Abstract
Multiple biological functions of Mentha pulegium extract were evaluated in the current work. Phytochemical components of the M. pulegium extract were detected by Gas Chromatography-Mass Spectrometry (GC-MS) and High-performance liquid chromatography (HPLC). Moreover, M. pulegium extract was estimated for antioxidant potential by 2,2-Diphenyl-1-picryl-hydrazyl-hydrate (DPPH) free radical scavenging, antimicrobial activity by well diffusion, and anticoagulant activity via prothrombin time (PT) and activated partial thromboplastin time (APTT). GC-MS analysis detected compounds including cholesterol margarate, stigmast-5-en-3-ol, 19-nor-4-androstenediol, androstan-17-one, pulegone-1,2-epoxide, isochiapin B, dotriacontane, hexadecanoic acid and neophytadiene. Chrysoeriol (15.36 µg/mL) was followed by kaempferol (11.14 µg/mL) and 7-OH flavone (10.14 µg/mL), catechin (4.11 µg/mL), hisperdin (3.05 µg/mL), and luteolin (2.36 µg/mL) were detected by HPLC as flavonoids, in addition to ferulic (13.19 µg/mL), cinnamic (12.69 µg/mL), caffeic (11.45 µg/mL), pyrogallol (9.36 µg/mL), p-coumaric (5.06 µg/mL) and salicylic (4.17 µg/mL) as phenolics. Antioxidant activity was detected with IC50 18 µg/mL, hemolysis inhibition was recorded as 79.8% at 1000 μg/mL, and PT and APTT were at 21.5 s and 49.5 s, respectively, at 50 μg/mL of M. pulegium extract. The acute toxicity of M. pulegium extract was recorded against PC3 (IC50 97.99 µg/mL) and MCF7 (IC50 80.21 µg/mL). Antimicrobial activity of M. pulegium extract was documented against Bacillus subtilis, Escherichia coli, Pseudomonas aureus, Candida albicans, Pseudomonas aeruginosa, but not against black fungus Mucor circinelloides. Molecular docking was applied using MOE (Molecular Operating Environment) to explain the biological activity of neophytadiene, luteolin, chrysoeriol and kaempferol. These compounds could be suitable for the development of novel pharmacological agents for treatment of cancer and bacterial infections.
1. Introduction
At the present time, with the spread and outbreak of diseases, and the resistance of pathogens to drugs, scientists in the medical and pharmaceutical fields have increased their search for safe alternative sources that can solve these problems [1]. Plants are one of the best sources of herbal preparations because they contain different and diverse phyto-constituents. Despite many biologically active compounds of plant origin having been discovered, there are many that so far remain undiscovered. There are many medicinal plants that have been traditionally used without any scientific basis, requiring further study for the conversion from traditional use to scientific validity [2,3].
Mentha (commonly named pennyroyal) of the family Lamiaceae contains 61 species with approximately 100 varieties which are disseminated across temperate regions including North America, Africa, Asia, Europe, and Australia [4]. The family Lamiaceae is considered a rich source of flavonoids, particularly flavones such as apigenin, luteolin and 6-hydroxyflavone [4]. According to Marzouk et al. [5] the genus Mentha is represented in the flora of Egypt by two species, namely Mentha longifolia and M. pulegium L. Numerous uses have been recorded for the M. pulegium plant [6] including for relief of colds, coughs, headaches, kidney problems, liver complaints, and gallbladder ailments; in addition, it has been applied in drink and food additives. Despite these therapeutic and nutritional uses, M. pulegium is well-known for its poisonousness [4]. Additionally, some reports have indicated the presence of certain toxic compounds in M. pulegium, although Caputo et al. [7] suggested that the soil conditions may influence the expression of toxic compounds such as pulegone and its derivatives. Numerous biological activities have previously been reported for M. pulegium extract as well as its essential oils, and occasionally the activity has differed based on cultivation conditions. Previous studies reported a number of biological activities including antimicrobial activity against many microorganisms [8,9], decremental inflammation with oral mucosa treatment, anti-hemolytic, and anticancer activities [10] of Mentha.
Recently, Alharbi et al. [11] demonstrated the bactericidal potential of M. pulegium extract towards four gram-positive and five gram-negative bacteria; also, the development of Aspergillus niger, A. flavus and Candida albicans was inhibited by M. pulegium extract. Baali et al. [9] suggested food and pharmaceutical applications of Algerian M. pulegium as a preservative, antimicrobial, and antioxidant agent. Moreover, the anthelmintic properties of M. pulegium extract were recently documented [12]. Some scientific papers have focused on the application of M. pulegium essential oils [13,14] in biological activities including antiviral and antifungal activities. Bektašević et al. [15] observed excellent antioxidant activity in addition to moderate cholinesterase inhibition using essential oils, and therefore recommended their use in Alzheimer disease. The most serious diseases worldwide in the last decade have been cancers, which in 2020 led to about 9.9 million deaths [16]. Promising sources of phenolic and flavonoid contents in plants can play a main role in cancer treatment and treatment of other diseases [17,18]. The inhibitory effect of plant flavonoids on the spread of cancer cells through apoptosis induction has been detected [19,20]. The existence in M. pulegium of several phenolic acids was reported, including syringic acid and ferulic acid, as well as several compounds of flavonoid including isorhamnetin-3-O-glucoside and kaempferol-3-O-rutinoside; these compounds reflected high antioxidant activity [12]. Positive correlations among the phenolic contents of certain Mentha species (M. rotundifolia and M. pulegium) and their biological activities, namely antimicrobial and antioxidant activities, were documented by Alharbi et al. [11].
A computer simulation technique such as molecular dynamic simulation (MDS) can be utilized [20] to screen and estimate the physical movements of molecules and atoms. This allows searchers to measure flexibility, rigidity, and secondary construction prediction in terms of gain or loss during the simulation time. Recently, the silico protocol was applied to select the most suitable structures for biological observation of active compounds; this protocol was dependent on the correlation among biological activity values of the input structures and the target proteins in pathogenic organisms, cancer cells, etc. [20,21,22,23,24,25]. The present investigation was designed to explore the phytochemical characterization of M. pulegium extract by GC-MS and HPLC, studying different biological applications comprising antitumor, antimicrobial, anti-hemolytic and anticoagulant activities.
2. Results and Discussion
2.1. GC-MS and HPLC Analysis of M. pulegium Extract
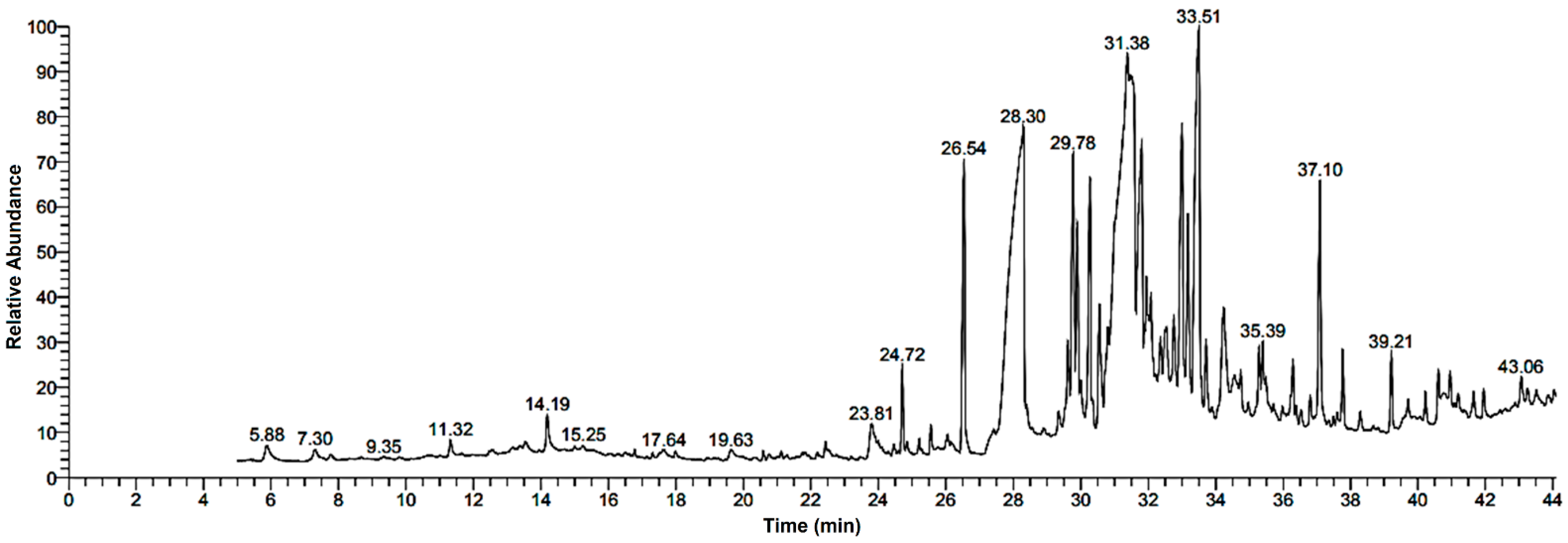
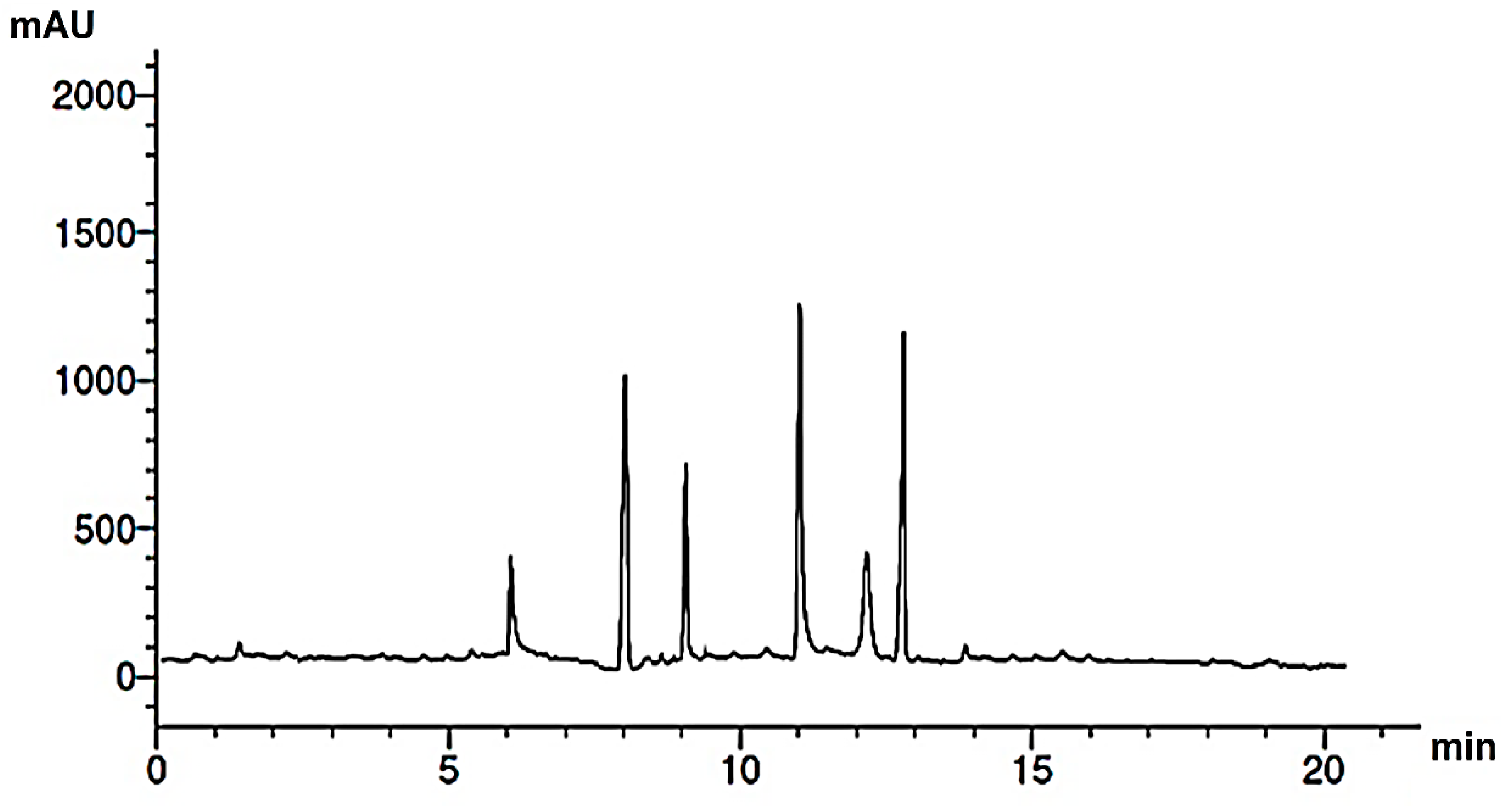
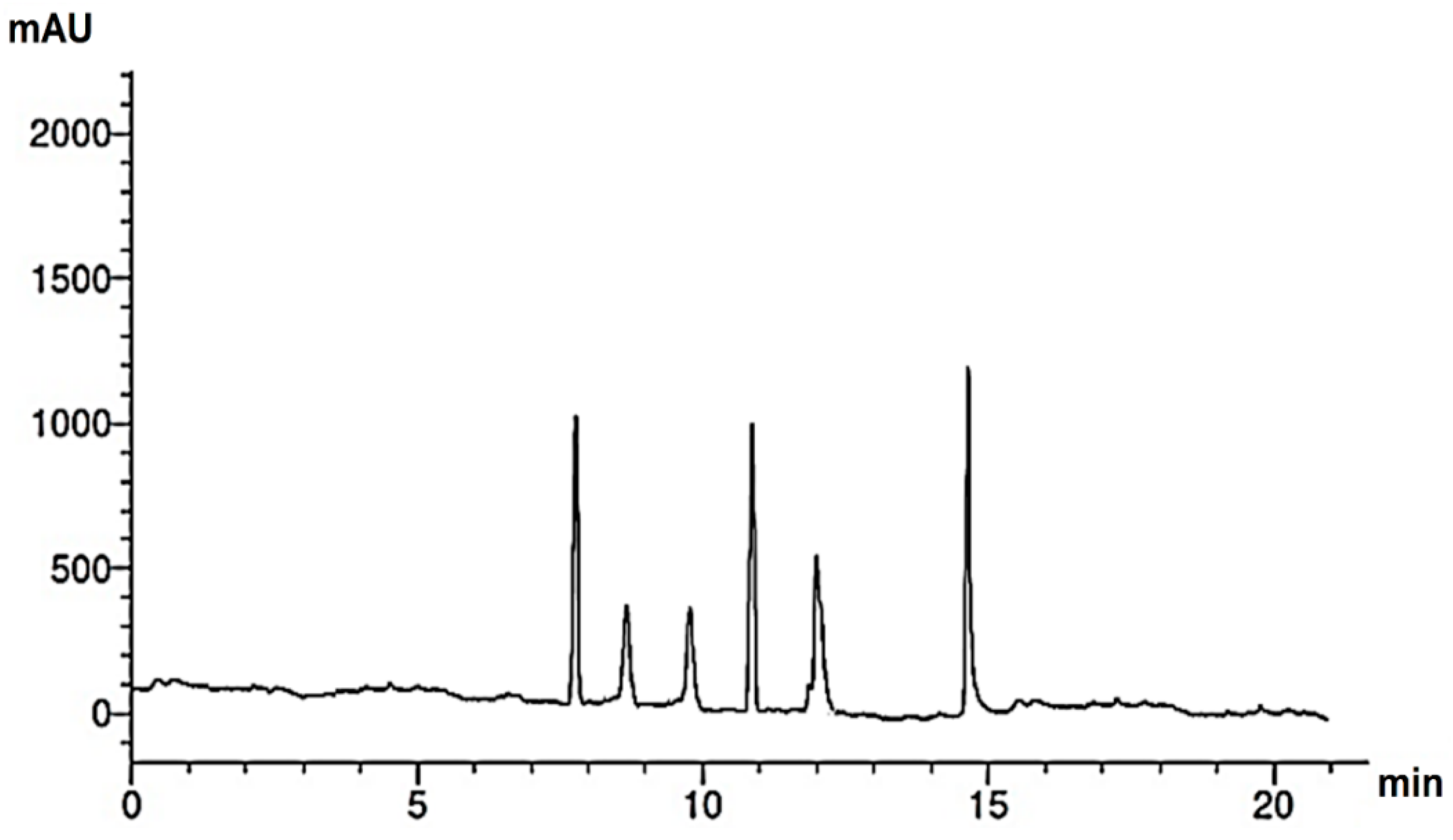
Research into the pharmaceutical value of medicinal plants depends on the detection and identification of phyto-constituents and their chemical structures, and understanding their pharmacodynamic action when used for the treatment of any illness. In the current study, extract of M. pulegium was chemically evaluated using GC-MS and HPLC analysis (Figure 1) for performing various biological activities. The GC-MS analysis of M. pulegium (Figure 2) revealed the existence of 38 compounds (Table 1) and, as indicated by chromatogram (Figure 2), the most abundant component by peak area was shown to be hexadecanoic acid (20.88%), followed by methyl 10-ketostearate (10.84%), cis-13-Octadecenoic acid (6.84%), stearic acid (4.87%), oxiraneoctanoic acid 3-octyl-,methyl ester (4.72%), methyl hexadecanoate (4.56%), elaidic acid, methyl ester (4.17%), and 1,2-benzenedicarboxylic acid (3.60%). Neophytadiene (diterpenoid) was identified as a constituent of M. pulegium extract (Table 1); this constituent has been found to include multiple functionalized agents according to to earlier reports [26,27], including anti-inflammatory, analgesic, antipyretic, antioxidant, and antimicrobial. Among the M. pulegium extract constituents, phytosterols including cholesterol margarate, stigmast-5-en-3-ol, 19-nor-4-androstenediol, and androstan-17-one (Table 1), can play an important biological role in protection against various cancers of the stomach, breast, prostate, lung, liver, and ovaries, as well as regulation of sugar uptake in diabetics, as mentioned by previous researchers [28,29,30]. Raju et al. [31] documented the safe properties (non-carcinogenic, non-poisonous, and non-mutagenic) of these phytosterols, and the anti-hypercholesterolemic qualities of stigmast-5-en-3-ol have also been reported [32]. Furthermore, the obtained results showed the presence of other compounds in M. pulegium extract, namely pulegone-1,2-epoxide (oxygenated terpene), which has vasorelaxant activity according to Lima et al. [33]. The non-hemolytic, non-toxic, and non-irritant qualities of pulegone-1,2-epoxide have been recognized [34], therefore its application for medicinal purposes was validated. Other important ingredients of M. pulegium extract (Table 1), namely isochiapin B (sesquiterpene lactone) and dotriacontane, have been identified as antioxidants [35] and bactriocidal agents [36], respectively. From the GC-MS, it is obvious that M. pulegium extract is rich in biologically active constituents. Different and various flavonoids and phenolic acids within M. pulegium extract were recognized by HPLC analysis (Table 2 and Figure 3 and Figure 4). Among the detected flavonoids, chrysoeriol was the most abundant(15.36 µg/mL), followed by kaempferol (11.14 µg/mL), and 7-OH flavone (10.14 µg/mL) (Table 2), while luteolin, hisperdin, and catechin were detected in the extract with low concentrations of 2.36, 3.05, and 4.11 µg/mL, respectively. From a scientific study [37] of thirteen Mentha species, the detected flavonoid levels varied within the species, particularly in the incidence of hesperidin, luteolin, and kaempferol, which ranged from 0.73 to 109.39 µg/g, 1.84 to 31.03 µg/g, and 1.30 to 33.68 µg/g, respectively; in contrast, levels of phenolic acids did not significantly differ. Six phenolic acids were identified in the M. pulegium extract, namely ferulic, cinnamic, caffeic, pyrogallol, p-coumaric, and salicylic with concentrations of 13.19, 12.69, 11.45, 9.36 5.06 and 4.17 µg/mL, respectively. Similar observations were recorded in a previous study [38], where caffeic acid represented the main compound (31.48 mg/g) in M. pulegium. Different flavonoids and phenolic acids were detected in M. pulegium, depending on the origin, as mentioned previously in the literature. Caffeic (as phenolic acid), acacetin 5-O-α-l-rhamnopyranosyl(1-2)-O-α-l-rhamnopyranoside, 7-O-α-rutinosides of apigenin and luteolin, vicenin, and 5-hydroxy-6,7,3′,4′-tetramethoxyflavone as flavonoids were detected in M. pulegium of Egyptian origin [39]. Caffeic, vanillic, and ferulic as phenolic acids, in addition to apigenin, luteolin, naringenin, and catechin as flavonoids, were detected in M. pulegium of Greek origin [40]. 4-Hydroxy benzoic, caffeic, p-coumaric, chlorogenic, and rosmarinic acids as phenolic acid, in addition to luteolin, diosmin, and kaempferol, were detected in M. pulegium of Algerian origin [41]. Hesperidin was recorded in M. pulegium at low concentrations compared with the other detected flavonoids (Table 2), was although it has been recorded in various levels in other species, for example 0.21 mg/g in M. piperita and 11.83 mg/g in M. spicata [42]. The main reason for the traditional therapeutic potential of Mentha spp. may be due to the existence of these flavonoids and phenolics that provide different health benefits. In a recent evaluation [43], multiple biological functions including anticancer, antioxidant, antimicrobial and anti-inflammatory properties of chrysoeriol and luteolin were documented. Moreover, Wei et al. [44] demonstrated in vivo activity of chrysoeriol against human lung carcinoma development. Cancer-preventive action without distracting normal cells and anti-inflammatory activities of kaempferol were recorded by Sharma et al. [45], in vivo and in vitro. Recently, Abbou et al. [46] identified different compounds, namely p-coumaric acid, gallic acid, naringenin, quercetin, and ferulic acid, in the extract of M. pulegium L aerial parts, which could be supportive in the regulation of oxidative stress leading to Alzheimer’s disease and diabetes.
Figure 1.
Diagram illustrating M. pulegium plants with the analysis methods and biological activities.
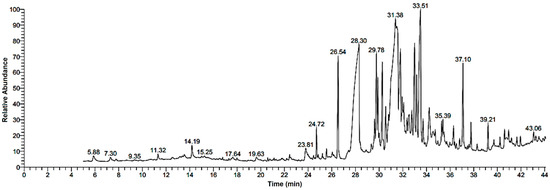
Figure 2.
GC-MS chromatogram analysis of M. pulegium extract.

Table 1.
Phyto-constituents of M. pulegium extract identified by GC-MS.

Table 2.
Flavonoids and phenolic acids contained in M. pulegium extract, identified by HPLC.
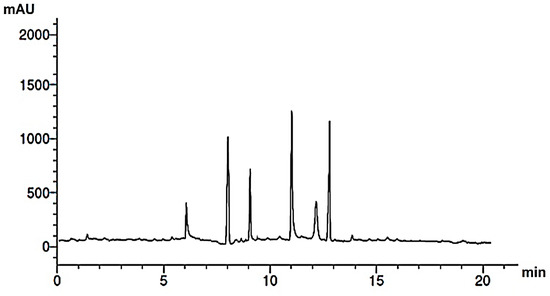
Figure 3.
HPLC chromatogram of phenolic acids of M. pulegium extract.
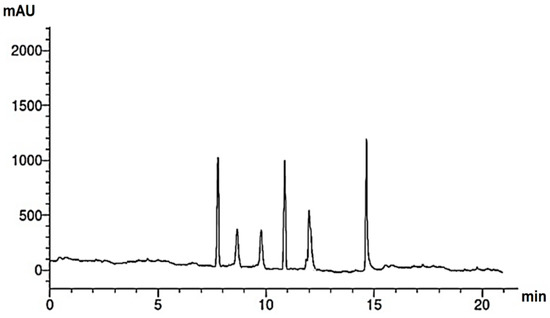
Figure 4.
HPLC chromatogram of flavonoids of M. pulegium extract.
2.2. Antimicrobial Potential of M. pulegium Extract
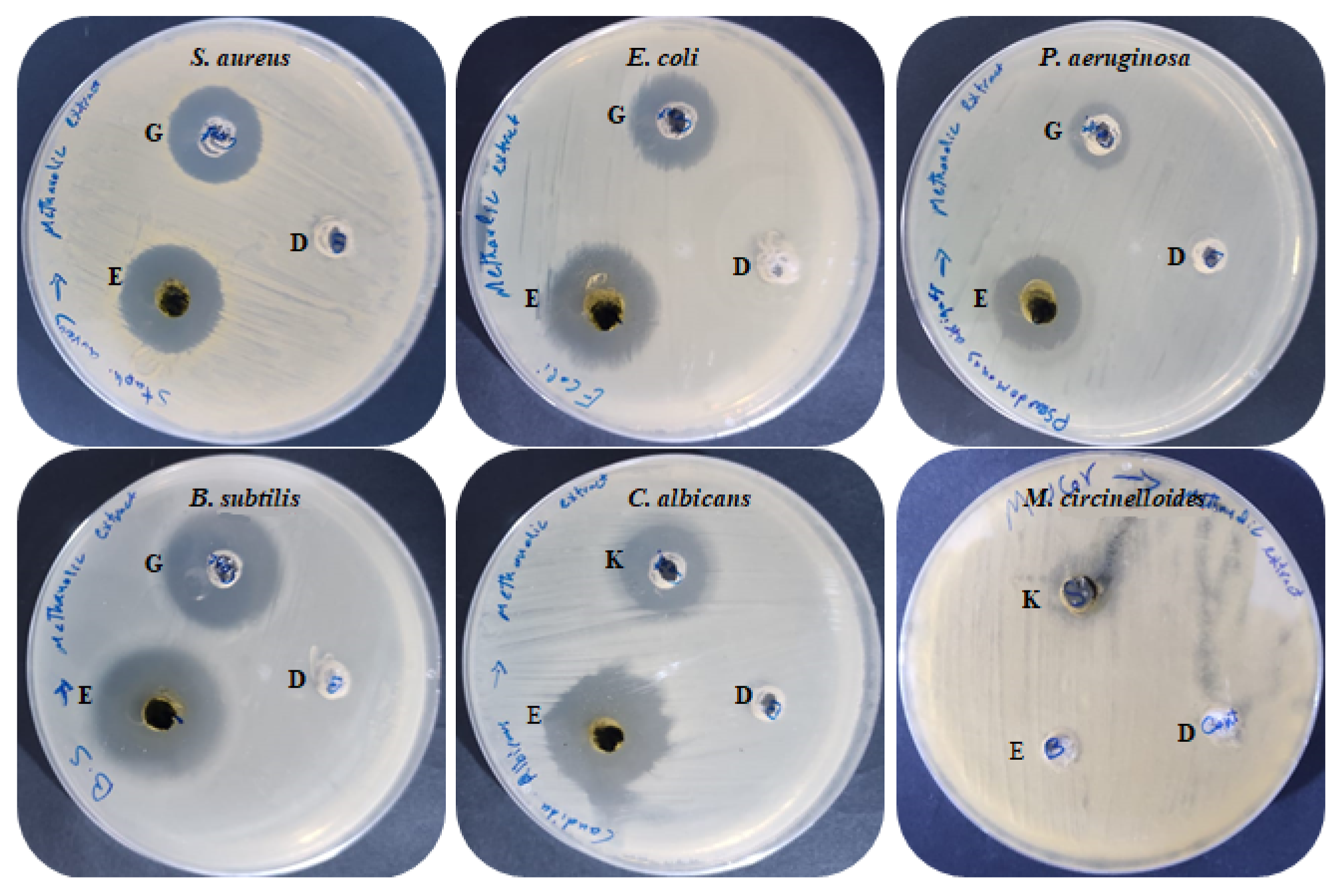
Antimicrobial activities of M. pulegium extract were assessed against four bacteria, one unicellular fungus, and one black mold as presented (Table 3 and Figure 5). The findings indicated that all the tested bacteria and C. albicans were sensitive to M. pulegium extract. Growth of B. subtilis (IZ, 27 mm) was more affected by M. pulegium extract than other bacteria, namely E. coli (IZ, 26 mm), S. aureus (IZ, 19 mm) and P. aeruginosa (IZ, 19 mm). Surprisingly, the extract was more effective on the tested organisms than the positive control (Gentamycin antibiotic). However, C. albicans was inhibited where the IZ was 25 mm, while Mucor circinelloides growth was not inhibited. These results may be due to differences in cell wall composition or genes responsible for extract resistance. According to a recent study [47], M. pulegium extract exhibited antibacterial potential against S. aureus only, and therefore it was applied for wound healing. Previous reports have indicated the antimicrobial activity of M. pulegium extract towards many bacteria (including five gram-positive and five gram-negative types) and fungi including six fungal species [7]. Numerous studies have shown that M. pulegium essential oil exerts antimicrobial activities on bacteria and fungi. A study by Aimad et al. [48] indicated that M. pulegium oil completely inhibited Aspergillus niger growth, and inhibited B. subtilis where the IZ was 25 mm. The antibacterial activity is perhaps attributable to the occurrence of natural ingredients of M. pulegium extract including neophytadiene, isochiapin B, and other constituents mentioned in Table 3. The current finding is in good agreement with the results of Ceyhan-Güvensen and Keskin [49], who reported the antimicrobial activity of M. pulegium extract and explained this activity by the presence of high neophytadiene content.

Table 3.
Antimicrobial activity of M. pulegium extract.
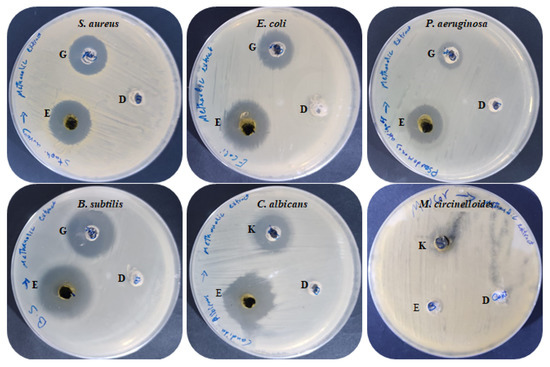
Figure 5.
Antimicrobial activities of M. pulegium extract against different bacteria and fungi: (E) M. pulegium extract; (D) methanolic loaded disc, (G) Gentamycin, (K) Ketoconazole.
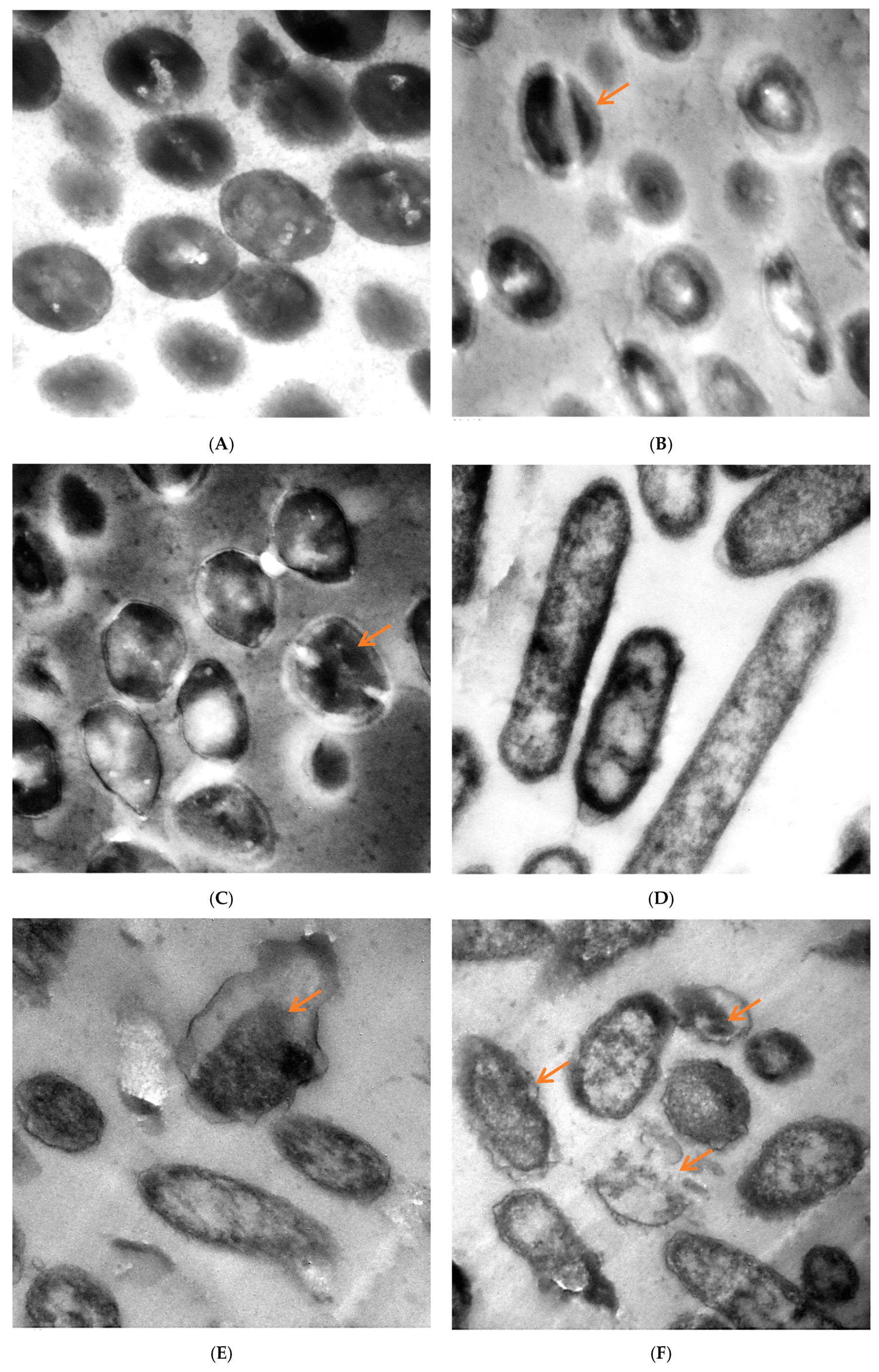
TEM of untreated and treated S. aureus and P. aeruginosa with M. pulegium extract are shown in Figure 6. The untreated cells of S. aureus had a distinct structure with cocci shape and intact cellular walls, and showed no signs of damage or morphological change (Figure 6A). Some deformations were observed in S. aureus treated by M. pulegium extract (Figure 6B,C), including partially digested cell wall and exfoliated fragments from the surface of bacterial cell, exposition of the patches in the cytoplasmic membrane, lack of cell wall or its detachment from the membrane, thickening of the outer membrane, and abnormal septa with the presence of ghost cells. Toxic effects of the M. pulegium extract on the structure and function of the membrane have generally been used to describe the antimicrobial action of the extract and its components. Untreated P. aeruginosa cells revealed a normal rod-shaped structure with an intact outer membrane and the cytoplasmic membrane near the cell wall, and the intracellular content was well-maintained. The periplasmic area was narrow and homogenous (Figure 6D). Several alterations in cell shape were detected after treatment with M. pulegium extract, and the periplasmic space indicated separation of the cell wall from the plasma membrane. The internal structures were disordered, and the components of the bacterial cell membrane were distorted and distributed from their original form. Draining of the intracellular contents was seen at particular sites within the cells, mostly at the septal and polar regions, due to breaches of the cell membrane. There were numerous cells without membranes and null cells of P. aeruginosa (Figure 6E,F). Approximately the same alteration was recorded in P. aeruginosa, leading to cell death [50].
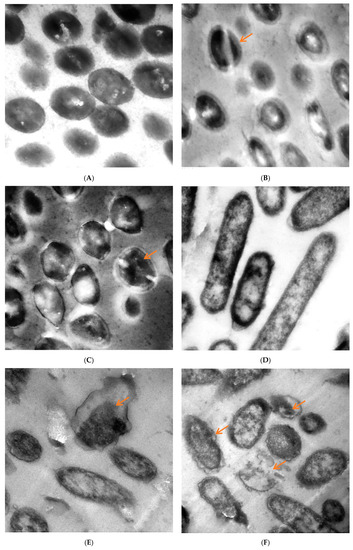
Figure 6.
TEM micrographs of (A) untreated S. aureus, (B) S. aureus treated at 62.50 µg/mL and (C) at 100 µg/mL, (D) untreated P. aeruginosa, (E) P. aeruginosa treated with M. pulegium extract at 15.62 µg/mL and (F) at 30 µg/mL. Scale Bar = 100 nm, 5000×.
2.3. Antioxidant Activity of M. pulegium Extract
The extract of M. pulegium showed excellent antioxidant activity according to the DPPH scavenging method. DPPH scavenging (%) increased with the increment of concentration, and reached 88.1% at 1000 μg/mL (Table 4). Surprisingly, the IC50 of M. pulegium extract was 18 μg/mL, compared with IC50 of 15.0 μg/mL for ascorbic acid. The current results can therefore confirm the antioxidant activity of this extract. The current study suggested that the main contributors to the antioxidant potential are associated with phenolic and flavonoid contents. Strong antioxidant activity of M. pulegium extract has been documented via numerous techniques including DPPH, trolox equivalent antioxidant capacity, ferric reducing antioxidant power, and oxygen radical absorbance capacity [47]. Many studies have reported relationships between phenolic content and antioxidative activity in plants [10,11]. However, the IC50 of the M. pulegium essential oil was 7.7 mg/mL [48] less than the current result (IC50, 18 μg/mL) for M. pulegium extract, although Aimad et al. [48] confirmed their obtained result using essential oil while our study dealt with the raw extract. Aimad et al. [48] suggested that the antioxidant activity of M. pulegium extract obtained by total antioxidant capacity and DPPH techniques may be due to the occurrence of pulegone.

Table 4.
Antioxidant activity of M. pulegium extract.
2.4. Hemolysis Inhibition by M. pulegium Extract
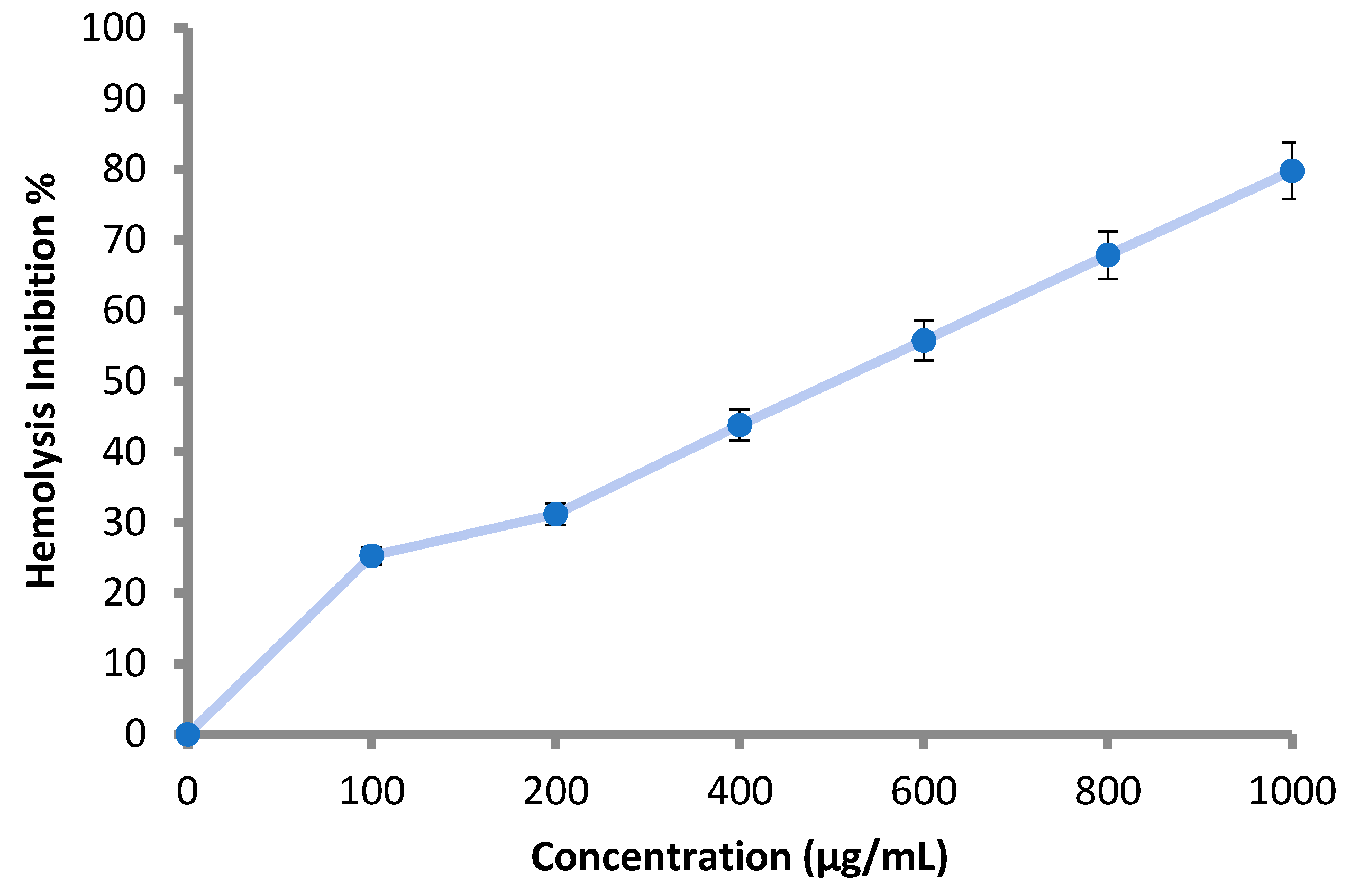
According to the obtained results, all the applied concentrations (100–1000 μg/mL) of the M. pulegium extract inhibited the lysis of the erythrocyte membrane as shown (Table 5 and Figure 7). Hemolysis inhibition increased with increment of the concentration, and reached 79.8% at 1000 μg/mL (Table 5). The result was compared using 200 μg/mL of indomethacin, where hemolysis inhibition was 91.0%. Hemolysis inhibition reflects the anti-inflammatory activity of M. pulegium extract, and the obtained results are in accordance with previous studies demonstrating that M. pulegium exhibited anti-inflammatory activities [41,51]. There are similarities between the membranes of red blood cells and lysosomal membrane contents; lysis of lysosomes occurs during inflammation. When the red blood cells are exposed to hypotonic conditions, cell membrane lysis is caused, leading to hemolysis in addition to haemoglobin oxidation. Prevention of hypo-tonicity leading to lysis of the red blood cell membrane that was performed to observe anti-inflammatory activity. The obtained results suggest a potential anti-inflammatory action that is probably due to the presence of pulegone-1,2-epoxide in the extract of the M. pulegium. This was noted in the GC-MS analysis, and it was previously demonstrated that pulegone can inhibit inflammation mechanisms [51,52]. Recently, Baali et al. [47] mentioned the promising activity of M. pulegium extract as a wound healing agent and documented a scientific rationale that confirmed the traditional application of this plant.

Table 5.
Effect of M. pulegium extract on hemolysis inhibition.
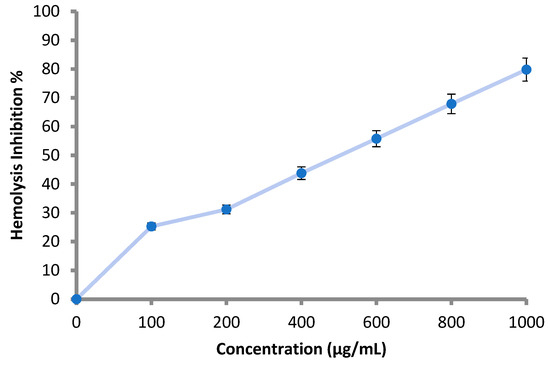
Figure 7.
Hemolysis inhibition (%) at different concentration of M. pulegium extract 3.5. Anticoagulant Activity of M. pulegium Extract.
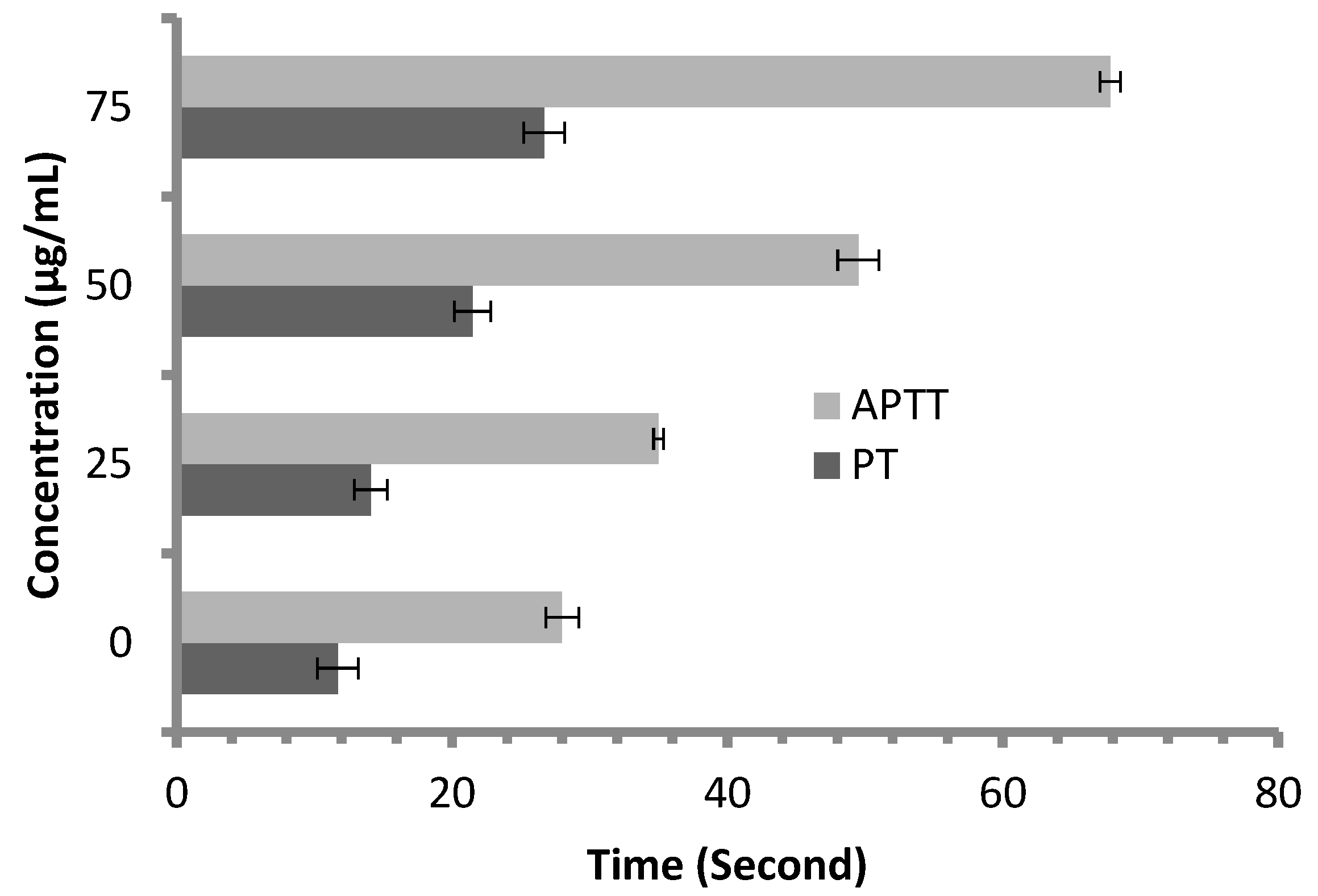
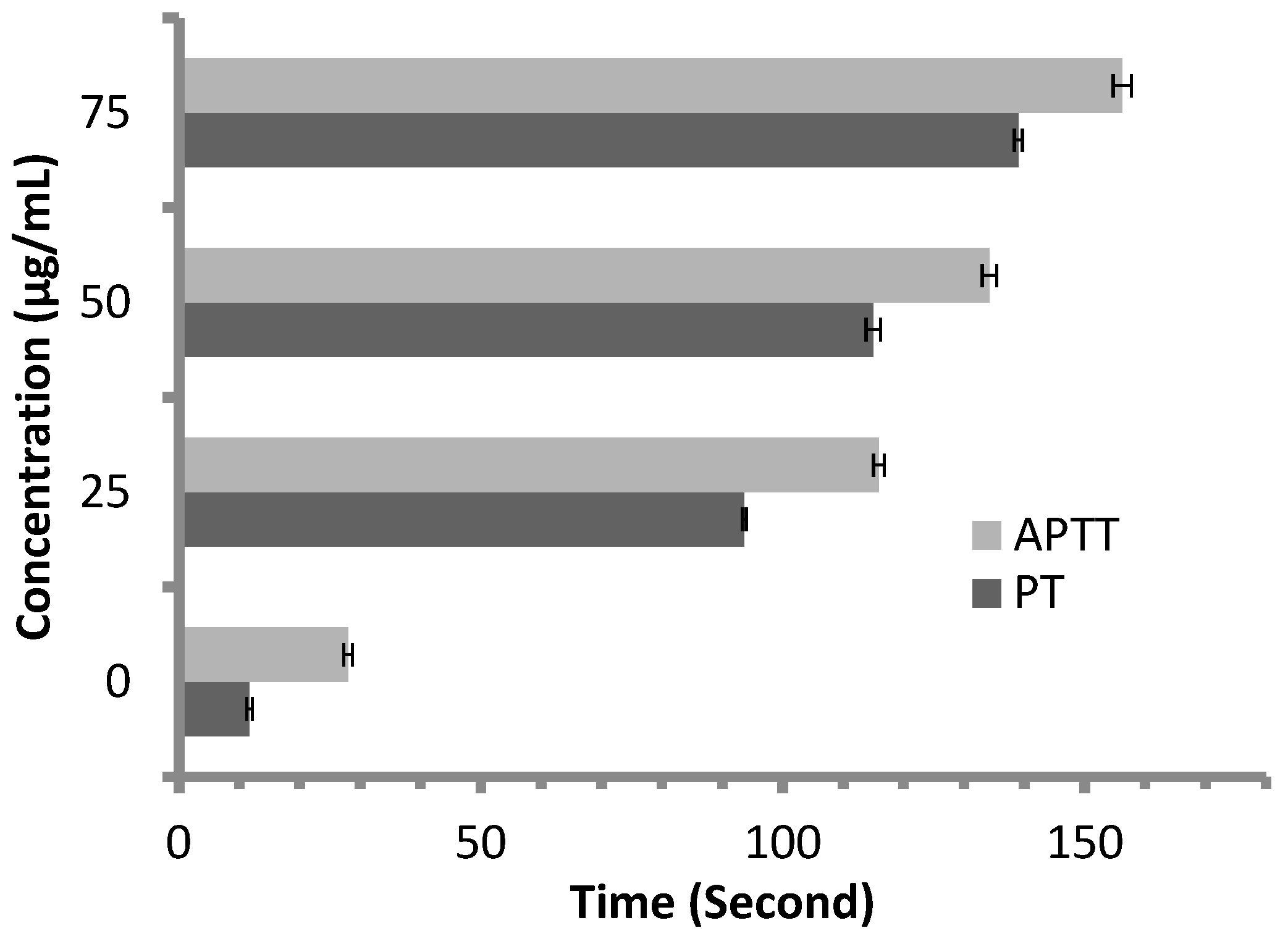
Anticoagulant activity of M. pulegium extract was evaluated using APTT assay, measuring the activity of all coagulation factors in the intrinsic pathway, and PT assay, measuring the activity of the extrinsic pathway. PT and APTT results increased with incremental concentrations of the M. pulegium extract (Figure 8). M. pulegium extract showed PT and APTT of 21.5 s and 49.5 s, respectively (Figure 8), while heparin as a positive control showed PT and APTT as 115 s and 134.2 s, respectively, at 50 μg/mL (Figure 9). At 75 μg/mL of M. pulegium extract, PT and APTT were at 26.7 s and 67.8 s, respectively. Recently Leite et al. [53] studied the anticoagulant activity of numerous plants, among which Mentha crispa (APTT = 51.25) showed greatest anticoagulant potential. Ku and Bae [54] suggested that the inhibition of intrinsic and common pathways was associated with the prolongation of APTT, while the inhibition of the extrinsic coagulation pathway was associated with the prolongation of PT. The anticoagulant activities exhibited by M. pulegium extract may be due to the presence of natural constituents as observed by GC-MS analysis in the current study. The obtained results proved that the M. pulegium extract was effective as an anticoagulation agent.
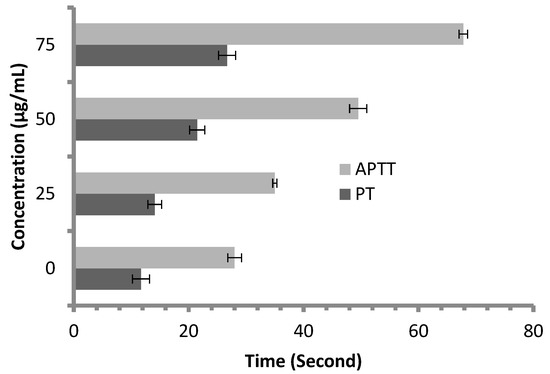
Figure 8.
Anticoagulant activity of M. pulegium at different concentrations, represented by PT and APTT.
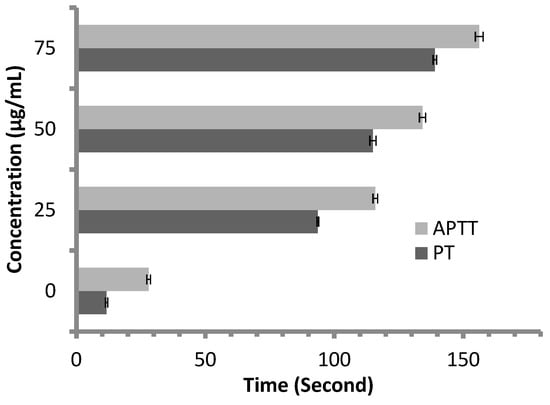
Figure 9.
Anticoagulant activity of heparin at different concentrations, represented by PT and APTT.
2.5. Anticancer Activity of M. pulegium Extract
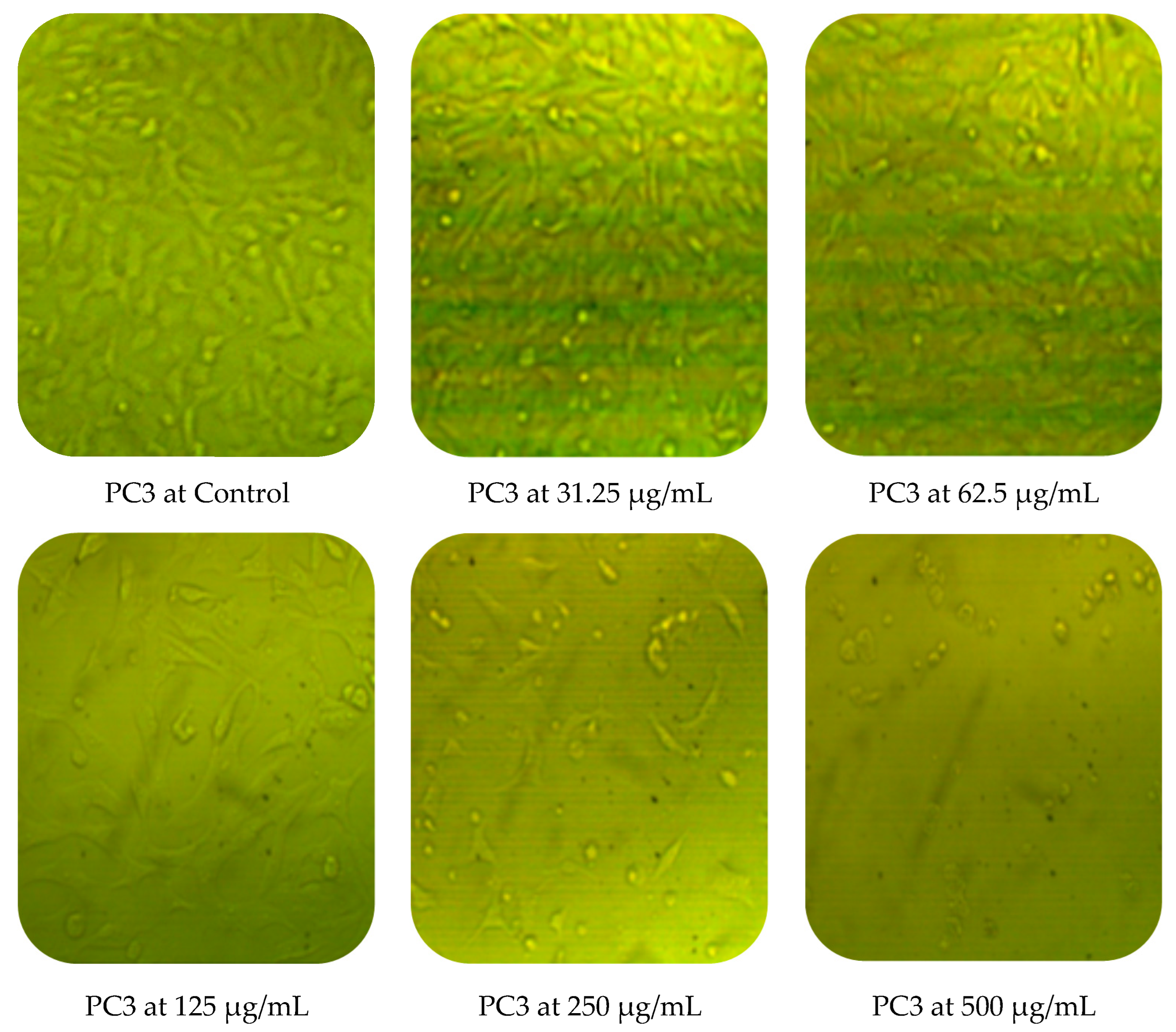
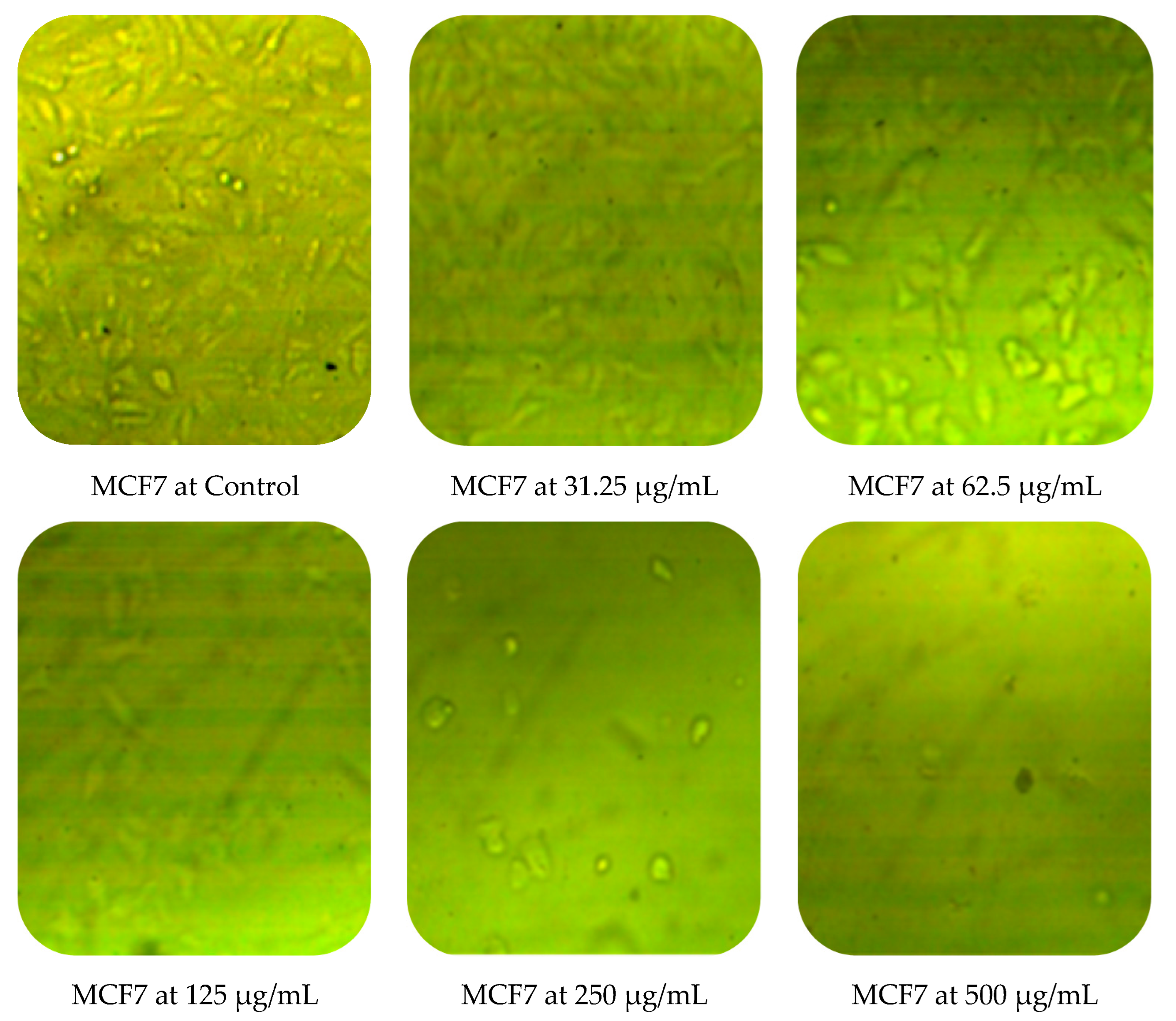
Cytotoxic activity of M. pulegium against two cancer cell lines (PC3 and MCF7) was assessed by MTT in vitro. The obtained results showed a dose-dependent decline in cancer cell line proliferation after exposure to M. pulegium extract. The highest cytotoxic activity was 97.17% and 96.71% against PC3 and MCF7, respectively, at 500 µg/mL (Table 6). PC3 was more resistant than MCF7 at low concentrations of M. pulegium extract, where acute toxicity was 0.60 and 10.34% for PC3 and MCF7, respectively, at 31.25 µg/mL. Toxicity was 28.88 and 48.46% for PC3 and MCF7, respectively, at 62.5 µg/mL. A similar study was conducted previously [55], where the anticancer potential of M. pulegium was documented against the MCF7 breast cancer cell line with acute toxicity of 88.547% at 500 μg/mL. However, the anticancer activity was recorded against the two tested cells, but different values of IC50 (97.99 and 80.21 µg/mL for PC3 and MCF7, respectively) were recorded according to the type of cell (Table 6) or plant origin, as reported in other literature. Low IC50 (59 and 48.86 µg/mL against MCF7 and A549, respectively) of M. pulegium L extract was recorded in another study [56]. Numerous authors have mentioned that the anti-proliferative and apoptotic activities of M. pulegium L extract, as well as those of other plants, may be due to the presence of flavanones, particularly hesperidin [10,20]. Cell death mechanism caused by the M. pulegium L extract was observed by cell morphology assay, where the acute toxicity was accompanied by a morphological change of PC3 and MCF7 (Figure 10), particularly at high concentrations. Cell shrinkage was observed with exposure to 125 µg/mL, in addition to the appearance of round cells and membrane breakdown with cell disruption at 250 and 500 µg/mL, possibly underlining a genotoxic interference, although this postulation requires further study.

Table 6.
Cytotoxicity of M. pulegium L extract against PC3 and MCF7 at different concentrations.
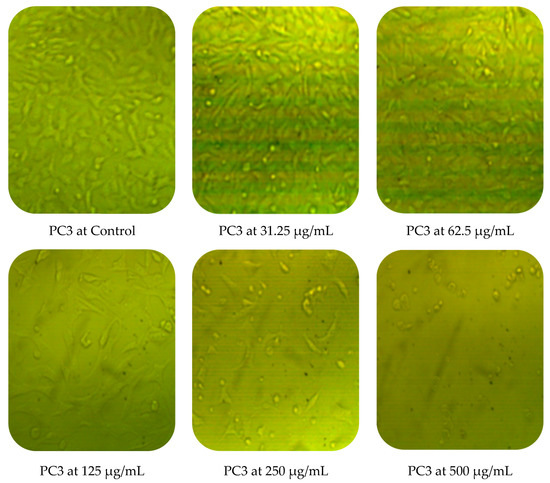
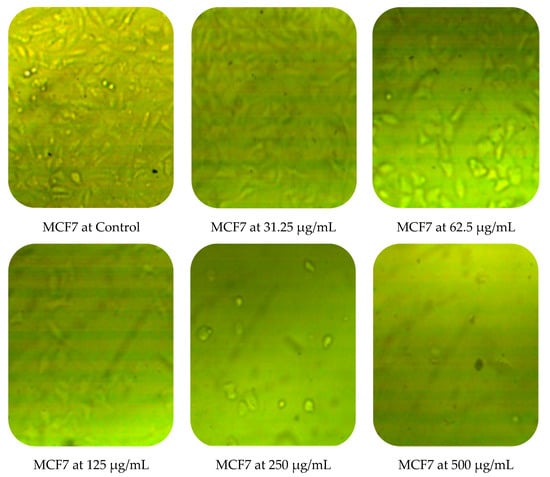
Figure 10.
Effects of different concentrations of M. pulegium extract on morphological changes of PC3 and MCF7.
2.6. Molecular Modeling: Docking Study
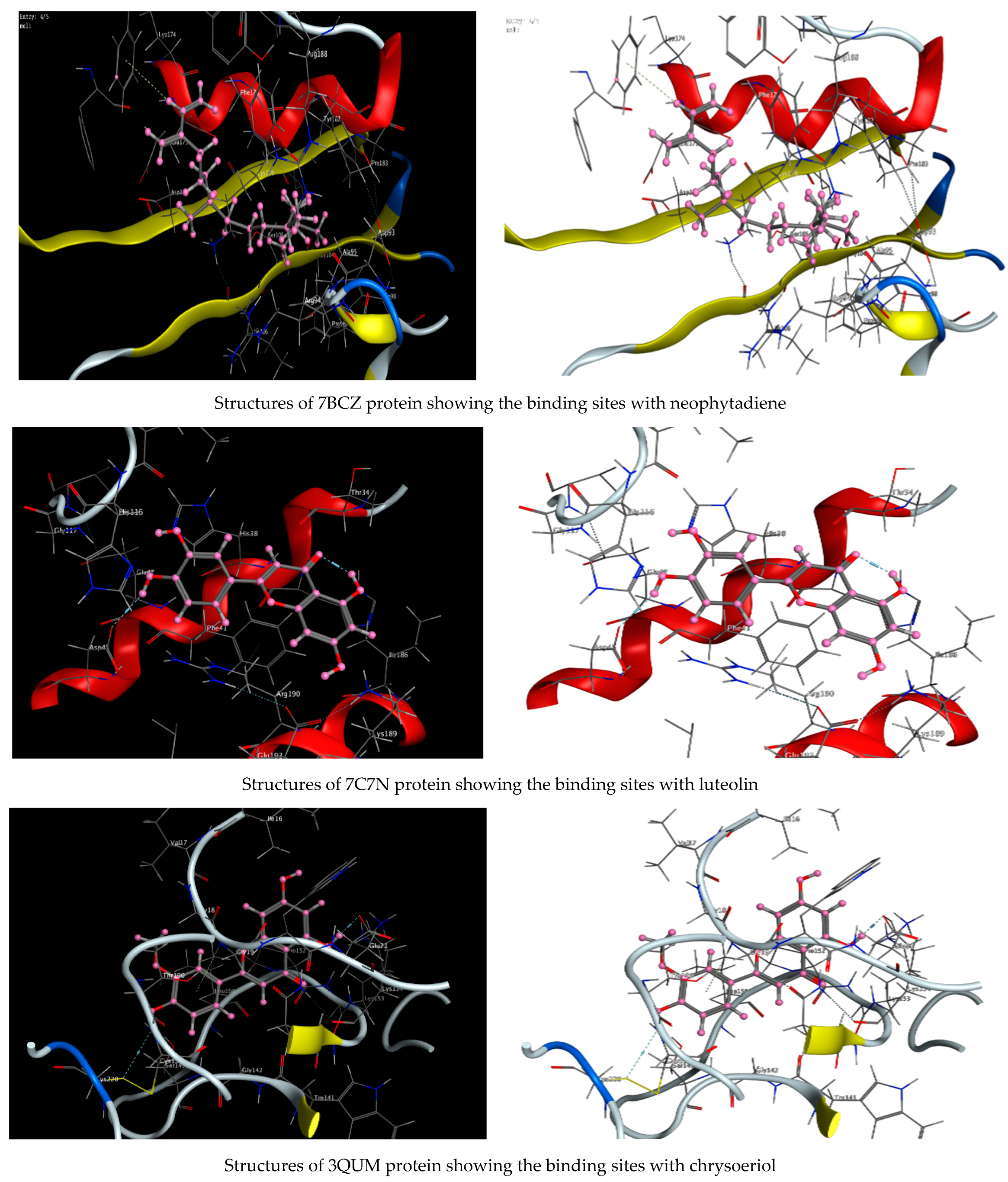
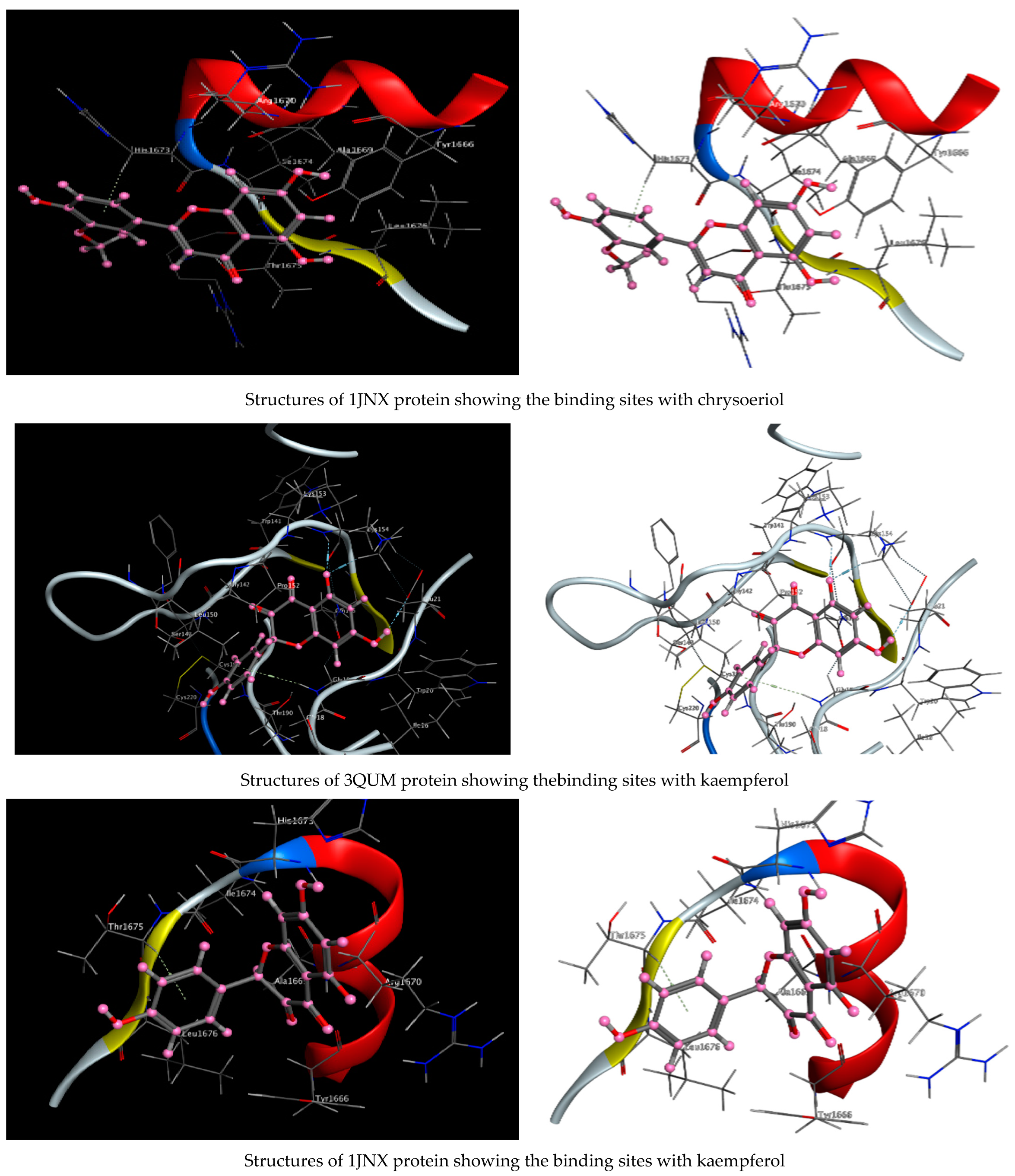
As described in the “Method” section, the current docking protocol was authenticated for all target receptors. Neophytadiene, luteolin, chrysoeriol, and kaempferol were analyzed for binding affinity with P. aeruginosa (PDB = 7BCZ), crystal structure of E. coli DNA (PDB = 7C7N), crystal structure of human prostate (PDB = 3QUM), and the crystal structure of the breast-cancer-associated protein (PDB = 1JNX) (Figure 11). The amino acid residue interactions of different proteins with active compounds are presented in Figure 12. Molecular modeling calculation was carried out to investigate the binding free energies of this inhibitor inside the target receptor, as mentioned previously [23].
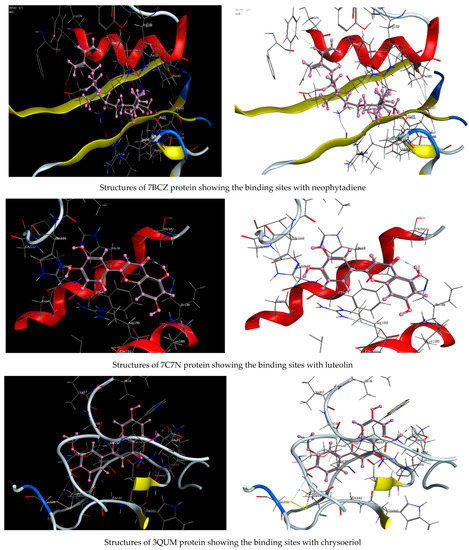
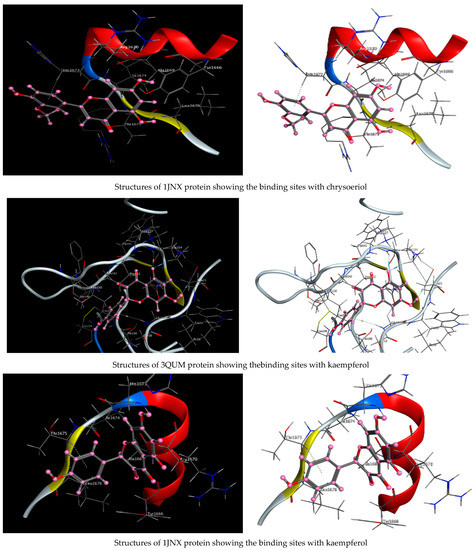
Figure 11.
Structures of different proteins showing their respective binding sites for active compounds.
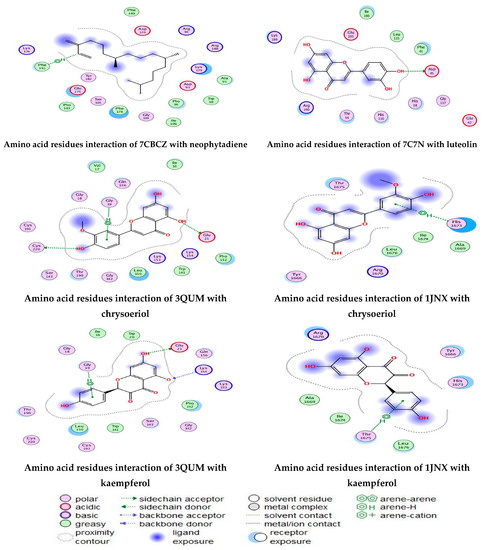
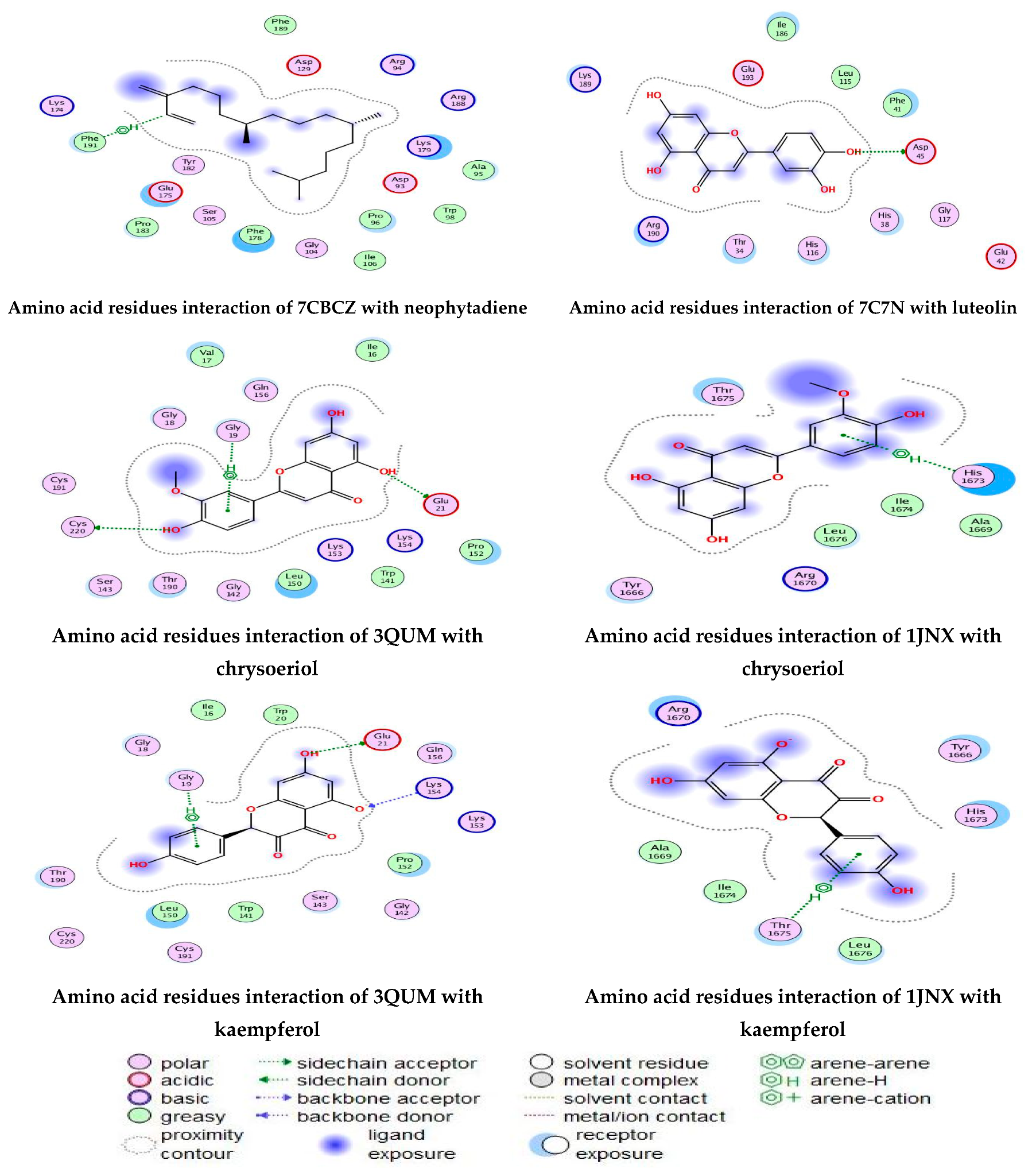
Figure 12.
Amino acid residue interactions of different proteins with active compounds, and the representative key of the types of interaction between active compounds and proteins.
Docking of neophytadiene with active receptor sites of (7BCZ) indicated the occurrence of a hydrogen bond among C1 atoms in the ligand with aromatic ring PHE191 amino acids residue at a distance of 4.37 oA. Also, the interaction between luteolin and the active site binding of E. coli DNA (7C7N) revealed the presence of a hydrogen donor atom between the O28 atom in the ligand and ASP 45 amino acid residue, at a distance of 3.26 oA. The docked chrysoeriol and kaempferol were superimposed on the crystal structure of human prostate (PDB = 3QUM) and the crystal structure of the breast-cancer-associated protein (PDB = 1JNX), with RMSD values (1.0946, 1.7265) and (1.7518, 1.3456), respectively, and binding free energies of (−6.3350 kcal·mol−1, −5.0644 kcal·mol−1) and (−6.3593 kcal·mol−1, −5.2916 kcal·mol−1), respectively. The total obtained results of docking scores and energies of compounds are presented in Table 7. The interactions between almost all atoms in the compounds and amino acid residues of enzymes are shown in Table 8. The effectiveness of the compounds tested in the current study was evaluated on the basis of docking scores, as described previously [24,25] for other compounds, namely deguelin and its derivatives.

Table 7.
Docking scores and energies of compounds with 7BCZ, 7C7N, 3QUM, and 1JNX proteins.

Table 8.
Interaction of active compounds with proteins.
3. Material and Methods
3.1. Collected Plant Samples and Process of Extraction
Leaves, stems, and flowers of M. pulegium were collected from Menofia Governorate throughout August 2021. The collected plant parts were washed with running tap water followed by sterile water to eliminate any dust, then dried and ground by electric mixer. Then, the obtained dried plants (140 g) were ground using the electric mixer into a fine powder, homogenized, then macerated in a stoppered container with 500 mL of 85% methanol for a period of 7 days. Then, for conventional extraction, the extract was processed in a sonicator at 40 °C for 60 min. This extract was filtered, then concentrated in a vacuum at 40 °C for 30 min using Rota vapor, to provide 7.0 g crude extract. Chemical analysis and biological activity analyses were performed on the extract.
3.2. Analysis of M. pulegium Extract by Gas Chromatography-Mass Spectrometry (GC-MS)
GC (THERMO Scientific Corp., Waltham, MA, USA)-MS (ISQ Single Quadrupole Mass Spectrometer) was used for M. pulegium extract analysis. Chromatographic conditions included film thickness of the capillary column TR-5MS of 30 m × 0.32 mm × 0.25 μm. Temperature cycling to 60 °C was used for the initial analysis, incrementing to 240 °C, followed by a gradual increase to the maximum temperature 290 °C by 30 °C/min, remaining isothermally constant up to 2 min. The protected temperature for injector was 250 °C and for MS transfer was 260 °C. One mL/min of high purity helium at constant flow was applied as carrier. One µL extract of M. pulegium was injected using the Auto sampler AS1300 linked to GC in the split manner. At a range of m/z 40–1000 achieved by electron energy with 70 eV application, the spectra of the electron ionization mass were collected in full scan mode. By calculation of mass spectra and retention time (RT) of the detected contents, these were identified by comparison with the existing evidence in the mass spectral library at the National Institute of Standards and Technology [1].
3.3. High-Performance Liquid Chromatography (HPLC) for Flavonoid and Phenolic Contents Determination
The analysis of flavonoid and phenolic contents of the extract was performed using HPLC. The chromatographic conditions included HPLC-(Agilent 1100) (Agilent, Santa Clara, CA, USA), equipped with two LC pumps, a UV/Vis detector, 125 mm × 4.60 mm, with 5 µm particle size of the C18 column. Twenty µL of the extract was injected in HPLC using methanol and 1/25 of acetic acid/water (60:40 v/v) as a mobile solvent for separation of phenolic acids. In case of flavonoids separation, the used mobile solvent with an isocratic flow rate of 1.0 mL min−1 was methanol/water in a 50:50 ratio v/v, adjusted using phosphoric acid to obtain pH 2.8. The obtained peaks of the separated flavonoid and phenolic content were compared with the different standard stock solutions of pure phenolic and flavonoids in methanol extract, using the RT (retention time) and UV-Vis spectra [57].
3.4. Antimicrobial Potential of M. pulegium Extract
Study of the antimicrobial potential of M. pulegium extract was carried out using well diffusion assay against bacteria including four bacterial species (Bacillus subtilis, Pseudomonas aeruginosa, Staphylococcus aureus, Escherichia coli), as well as yeast (Candida albicans), and filamentous black mold (Mucor circinelloides). Ain Shams University Hospitals, Egypt were the source of the tested bacteria, while Assiut University Mycological Centre provided the tested fungi. A hole (6 mm) was cut aseptically into agar using a sterile cork borer; extract solution (100 ul) was poured into the hole. Next, the plates were inoculated, then kept for 30 min in the refrigerator for proper diffusion of the extract, followed by incubation at 28 °C for fungi and 37 °C for bacteria and yeast. The diameter of the apparent inhibition zone (IZ) surrounding the wells was measured (mm) [58]. Gentamycin and ketoconazole were used as antibiotic and antifungal, respectively, as positive control. Dimethyl sulfoxide (DMSO) as a solvent of plant extract was applied as control. MIC was detected by microdilution protocol by making serial dilutions of 1 mL of M. pulegium extract (dissolved in DMSO) in broth, using a new pipette for each subsequent dilution step. The antimicrobial and broth solutions were dispensed into the plastic microdilution trays. The inoculum was prepared by making saline suspension of bacterial or yeast colonies (2 × 108 colony-forming units/mL) from 20 h old, cultivated agar plates; 100 μL from the relevant suspension was inoculated into each well. Then the inoculated macro-dilution tubes were incubated (35 ± 2 °C) for 18 h (specific for bacteria) or for 24 h (specific for yeast) The MIC of M. pulegium extract was detected by measuring OD at 600 nm, and compared with growth without treatment as control [18].
3.5. Antioxidant Activity of M. pulegium Extract
The antioxidant activity of the extract was observed using a 2, 2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging test. Different concentrations of the dried methanolic extract of M. pulegium were dissolved in DMSO. Then the different concentrations (15–1000 μg/mL) were prepared by dilution, and 2 mL of 0.1 mM prepared DPPH in methanol as solvent solution was added to each of the diluted extracts, then mixed by vortex, followed by keeping under shade for 1 h. The addition of 2 mL of the DPPH solution to 1 mL of methanol was utilized as a negative control [59]. The mean of three absorbance readings of the plant extract and negative control at 514 nm was recorded, then the activity of DPPH radical scavenging (%) was determined by the following formulation:
Inhibitory concentration 50% (IC50) of the extract was determined.
Ascorbic acid at different amounts from 5 to 80 μg/mL was used to determine antioxidant activity as a positive control, as mentioned in the description of the antioxidant activity of the extract.
3.6. Anti-Hemolytic Activity Evaluation of M. pulegium Extract In Vitro
For the erythrocyte preparation, 3 mL of fresh whole blood was collected from one of the current research authors, in heparin blood collection tubes. The collected red blood cells from the blood sample obtained via centrifugation at 3000 rpm for 10 min were dissolved using normal saline solution (v/v supernatant), then reconstituted up to 40% v/v suspension by sodium phosphate (10 mM) as isotonic buffer solution adjusted to pH 7.4. Successive dilutions of plant extract in DMSO (from 100 to 1000 μg/mL) were added to 5 mL of distilled water to obtain the hypotonic solution.
Suspension of erythrocyte (0.1 mL) was added to the prepared hypotonic solution, followed by gentle mixing and incubation (37 °C) for 60 min. For the next incubation period it was centrifuged at 1200× g for 5 min, and the collected supernatant was subjected to assessment of its haemoglobin content via spectrophotometer (Milton Roy) at 540 nm. Indomethacin at 200 μg/mL was used as a positive control. Haemolysis inhibition (%) caused by the plant extract was calculated by the equation:
where AD1 is the absorbance of the treated sample in the isotonic solution, AD2 is the absorbance of the treated sample in the hypotonic solution, AD3 is absorbance of the control sample in the hypotonic solution. Hemolysis % was assessed with regard to hemolysis in distilled water (100%) [60].
3.7. Coagulate Activity Evaluation of M. pulegium Extract In Vitro
The dried methanolic extract of M. pulegium was dissolved in dimethyl sulfoxide (DMSO) then evaluated as an anticoagulant agent using prothrombin time (PT) and activated partial thromboplastin time (APTT) as a classical coagulant test. Nine parts of blood collected from an author of the current research were added to one part of 3.2% sodium citrate, followed by centrifugation (5000× g rpm) for 10 min, and the collected supernatant was utilized for carrying out the PT and APTT tests. Citrated normal human plasma was combined with different dilutions of the extract and incubated for 3 min at 37 °C. The reagents of the PT and APTT were incubated at 37 °C for 3 min before carrying out the tests, then 0.10 mL of the APTT reagent was added to each mixture of plasma and extract, then incubated at 37 °C for 5 min, then 0.10 mL of 0.025 mol/L CaCl2 (pre-incubated at 37 °C for 3 min) was added, and the clotting time was recorded. Mixtures of citrated normal human plasma with different dilutions of the extract were amended with 0.20 mL PT reagent, then clotting times were recorded to assess PT. These procedures were carried out using heparin as control [57].
3.8. Antitumor Assay and Morphological Characteristics of the Treated Cancer Cells
Cytotoxicity of the extract against MCF7 and PC3 cells was performed via 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay. The solution of MTT was prepared in phosphate buffered saline (PBS) as 5 mg/mL. The cells (1 × 105 cells/mL) were inoculated in the 96-well tissue culture plate, followed by incubation at 37 °C for 24 h to develop a complete monolayer sheet. Then, the growth medium was decanted from the 96-well micro titer plates after confluent sheets of cells were formed, and the cell monolayer was washed twice with wash media. Six-fold dilutions of the extract were made in Roswell Park Memorial Institute (RPMI) 1640 medium with serum (2%) as maintenance medium. Each dilution (0.1 mL) was tested in different wells, leaving three wells as control receiving only maintenance medium, then incubated at 37 °C and scanned for any physical signs of toxicity. To each well, 20 µL MTT solution was added, and the MTT was mixed into the media. It was placed on a shaking desk for 5 min at around 150 rpm, then incubated at 37 °C under 5% CO2 for 1–5 h to permit the metabolization of MTT. The resulting MTT metabolic product was re-suspended in 200 µL of DMSO, followed by shaking at 150 rpm for 5 min to mix it into the solvent. The optical density (correlated directly with cell numbers) was measured at 560 nm with subtract background at 620 nm. Cell lines in growth medium without plant extract were studied as a control [59].
3.9. Molecular Docking
A molecular modeling study was undertaken using the Molecular Operating Environment (MOE) [61], a drug discovery software platform that integrates visualization, modeling and simulations, as well as methodology development, into one package. Molecular modeling studies were performed using Molecular Operating Environment (MOE, 2019) software on Dell Core i7 processor with 1.9 GH, 16 GB memory, and a Windows 10, 64-bit operating system. Energy minimizations were performed using MOE, with a RMSD (root mean square deviation) gradient of 0.05 kcal/mol and MMFF94X force field, and partial charges were automatically calculated. The X-ray crystallographic structures were obtained from the Protein Data Bank (PDB) (www.rcsb.org/pdb accessed on 19 July 2022); the PDB files were (7BCZ), (7C7N), (3QUM) and (1JNX).
The target proteins were prepared for docking by:
- Removing the water molecules and co-ligand from the active site of the protein.
- Addition of hydrogen atoms to the structure, with standard geometry.
- Using the MOE site finder to generate the active binding sites, to create the dummy sites as the binding pocket.
- Saving the obtained pocket was saved in MOE, to be used for predicting the ligand protein.
Moreover, the native ligands were minimized to their lowest energy using the MMFF94 force field. Then, the final form was obtained after 3D protonation and the correction process. The general docking scenario was run to 100ns on the rigid receptor atoms, and the ligands were placed in the site using the triangle matcher method. London dG was utilized as a scoring function and the GBVI/WSA dG methods were used for rescoring. The five best poses were ranked by their binding free energy (S, kcal/mol), and the lengths of hydrogen bonds between the compounds and amino acids in the protein did not exceed 3.5 A°. RMSD and RMSD-refine fields were used for pose-with-pose comparison of the results in the co-crystal ligand position before and after amendment, respectively.
3.10. Statistical Analysis
The current experiments were realized in replicate, and results were therefore calculated as ± standard deviation (SD) or standard error (SE) means. GraphPad Prism® (version 5.0, San Diego, CA, USA) software was applied to obtain graphs for the IC50 values of DPPH radical scavenging activity.
4. Conclusions
The obtained findings highlight the phytochemical and pharmaceutical application of M. pulegium extracts. In the current study, the extract was rich in several active components that showed biological activity including anticancer, antimicrobial, antioxidant, and as anti-hemolytic activity. These data suggest that this plant extract is a potential candidate for further experiments towards its use as an alternative drug. The MOE molecular modeling environment was used to study inhibitor activity for (7BCZ), (7C7N), (3QUM) and (1JNX). It was found that the energy scores of the molecular docking study were in good agreement with the experimental results. In conclusion, the antibacterial and antifungal activities of M. pulegium extract obtained from plants growing in Egypt were more effective than traditional antibiotics against the microorganisms studied.
Author Contributions
A.M.H.A.-R. carried out some of the experiments, review and editing; H.Q. carried out some experiments and wrote the first draft of the manuscript; M.S.A. designed some experiments, performed formal analysis, and edited the manuscript; S.K.A.J. carried out some experiments, wrote the paper introduction, and undertook review and editing; M.M.B. assisted in some cell culture experiments, and undertook review and editing; M.G. designed some experiments and wrote the paper; H.M.S. designed the docking studies; S.S. carried out some experiments, wrote final draft, and undertook review and editing; T.M.A. carried out some experiments, wrote the paper, and undertook review and editing; All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding from Princess Nourah bint Abdulrahman University, through Researchers Supporting Project number PNURSP2022R217.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data that support the findings of this study are available within the article.
Acknowledgments
All authors thanks Princess Nourah bint Abdulrahman University for their grant through Researchers Supporting Project number PNURSP2022R217, Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the corresponding authors.
Abbreviations
| HPLC | High-performance liquid chromatography |
| GC-MS | Gas Chromatography-Mass Spectrometry |
| RT | Retention Time |
| MIC | Minimum inhibitory concentration |
| DPPH | 2-Diphenyl-1-picryl-hydrazyl-hydrate |
| PT | Prothrombin time |
| APTT | Activated partial thromboplastin time |
| IZ | Inhibition zone |
| DMSO | Dimethyl sulfoxide |
| TEM | Transmission Electron Microscopy |
| SD | Standard deviation |
| SE | Standard error |
| AD1 | Absorbance of treated sample in the isotonic solution |
| AD2 | Absorbance of treated sample in the hypotonic solution |
| PBS | phosphate buffered saline |
| AD3 | Absorbance of control treated sample in the hypotonic solution |
| O.D | Optical density |
| RPMI | Roswell Park Memorial Institute |
| MCF7 | Michigan Cancer Foundation-7 |
| PC3 | Prostate cancer cell |
| MOE | Molecular Operating Environment |
| RMSD | Root mean square deviation |
| GBVI/WSA | Generalized-Born Volume Integral/Weighted Surface area |
References
- Abdelghany, T.M.; Eman, M.E.T.; El-Sheikh, H.H. Efficacy of fungal rust disease on willow plant in Egypt. Aust. J. Basic Appl. Sci. 2009, 3, 1527–1539. [Google Scholar]
- Abdelghany, T.M. Eco-friendly and safe role of Juniperus procera in controlling of fungal growth and secondary metabolites. J. Plant Pathol. Microbiol. 2014, 5, 231. [Google Scholar]
- Abdelghany, T.M. Safe food additives: A review. J. Biol. Chem. Res. 2015, 32, 402–437. [Google Scholar]
- Brahmi, F.; Khodir, M.; Mohamed, C.; Pierre, D. Chemical Composition and Biological Activities of Mentha Species. In Aromatic and Medicinal Plants-Back to Nature; London, UK, 2017; pp. 47–49. Available online: https://www.intechopen.com/books/5612 (accessed on 19 July 2022).
- Marzouk, M.M.; Hussein, S.R.; Elkhateeb, A.; El-shabrawy, M.; Abdel-Hameed, E.S.; Kawashty, S.A. Comparative study of Mentha species growing wild in Egypt: LC-ESI-MS analysis and chemosystematic significance. J. App. Pharm. Sci. 2018, 8, 116–122. [Google Scholar] [CrossRef]
- Teixeira, B.; Marques, A.; Ramos, C.; Batista, I.; Serrano, C.; Matos, O.; Neng, N.R.; Nogueira, J.M.; Saraiva, J.A.; Nunes, M.L. European pennyroyal (Mentha pulegium) from Portugal: Chemical composition of essential oil and antioxidant and antimicrobial properties of extracts and essential oil. Ind. Crop. Prod. 2012, 36, 81–87. [Google Scholar] [CrossRef]
- Caputo, L.; Cornara, L.; Raimondo, F.; De Feo, V.; Vanin, S.; Denaro, M.; Trombetta, D.; Smeriglio, A. Mentha pulegium L.: A Plant Underestimated for Its Toxicity to Be Recovered from the Perspective of the Circular Economy. Molecules 2021, 26, 2154. [Google Scholar] [CrossRef] [PubMed]
- Boukhebti, H.; Chaker, A.N.; Belhadj, H.; Sahli, F.; Ramdhani, M.; Laouer, H.; Harzallah, D. Chemical composition and antibacterial activity of Mentha pulegium L. and Mentha spicata L. essential oils. Der Pharm. Lett. 2011, 3, 267–275. [Google Scholar]
- Baali, F.; Boumerfeg, S.; Napoli, E.; Boudjelal, A.; Righi, N.; Deghima, A.; Baghiani, A.; Ruberto, G. Chemical Composition and Biological Activities of Essential Oils from Two Wild Algerian Medicinal Plants: Mentha pulegium L. and Lavandula stoechas L. J. Essent. Oil Bear. Plants 2019, 22, 821–837. [Google Scholar] [CrossRef]
- Patti, F.; Palmioli, A.; Vitalini, S.; Bertazza, L.; Redaelli, M.; Zorzan, M.; Rubin, B.; Mian, C.; Bertolini, C.; Iacobone, M.; et al. Anticancer Effects of Wild Mountain Mentha longifolia Extract in Adrenocortical Tumor Cell Models. Front. Pharmacol. 2020, 10, 1647. [Google Scholar] [CrossRef] [PubMed]
- Alharbi, N.K.; Naghmouchi, S.; Al-Zaban, M. Evaluation of Antimicrobial Potential and Comparison of HPLC Composition, Secondary Metabolites Count, and Antioxidant Activity of Mentha rotundifolia and Mentha pulegium Extracts. Evid.-Based Complement. Altern. Med. 2021, 2021, 9081536. [Google Scholar] [CrossRef]
- Sebai, E.; Serairi, R.; Saratsi, K.; Abidi, A.; Sendi, N.; Darghouth, M.A.; Wilson, M.S.; Sotiraki, S.; Akkari, H. Hydro-Ethanolic Extract of Mentha pulegium Exhibit Anthelmintic and Antioxidant Proprieties In Vitro and In Vivo. Acta Parasitol. 2020, 65, 375–387. [Google Scholar] [CrossRef] [PubMed]
- El Hassani, F.Z. Characterization, activities, and ethnobotanical uses of Mentha species in Morocco. Heliyon 2020, 6, e05480. [Google Scholar] [CrossRef] [PubMed]
- Piras, A.; Porcedda, S.; Falconieri, D.; Maxia, A.; Gonçalves, M.; Cavaleiro, C.; Salgueiroet, L. Antifungal activity of essential oil from Mentha spicata L. and Mentha pulegium L. growing wild in Sardinia island (Italy). Nat. Prod. Res. 2021, 35, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Bektašević, M.; Politeo, O.; Carev, I. Comparative Study of Chemical Composition, Cholinesterase Inhibition and Antioxidant Potential of Mentha pulegium L. Essential Oil. Chem. Biodivers. 2021, 18, e2000935. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Oliveira, P.; Otero, P.; Pereira, A.; Chamorro, F.; Carpena, M.; Echave, J.; Fraga-Corral, M.; Simal-Gandara, J.; Prieto, M. Status and Challenges of Plant-Anticancer Compounds in Cancer Treatment. Pharmaceuticals 2021, 14, 157. [Google Scholar] [CrossRef] [PubMed]
- Bakri, M.M.; El-Naggar, M.A.; Helmy, E.A.; Ashoor, M.S.; Ghany, T.M.A. Efficacy of Juniperus procera Constituents with Silver Nanoparticles Against Aspergillus fumigatus and Fusarium chlamydosporum. BioNanoScience 2020, 10, 62–72. [Google Scholar] [CrossRef]
- Abdelghany, T.; Yahya, R.; Bakri, M.M.; Ganash, M.; Amin, B.H.; Qanash, H. Effect of Thevetia peruviana Seeds Extract for Microbial Pathogens and Cancer Control. Int. J. Pharmacol. 2021, 17, 643–655. [Google Scholar] [CrossRef]
- Cao, X.; He, Y.; Kong, Y.; Mei, X.; Huo, Y.; He, Y.; Liu, J. Elucidating the interaction mechanism of eriocitrin with β-casein by multi-spectroscopic and molecular simulation methods. Food Hydrocoll. 2019, 94, 63–70. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Ballav, S.; Yadav, R.S.; Swamy, K.V.; Basu, S. Probing intermolecular interactions and binding stability of kaempferol, quercetin and resveratrol derivatives with PPAR-γ: Docking, molecular dynamics and MM/GBSA approach to reveal potent PPAR-γ agonist against cancer. J. Biomol. Struct. Dyn. 2020, 40, 971–981. [Google Scholar] [CrossRef]
- Yahya, R.; Al-Rajhi, A.M.H.; Alzaid, S.Z.; Al Abboud, M.A.; Almuhayawi, M.S.; Al Jaouni, S.K.; Selim, S.; Ismail, K.S.; Abdelghany, T.M. Molecular Docking and Efficacy of Aloe vera Gel Based on Chitosan Nanoparticles against Helicobacter pylori and Its Antioxidant and Anti-Inflammatory Activities. Polymers 2022, 14, 2994. [Google Scholar] [CrossRef]
- Al Abboud, M.A.; Al-Rajhi, A.M.; Shater, A.R.; Alawlaqi, M.M.; Mashraqi, A.; Selim, S.; Al Jaouni, S.K.; Ghany, T.M. Halostability and Thermostability of Chitinase Produced by Fungi Isolated from Salt Marsh Soil in Subtropical Region of Saudi Arabia. BioResources 2022, 17, 4763–4780. [Google Scholar] [CrossRef]
- Lokhande, K.B.; Ballav, S.; Thosar, N.; Swamy, K.V.; Basu, S. Exploring conformational changes of PPAR-Ɣ complexed with novel kaempferol, quercetin, and resveratrol derivatives to understand binding mode assessment: A small-molecule checkmate to cancer therapy. J. Mol. Model. 2020, 26, 242. [Google Scholar] [CrossRef] [PubMed]
- Qanash, H.; Yahya, R.; Bakri, M.M.; Bazaid, A.S.; Qanash, S.; Shater, A.F.; Abdelghany, T.M. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022, 12, 5914. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajhi, A.M.H.; Yahya, R.; Abdelghany, T.M.; Fareid, M.A.; Mohamed, A.M.; Amin, B.H.; Masrahi, A.S. Anticancer, Anticoagulant, Antioxidant and Antimicrobial Activities of Thevetia peruviana Latex with Molecular Docking of Antimicrobial and Anticancer Activities. Molecules 2022, 27, 3165. [Google Scholar] [CrossRef]
- Venkata, R.B.; Samuel, L.A.; Pardha Saradhi, M. Antibacterial, antioxidant activity and GC-MS analysis of Eupatorium odoratum. Asian J. Pharm. Clin. Res. 2012, 5, 99–106. [Google Scholar]
- Mustapa, A.N.; Martín, A.; Mato, R.B.; Cocero, M.J. Extraction of phytocompounds from the medicinal plant Clinacanthus nutans Lindau by microwave-assisted extraction and supercritical carbon dioxide extraction. Ind. Crops Prod. 2015, 74, 83–94. [Google Scholar] [CrossRef]
- Moon, D.-O.; Kim, M.-O.; Choi, Y.H.; Kim, G.-Y. β-Sitosterol induces G2/M arrest, endoreduplication, and apoptosis through the Bcl-2 and PI3K/Akt signaling pathways. Cancer Lett. 2008, 264, 181–191. [Google Scholar] [CrossRef]
- Sujatha, S.; Anand, S.; Sangeetha, K.; Shilpa, K.; Lakshmi, J.; Balakrishnan, A.; Lakshmi, B. Biological evaluation of (3β)-STIGMAST-5-EN-3-OL as potent anti-diabetic agent in regulating glucose transport using in vitro model. Int. J. Diabetes Mellit. 2010, 2, 101–109. [Google Scholar] [CrossRef]
- Uddin, M.S.; Ferdosh, S.; Akanda, J.H.; Ghafoor, K.; Rukshana, A.H.; Ali, E.; Kamaruzzaman, B.Y.; Fauzi, M.B.; Hadijah, S.; Shaarani, S.; et al. Techniques for the extraction of phytosterols and their benefits in human health: A review. Sep. Sci. Technol. 2018, 53, 2206–2223. [Google Scholar]
- Raju, L.; Lipin, R.; Eswaran, R. Identification, ADMET evaluation and molecular docking analysis of Phytosterols from Banaba (Lagerstroemia speciosa (L.)Pers) seed extract against breast cancer. Silico Pharmacol. 2021, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Al-Rubaye, A.F.; Kadhim, M.J.; Hameed, I.H. Determination of Bioactive Chemical Composition of Methanolic Leaves Extract of Sinapis arvensis Using GC-MS Technique. Int. J. Toxicol. Pharmacol. Res. 2017, 9, 163–178. [Google Scholar] [CrossRef]
- Lima, T.C.; Mota, M.M.; Barbosa-Filho, J.M.; Dos Santos, M.R.V.; de Sousa, D. Structural relationships and vasorelaxant activity of monoterpenes. DARU J. Pharm. Sci. 2012, 20, 23. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Muhammad, F.; de Sousa, D.P.; Khaliq, T.; Aslam, B.; Andrade, L.; Bashir, S.; Anwar, M.I.; Shahid, M.; Qamar, M. In vitro and in vivo toxicological evaluations of methyl ferulate, methyl p-coumarate, and pulegone 1,2-epoxide. Pharm. Biol. 2016, 54, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Pinedo-Espinoza, J.M.; Gutiérrez-Tlahque, J.; Saenz, Y.O.S.; Aguirre-Mancilla, C.L.; Reyes-Fuentes, M.; López-Palestina, C.U. Nutritional Composition, Bioactive Compounds and Antioxidant Activity of Wild Edible Flowers Consumed in Semiarid Regions of Mexico. Mater. Veg. 2020, 75, 413–419. [Google Scholar] [CrossRef]
- Nithya, T.G.; Jayanthi, J.; Raghunathan, M.G. Phytochemical, antibacterial and GC MS analysis of a floating fern Salvinia molesta D.S.Mitchell (1972). Int. J. PharmTech Res. 2015, 8, 85–90. [Google Scholar]
- Ćavar, Z.S.; Šišková, J.; Komzáková, K.; De Diego, N.; Kaffková, K.; Tarkowski, P. Phenolic Compounds and Biological Activity of Selected Mentha Species. Plants 2021, 10, 550. [Google Scholar] [CrossRef]
- Tahira, R.; Naeemullah, M.; Akbar, F.; Masood, M.S. Major phenolic acids of local and exotic mint germplasm grown in Islamabad. Pak. J. Bot. 2011, 43, 151–154. [Google Scholar]
- Shalaby, N.M.M.; Moharram, F.A.; El-Toumy, S.A.A.; Marzoyk, M.S.A.; Ahmed, A.A.E. Phytochemical and Pharmacological Studies of Mentha Pulegium L. Bull. Fac. Pharm. 2000, 38, 143–151. [Google Scholar]
- Proestos, C.; Chorianopoulos, N.; Nychas, G.J.E.; Komaitis, M. RP-HPLC analysis of the phenolic compounds of plant extracts: Investigation of their antioxidant capacity and antimicrobial activity. J. Agric. Food Chem. 2005, 53, 1190–1195. [Google Scholar] [CrossRef]
- Fatiha, B.; Didier, H.; Naima, G.; Khodir, M.; Martin, K.; Léocadie, K.; Caroline, S.; Mohamed, C.; Pierre, D. Phenolic composition, in vitro antioxidant effects and tyrosinase inhibitory activity of three Algerian Mentha species: M. spicata (L.) M. pulegium (L.) and M. rotundifolia (L.) Huds (Lamiaceae). Ind. Crops Prod. 2015, 74, 722–730. [Google Scholar] [CrossRef]
- Erenler, R.; Telci, I.; Elmastaş, M.; Aksit, H.; Gül, F.; Tüfekçi, A.R.; Demirtaş, I.; Kayir, O. Quantification of flavonoids isolated from Mentha spicata in selected clones of Turkish mint landraces. Turk. J. Chem. 2018, 42, 1695–1705. [Google Scholar] [CrossRef]
- Barreca, D.; Mandalari, G.; Calderaro, A.; Smeriglio, A.; Trombetta, D.; Felice, M.R.; Gattuso, G. Citrus Flavones: An Update on Sources, Biological Functions, and Health Promoting Properties. Plants 2020, 9, 288. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; He, J.; Ruan, H.; Wang, Y. In vitro and in vivo cytotoxic effects of chrysoeriol in human lung carcinoma are facilitated through activation of autophagy, sub-G1/G0 cell cycle arrest, cell migration and invasion inhibition and modulation of MAPK/ERK signaling pathway. J. BUON 2019, 24, 936–942. [Google Scholar] [PubMed]
- Sharma, N.; Biswas, S.; Al-Dayan, N.; Alhegaili, A.S.; Sarwat, M. Antioxidant role of kaempferol in prevention of hepatocellular carcinoma. Antioxidants 2021, 10, 1419. [Google Scholar] [CrossRef]
- Abbou, F.; Azzi, R.; Ouffai, K.; El Haci, I.A.; Belyagoubi-Benhammou, N.; Bensouici, C.; Benamar, H. Phenolic profile, antioxidant and enzyme inhibitory properties of phenolic-rich fractions from the aerial parts of Mentha pulegium L. S. Afr. J. Bot. 2022, 146, 196–204. [Google Scholar] [CrossRef]
- Baali, F.; Boumerfeg, S.; Boudjelal, A.; Denaro, M.; Ginestra, G.; Baghiani, A.; Righi, N.; Deghima, A.; Benbacha, F.; Smeriglio, A.; et al. Wound-healing activity of Algerian Lavandula stoechas and Mentha pulegium extracts: From traditional use to scientific validation. Plant Biosyst.—Int. J. Deal. All Asp. Plant Biol. 2021, 156, 427–439. [Google Scholar] [CrossRef]
- Aimad, A.; Sanae, R.; Anas, F.; Abdelfattah, E.M.; Bourhia, M.; Salamatullah, A.M.; Alzahrani, A.; Alyahya, H.K.; Albadr, N.A.; Abdelkrim, A.; et al. Chemical Characterization and Antioxidant, Antimicrobial, and Insecticidal Properties of Essential Oil from Mentha pulegium L. Evid.-Based Complement. Altern. Med. 2021, 2021, 1108133. [Google Scholar] [CrossRef]
- Ceyhan-Güvensen, N.; Keskin, D. Chemical content and antimicrobial properties of three different extracts of Mentha pulegium leaves from Mugla Region, Turkey. J. Environ. Biol. 2016, 37, 1341–1346. [Google Scholar]
- Bouhdid, S.; Abrini, J.; Amensour, M.; Zhiri, A.; Espuny, M.J.; Manresa, A. Functional and ultrastructural changes in Pseudomonas aeruginosa and Staphylococcus aureus cells induced by Cinnamomum verum essential oil. J. Appl. Microbiol. 2010, 109, 1139–1149. [Google Scholar] [CrossRef] [PubMed]
- Rocha, J.; Direito, R.; Lima, A.; Mota, J.; Gonçalves, M.; Duarte, M.P.; Solas, J.; Peniche, B.F.; Fernandes, A.; Pinto, R.; et al. Reduction of inflammation and colon injury by a Pennyroyal phenolic extract in experimental inflammatory bowel disease in mice. Biomed. Pharmacother. 2019, 118, 109351. [Google Scholar] [CrossRef]
- Yang, Q.; Liu, Q.; Lv, H.; Wang, F.; Liu, R.; Zeng, N. Effect of pulegone on the NLPR3 inflammasome during inflammatory activation of THP-1 cells. Exp. Ther. Med. 2019, 19, 1304–1312. [Google Scholar] [CrossRef] [PubMed]
- Leite, P.M.; Freitas, A.; Amorim, J.; Figueiredo, R.C.D.; Bertolucci, S.; Faraco, A.; Martins, M.; Carvalho, M.G.; Castilho, R. In vitro anticoagulant activity of selected medicinal plants: Potential interactions with warfarin and development of new anticoagulants. J. Basic Clin. Physiol. Pharmacol. 2021. [Google Scholar] [CrossRef]
- Ku, S.-K.; Bae, J.-S. Antiplatelet and antithrombotic activities of purpurogallin in vitro and in vivo. BMB Rep. 2014, 47, 376–381. [Google Scholar] [CrossRef]
- Mohebi, A.; Yazdani, H.; Nourian, S.; Torabi Godarzi, R.; Sarveahrabi, Y. Cytotoxicity of hydroalcoholic extracts of Mentha pulegium L. and Satureja hortensis L. on human breast cancer cell lines (MCF-7 and MCF-10). Navid No 2021, 24, 50–58. [Google Scholar] [CrossRef]
- Farnam, G.; Aryanpour, N.; Behtaj, R.; Shirazi, H. Cytotoxic and cell progression effects of Mentha pulegium L. extract on selected cancer cell lines. Iran. J. Pharm. Sci. 2020, 16, 27–34. [Google Scholar]
- Mizzi, L.; Chatzitzika, C.; Gatt, R.; Valdramidis, V. HPLC Analysis of Phenolic Compounds and Flavonoids with Overlapping Peaks. Food Technol. Biotechnol. 2020, 58, 12–19. [Google Scholar] [CrossRef]
- Abdelghany, T.M. Stachybotrys chartarum: A novel biological agent for the extracellular synthesis of silver nanoparticles and their antimicrobial activity. Indonesian. J. Biotechnol. 2013, 18, 75–82. [Google Scholar]
- Abdelghany, T.M.; Ganash, M.; Alawlaqi, M.M.; Al-Rajhi, A.M.H. Antioxidant, Antitumor, Antimicrobial Activities Evaluation of Musa paradisiaca L. Pseudostem Exudate Cultivated in Saudi Arabia. BioNanoScience 2019, 9, 172–178. [Google Scholar] [CrossRef]
- Meziti, H.; Bouriche, H.; Kada, S.; Demirtas, I.; Murat Kizil, M.; Senator, A. Phytochemical analysis, and antioxidant, anti-hemolytic and genoprotective effects of Quercus ilex L. and Pinus halepensis Mill. methanolic extracts. J. Pharm. Pharmacogn. Res. 2019, 7, 260–272. [Google Scholar]
- Lokhande, K.B.; Nagar, S.; Swamy, K.V. Molecular interaction studies of Deguelin and its derivatives with Cyclin D1 and Cyclin E in cancer cell signaling pathway: The computational approach. Sci. Rep. 2019, 9, 1778. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).