A Novel Turn-On Fluorescence Probe Based on Cu(II) Functionalized Metal–Organic Frameworks for Visual Detection of Uric Acid
Abstract
:1. Introduction
2. Results and Discussion
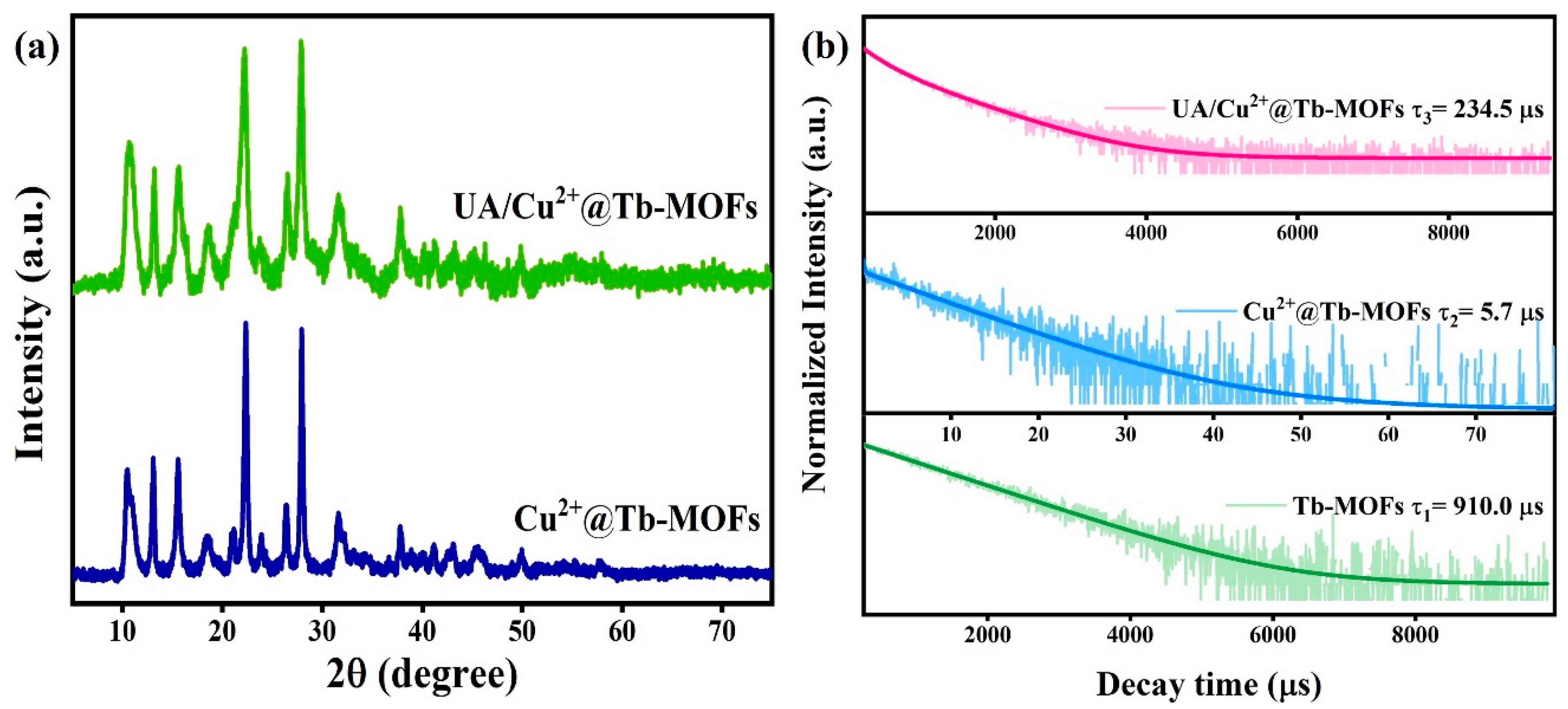
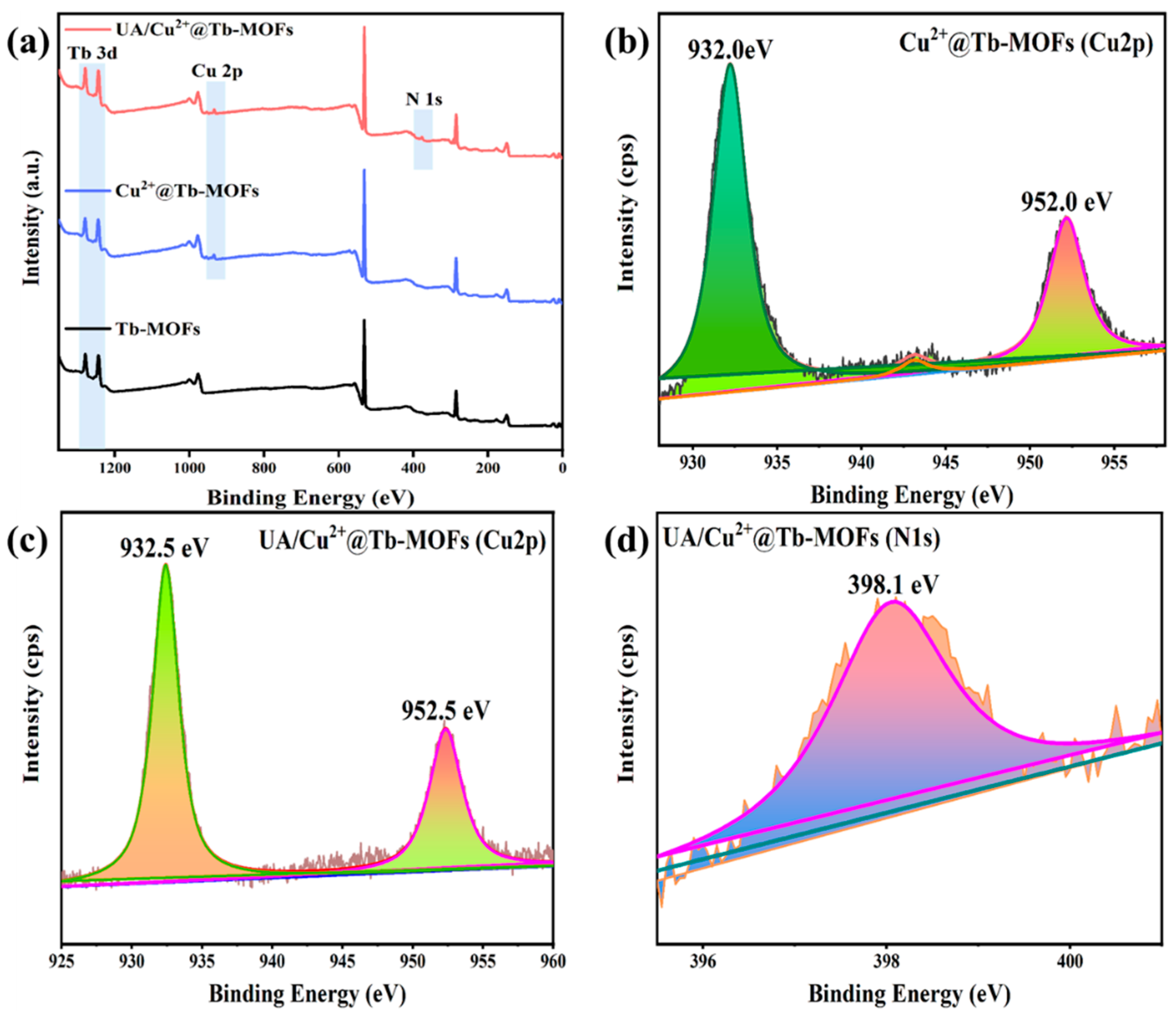
2.1. Characterization of Cu2+@Tb-MOFs Fluorescent Probe
2.2. Detection of Uric Acid in Aqueous Solutions
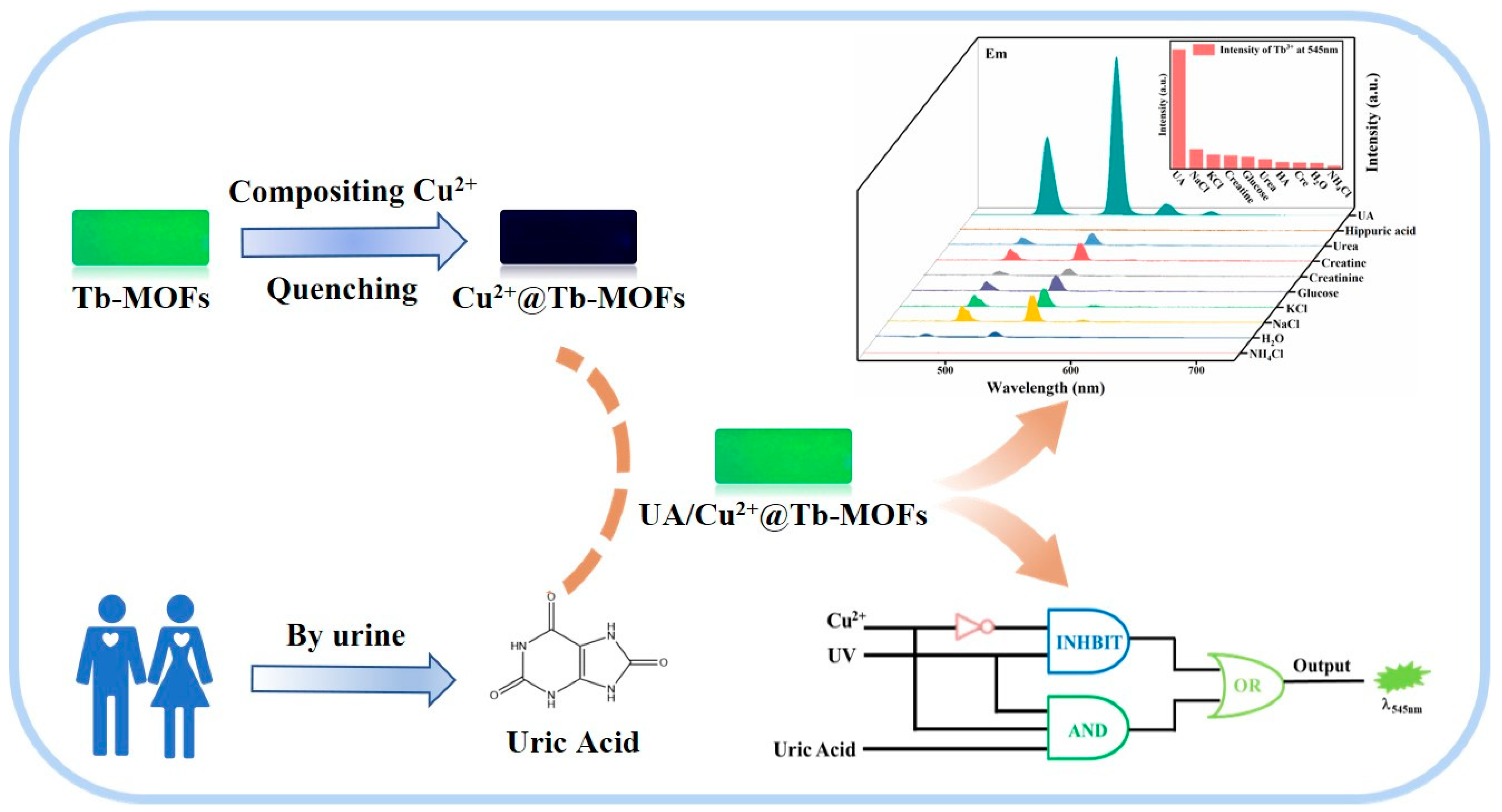
2.3. Sensing Mechanism
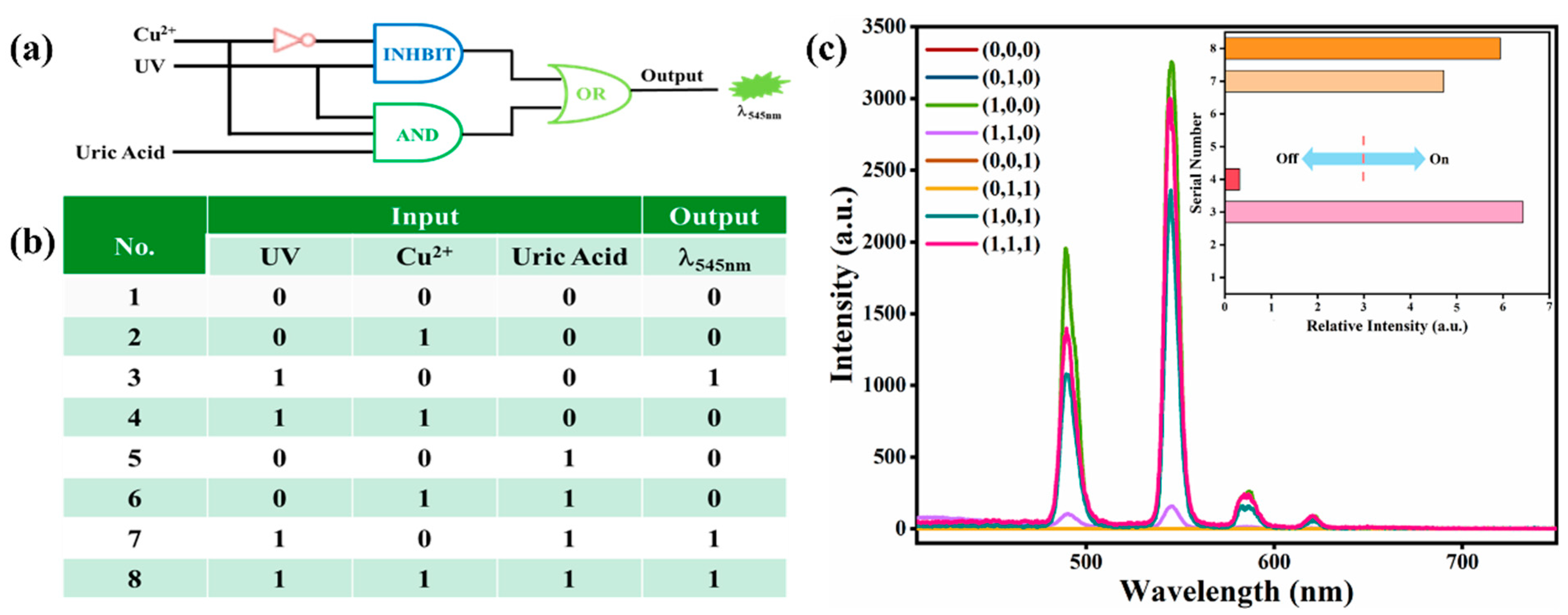
2.4. Construction of Luminescent Logic Gate
3. Experimental Section
3.1. Reagents and Instruments
3.2. Synthesis of Tb-MOFs and Cu2+@Tb-MOFs
3.3. Fluorescence Sensing of Detection UA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Dincer, H.E.; Dincer, A.P.; Levinson, D.J. Asymptomatic Hyperuricemia: To Treat or Not to Treat. Clevel. Clin. J. Med. 2002, 69, 594–608. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitart, V.; Rudan, I.; Hayward, C.; Gray, N.K.; Floyd, J.; Palmer, C.N.; Knott, S.A.; Kolcic, I.; Polasek, O.; Graessler, J. SLC2A9 is a newly identified urate transporter influencing serum urate concentration, urate excretion and gout. Nat. Genet. 2008, 40, 437–442. [Google Scholar] [CrossRef] [PubMed]
- Westley, C.; Xu, Y.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Label-free surface enhanced Raman scattering approach for high-throughput screening of biocatalysts. Anal. Chem. 2016, 88, 5898–5903. [Google Scholar] [CrossRef] [PubMed]
- Ragab, G.; Elshahaly, M.; Bardin, T. Gout: An old disease in new perspective—A review. J. Adv. Res. 2017, 8, 495–511. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Xiong, L.; Yu, L.; Wu, D.; Yang, C.; Xiao, Y. An enzyme-free fluorescent sensing platform for the detection of uric acid in human urine. J. Lumin. 2021, 236, 118076. [Google Scholar] [CrossRef]
- Kim, M.-C.; Kwak, J.; Lee, S.-Y. Sensing of uric acid via cascade catalysis of uricase and a biomimetic catalyst. Sens. Actuators B 2016, 232, 744–749. [Google Scholar] [CrossRef]
- Liu, X.; Qi, W.; Wang, Y.; Lin, D.; Yang, X.; Su, R.; He, Z. Rational design of mimic multienzyme systems in hierarchically porous biomimetic metal–organic frameworks. ACS Appl. Mater. Interfaces 2018, 10, 33407–33415. [Google Scholar] [CrossRef] [PubMed]
- Guo, J. Uric acid monitoring with a smartphone as the electrochemical analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef] [Green Version]
- Su, C.-H.; Sun, C.-L.; Liao, Y.-C. Printed combinatorial sensors for simultaneous detection of ascorbic acid, uric acid, dopamine, and nitrite. ACS Omega 2017, 2, 4245–4252. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Hu, A.; Feng, F. Synthesis of a sulfonated methylene blue-backboned polymer for biodetections. Dyes Pigm. 2022, 203, 110360. [Google Scholar] [CrossRef]
- Samoson, K.; Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Lim Abdullah, A.F.; Tayayuth, K.; et al. Facile fabrication of a flexible laser induced gold nanoparticle/chitosan/porous graphene electrode for uric acid detection. Talanta 2022, 243, 123319. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Liang, P. Determination of Cr (III) and total chromium in water samples by cloud point extraction and flame atomic absorption spectrometry. Microchim. Acta 2008, 162, 121–125. [Google Scholar] [CrossRef]
- Dey, N.; Bhattacharya, S. Nanomolar level detection of uric acid in blood serum and pest-infested grain samples by an amphiphilic probe. Anal. Chem. 2017, 89, 10376–10383. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Zhang, M.; Zhao, J.; Han, C.; Liu, X.; Xiao, Z.; Zhang, L.; Xu, B.; Guo, W.; Wang, R. Fluorescence turn-on detection of uric acid by a water-stable metal–organic nanotube with high selectivity and sensitivity. J. Mater. Chem. C 2017, 5, 601–606. [Google Scholar] [CrossRef]
- Gavrilenko, N.A.; Volgina, T.N.; Pugachev, E.V.; Gavrilenko, M.A. Visual determination of malachite green in sea fish samples. Food Chem. 2019, 274, 242–245. [Google Scholar] [CrossRef]
- Westley, C.; Xu, Y.; Thilaganathan, B.; Carnell, A.J.; Turner, N.J.; Goodacre, R. Absolute quantification of uric acid in human urine using surface enhanced Raman scattering with the standard addition method. Anal. Chem. 2017, 89, 2472–2477. [Google Scholar] [CrossRef] [Green Version]
- Cheng, F.-C.; Kuo, J.-S.; Chang, W.-H.; Juang, D.-J.; Shih, Y.; Lai, J.-S. Rapid and reliable high-performance liquid chromatographic method for analysing human plasma serotonin, 5-hydroxyindoleacetic acid, homovanillic acid and 3,4-dihydroxyphenylacetic acid. J. Chromatogr. B 1993, 617, 227–232. [Google Scholar] [CrossRef]
- Dai, X.; Fang, X.; Zhang, C.; Xu, R.; Xu, B. Determination of serum uric acid using high-performance liquid chromatography (HPLC)/isotope dilution mass spectrometry (ID-MS) as a candidate reference method. J. Chromatogr. B 2007, 857, 287–295. [Google Scholar] [CrossRef]
- Zhou, S.; Zuo, R.; Zhu, Z.; Wu, D.; Vasa, K.; Deng, Y.; Zuo, Y. An eco-friendly hydrophilic interaction HPLC method for the determination of renal function biomarkers, creatinine and uric acid, in human fluids. Anal. Methods 2013, 5, 1307–1311. [Google Scholar] [CrossRef]
- Shi, Q.; Shen, L.-Y.; Xu, H.; Wang, Z.-Y.; Yang, X.-J.; Huang, Y.-L.; Redshaw, C.; Zhang, Q.-L. A 1-Hydroxy-2,4-Diformylnaphthalene-Based Fluorescent Probe and Its Detection of Sulfites/Bisulfite. Molecules 2021, 26, 3064. [Google Scholar] [CrossRef]
- Dalapati, R.; Biswas, S. A Pyrene-Functionalized Metal–Organic Framework for Nonenzymatic and Ratiometric Detection of Uric Acid in Biological Fluid via Conformational Change. Inorg. Chem. 2019, 58, 5654–5663. [Google Scholar] [CrossRef] [PubMed]
- Geng, L.-Y.; Zhao, Y.; Kamya, E.; Guo, J.-T.; Sun, B.; Feng, Y.-K.; Zhu, M.-F.; Ren, X.-K. Turn-off/on fluorescent sensors for Cu2+ and ATP in aqueous solution based on a tetraphenylethylene derivative. J. Mater. Chem. C 2019, 7, 2640–2645. [Google Scholar] [CrossRef]
- Huang, L.-X.; Guo, Q.; Chen, Y.; Verwilst, P.; Son, S.; Wu, J.-B.; Cao, Q.-Y.; Kim, J.S. Nanomolar detection of adenosine triphosphate (ATP) using a nanostructured fluorescent chemosensing ensemble. Chem. Commun. 2019, 55, 14135–14138. [Google Scholar] [CrossRef]
- Lobas, M.A.; Tao, R.; Nagai, J.; Kronschläger, M.T.; Borden, P.M.; Marvin, J.S.; Looger, L.L.; Khakh, B.S. A genetically encoded single-wavelength sensor for imaging cytosolic and cell surface ATP. Nat. Commun. 2019, 10, 711. [Google Scholar] [CrossRef] [Green Version]
- Han, L.-J.; Kong, Y.-J.; Zhang, X.-M.; Hou, G.-Z.; Chen, H.-C.; Zheng, H.-G. Fluorescence recognition of adenosine triphosphate and uric acid by two Eu-based metal–organic frameworks. J. Mater. Chem. C 2021, 9, 6051–6061. [Google Scholar] [CrossRef]
- Ma, C.; Li, P.; Xia, L.; Qu, F.; Kong, R.-M.; Song, Z.-L. A novel ratiometric fluorescence nanoprobe for sensitive determination of uric acid based on CD@ZIF-CuNC nanocomposites. Microchim. Acta 2021, 188, 259. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Dong, C.; Li, M.; Li, L.; Jiang, X.; Gao, R.; Wang, R.; Zhang, L.; Ning, Z.; Gao, D.; et al. Terbium-based metal-organic frameworks: Highly selective and fast respond sensor for styrene detection and construction of molecular logic gate. J. Hazard. Mater. 2020, 388, 121816. [Google Scholar] [CrossRef]
- Dong, C.-L.; Li, M.-F.; Yang, T.; Feng, L.; Ai, Y.-W.; Ning, Z.-L.; Liu, M.-J.; Lai, X.; Gao, D.-J. Controllable synthesis of Tb-based metal–organic frameworks as an efficient fluorescent sensor for Cu2+ detection. Rare Met. 2021, 40, 505–512. [Google Scholar] [CrossRef]
- Zhu, Z.-X.; Wang, C.-J.; Luo, D.; Liu, C.; Liu, D.-N.; Xiao, Y.-M.; Chen, S.; Wang, Y.-Y. Six new lanthanide metal–organic frameworks as luminescent sensors for the detection of 1-N, TDGA, UA, and HA in urine. J. Coord. Chem. 2019, 72, 3526–3543. [Google Scholar] [CrossRef]
- Han, L.-J.; Kong, Y.-J.; Hou, G.-Z.; Chen, H.-C.; Zhang, X.-M.; Zheng, H.-G. A europium-based MOF fluorescent probe for efficiently detecting malachite green and uric acid. Inorg. Chem. 2020, 59, 7181–7187. [Google Scholar] [CrossRef]
- Liu, K.; Chen, Y.; Dong, X.; Huang, H. Simultaneous voltammetric determination of dopamine and uric acid based on MOF-235 nanocomposite. Inorg. Chem. Commun. 2022, 142, 109584. [Google Scholar] [CrossRef]
- Erbas-Cakmak, S.; Kolemen, S.; Sedgwick, A.C.; Gunnlaugsson, T.; James, T.D.; Yoon, J.; Akkaya, E.U. Molecular logic gates: The past, present and future. Chem. Soc. Rev. 2018, 47, 2228–2248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawar, S.; Duadi, H.; Fleger, Y.; Fixler, D. Carbon Dots-Based Logic Gates. Nanomaterials 2021, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Liu, P.; Ga, L.; Ai, J. Advances in Applications of Molecular Logic Gates. ACS Omega 2021, 6, 30189–30204. [Google Scholar] [CrossRef]
- Bu, X.; Fu, Y.; Jiang, X.; Jin, H.; Gui, R. Self-assembly of DNA-templated copper nanoclusters and carbon dots for ratiometric fluorometric and visual determination of arginine and acetaminophen with a logic-gate operation. Microchim. Acta 2020, 187, 154. [Google Scholar] [CrossRef]
- Fu, L.; Yu, D.; Zou, D.; Qian, H.; Lin, Y. Engineering the Stability of Nanozyme-Catalyzed Product for Colorimetric Logic Gate Operations. Molecules 2021, 26, 6494. [Google Scholar] [CrossRef]
- Li, B.; Zhao, D.; Wang, F.; Zhang, X.; Li, W.; Fan, L. Recent advances in molecular logic gate chemosensors based on luminescent metal organic frameworks. Dalton Trans. 2021, 50, 14967–14977. [Google Scholar] [CrossRef]
- Yuan, X.; Bai, F.; Ye, H.; Zhao, H.; Zhao, L.; Xiong, Z. Smartphone-assisted ratiometric fluorescence sensing platform and logical device based on polydopamine nanoparticles and carbonized polymer dots for visual and point-of-care testing of glutathione. Anal. Chim. Acta 2021, 1188, 339165. [Google Scholar] [CrossRef]
- Li, B.-N.; Liu, Y.-Y.; Wang, Y.-P.; Pan, M. Visual Detection of Triethylamine and a Dual Input/Output Logic Gate Based on a Eu3+-Complex. Molecules 2021, 26, 3244. [Google Scholar] [CrossRef]
- Wong, K.-L.; Law, G.-L.; Yang, Y.-Y.; Wong, W.-T. A Highly Porous Luminescent Terbium–Organic Framework for Reversible Anion Sensing. Adv. Mater. 2006, 18, 1051–1054. [Google Scholar] [CrossRef]
- Wang, H.; Yang, T.; Feng, L.; Ning, Z.; Liu, M.; Lai, X.; Gao, D.; Bi, J. Energy Transfer and Multicolor Tunable Luminescence Properties of NaGd0.5Tb0.5−xEux(MoO4)2 Phosphors for UV-LED. J. Electron. Mater. 2018, 47, 6494–6506. [Google Scholar] [CrossRef]
- Wu, S.; Zhu, M.; Zhang, Y.; Kosinova, M.; Fedin, V.P.; Gao, E. A Water-Stable Lanthanide Coordination Polymer as Multicenter Platform for Ratiometric Luminescent Sensing Antibiotics. Chem.—Eur. J. 2020, 26, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhu, M.; Zhang, Y.; Kosinova, M.; Fedin, V.P.; Gao, E. Luminescent sensors based on coordination polymers with adjustable emissions for detecting biomarker of pollutant ethylbenzene and styrene. Appl. Organomet. Chem. 2021, 35, e6058. [Google Scholar] [CrossRef]
- Gontcharenko, V.E.; Lunev, A.M.; Taydakov, I.V.; Korshunov, V.M.; Drozdov, A.A.; Belousov, Y.A. Luminescent Lanthanide-Based Sensor for H2O Detection in Aprotic Solvents and D2O. IEEE Sens. J. 2019, 19, 7365–7372. [Google Scholar] [CrossRef]
- Lian, X.; Yan, B. Phosphonate MOFs Composite as Off–On Fluorescent Sensor for Detecting Purine Metabolite Uric Acid and Diagnosing Hyperuricuria. Inorg. Chem. 2017, 56, 6802–6808. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qi, W.; Wang, Y.; Su, R.; He, Z. A facile strategy for enzyme immobilization with highly stable hierarchically porous metal–organic frameworks. Nanoscale 2017, 9, 17561–17570. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.; Wen, K.; Han, H.; Li, Q. Urate oxidase loaded in PCN-222(Fe) with peroxidase-like activity for colorimetric detection of uric acid. J. Mater. Chem. B 2021, 9, 6811–6817. [Google Scholar] [CrossRef]
- Li, A.; Chu, Q.; Zhou, H.; Yang, Z.; Liu, B.; Zhang, J. Effective nitenpyram detection in a dual-walled nitrogen-rich In(iii)/Tb(iii)–organic framework. Inorg. Chem. Front. 2021, 8, 2341–2348. [Google Scholar] [CrossRef]
- Jin, Y.; Yan, B. A bi-functionalized metal-organic framework based on N-methylation and Eu3+ post-synthetic modification for highly sensitive detection of 4-Aminophenol (4-AP), a biomarker for aniline in urine. Talanta 2021, 227, 122209. [Google Scholar] [CrossRef]
- Sahoo, J.; Arunachalam, R.; Subramanian, P.S.; Suresh, E.; Valkonen, A.; Rissanen, K.; Albrecht, M. Coordinatively Unsaturated Lanthanide(III) Helicates: Luminescence Sensors for Adenosine Monophosphate in Aqueous Media. Angew. Chem. Int. Ed. 2016, 55, 9625–9629. [Google Scholar] [CrossRef]
- Zhang, Y.; Qu, X.; Yan, B. A visual logic alarm sensor for diabetic patients towards diabetic polyneuropathy based on a metal–organic framework functionalized by dual-cation exchange. J. Mater. Chem. C 2021, 9, 3440–3446. [Google Scholar] [CrossRef]
- Zhang, L.; Xu, Y.; Xu, J.; Zhang, H.; Zhao, T.; Jia, L. Intelligent multicolor nano-sensor based on nontoxic dual fluoroprobe and MOFs for colorful consecutive detection of Hg2+ and cysteine. J. Hazard. Mater. 2022, 430, 128478. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Feng, L.; Chen, B.; Tang, L.; Wang, J.; Ning, Z.; Bi, J.; Gao, D.; Lai, X.; Li, W. Magnolia-like terbium-based metal-organic frameworks hierarchical architectures: Solvent-induced synthesis, microstructure and enhanced luminescent properties. Opt. Mater. 2018, 81, 64–70. [Google Scholar] [CrossRef]


| Probe | Work Range (μM) | LOD (μM) | Ref. |
|---|---|---|---|
| Eu-BDC@FM | 0~200 | 0.6 | [5] |
| MBP-SO3@Nafion | 30~3000 | 11.3 | [10] |
| CD-MONT-2 | 1.5~9.1 | 4.3 | [14] |
| Hf-UiO-66-Py | 0~30 | 1.4 | [21] |
| Eu-TDA | 0~327 | 0.689 | [30] |
| MOF-235 | 10~90 | 3.46 | [31] |
| Cu2+@MIL-91(Al:Eu) | 0~1200 | 1.6 | [45] |
| URICASE and HRP@HP-DUT-5 | 5~100 | 0.8 | [46] |
| PCN-222(Fe) | 10~800 | 3.5 | [47] |
| Cu2+@Tb-MOFs | 0~104 | 0.65 | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, J.; Che, J.; Jiang, X.; Fan, Y.; Gao, D.; Bi, J.; Ning, Z. A Novel Turn-On Fluorescence Probe Based on Cu(II) Functionalized Metal–Organic Frameworks for Visual Detection of Uric Acid. Molecules 2022, 27, 4803. https://doi.org/10.3390/molecules27154803
Yang J, Che J, Jiang X, Fan Y, Gao D, Bi J, Ning Z. A Novel Turn-On Fluorescence Probe Based on Cu(II) Functionalized Metal–Organic Frameworks for Visual Detection of Uric Acid. Molecules. 2022; 27(15):4803. https://doi.org/10.3390/molecules27154803
Chicago/Turabian StyleYang, Jie, Jie Che, Xin Jiang, Yangchun Fan, Daojiang Gao, Jian Bi, and Zhanglei Ning. 2022. "A Novel Turn-On Fluorescence Probe Based on Cu(II) Functionalized Metal–Organic Frameworks for Visual Detection of Uric Acid" Molecules 27, no. 15: 4803. https://doi.org/10.3390/molecules27154803
APA StyleYang, J., Che, J., Jiang, X., Fan, Y., Gao, D., Bi, J., & Ning, Z. (2022). A Novel Turn-On Fluorescence Probe Based on Cu(II) Functionalized Metal–Organic Frameworks for Visual Detection of Uric Acid. Molecules, 27(15), 4803. https://doi.org/10.3390/molecules27154803






