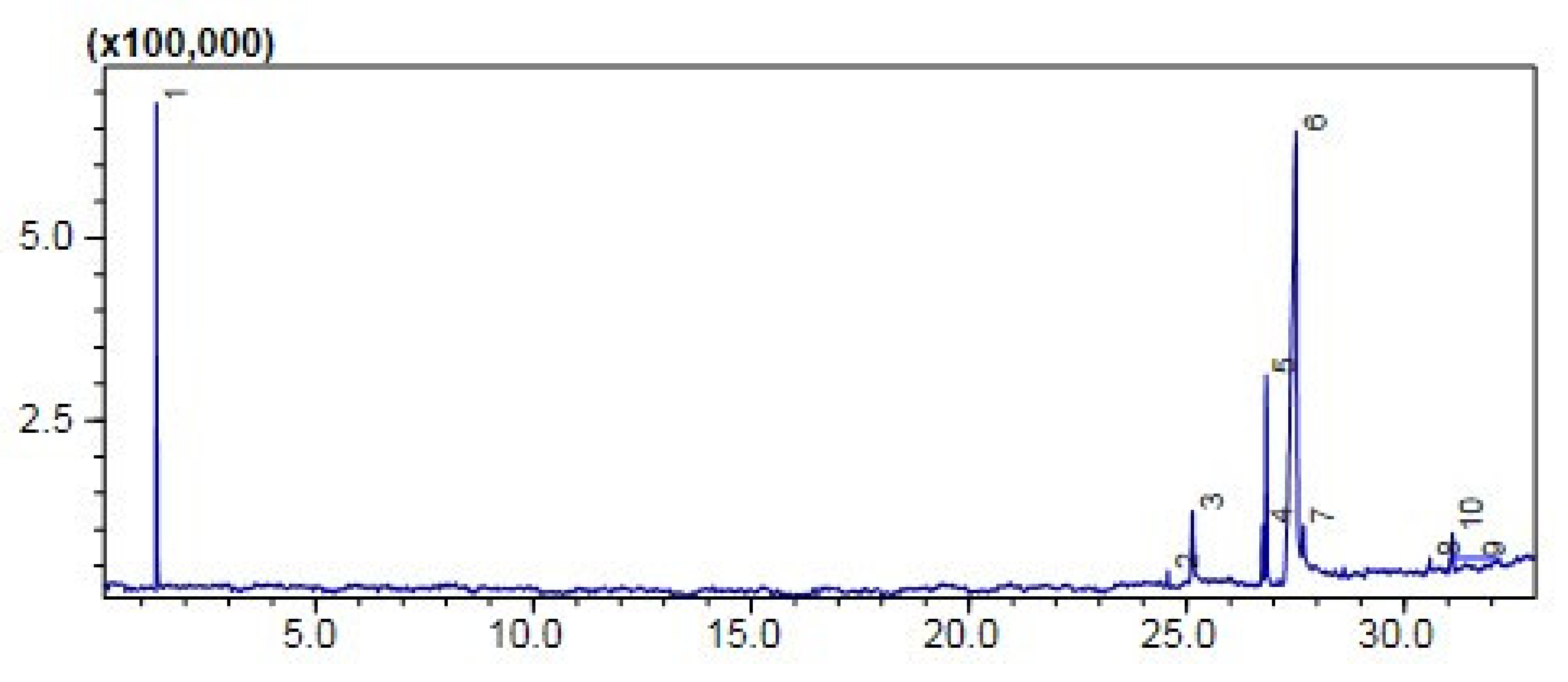
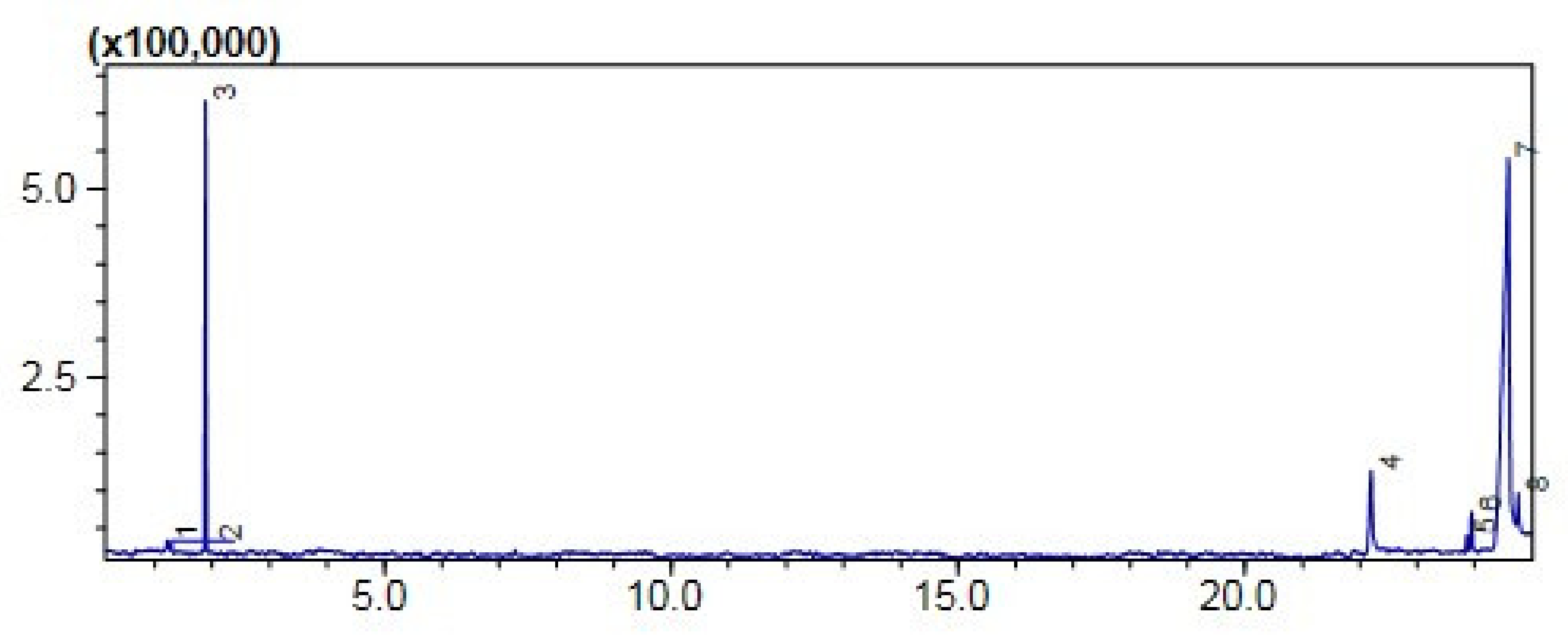
2.7.1. Gas Chromatography-MS Analysis
2.7.2. Magnetic Resonance Spectroscopy (MRS) or Nuclear–Magnetic Resonance (NMR)
2.7.3. FT-IR
2.7.4. Methyl Esters Physical-Fuel Properties
3. Results and Discussion
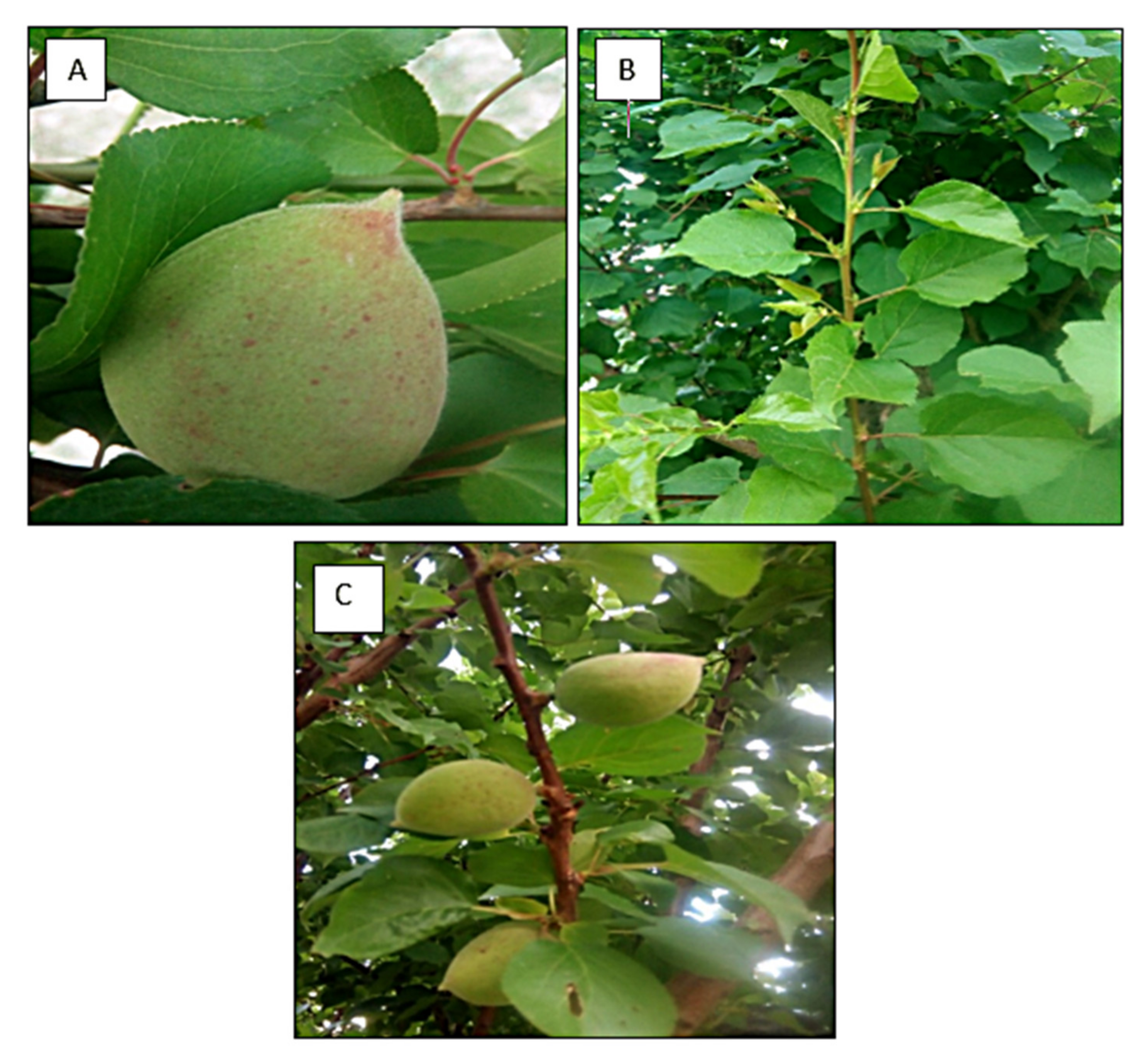
3.1. Biofuel Production of Seeds of Prunus aitchisonii
3.2. Representation of Heterogeneous Nano-Catalysts, CPC, KPC as Well as TiO2
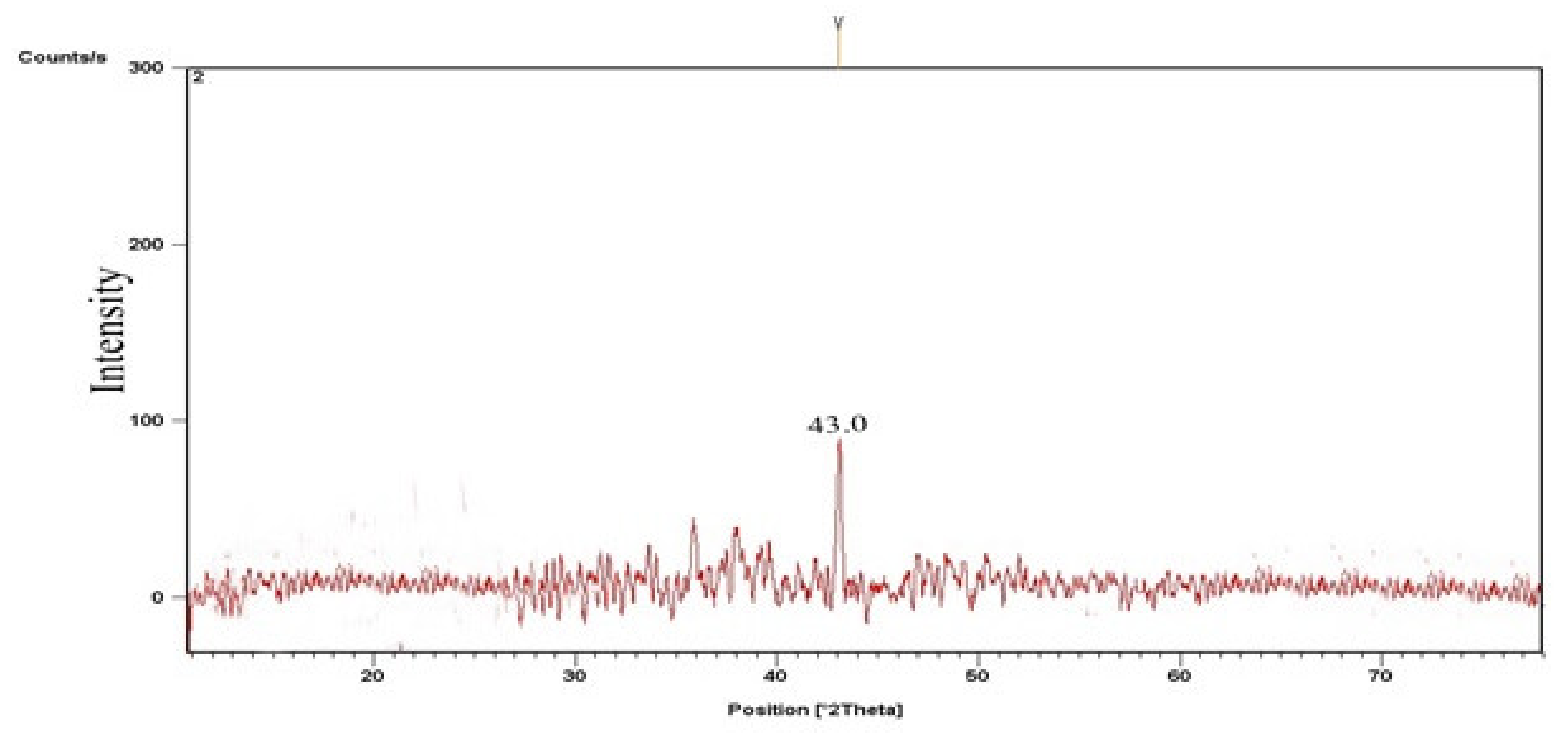
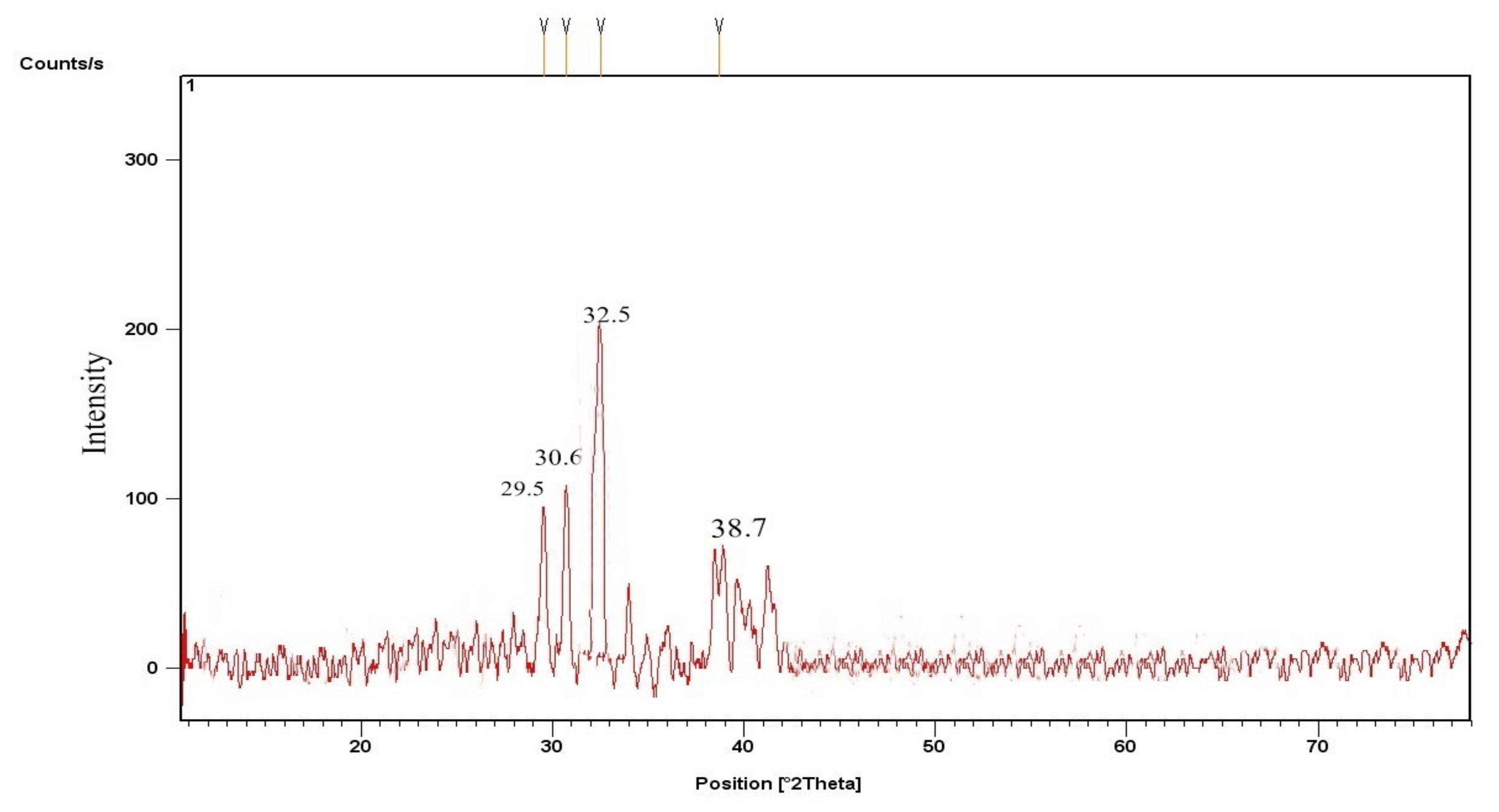
3.2.1. CPC and KPC X-ray Diffraction (XRD)
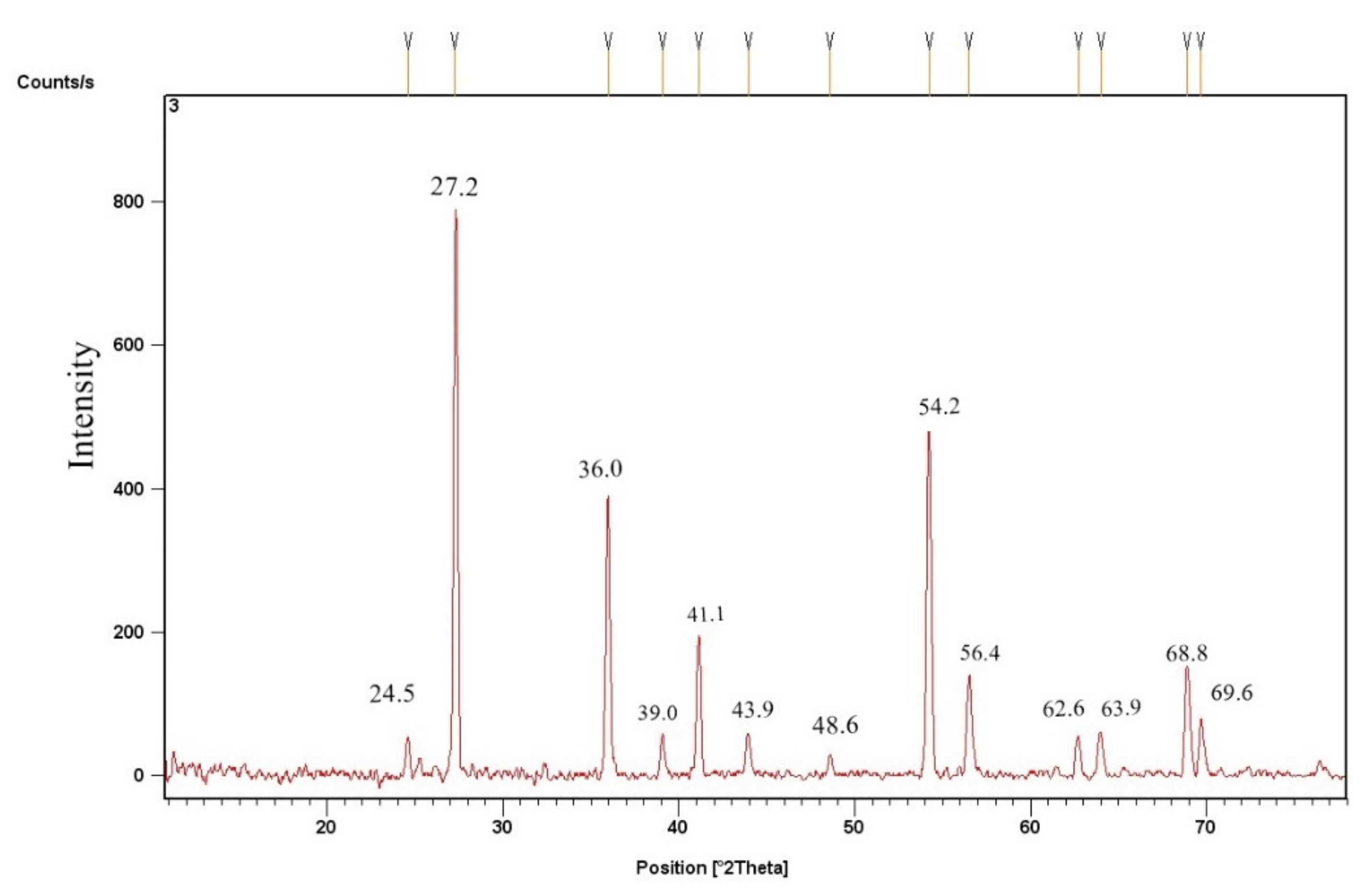
3.2.2. TiO2 Catalyst’s X-ray Diffraction
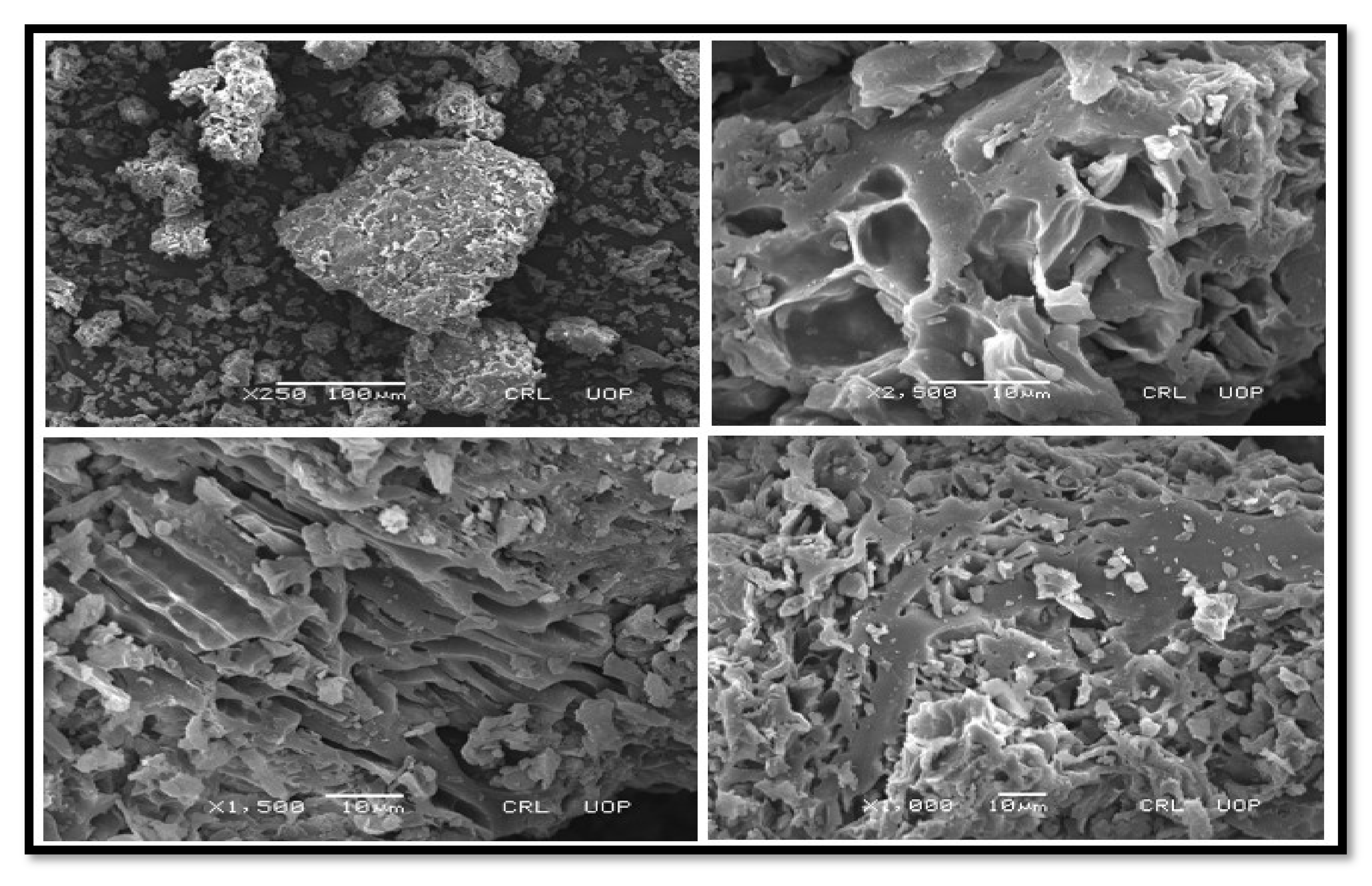
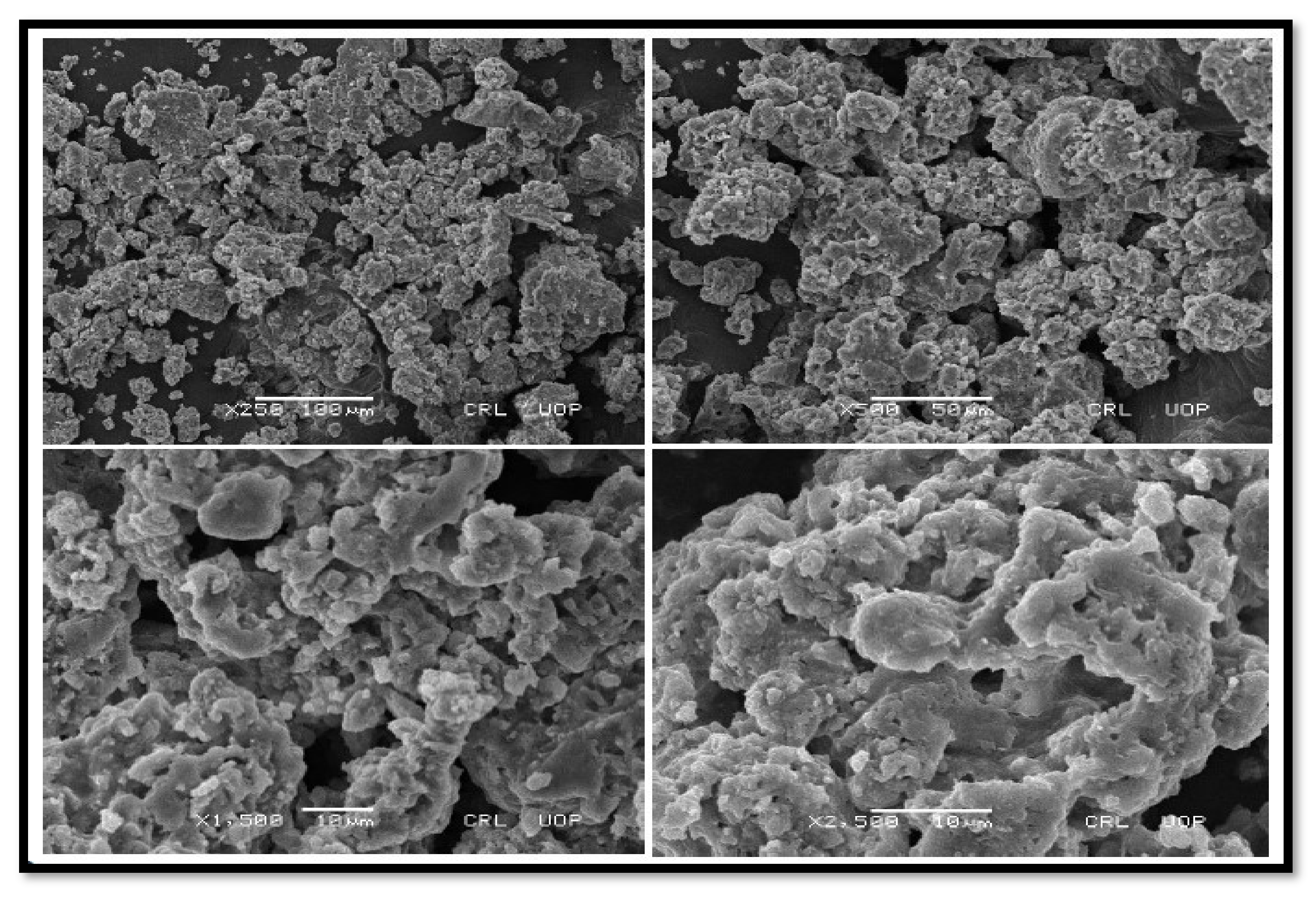
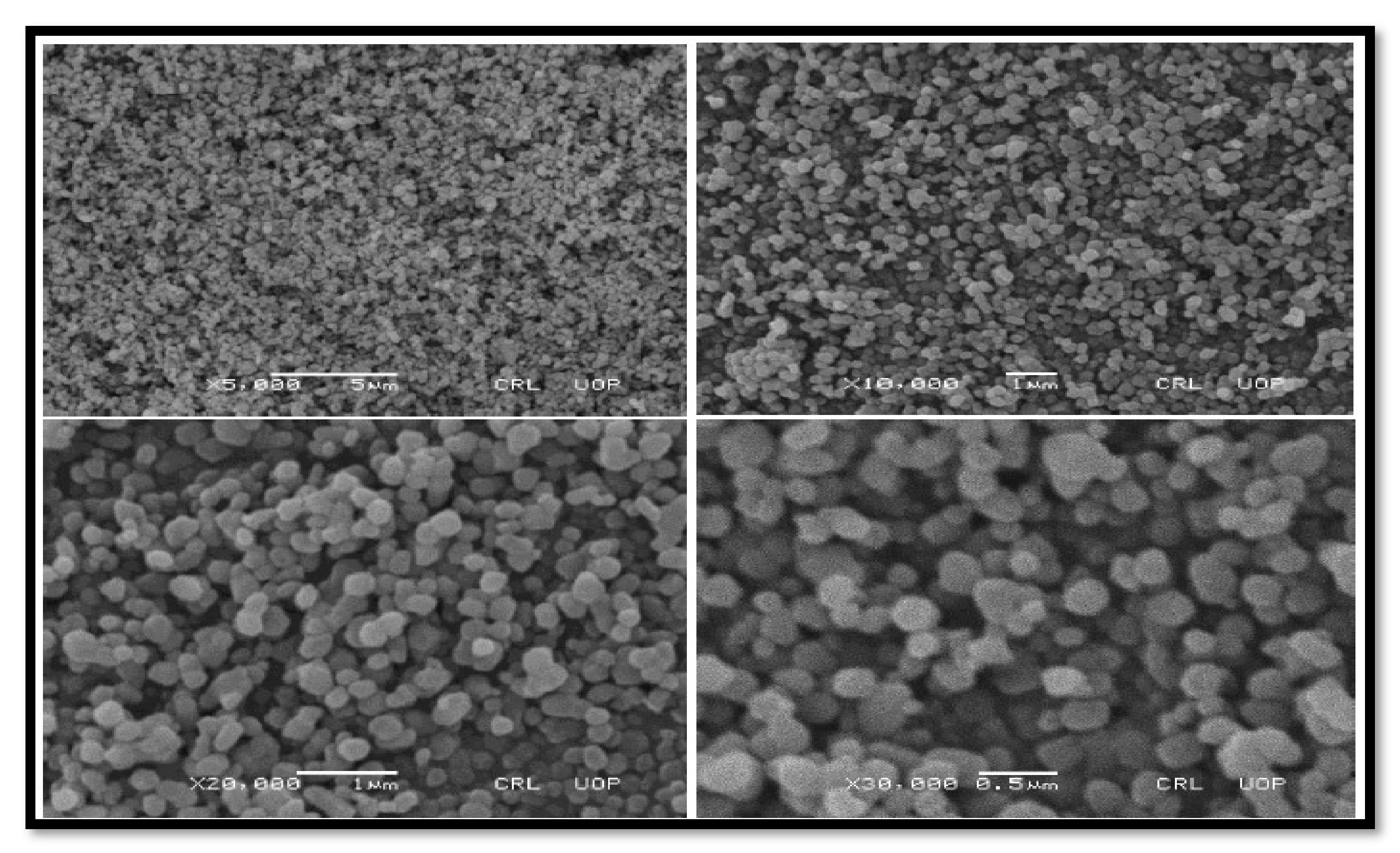
3.2.3. SEM Findings of Nano-Catalysts
SEM of CPC and KPC
SEM of TiO2
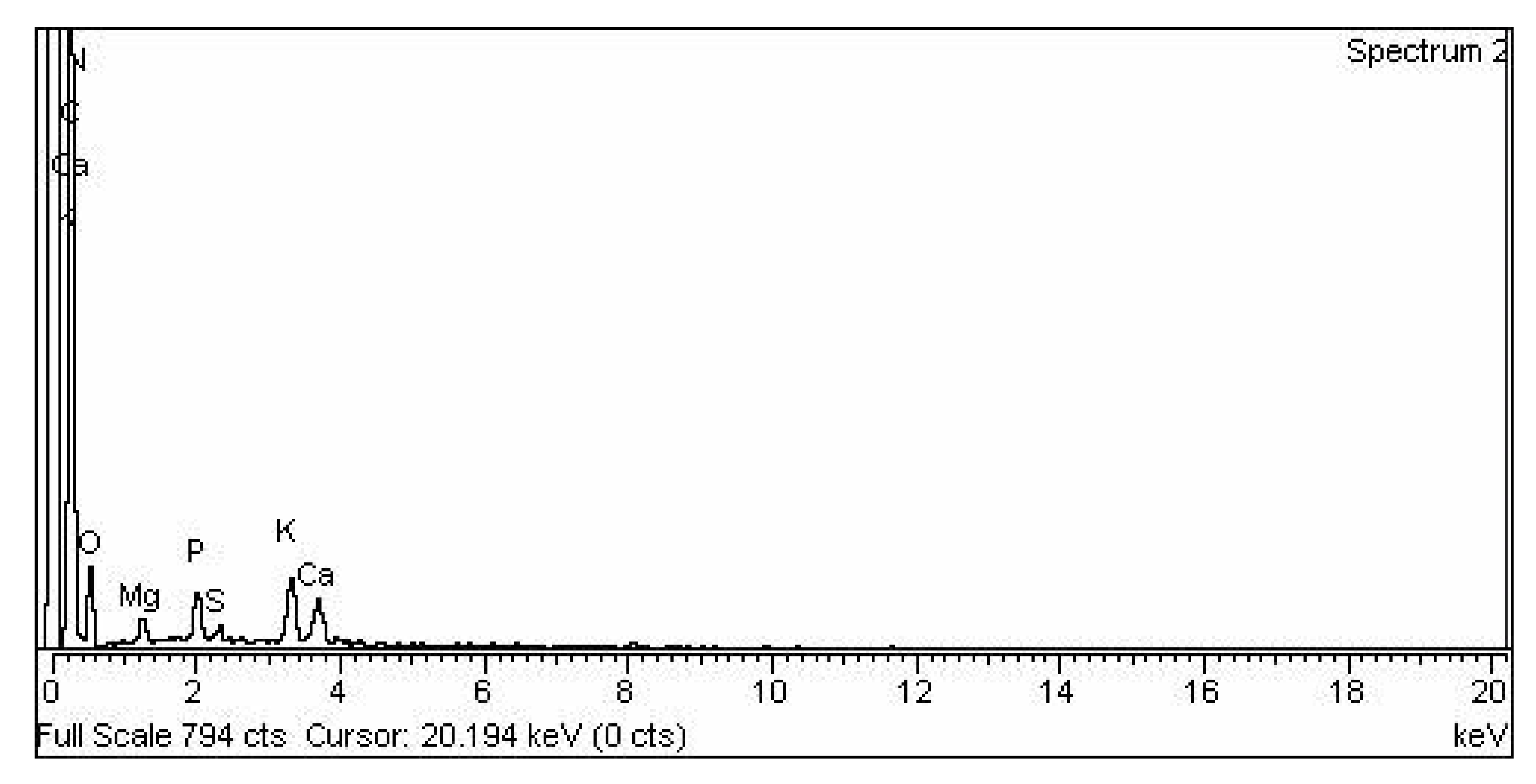
3.2.4. Representation of (EDX) Analysis of CPC Heterogenous Nano-Catalyst
3.2.5. KPC Heterogenous Nano-Catalyst EDX
3.2.6. TiO2 Nano-Catalyst Energy Dispersive X-ray EDX Analysis
3.3. Characterizations of Synthesized FAMEs (Fatty Acid Methyl Esters)
Comparative (GC-MS) Investigation of P. aitchisonii Biodiesel
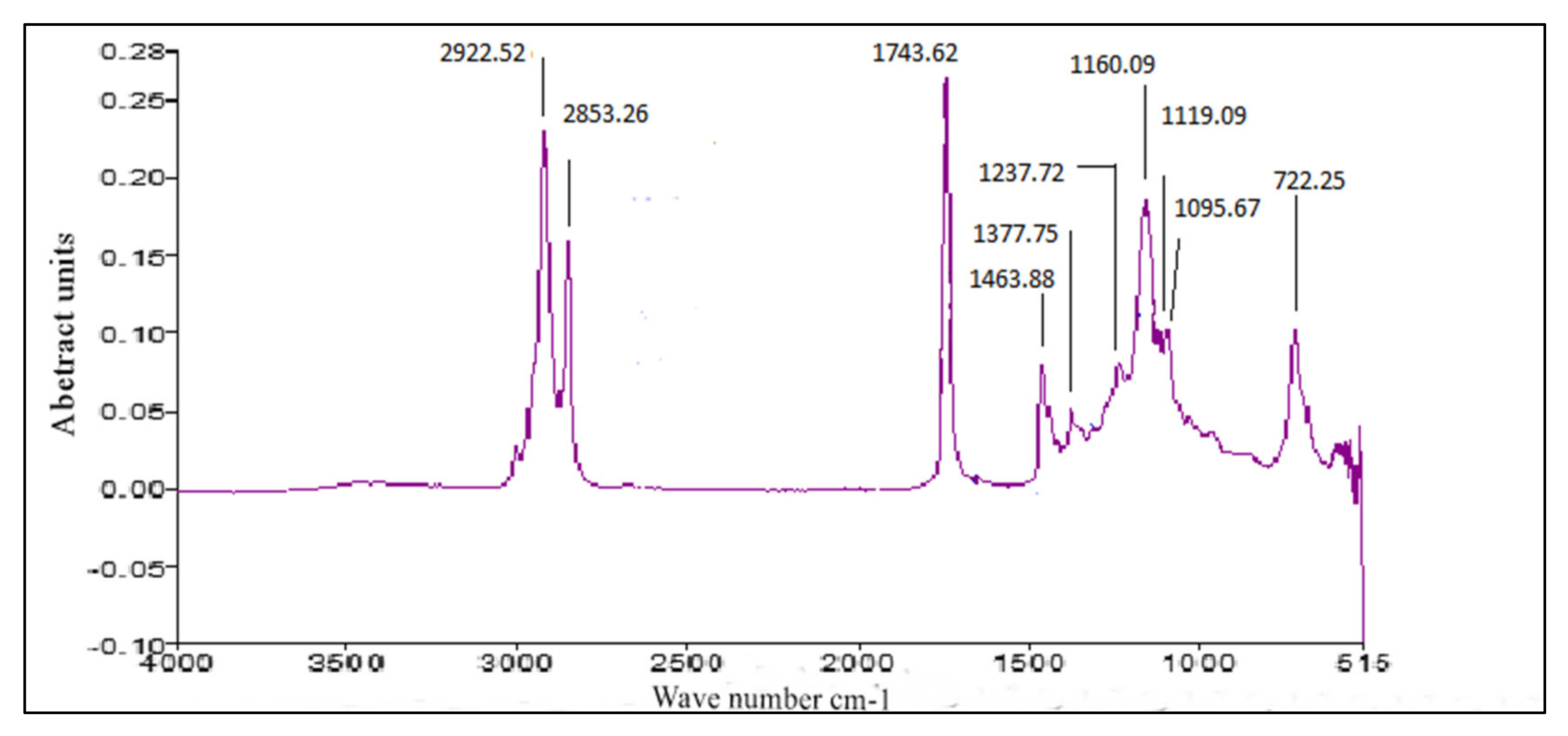
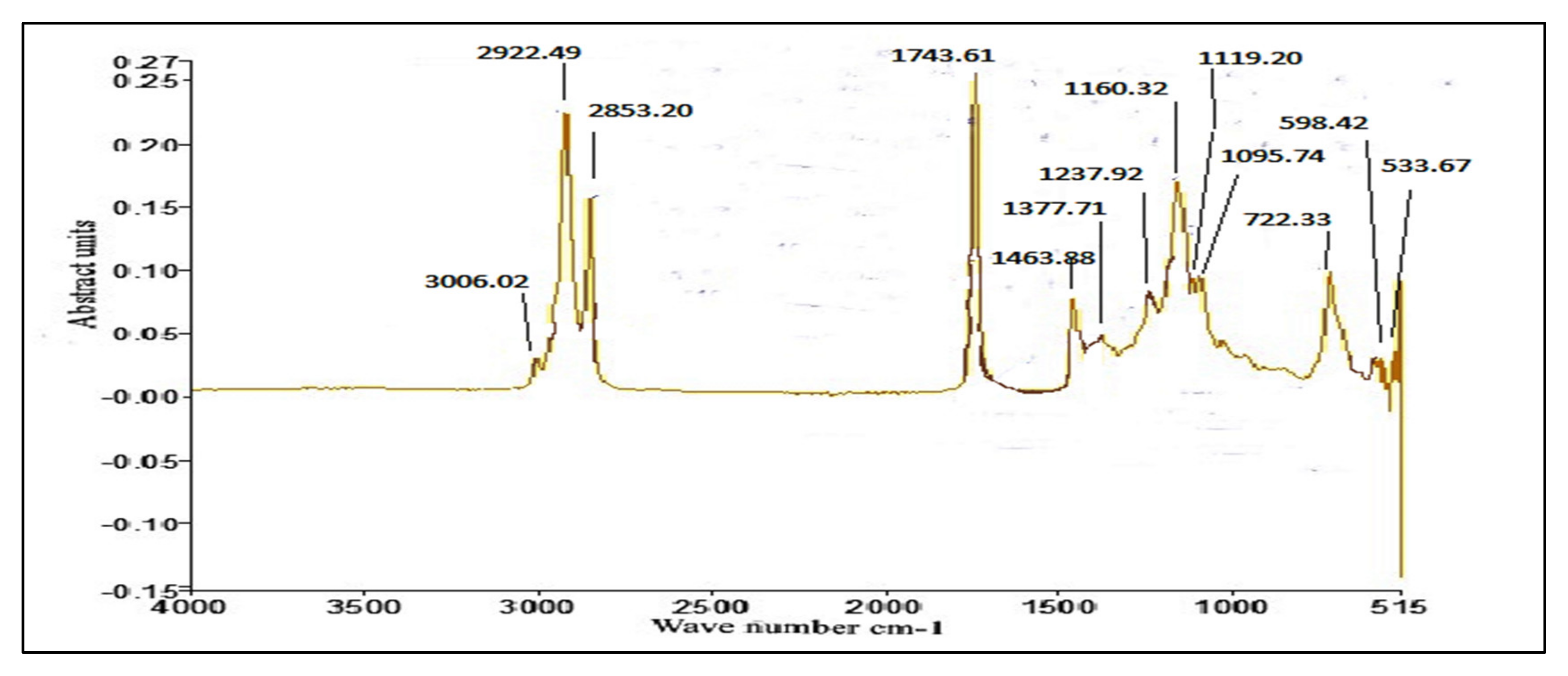
3.4. FT-IR of P. aitchisonii Seed Oil Biodiesel (PAOB)
Comparative Analysis of FT-IR of P. aitchisonii FAMEs
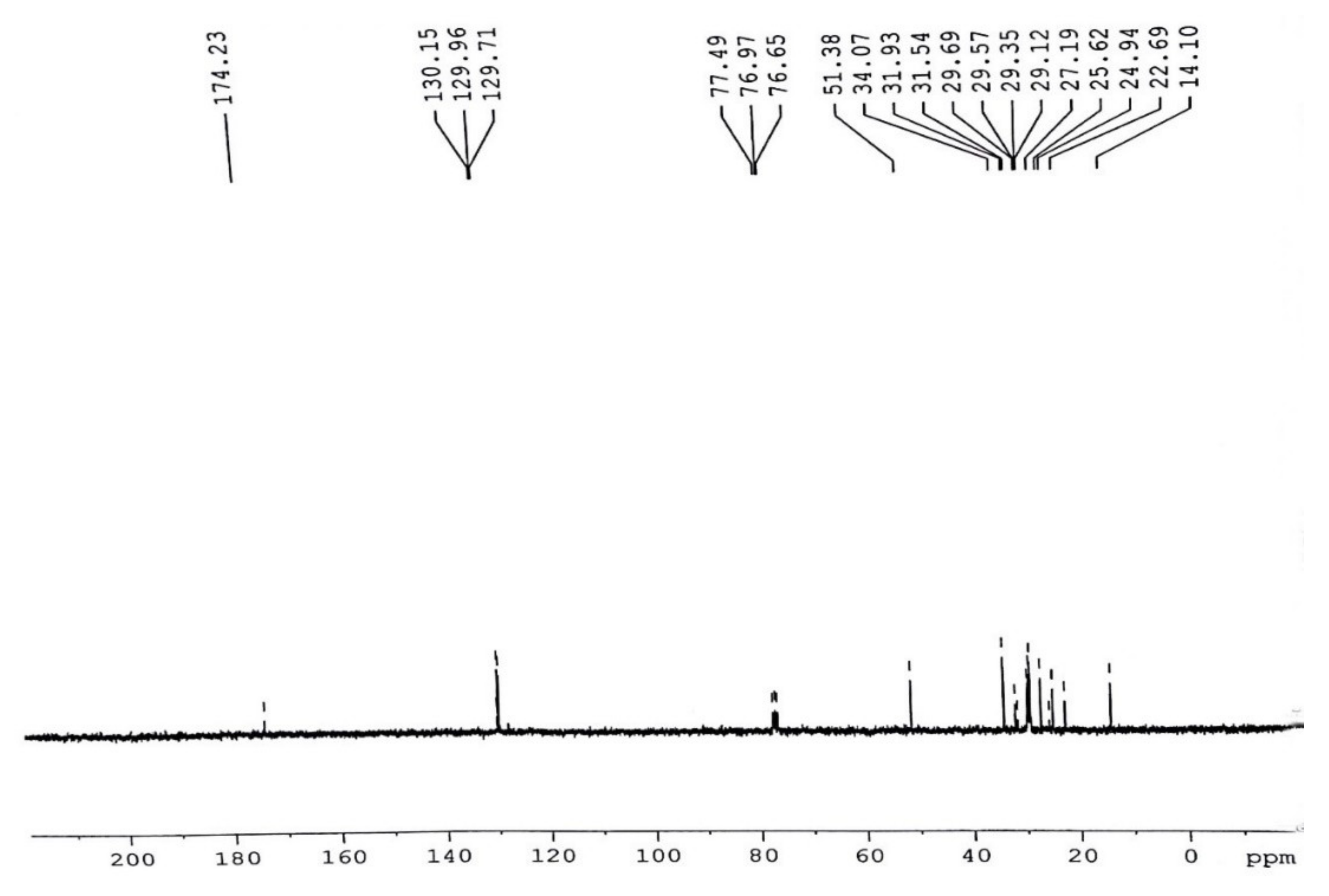
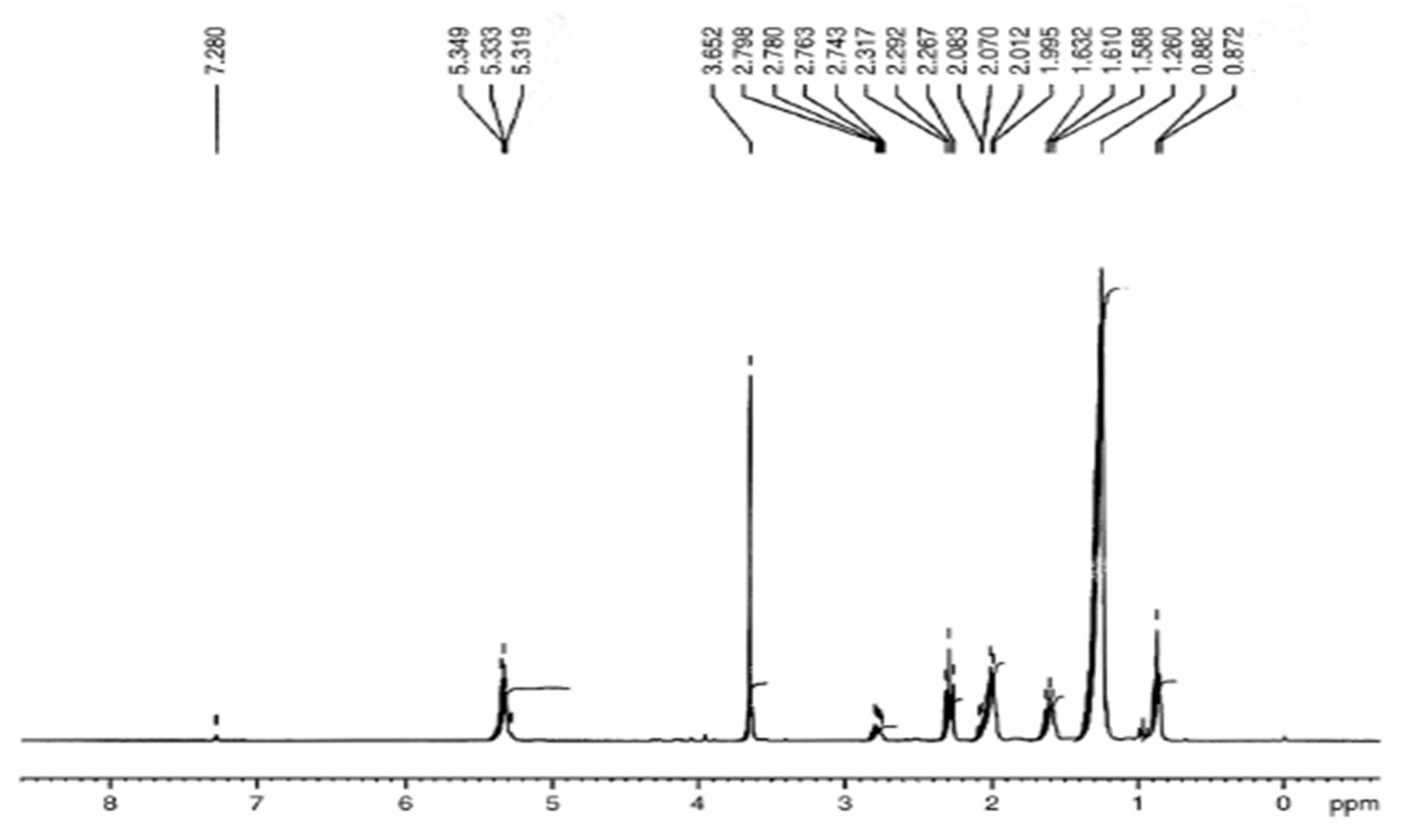
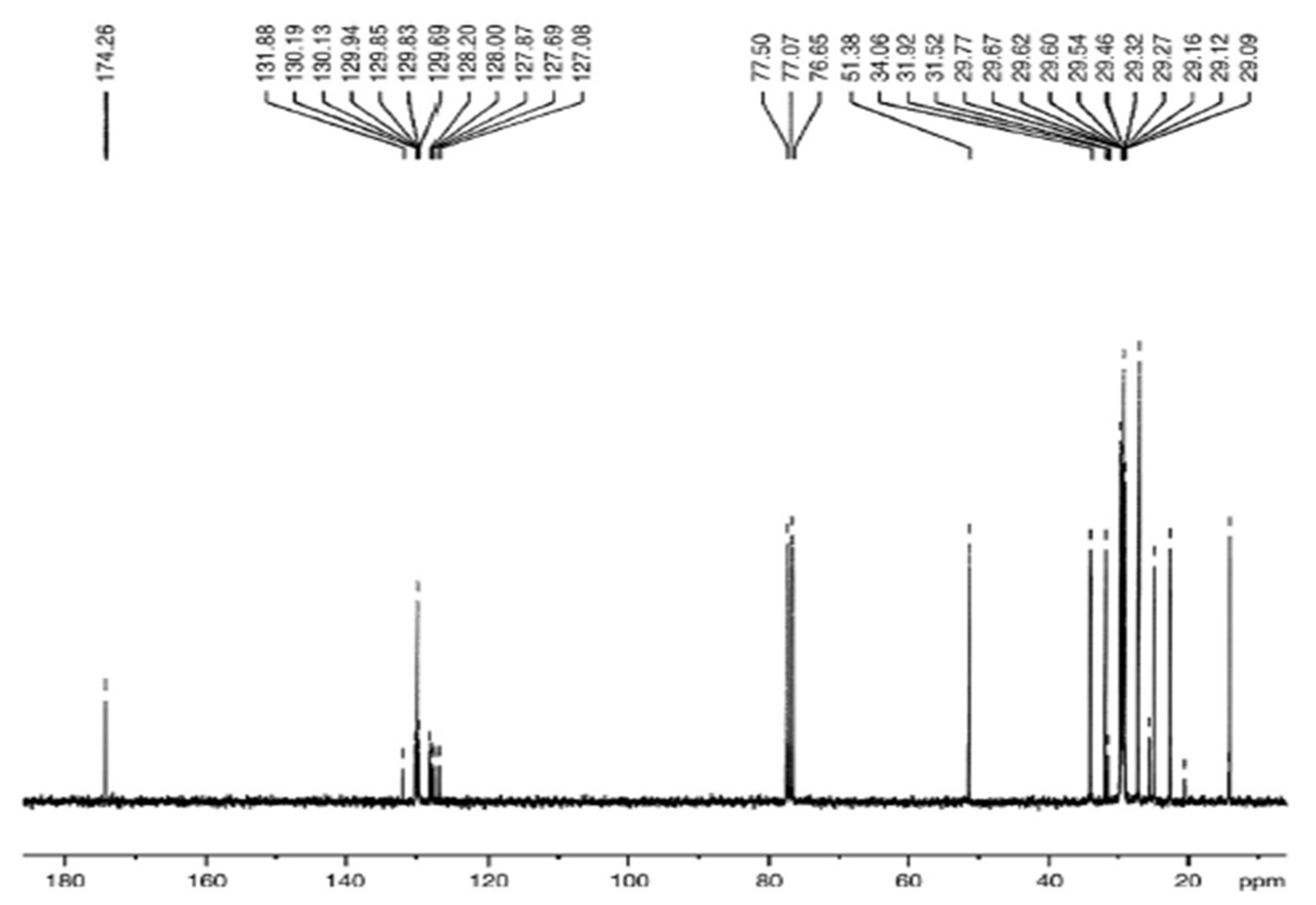
3.5. Comparative NMR Analysis of Prunus aitchisonii FAMEs
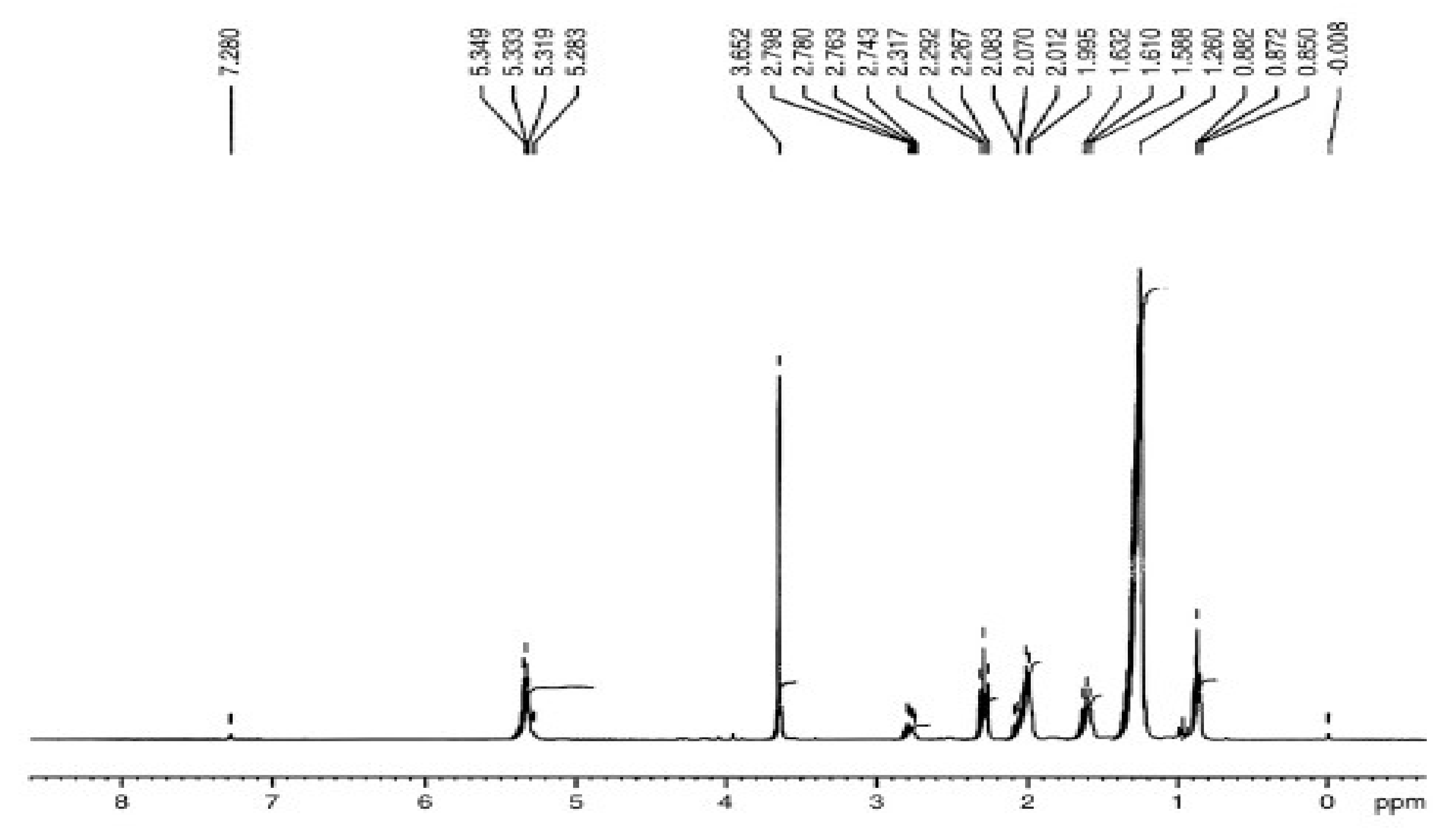
3.5.1. H NMR Assessment of P. aitchisonii Biodiesel Deal with TiO2
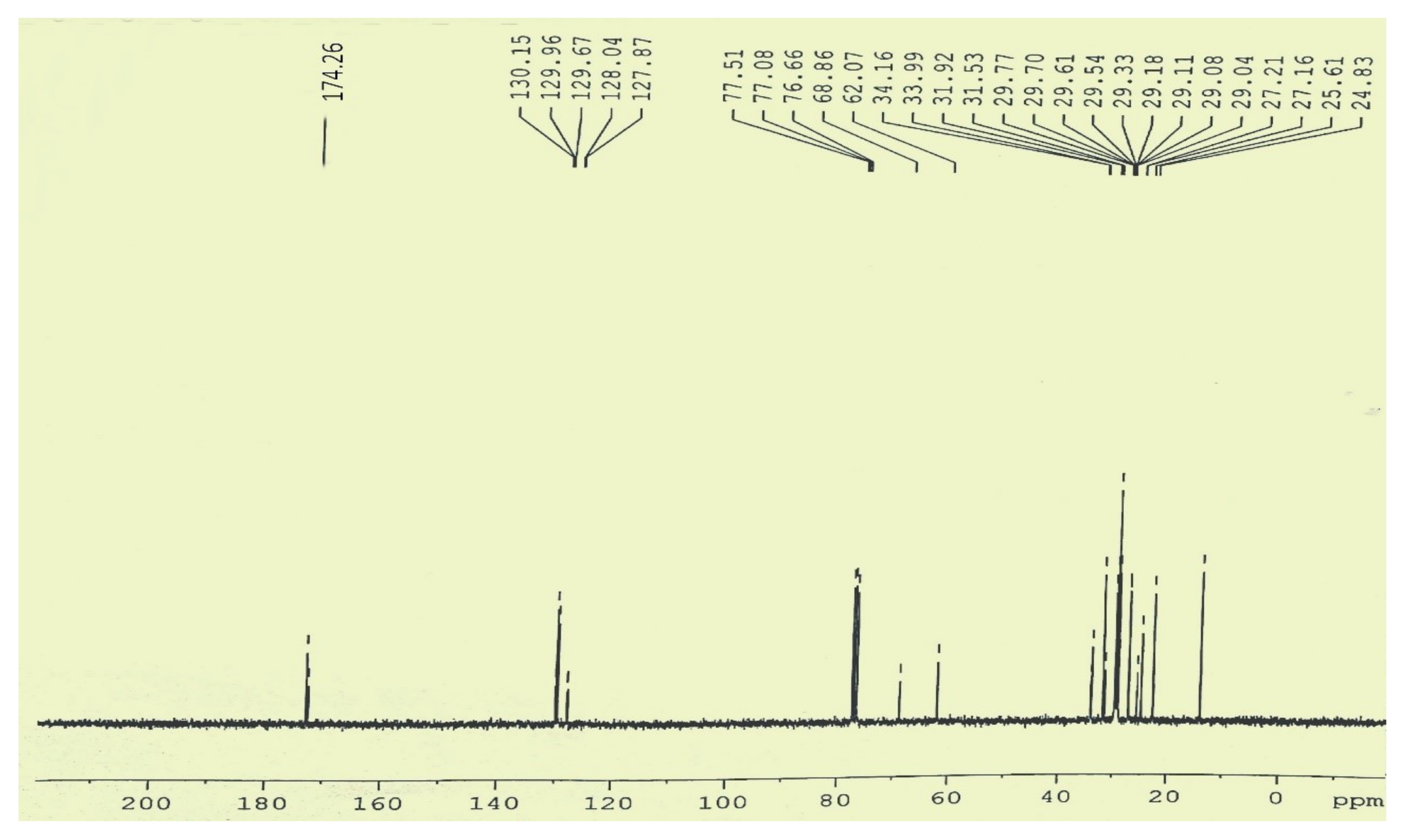
3.5.2. C-NMR Examination of P. aitchisonii FAME Deals with (TiO2)
3.5.3. “1. H NMR” Analysis of P. aitchisonii FAMEs Resulted from CPC
3.5.4. C NMR Analysis of P. aitchisonii FAMEs Treated with CPC
3.5.5. H NMR Analysis of P. aitchisonii FAMEs Treated with KPC
3.5.6. C NMR Analysis of Prunus aitchisonii Biodiesel Treated with KPC
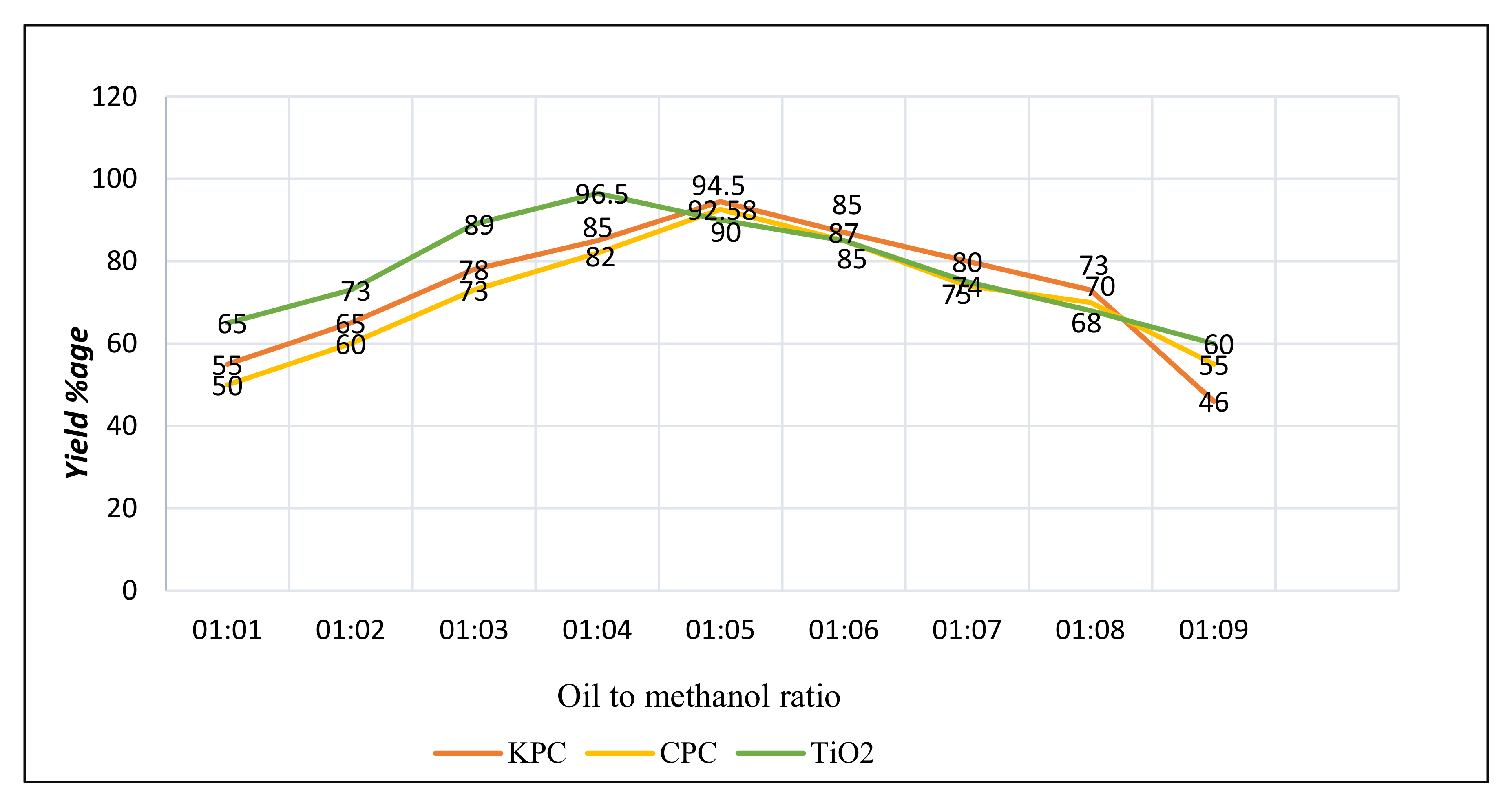
3.6. Biodiesel Optimization
- Oil and methanol proportion;
- Amount of catalysts;
- The temperature of the chemical reaction;
- The time period of reaction.
3.6.1. Oil and Methanol Proportion
3.6.2. Amount of Catalysts
3.6.3. Temperature of Reaction
3.6.4. Time Period of Reaction
3.7. Physical Properties of P. aitchisonii Oil Biodiesel (PAOB)
3.7.1. Flash-Point
3.7.2. Density
3.7.3. Kinematic Viscosity
3.7.4. Pour Point and Cloud Point
3.7.5. Sulphur Weight %
3.7.6. Acid-Value
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marriboina, S.; Sengupta, D.; Kumar, S.; Reddy, A.R. Physiological and molecular insights into the high salinity tolerance of Pongamia pinnata (L.) pierre, a potential biofuel tree species. Plant Sci. 2017, 258, 102–111. [Google Scholar] [PubMed]
- Mardhiah, H.H.; Ong, H.C.; Masjuki, H.; Lim, S.; Pang, Y.L. Investigation of carbon-based solid acid catalyst from Jatropha curcas biomass in biodiesel production. Energy Convers. Manag. 2017, 144, 10–17. [Google Scholar] [CrossRef]
- García-Martínez, N.; Andreo-Martínez, P.; Quesada-Medina, J.; de los Ríos, A.P.; Chica, A.; Beneito-Ruiz, R.; Carratalá-Abril, J. Optimization of non-catalytic transesterification of tobacco (Nicotiana tabacum) seed oil using supercritical methanol to biodiesel production. Energy Convers. Manag. 2017, 131, 99–108. [Google Scholar] [CrossRef]
- Dhawane, S.H.; Kumar, T.; Halder, G. Biodiesel synthesis from Hevea brasiliensis oil employing carbon supported heterogeneous catalyst: Optimization by Taguchi method. Renew. Energy 2016, 89, 506–514. [Google Scholar] [CrossRef]
- Maran, J.P.; Priya, B. Modeling of ultrasound assisted intensification of biodiesel production from neem (Azadirachta indica) oil using response surface methodology and artificial neural network. Fuel 2015, 143, 262–267. [Google Scholar] [CrossRef]
- Fadhil, A.B.; Ahmed, K.M.; Dheyab, M.M. Silybum marianum L. seed oil: A novel feedstock for biodiesel production. Arab. J. Chem. 2017, 10, S683–S690. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Caldwell, C.; Corscadden, K.; He, Q.S.; Li, J. An evaluation of biodiesel production from Camelina sativa grown in Nova Scotia. Ind. Crops Prod. 2016, 81, 162–168. [Google Scholar]
- Mumtaz, M.W.; Mukhtar, H.; Dilawer, U.A.; Hussain, S.M.; Hussain, M.; Iqbal, M.; Adnan, A.; Nisar, J. Biocatalytic transesterification of Eruca sativa oil for the production of biodiesel. Biocatal. Agric. Biotechnol. 2016, 5, 162–167. [Google Scholar] [CrossRef]
- Shaheen, A.; Sultana, S.; Lu, H.; Ahmad, M.; Asma, M.; Mahmood, T. Assessing the potential of different nano-composite (MgO, Al2O3-CaO and TiO2) for efficient conversion of Silybum eburneum seed oil to liquid biodiesel. J. Mol. Liq. 2018, 249, 511–521. [Google Scholar] [CrossRef]
- Singh, B.; Guldhe, A.; Rawat, I.; Bux, F. Towards a sustainable approach for development of biodiesel from plant and microalgae. Renew. Sustain. Energy Rev. 2014, 29, 216–245. [Google Scholar]
- Okitsu, K.; Maeda, Y.; Bandow, H. Ultrasound assisted production of fatty acid methyl esters from transesterification of triglycerides with methanol in the presence of KOH catalyst: Optimization, mechanism and kinetics. Ultrason. Sonochem. 2014, 21, 467–471. [Google Scholar]
- Kumar, G.; Kumar, D.; Johari, R.; Singh, C. Enzymatic transesterification of Jatropha curcas oil assisted by ultrasonication. Ultrason. Sonochem. 2011, 18, 923–927. [Google Scholar] [CrossRef]
- Dosado, A.G.; Chen, W.-T.; Chan, A.; Sun-Waterhouse, D.; Waterhouse, G.I. Novel Au/TiO2 photocatalysts for hydrogen production in alcohol–water mixtures based on hydrogen titanate nanotube precursors. J. Catal. 2015, 330, 238–254. [Google Scholar] [CrossRef]
- Kumar, A.; Osembo, S.O.; Namango, S.S.; Kiriamiti, K.H. Heterogeneous basic catalysts for transesterification of vegetable oils: A review. In Proceedings of the Sustainable Research and Innovation Conference, Barcelona, Spain, 22–23 October 2012; pp. 59–68. [Google Scholar]
- Sotoft, L.F.; Rong, B.-G.; Christensen, K.V.; Norddahl, B. Process simulation and economical evaluation of enzymatic biodiesel production plant. Bioresour. Technol. 2010, 101, 5266–5274. [Google Scholar] [CrossRef]
- Guldhe, A.; Moura, C.V.; Singh, P.; Rawat, I.; Moura, E.M.; Sharma, Y.; Bux, F. Conversion of microalgal lipids to biodiesel using chromium-aluminum mixed oxide as a heterogeneous solid acid catalyst. Renew. Energy 2017, 105, 175–182. [Google Scholar] [CrossRef]
- Prabu, S.S.; Asokan, M.; Roy, R.; Francis, S.; Sreelekh, M. Performance, combustion and emission characteristics of diesel engine fuelled with waste cooking oil bio-diesel/diesel blends with additives. Energy 2017, 122, 638–648. [Google Scholar] [CrossRef]
- Gutiérrez-Zapata, C.; Martínez, D.B.; Collazos, C.; Acuña, H.C.; Cuervo, J.; Fernandez, C. Productions of sunflower oil biodiesel and used cooking oil through heterogeneous catalysts compared to conventional homogeneous catalysts. J. Phys. Conf. Ser. 2017, 786, 012025. [Google Scholar] [CrossRef]
- Gürsel, I.V.; Noël, T.; Wang, Q.; Hessel, V. Separation/recycling methods for homogeneous transition metal catalysts in continuous flow. Green Chem. 2015, 17, 2012–2026. [Google Scholar] [CrossRef]
- Hu, S.; Guan, Y.; Wang, Y.; Han, H. Nano-magnetic catalyst KF/CaO–Fe3O4 for biodiesel production. Appl. Energy 2011, 88, 2685–2690. [Google Scholar] [CrossRef]
- Chen, L.; Liu, T.; Zhang, W.; Chen, X.; Wang, J. Biodiesel production from algae oil high in free fatty acids by two-step catalytic conversion. Bioresour. Technol. 2012, 111, 208–214. [Google Scholar] [CrossRef]
- Chaudhry, B.; Akhtar, M.S.; Ahmad, M.; Munir, M.; Zafar, M.; Alhajeri, N.S.; Ala’a, H.; Ahmad, Z.; Hasan, M.; Bokhari, A. Membrane based reactors for sustainable treatment of Coronopus didymus L. by developing Iodine doped potassium oxide membrane under Dynamic conditions. Chemosphere 2022, 303, 135138. [Google Scholar] [CrossRef]
- Dawood, S.; Koyande, A.K.; Ahmad, M.; Mubashir, M.; Asif, S.; Klemeš, J.J.; Bokhari, A.; Saqib, S.; Lee, M.; Qyyum, M.A. Synthesis of biodiesel from non-edible (Brachychiton populneus) oil in the presence of nickel oxide nanocatalyst: Parametric and optimisation studies. Chemosphere 2021, 278, 130469. [Google Scholar] [CrossRef] [PubMed]
- Tantirungrotechai, J.; Thepwatee, S.; Yoosuk, B. Biodiesel synthesis over Sr/MgO solid base catalyst. Fuel 2013, 106, 279–284. [Google Scholar] [CrossRef]
- Akia, M.; Yazdani, F.; Motaee, E.; Han, D.; Arandiyan, H. A review on conversion of biomass to biofuel by nanocatalysts. Biofuel Res. J. 2014, 1, 16–25. [Google Scholar] [CrossRef]
- Ullah, K.; Ahmad, M.; Sharma, V.K.; Lu, P.; Harvey, A.; Zafar, M.; Sultana, S. Assessing the potential of algal biomass opportunities for bioenergy industry: A review. Fuel 2015, 143, 414–423. [Google Scholar] [CrossRef]
- Khalil, H.A.; Aprilia, N.S.; Bhat, A.; Jawaid, M.; Paridah, M.; Rudi, D. A Jatropha biomass as renewable materials for biocomposites and its applications. Renew. Sustain. Energy Rev. 2013, 22, 667–685. [Google Scholar] [CrossRef]
- Theivasanthi, T.; Alagar, M. Titanium dioxide (TiO2) Nanoparticles XRD Analyses: An Insight. arXiv 2013, arXiv:1307.1091. [Google Scholar]
- Ahmad, M.; Sultana, S.; Keat Teong, L.; Abdullah, A.Z.; Sadia, H.; Zafar, M.; Hadda, T.B.; Ashraf, M.A.; Tareen, R.B. Distaff Thistle Oil: A possible new non-edible feedstock for bioenergy. Int. J. Green Energy 2015, 12, 1066–1075. [Google Scholar] [CrossRef]
- Ahmad, M.; Teong, L.K.; Zafar, M.; Sultana, S.; Sadia, H.; Khan, M.A. Prospects and potential of green fuel from some non traditional seed oils used as biodiesel. In Biodiesel—Feedstocks, Production and Applications; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Rashid, U.; Ibrahim, M.; Yasin, S.; Yunus, R.; Taufiq-Yap, Y.; Knothe, G. Biodiesel from Citrus reticulata (mandarin orange) seed oil, a potential non-food feedstock. Ind. Crops Prod. 2013, 45, 355–359. [Google Scholar] [CrossRef]
- Moser, B.R. Camelina (Camelina sativa L.) oil as a biofuels feedstock: Golden opportunity or false hope? Lipid Technol. 2010, 22, 270–273. [Google Scholar] [CrossRef]
- Joshi, H.; Moser, B.R.; Toler, J.; Walker, T. Preparation and fuel properties of mixtures of soybean oil methyl and ethyl esters. Biomass Bioenergy 2010, 34, 14–20. [Google Scholar] [CrossRef]
- Munir, M.; Ahmad, M.; Saeed, M.; Waseem, A.; Nizami, A.-S.; Sultana, S.; Zafar, M.; Rehan, M.; Srinivasan, G.R.; Ali, A.M. Biodiesel production from novel non-edible caper (Capparis spinosa L.) seeds oil employing Cu–Ni doped ZrO2 catalyst. Renew. Sustain. Energy Rev. 2020, 138, 110558. [Google Scholar] [CrossRef]
- Demirbas, A. Biodiesel from waste cooking oil via base-catalytic and supercritical methanol transesterification. Energy Convers. Manag. 2009, 50, 923–927. [Google Scholar] [CrossRef]
- Dhar, A.; Kevin, R.; Agarwal, A.K. Production of biodiesel from high-FFA neem oil and its performance, emission and combustion characterization in a single cylinder DICI engine. Fuel Process. Technol. 2012, 97, 118–129. [Google Scholar] [CrossRef]
- Wang, Y.; Ou, S.; Liu, P.; Zhang, Z. Preparation of biodiesel from waste cooking oil via two-step catalyzed process. Energy Convers. Manag. 2007, 48, 184–188. [Google Scholar] [CrossRef]
- Naik, M.; Meher, L.; Naik, S.; Das, L. Production of biodiesel from high free fatty acid Karanja (Pongamia pinnata) oil. Biomass Bioenergy 2008, 32, 354–357. [Google Scholar] [CrossRef]
- Vicente, G.; Coteron, A.; Martinez, M.; Aracil, J. Application of the factorial design of experiments and response surface methodology to optimize biodiesel production. Ind. Crops Prod. 1998, 8, 29–35. [Google Scholar] [CrossRef]
- Gurunathan, B.; Ravi, A. Biodiesel production from waste cooking oil using copper doped zinc oxide nanocomposite as heterogeneous catalyst. Bioresour. Technol. 2015, 188, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Ahmad, M.; Sultana, S.; Teong, L.K.; Sharma, V.K.; Abdullah, A.Z.; Zafar, M.; Ullah, Z. Experimental analysis of di-functional magnetic oxide catalyst and its performance in the hemp plant biodiesel production. Appl. Energy 2014, 113, 660–669. [Google Scholar] [CrossRef]
- Ali, O.M.; Mamat, R.; Abdullah, N.R.; Abdullah, A.A. Analysis of blended fuel properties and engine performance with palm biodiesel–diesel blended fuel. Renew. Energy 2016, 86, 59–67. [Google Scholar] [CrossRef] [Green Version]
- Krishna, M.; Chowdary, R. Comparative Studies on Performance Evaluation of Waste Fried Vegetable Oil in Crude Form and Biodiesel Form in Conventional Diesel Engine; SAE Technical Paper 0148-7191; SAE: Warrendale, PA, USA, 2014. [Google Scholar]
- Lam, M.K.; Lee, K.T.; Mohamed, A.R. Homogeneous, heterogeneous and enzymatic catalysis for transesterification of high free fatty acid oil (waste cooking oil) to biodiesel: A review. Biotechnol. Adv. 2010, 28, 500–518. [Google Scholar] [CrossRef] [PubMed]
- Shajudheen, V.M.; Vishwanathan, K.; Rani, K.A.; Maheswari, A.U.; Kumar, S.S. A simple chemical precipitation method of titanium dioxide nanoparticles using polyvinyl pyrrolidone as a capping agent and their characterization. Int. Sci. Index Chem. Mol. Eng. 2016, 10, 556–559. [Google Scholar]
- Kamel, D.A.; Farag, H.A.; Amin, N.K.; Zatout, A.A.; Ali, R.M. Smart utilization of jatropha (Jatropha curcas Linnaeus) seeds for biodiesel production: Optimization and mechanism. Ind. Crops Prod. 2018, 111, 407–413. [Google Scholar] [CrossRef]
- Mello, V.M.; Oliveira, F.C.; Fraga, W.G.; do Nascimento, C.J.; Suarez, P.A. Determination of the content of fatty acid methyl esters (FAME) in biodiesel samples obtained by esterification using 1H-NMR spectroscopy. Magn. Reson. Chem. 2008, 46, 1051–1054. [Google Scholar] [CrossRef]
- Samios, D.; Pedrotti, F.; Nicolau, A.; Reiznautt, Q.; Martini, D.; Dalcin, F. A transesterification double step process—TDSP for biodiesel preparation from fatty acids triglycerides. Fuel Processing Technol. 2009, 90, 599–605. [Google Scholar] [CrossRef]
- Gelbard, G.; Bres, O.; Vargas, R.; Vielfaure, F.; Schuchardt, U. 1H nuclear magnetic resonance determination of the yield of the transesterification of rapeseed oil with methanol. J. Am. Oil Chem. Soc. 1995, 72, 1239–1241. [Google Scholar] [CrossRef]
- Knothe, G. Monitoring a progressing transesterification reaction by fiber-optic near infrared spectroscopy with correlation to 1H nuclear magnetic resonance spectroscopy. J. Am. Oil Chem. Soc. 2000, 77, 489–493. [Google Scholar] [CrossRef]
- Ahmad, M.; Ullah, K.; Khan, M.; Ali, S.; Zafar, M.; Sultana, S. Quantitative and qualitative analysis of sesame oil biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1239–1249. [Google Scholar] [CrossRef]
- Menon, V.; Rao, M. Trends in bioconversion of lignocellulose: Biofuels, platform chemicals & biorefinery concept. Prog. Energy Combust. Sci. 2012, 38, 522–550. [Google Scholar]
- Sultana, S.; Khalid, A.; Ahmad, M.; Zuhairi, A.A.; Teong, L.K.; Zafar, M.; ul Hassan, F. The production, optimization, and characterization of biodiesel from a novel source: Sinapis alba L. Int. J. Green Energy 2014, 11, 280–291. [Google Scholar] [CrossRef]
- Khan, N.; Dessouky, H. Biodiesel production from corn oil by transesterification process. Nucleus 2009, 46, 241–252. [Google Scholar]
- Rashid, U.; Anwar, F.; Knothe, G. Evaluation of biodiesel obtained from cottonseed oil. Fuel Processing Technol. 2009, 90, 1157–1163. [Google Scholar] [CrossRef]
- Shah, M.; Tariq, M.; Ali, S.; Guo, Q.-X.; Fu, Y. Transesterification of jojoba oil, sunflower oil, neem oil, rocket seed oil and linseed oil by tin catalysts. Biomass Bioenergy 2014, 70, 225–229. [Google Scholar] [CrossRef]
- El-Enin, S.A.; Attia, N.; El-Ibiari, N.; El-Diwani, G.; El-Khatib, K. In-situ transesterification of rapeseed and cost indicators for biodiesel production. Renew. Sustain. Energy Rev. 2013, 18, 471–477. [Google Scholar] [CrossRef]
- Patil, P.D.; Deng, S. Optimization of biodiesel production from edible and non-edible vegetable oils. Fuel 2009, 88, 1302–1306. [Google Scholar] [CrossRef]
- Sinha, S.; Agarwal, A.K.; Garg, S. Biodiesel development from rice bran oil: Transesterification process optimization and fuel characterization. Energy Convers. Manag. 2008, 49, 1248–1257. [Google Scholar] [CrossRef]
- Khan, N.A.; el Dessouky, H. Prospect of biodiesel in Pakistan. Renew. Sustain. Energy Rev. 2009, 13, 1576–1583. [Google Scholar] [CrossRef]
- Candeia, R.; Silva, M.; Carvalho Filho, J.; Brasilino, M.; Bicudo, T.; Santos, I.; Souza, A. Influence of soybean biodiesel content on basic properties of biodiesel–diesel blends. Fuel 2009, 88, 738–743. [Google Scholar] [CrossRef]
- Morshed, M.; Ferdous, K.; Khan, M.R.; Mazumder, M.; Islam, M.; Uddin, M.T. Rubber seed oil as a potential source for biodiesel production in Bangladesh. Fuel 2011, 90, 2981–2986. [Google Scholar] [CrossRef]
- Karmee, S.K.; Chadha, A. Preparation of biodiesel from crude oil of Pongamia pinnata. Bioresour. Technol. 2005, 96, 1425–1429. [Google Scholar] [CrossRef]
- Ullah, F.; Nosheen, A.; Hussain, I.; Banon, A. Base catalyzed transesterification of wild apricot kernel oil for biodiesel production. Afr. J. Biotechnol. 2009, 8, 14. [Google Scholar]
- Chamola, R.; Khan, M.F.; Raj, A.; Verma, M.; Jain, S. Response surface methodology based optimization of in situ transesterification of dry algae with methanol, H2SO4 and NaOH. Fuel 2019, 239, 511–520. [Google Scholar] [CrossRef]
- Ahmad, M.; Ullah, K.; Khan, M.; Zafar, M.; Tariq, M.; Ali, S.; Sultana, S. Physicochemical analysis of hemp oil biodiesel: A promising non edible new source for bioenergy. Energy Sources Part A Recovery Util. Environ. Eff. 2011, 33, 1365–1374. [Google Scholar] [CrossRef]
- Ilham, Z.; Saka, S. Two-step supercritical dimethyl carbonate method for biodiesel production from Jatropha curcas oil. Bioresour. Technol. 2010, 101, 2735–2740. [Google Scholar] [CrossRef] [Green Version]
- Murshid, G.; Shariff, A.M.; Keong, L.K.; Bustam, M.A. Physical Properties of Aqueous Solutions of Piperazine and (2-Amino-2-methyl-1-propanol+ Piperazine) from (298.15 to 333.15) K. J. Chem. Eng. Data 2011, 56, 2660–2663. [Google Scholar] [CrossRef]
- Saka, S.; Isayama, Y. A new process for catalyst-free production of biodiesel using supercritical methyl acetate. Fuel 2009, 88, 1307–1313. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, F.; Khan, A.U.; Yasar, A. Transesterification of oil extracted from different species of algae for biodiesel production. Afr. J. Environ. Sci. Technol. 2013, 7, 358–364. [Google Scholar]
- Ahmad, M.; Zafar, M.; Rashid, S.; Sultana, S.; Sadia, H.; Ajab Khan, M. Production of methyl ester (biodiesel) from four plant species of Brassicaceae: Optimization of the transesterification process. Int. J. Green Energy 2013, 10, 362–369. [Google Scholar] [CrossRef]
- Rashid, U.; Rehman, H.A.; Hussain, I.; Ibrahim, M.; Haider, M.S. Muskmelon (Cucumis melo) seed oil: A potential non-food oil source for biodiesel production. Energy 2011, 36, 5632–5639. [Google Scholar] [CrossRef]
- Ahmad, M.; Khan, M.; Zafar, M.; Sultana, S. Environment-friendly renewable energy from sesame biodiesel. Energy Sources Part A Recovery Util. Environ. Eff. 2009, 32, 189–196. [Google Scholar] [CrossRef]
- Wang, R.; Hanna, M.A.; Zhou, W.-W.; Bhadury, P.S.; Chen, Q.; Song, B.-A.; Yang, S. Production and selected fuel properties of biodiesel from promising non-edible oils: Euphorbia lathyris L., Sapium sebiferum L. and Jatropha curcas L. Bioresour. Technol. 2011, 102, 1194–1199. [Google Scholar] [CrossRef] [PubMed]




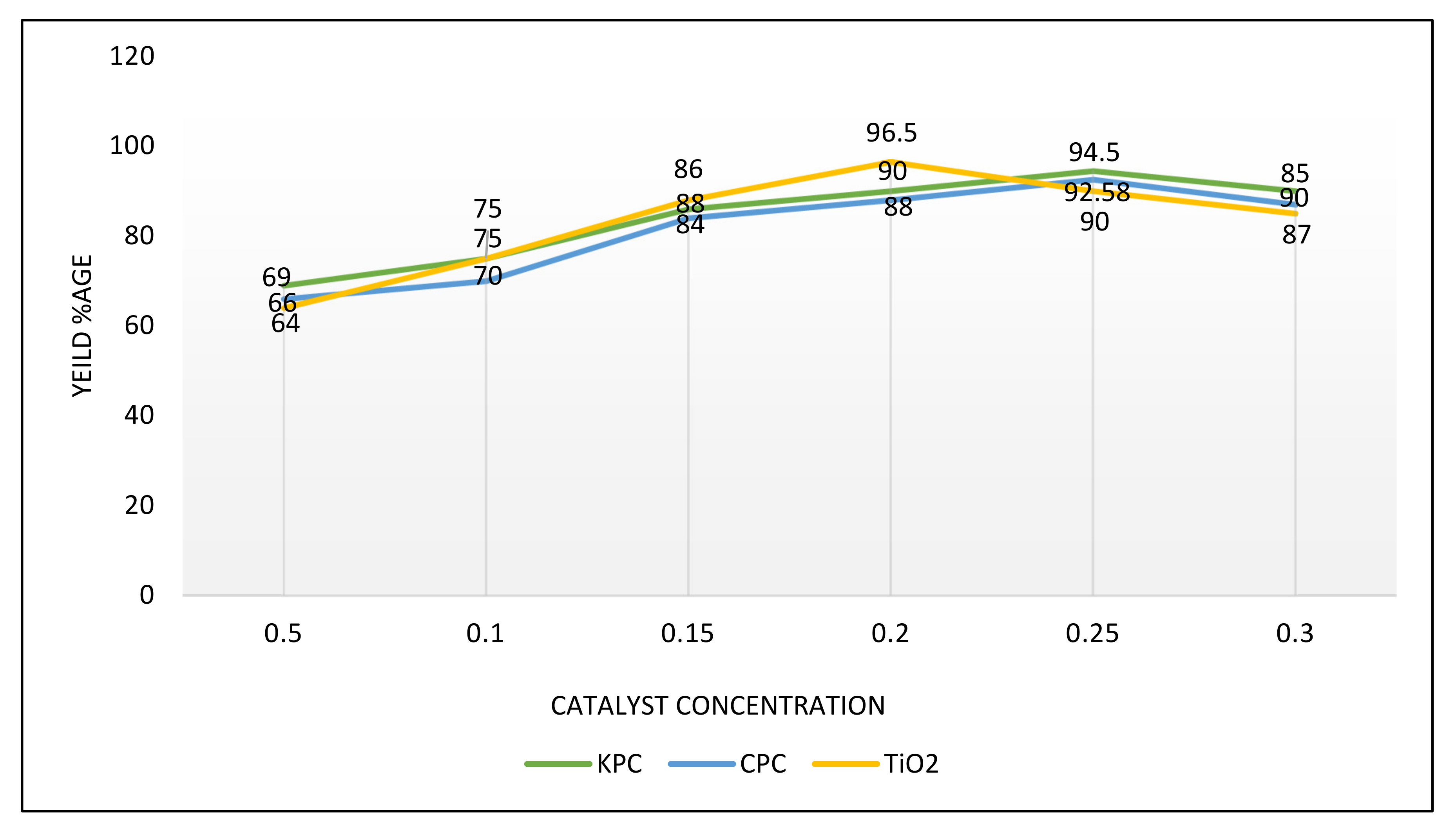
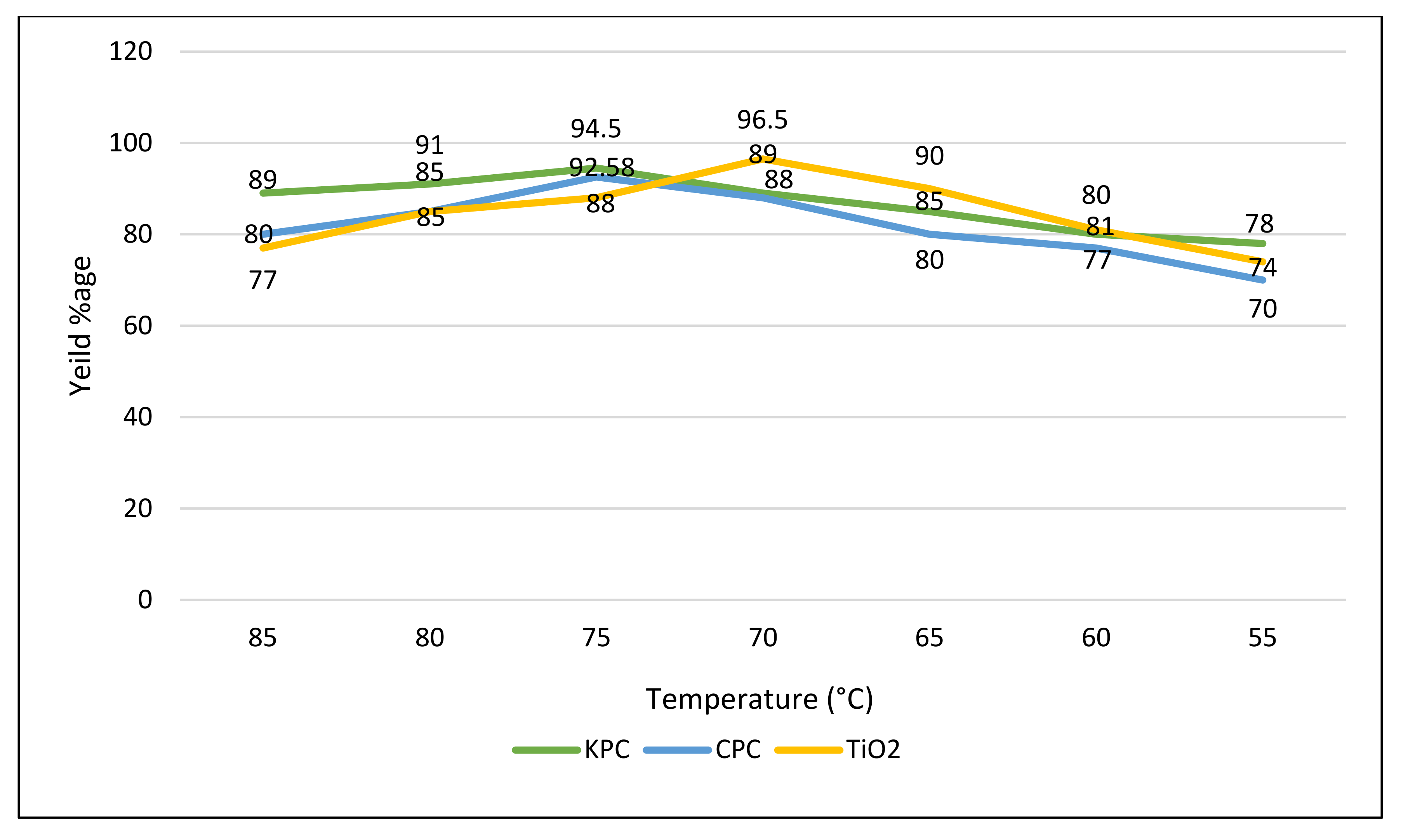

| S. No | Name of Catalyst | Amount of Catalysts (gm) | Oil to Methanol Ratios | Time Period (h) | Temperature (°C) | Conversion %Age |
|---|---|---|---|---|---|---|
| 1 | CPC | 0.25 | 1:5 | 2 | 70 | 92.58 |
| 2 | KPC | 0.25 | 1:5 | 2 | 70 | 94.5 |
| 3 | TiO2 | 0.20 | 1:4 | 2 | 75 | 96.5 |
| S. No | Element | Weight % | Atomic ta% |
|---|---|---|---|
| 1 | C K | 60.45 | 67.91 |
| 2 | N K | 13.22 | 12.74 |
| 3 | O K | 20.06 | 16.92 |
| 4 | Mg K | 0.62 | 0.35 |
| 5 | P K | 1.43 | 0.62 |
| 6 | S K | 0.30 | 0.13 |
| 7 | K K | 2.35 | 0.81 |
| 8 | Ca K | 1.57 | 0.53 |
| 9 | Total | 100.00 | 100.00 |
| S. No | Element | Weight % | Atomic % |
|---|---|---|---|
| 1 | C K | 26.05 | 39.15 |
| 2 | N K | 4.54 | 5.85 |
| 3 | O K | 34.38 | 38.79 |
| 4 | P K | 0.38 | 0.22 |
| 5 | K K | 34.65 | 16.00 |
| 6 | Total | 100.00 | 100.00 |
| S. No | Element | Weight % | Atomic % |
|---|---|---|---|
| 1 | O K | 41.57 | 67.61 |
| 2 | Ti K | 58.43 | 32.39 |
| 3 | Total | 100.00 | 100.00 |
| Heterogenous Nano-Catalysts | Peak # | Possible Compounds | Retention Time (min) | Formula | Molecular Weight g/mole | Base Peak | Percentage % | Total Percentage of Methyl Ester |
|---|---|---|---|---|---|---|---|---|
| TiO2 | 2 | Dodecanoic acid methyl ester | 18.658 | C13H26O2 | 214 | 74 | 0.78 | 74.5% |
| 3 | 9-Hexadecenoic acid methyl ester | 24.291 | C17H32O2 | 268 | 55 | 1.33 | ||
| 4 | (Hexadecanoic acid methyl ester | 24.579 | C17H34O2 | 270 | 74 | 11.42 | ||
| 6 | 9,12-Octadecadienoic acid methyl ester | 26.783 | C19H34O2 | 294 | 67 | 20.70 | ||
| 7 | 9-Octadecenoic acid methyl ester | 26.928 | C19H36O2 | 296 | 55 | 35.56 | ||
| 8 | Octadecenoic acid methyl ester | 27.139 | C19H38O2 | 298 | 74 | 4.36 | ||
| 10 | 9-Octadecenoic acid, 1,2,3-dihydroxypropyl ester | 31.094 | C21H40O4 | 356 | 129 | 1.40 | ||
| KPC | 2 | Hexadecanoic acid methyl ester | 24.570 | C17H34O2 | 270 | 74 | 1.17 | 58% |
| 4 | 9,12-Octadecadienoic acid methyl ester | 26.741 | C19H34O2 | 294 | 67 | 4.32 | ||
| 5 | 9-Octadecenoic acid methyl ester | 26.826 | C19H36O2 | 296 | 55 | 15.05 | ||
| 6 | 9,12,15-Octadecatrienoic acid ethyl ester- | 27.515 | C20H34O2 | 306 | 67 | 31.68 | ||
| 8 | 9-Octadecenoic acid 1,2,3 propanetriyl ester | 30.587 | C57H104O6 | 884 | 55 | 0.97 | ||
| 10 | 9-Octadecenoic acid, 2,3-dihydroxypropyl ester | 31.102 | C21H40O4 | 356 | 129 | 2.86 | ||
| CPC | 3 | Carbonochloridic acid ethyl ester | 1.884 | C3H5ClO | 108 | 45 | 43.78 | 50% |
| 5 | 9-12-Octadecadienoic acid methyl esters | 23.867 | C19H34O2 | 294 | 73 | 1.72 | ||
| 6 | 9-Octadecenoic acid methyl esters | 23.949 | C19H36O2 | 296 | 67 | 3.78 |
| S. No | Fuel Properties | Testing Methods | ASTM Standards | Results |
|---|---|---|---|---|
| 1 | Color | ASTM D-1500 | 2 | Visual |
| 2 | Flashpoint (°C) | ASTM D-93 | 60–100 | 71.5 |
| 3 | Density @ 15 °C | ASTM D-1298 | 0.86–0.90 | 0.836 |
| 4 | Kinematic viscosity @ 40 °C | ASTM D-445 | 1.9–6.0 | 4.33 |
| 5 | Pour point °C | ASTM D-97 | −15 to 16 | −7 |
| 6 | Cloud point °C | ASTM D-2500 | −3 to 12 | −8 |
| 7 | Sulfur % wt | ASTM D-4294 | 0.05 | 0.00015 |
| 8 | Total acid number mg KOH/gm | ASTM D-974 | 0.5 | 0.114 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nisa, B.; Ullah, F.; Nisa, I.; Ahmad, M.; Zafar, M.; Munir, M.; Sultana, S.; Zaman, W.; Manghwar, H.; Ullah, F.; et al. Biodiesel Production Using Wild Apricot (Prunus aitchisonii) Seed Oil via Heterogeneous Catalysts. Molecules 2022, 27, 4752. https://doi.org/10.3390/molecules27154752
Nisa B, Ullah F, Nisa I, Ahmad M, Zafar M, Munir M, Sultana S, Zaman W, Manghwar H, Ullah F, et al. Biodiesel Production Using Wild Apricot (Prunus aitchisonii) Seed Oil via Heterogeneous Catalysts. Molecules. 2022; 27(15):4752. https://doi.org/10.3390/molecules27154752
Chicago/Turabian StyleNisa, Batool, Fazal Ullah, Iqbal Nisa, Mushtaq Ahmad, Muhammad Zafar, Mamoona Munir, Shazia Sultana, Wajid Zaman, Hakim Manghwar, Farman Ullah, and et al. 2022. "Biodiesel Production Using Wild Apricot (Prunus aitchisonii) Seed Oil via Heterogeneous Catalysts" Molecules 27, no. 15: 4752. https://doi.org/10.3390/molecules27154752









