Rapid Identification for the Pterocarpus Bracelet by Three-Step Infrared Spectrum Method
Abstract
:1. Introduction
2. Results and Discussion
2.1. FTIR Analysis
2.2. SDIR Analysis
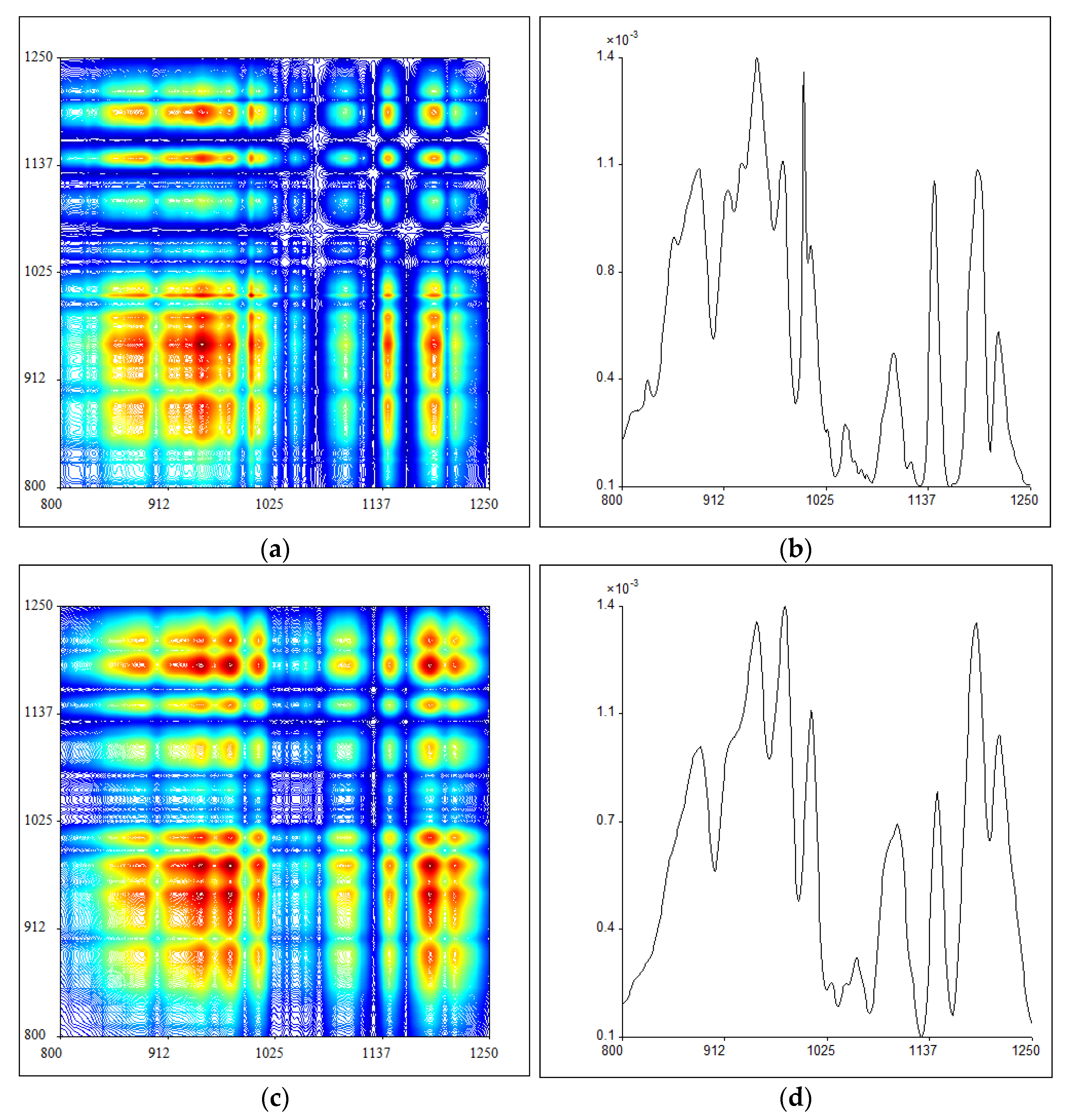
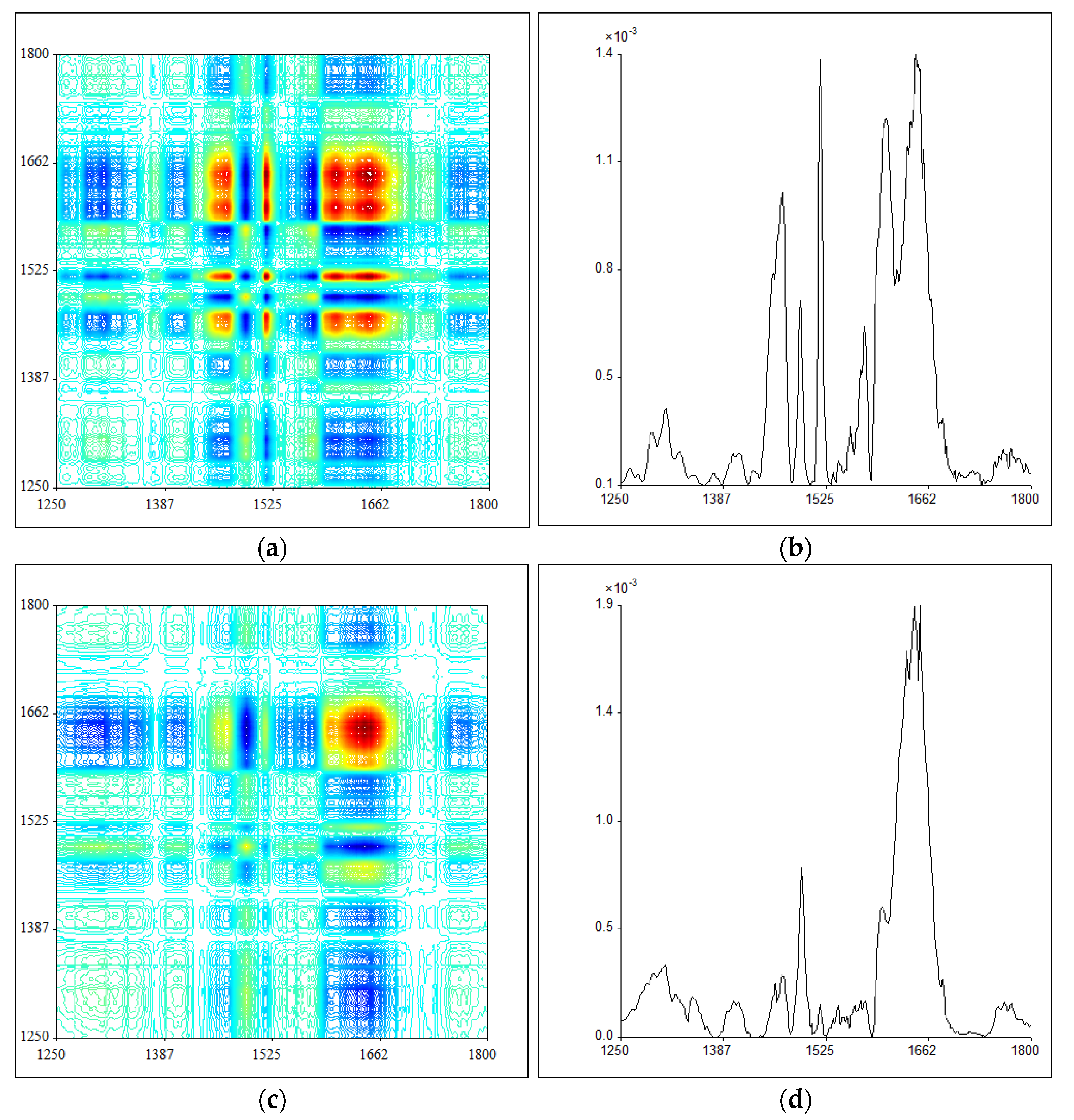
2.3. 2DIR Analysis
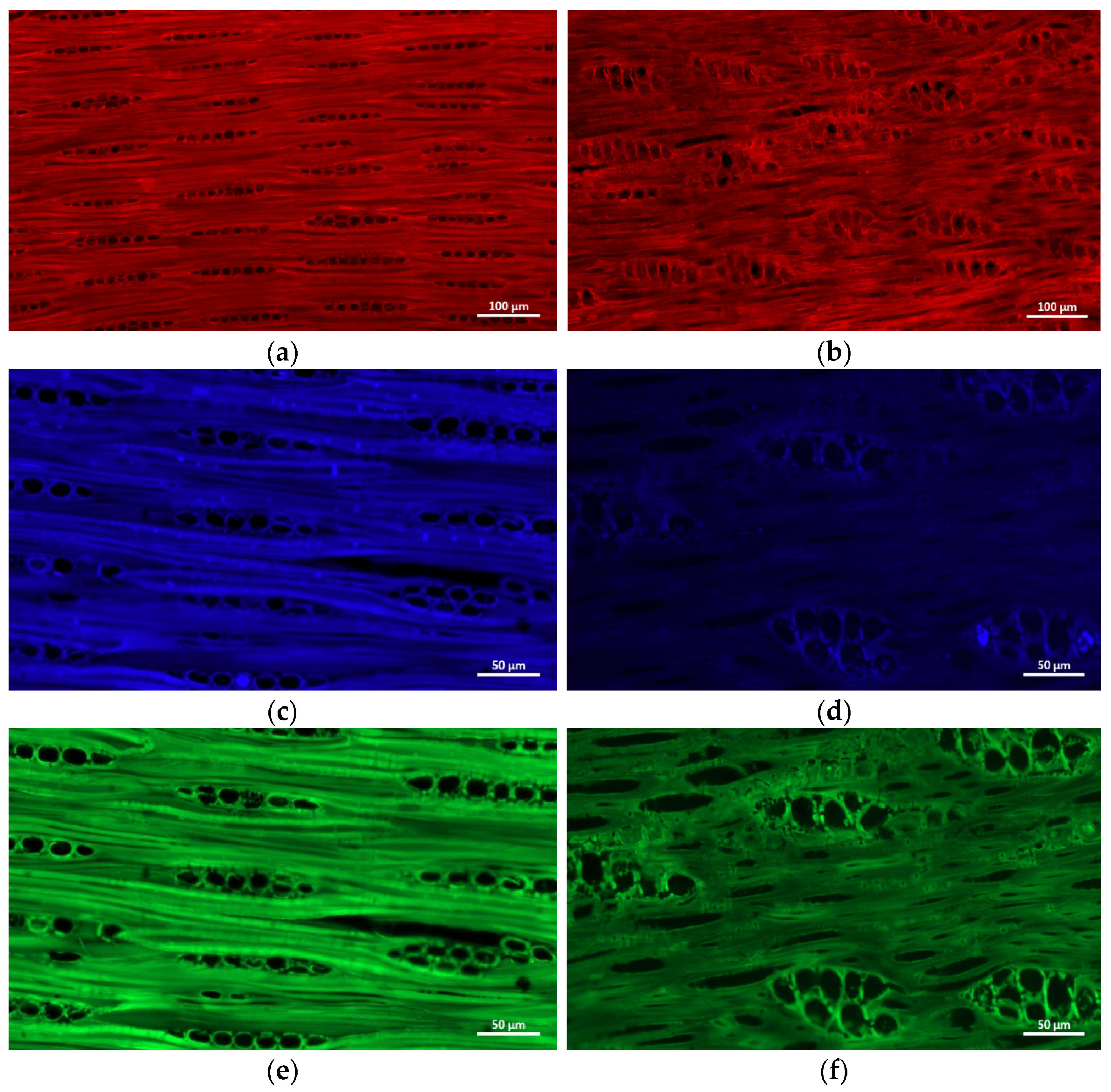
2.4. Fluorescence Microscopy
3. Materials and Methods
3.1. Materials and Samples Preparation
3.2. FTIR Analysis
3.3. SDIR Analysis
3.4. 2DIR Analysis
3.5. Fluorescence Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Maclachlan, I.R.; Gasson, P. PCA of cites listed pterocarpus santalinus (leguminosae) wood. IAWA J. 2010, 31, 121–138. [Google Scholar] [CrossRef] [Green Version]
- IUCN. The IUCN Red List of Threatened Species. Version 2017; IUCN: Gland, Switzerland, 2017; p. 3. [Google Scholar]
- Price, E.R.; Miles-Bunch, I.A.; Gasson, P.E.; Lancaster, C.A. Pterocarpus wood identification by independent and complementary analysis of DARTTOFMS, microscopic anatomy, and fluorescence spectrometry. IAWA J. 2021, 42, 397–418. [Google Scholar] [CrossRef]
- Liu, Y.; Huang, P.; Lin, F.; Yang, W.; Kettle, C.J. MaxEnt modelling for predicting the potential distribution of a near threatened rosewood species (Dalbergia cultrata Graham ex Benth). Ecol. Eng. 2019, 141, 105612. [Google Scholar] [CrossRef]
- Zhu, A.L. China’s rosewood boom: A cultural fix to capital overaccumulation. Ann. Am. Assoc. Geogr. 2020, 110, 277–296. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, J.; Cui, T.; Chen, Q.; Ma, Y. Introduction, growth performance and ecological adaptability of hongmu tree species (Pterocarpus Spp.) in China. J. Trop. For. Sci. 2016, 28, 260–267. [Google Scholar] [CrossRef]
- Yu, W.; Sun, Z.; Liu, Y. Rapid detection and identification of objects using a self-designed methodology based on LIBS and PCA-DVSM-taking rosewood for example. Optik 2021, 248, 168069. [Google Scholar] [CrossRef]
- Yang, X.; Zhao, Z.; Wang, Z.; Ge, Z.; Zhou, Y. Microstructure identification based on vessel pores feature extraction of high-value hardwood species. Bioresources 2021, 16, 5329–5340. [Google Scholar] [CrossRef]
- Gasson, P.; Baas, P.; Wheeler, E. Anatomy of CITES listed tree species. IAWA J. 2011, 32, 155–198. [Google Scholar] [CrossRef]
- Koch, G.; Haag, V.; Heinz, I.; Richter, H.G.; Schmitt, U. Control of internationally traded timber-the role of macroscopic and microscopic wood identification against illegal logging. J. Forensic Res. 2015, 6, 1–4. [Google Scholar] [CrossRef]
- Yin, Y.F.; Wiedenhoeft, A.C.; Donaldson, L. Advancing wood identification-anatomical and molecular techniques. IAWA J. 2020, 41, 391–392. [Google Scholar] [CrossRef]
- Gasson, P. How precise can wood identification be? Wood anatomy’s role in support of the legal timber trade, especially CITES. IAWA J. 2011, 32, 137–154. [Google Scholar] [CrossRef]
- Yu, M.; Jiao, L.; Guo, J.; Wiedenhoeft, A.C.; He, T.; Jiang, X.; Yin, Y. DNA barcoding of vouchered xylarium wood specimens of nine endangered Dalbergia species. Planta 2017, 246, 1165–1176. [Google Scholar] [CrossRef]
- Jiao, L.; Yu, M.; Wiedenhoeft, A.C.; He, T.; Li, J.; Liu, B.; Jiang, X.; Yin, Y. DNA barcode authentication and library development for the wood of six commercial Pterocarpus species: The critical role of Xylarium specimens. Sci. Rep. 2018, 8, 1945. [Google Scholar] [CrossRef] [PubMed]
- Hartvig, I.; Czako, M.; Kjær, E.D.; Nielsen, L.R.; Theilade, I. The use of DNA barcoding in identification and conservation of rosewood (Dalbergia spp.). PLoS ONE 2015, 10, e0138231. [Google Scholar] [CrossRef] [Green Version]
- Dickson, A.; Nanayakkara, B.; Sellier, D.; Meason, D.; Donaldson, L.; Brownlie, R. Fluorescence imaging of cambial zones to study wood formation in Pinus radiata D. Don. Trees 2017, 31, 479–490. [Google Scholar] [CrossRef]
- Wang, J.Z.; Liu, B.; Du, H.H.; Liang, Z.; Zhao, Y.F.; Zhao, Y.; Zhang, M.Z.; Wang, L.K. New method for identification of Dalbergia cochinchinensis Pierre and Dalbergia oliveri Prain by fluorescence spectroscopy. Spectrosc. Spect. Anal. 2019, 39, 2182–2189. [Google Scholar] [CrossRef]
- Sun, L.; Wang, M.; Ren, X.; Jiang, M.; Deng, Y. Journal of pharmaceutical and biomedical analysis rapid authentication and differentiation of herbal medicine using 1H NMR fingerprints coupled with chemometrics. J. Pharm. Biomed. Anal. 2018, 160, 323–329. [Google Scholar] [CrossRef]
- Emmanuel, V.; Odile, B.; Céline, R. FTIR spectroscopy of woods: A new approach to study the weathering of the carving face of a sculpture. Spectrochim. Acta Mol. Biomol. Spectrosc. 2015, 136, 1255–1259. [Google Scholar] [CrossRef]
- Traoré, M.; Kaal, J.; Cortizas, A.M. Application of FTIR spectroscopy to the characterization of archeological wood. Spectrochim. Acta Mol. Biomol. Spectrosc. 2016, 153, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Chen, B.; Ren, C.; Guo, M.; Feng, Y. Rapid identification model based on decision tree algorithm coupling with 1H NMR and feature analysis by UHPLC-QTOFMS spectrometry for sandalwood. J. Chromatogr. B 2020, 1161, 122449. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Zhao, G.; Guo, J.; Wiedenhoeft, A.C.; Liu, C.C.; Yin, Y. Timber species identification from chemical fingerprints using direct analysis in real time (DART) coupled to Fourier transform ion cyclotron resonance mass spectrometry (FTICR-MS): Comparison of wood samples subjected to different treatments. Holzforschung 2019, 73, 975–985. [Google Scholar] [CrossRef]
- Yin, X.; Huang, A.; Zhang, S.; Ru, L.; Ma, F. Identification of three Dalbergia species based on differences in extractive components. Molecules 2018, 23, 2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaw, J.M.; Sevilla, F.B., III. Discrimination of wood species based on a carbon nanotube/polymer composite chemiresistor array. Holzforschung 2017, 72, 215–223. [Google Scholar] [CrossRef]
- Du, H.; Wang, J.; Liu, B.; Liang, Z.; Liu, Z.; Zhang, M.; Zhao, Y.; Luo, J. A novel wood identification method for Pterocarpus santalinus L.f. species based on fluorescence features. J. Wood Chem. Technol. 2021, 41, 321–328. [Google Scholar] [CrossRef]
- Yang, F.; Chu, T.; Zhang, Y.; Liu, X.; Sun, G.; Chen, Z. Quality assessment of licorice (Glycyrrhiza glabra L.) from different sources by multiple fingerprint profiles combined with quantitative analysis, antioxidant activity and chemometric methods. Food Chem. 2020, 324, 126854. [Google Scholar] [CrossRef] [PubMed]
- Hua, R.; Sun, S.Q.; Zhou, Q.; Noda, I.; Wang, B.Q. Discrimination of fritillary according to geographical origin with Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. J. Pharmaceut. Biomed. Anal. 2003, 33, 199–209. [Google Scholar] [CrossRef]
- Zhou, Q.; Sun, S.Q.; Zuo, L. Study on traditional chinese medicine “Qing Kai Ling” injections from different manufactures by 2D IR correlation spectroscopy. Vib. Spectrosc. 2004, 36, 207. [Google Scholar] [CrossRef]
- Sun, S.Q.; Li, C.W.; Wei, J.P.; Zhou, Q.; Noda, I. Discrimination of chinese sauce liquor using FTIR and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 2006, 799, 72–76. [Google Scholar] [CrossRef]
- Noda, I.; Dowrey, A.E.; Marcott, C. Recent developments in two-dimensional infrared (2DIR) correlation spectroscopy. Appl. Spectrosc. 1993, 47, 1317–1323. [Google Scholar] [CrossRef]
- Zhou, Q.; Li, J.; Liu, J.; Huang, H.; Sun, S.Q. Two-dimensional correlation infrared spectroscopy of standard and false Dahuang. Chin. J. Anal. Chem. 2003, 31, 1058–1061. [Google Scholar]
- Huang, A.; Wang, G.; Zhou, Q.; Liu, J.; Sun, S. Study of thermal perturbation of natural bamboo fiber by two dimensional correlation analysis and Fourier transform infrared spectroscopy. Spectrosc. Spect. Anal. 2008, 28, 1237–1241. [Google Scholar]
- Huang, A.; Zhou, Q.; Liu, J.; Fei, B.; Sun, S. Distinction of three wood species by Fourier transform infrared spectroscopy and two-dimensional correlation IR spectroscopy. J. Mol. Struct. 2008, 883–884, 160–166. [Google Scholar] [CrossRef]
- Zhang, F.D.; Xu, C.H.; Li, M.Y.; Huang, A.M.; Sun, S.Q. Rapid identification of Pterocarpus Santalinus and Dalbergia Louvelii by FTIR and 2D correlation IR spectroscopy. J. Mol. Struct. 2014, 1069, 89–95. [Google Scholar] [CrossRef]
- Zhang, F.D.; Xu, C.H.; Li, M.Y.; Chen, X.D.; Zhou, Q.; Huang, A.M. Identification of Dalbergia cochinchinensis (CITES Appendix II) from other three Dalbergia species using FT-IR and 2D correlation IR spectroscopy. Wood Sci. Technol. 2016, 50, 693–704. [Google Scholar] [CrossRef]
- Wang, S.N.; Zhang, F.D.; Huang, A.M.; Zhou, Q. Distinction of four dalbergia species by FTIR, 2nd derivative IR, and 2D-IR spectroscopy of their ethanol-benzene extractives. Holzforschung 2016, 70, 503–510. [Google Scholar] [CrossRef]
- Liu, J.; Huang, A.; Zhang, Q. Impact analysis of infrared spectra in pterocarpus santalinus and confused species coated with wood wax oil. Spectrosc. Spect. Anal. 2019, 39, 3816–3820. [Google Scholar]
- Weng, S.F. Fourier Transform Infrared Spectroscopy Analysis; Chemical Industry Press: Beijing, China, 2010. [Google Scholar]
- Qu, L.; Chen, J.; Zhang, G.J.; Sun, S.; Zheng, J. Chemical profiling and adulteration screening of Aquilariae Lignum Resinatum by Fourier transform infrared (FT-IR) spectroscopy and two-dimensional correlation infrared (2D-IR) spectroscopy. Spectrochim. Acta A 2017, 174, 177. [Google Scholar] [CrossRef] [PubMed]
- Haberhauer, G.; Gerzabek, M.H. Drift and transmission FT-IR spectroscopy of forest soils: An approach to determine decomposition processes of forest litter. Vib. Spectrosc. 1999, 19, 413–417. [Google Scholar] [CrossRef]
- Schwanninger, M.; Rodrigues, J.C.; Pereira, H.; Hinterstoisser, B. Effects of short-time vibratory milling on the shape of FT-IR spectra of wood and cellulose. Vib. Spectrosc. 2004, 36, 23–40. [Google Scholar] [CrossRef]
- Liu, B.; Wu, R.; Lu, Y.; Zhang, M.; Jiang, X.; Yin, Y. Interspecies differences and differences between heartwood and sapwood in wood fluorescence properties of pterocarpus species. China Wood Industry 2019, 33, 14–17. [Google Scholar] [CrossRef]


| Wavenumber (cm−1) | Band Assignment | |
|---|---|---|
| A | B | |
| 3399 | 3399 | O–H stretching vibration of carbohydrate C–OH |
| 2935 | 2934 | C–H asymmetric stretching in alkanes (methyl or methylene) |
| 1736 | 1735 | Unconjugated C=O stretching vibration of xylan |
| 1594 | 1596 | Stretching vibration of carbon atoms in the aromatic framework |
| 1513 | 1508 | Aromatic skeletal vibrations in extractives and lignin |
| 1459 | 1459 | C–H bending vibration; aromatic skeletal vibrations |
| 1425 | 1426 | Aromatic skeletal vibration in lignin and C–H deformation in-plane deforming |
| 1370 | 1373 | C–H deformation, CH3 symmetric deformation in holocellulose |
| 1322 | 1328 | C–O stretch of acetate group in hemicelluloses and interaction band involving C–OH bend |
| 1267 | 1267 | O–C–O and Guaiacyl ring Stretching vibration in lignin and xylan |
| 1231 | 1234 | O–C–O and Syringyl ring Stretching vibration in lignin and xylan |
| 1205 | 1205 | C–O–C stretching vibration in extractives |
| 1155 | 1157 | C–O–C stretching or frame vibration in holocellulose |
| 1106 | 1112 | C–C, C–O stretching in holocellulose |
| 1058 | 1059 | C–O stretching vibration in holocellulose |
| 1034 | 1034 | C–O stretching vibration |
| 897 | 898 | C1–H deformation in cellulose |
| 836 | 834 | C–H out-of-plane deformation of aromatic ring in extractives and lignin |
| 828 | 885 | 916 | 949 | 977 | 1000 | 1008 | 1045 | 1057 | 1100 | 1144 | 1190 | 1214 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | + | ++ | ++ | +++ | ++ | +++ | + | + | − | + | ++ | ++ | + |
| B | − | ++ | ++ | +++ | +++ | ++ | ++ | + | + | ++ | + | +++ | ++ |
| 1292 | 1311 | 1456 | 1467 | 1491 | 1518 | 1558 | 1576 | 1605 | 1645 | |
|---|---|---|---|---|---|---|---|---|---|---|
| A | + | + | ++ | +++ | ++ | +++ | + | ++ | +++ | +++ |
| B | + | + | + | + | ++ | − | − | − | + | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, Z.; Cui, W.; Zhang, F.; Wang, F.; Cheng, S.; Fu, Y.; Huang, A. Rapid Identification for the Pterocarpus Bracelet by Three-Step Infrared Spectrum Method. Molecules 2022, 27, 4793. https://doi.org/10.3390/molecules27154793
Jin Z, Cui W, Zhang F, Wang F, Cheng S, Fu Y, Huang A. Rapid Identification for the Pterocarpus Bracelet by Three-Step Infrared Spectrum Method. Molecules. 2022; 27(15):4793. https://doi.org/10.3390/molecules27154793
Chicago/Turabian StyleJin, Zhi, Weili Cui, Fangda Zhang, Fang Wang, Shichao Cheng, Yuejin Fu, and Anmin Huang. 2022. "Rapid Identification for the Pterocarpus Bracelet by Three-Step Infrared Spectrum Method" Molecules 27, no. 15: 4793. https://doi.org/10.3390/molecules27154793
APA StyleJin, Z., Cui, W., Zhang, F., Wang, F., Cheng, S., Fu, Y., & Huang, A. (2022). Rapid Identification for the Pterocarpus Bracelet by Three-Step Infrared Spectrum Method. Molecules, 27(15), 4793. https://doi.org/10.3390/molecules27154793






