Ultrasonic-Assisted Extraction of Flavonoids from Juglans mandshurica Maxim.: Artificial Intelligence-Based Optimization, Kinetics Estimation, and Antioxidant Potential
Abstract
:1. Introduction
2. Results
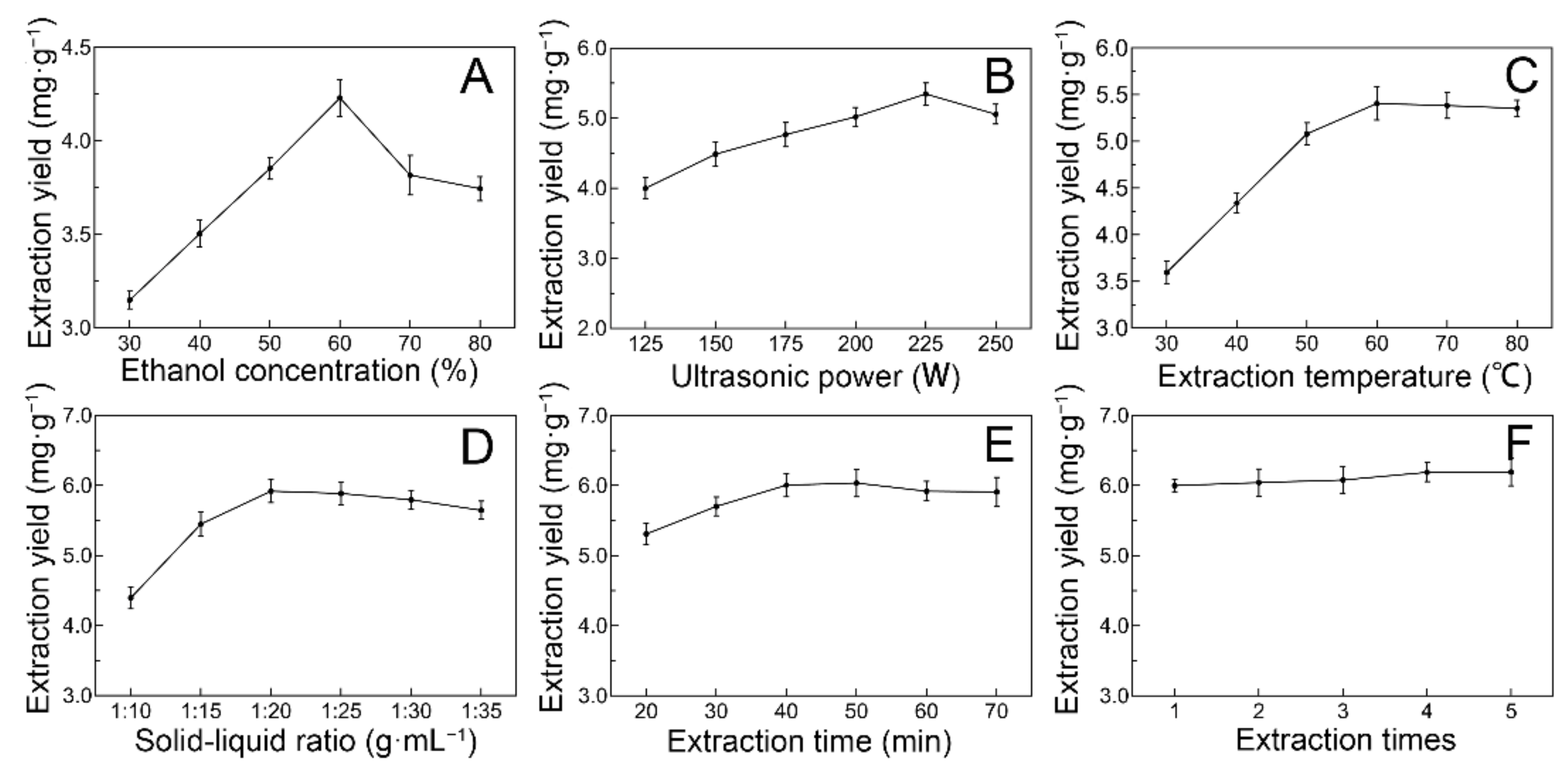
2.1. Selection of Optimal Ultrasonic Extraction Parameters of JMBF by Single-Factor Test
2.2. Contribution of Different Parameters to the Extraction Yield of JMBF
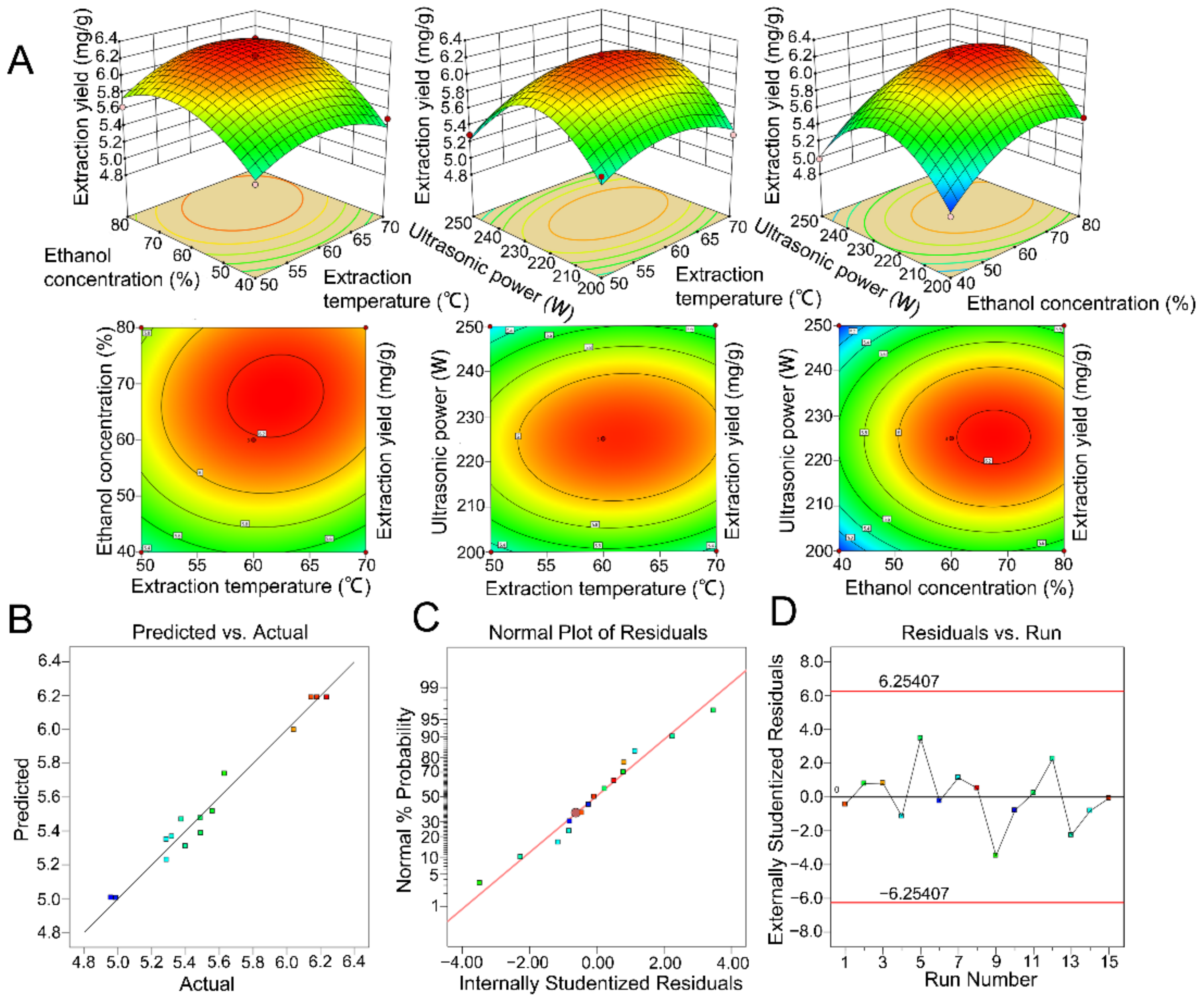
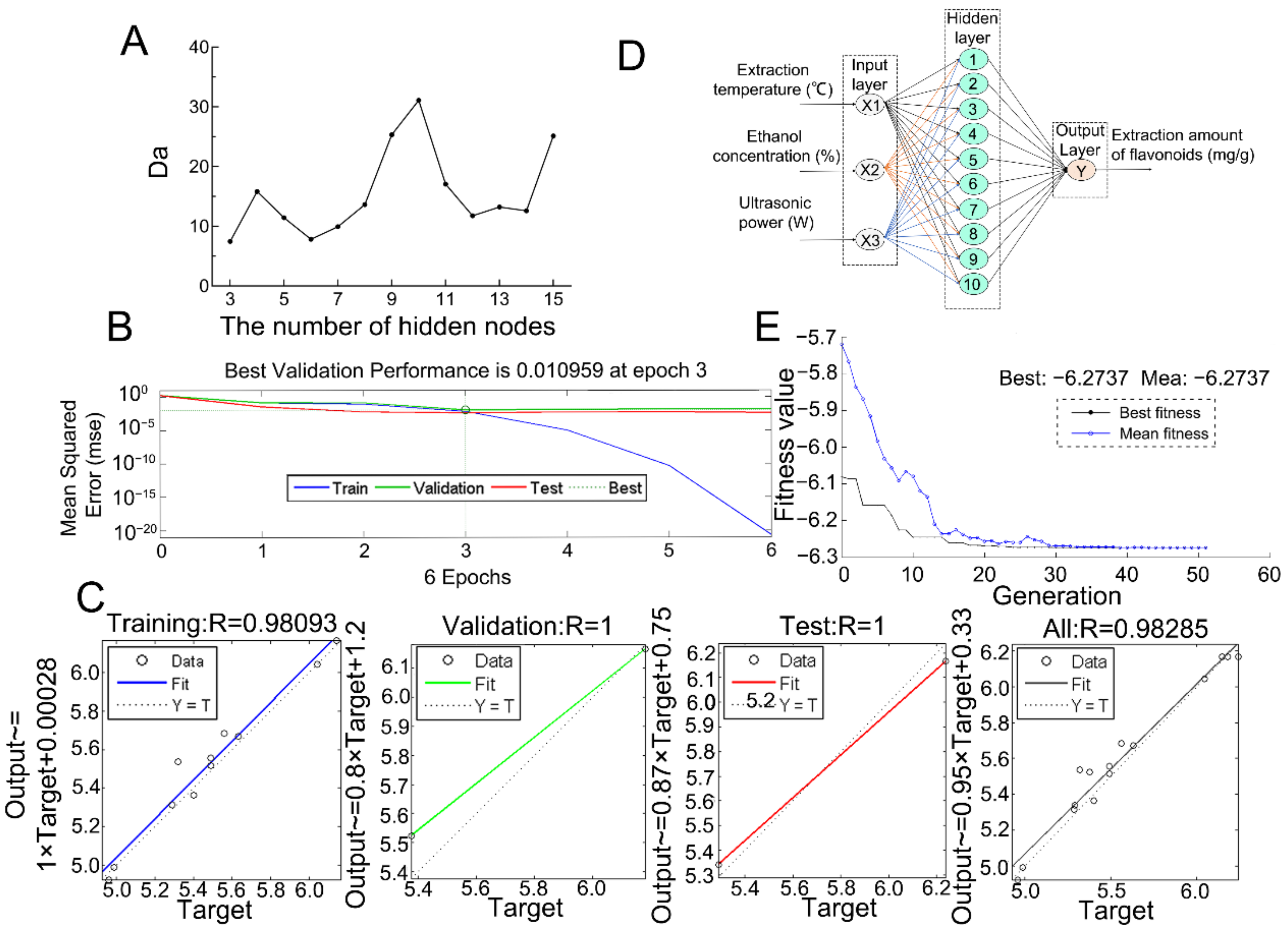
2.3. Optimization of the Significant Parameters Using Response Surface Methodology and Artificial Neural Network–Genetic Algorithm
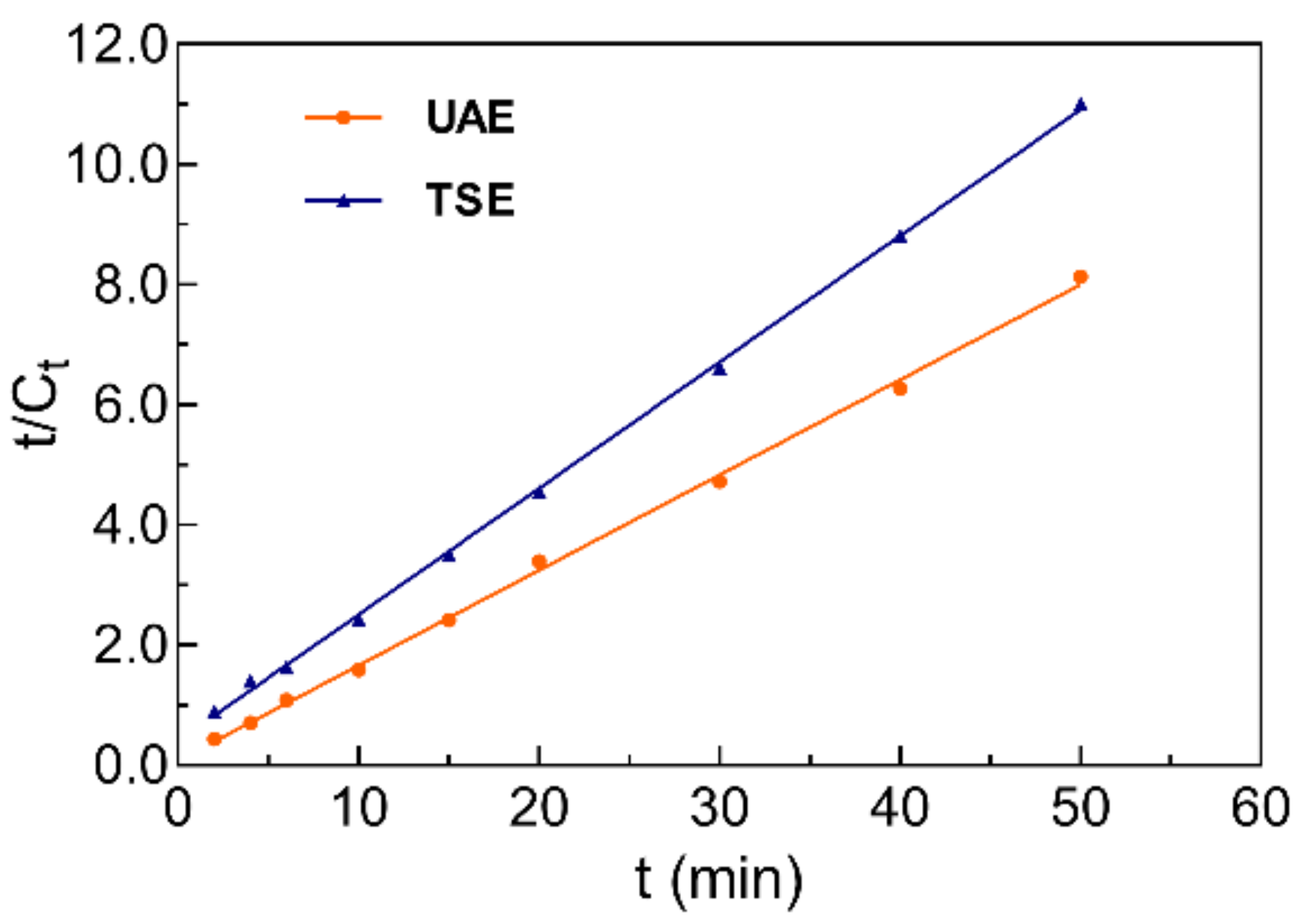
2.4. Kinetic Study of Bath Ultrasonic-Assisted Extraction and Traditional Solvent Extraction Procedure
2.5. Comparison of the Optimal UAE with TSE Procedure
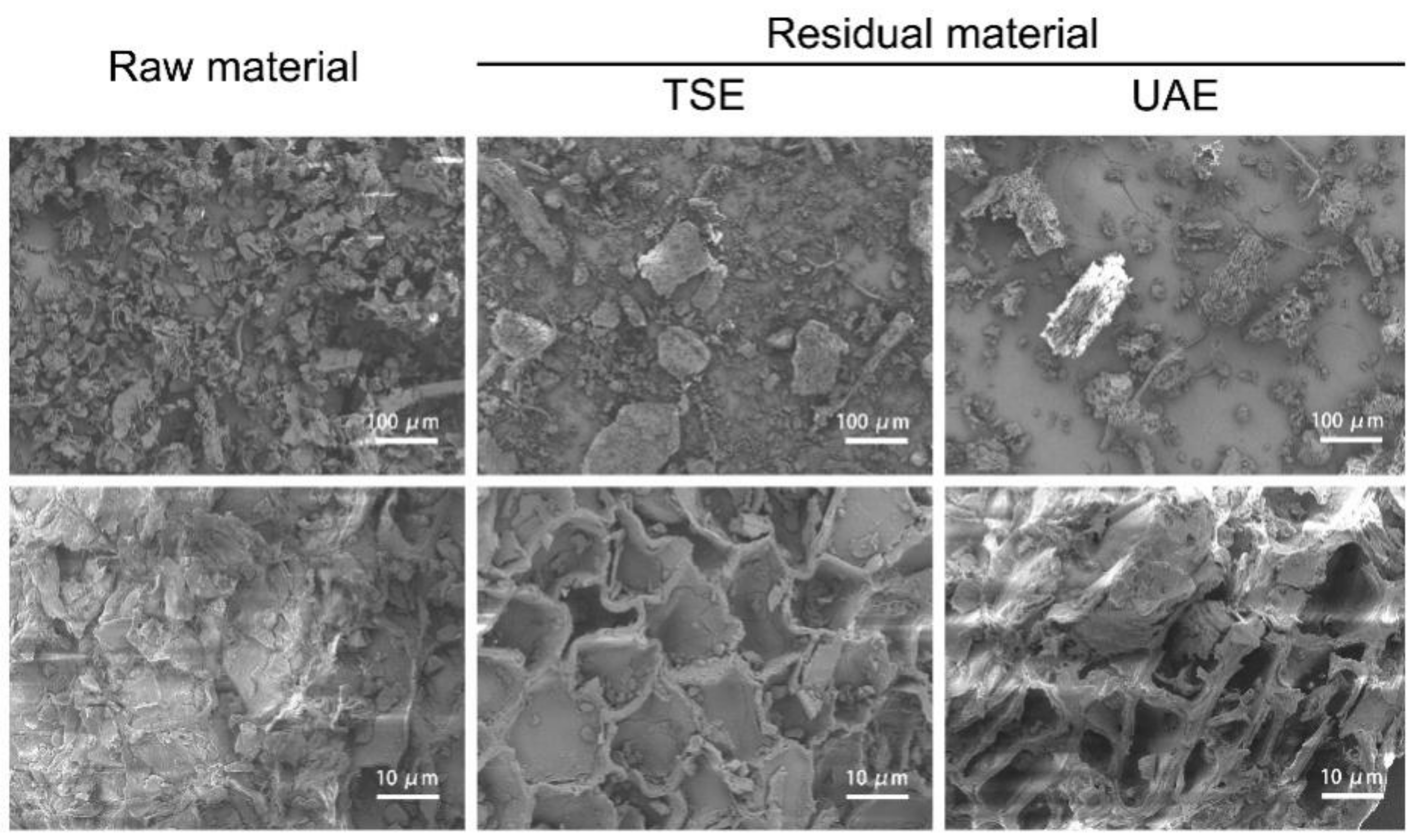
2.5.1. Microscopic Structures of J. mandshurica Treated with Different Extraction Techniques
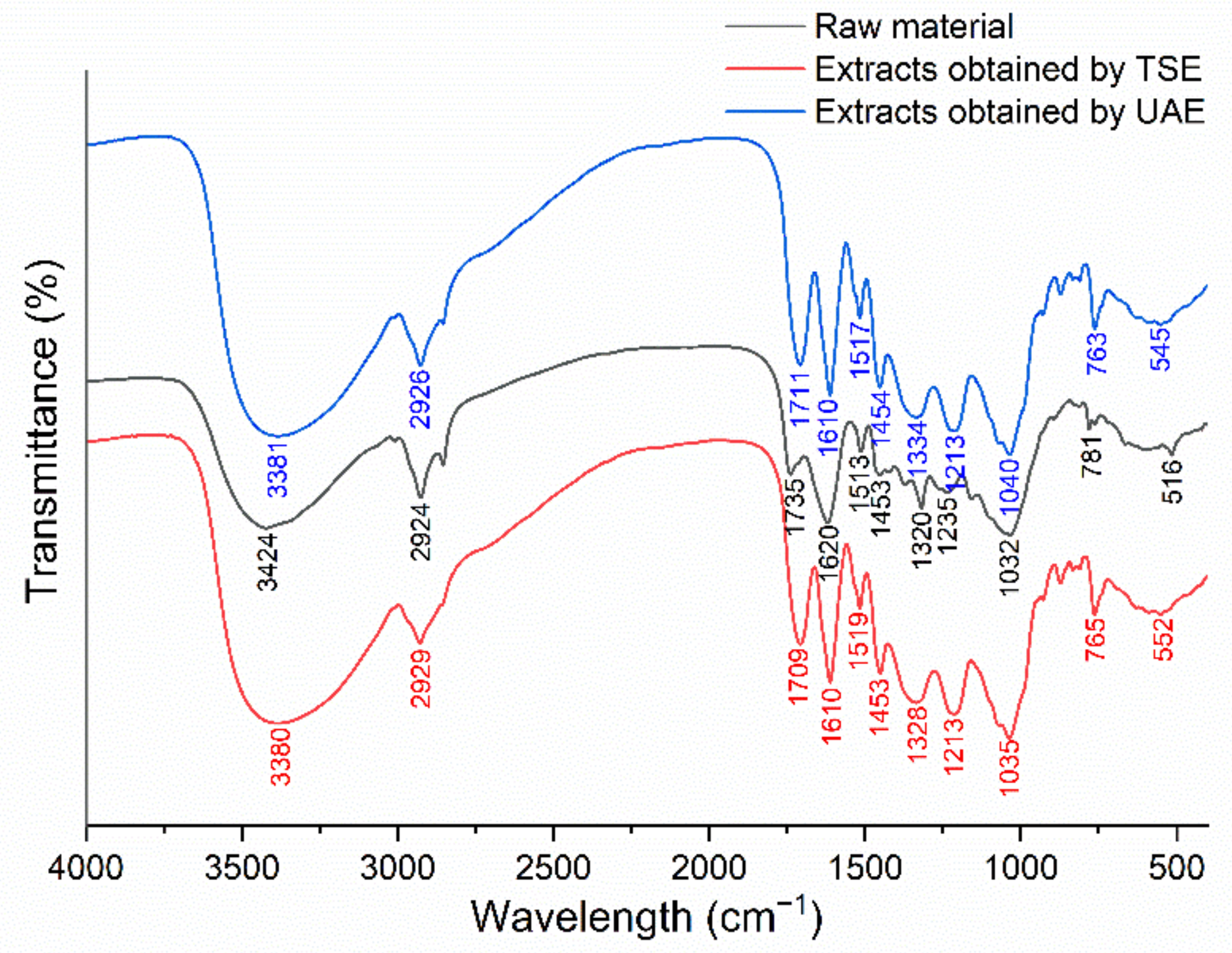
2.5.2. FTIR Spectroscopy Analysis of J. mandshurica-Derived Flavonoids Obtained Using Different Extraction Techniques
2.6. Antioxidant Activity of J. mandshurica-Derived Flavonoids
3. Materials and Methods
3.1. Material
3.2. Optimization of UAE Conditions of Flavonoids from J. mandshurica
3.2.1. Extraction Procedures
3.2.2. Single-Factor Test
3.2.3. Plackett–Burman Design Experiments
3.2.4. Box–Behnken Design Experiments
3.2.5. Verification Test
3.2.6. Analysis of Flavonoid Amount in Extracts
3.3. Traditional Solvent Extraction (TSE)
3.4. Study on Kinetics of Batch Extraction
3.5. Scanning Electron Microscopy (SEM)
3.6. Fourier-Transform Infrared Spectroscopy (FTIR)
3.7. Antioxidant Activity of the J. mandshurica-Derived Flavonoids
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Appendix A
| Source | DF | SS | MS | F | Pr > F |
|---|---|---|---|---|---|
| X1 | 1 | 1.1312 | 1.1312 | 17.9156 | 0.0241 |
| X2 | 1 | 4.3681 | 4.3681 | 69.1791 | 0.0036 |
| X3 | 1 | 0.0081 | 0.0081 | 0.1283 | 0.7439 |
| X4 | 1 | 0.8740 | 0.8740 | 13.8424 | 0.0338 |
| X5 | 1 | 0.2679 | 0.2679 | 4.2429 | 0.1315 |
| X6 | 1 | 0.1347 | 0.1347 | 2.1327 | 0.2403 |
| X7 | 1 | 0.0296 | 0.0296 | 0.4694 | 0.5424 |
| X8 | 1 | 0.0536 | 0.0536 | 0.8485 | 0.4249 |
| Model | 8 | 6.8673 | 0.8584 | 13.5949 | 0.0275 |
| Error | 3 | 0.1894 | 0.0631 | ||
| Total | 11 | 7.0567 |
| Level | Factors | ||||
|---|---|---|---|---|---|
| Ethanol Concentration (X1, %) | Extraction Temperature (X2, °C) | Extraction Time (X3, min) | Ultrasonic Power (X4, W) | Solid–Liquid Ratio (X5, g·mL−1) | |
| −1 | 40 | 50 | 40 | 200 | 1:10 |
| 1 | 70 | 75 | 60 | 250 | 1:30 |
References
- Zhou, Y.Y.; Guo, S.; Wang, Y.; Song, H.J.; Gao, H.R.; Zhang, X.J.; Sun, Y.P.; Liu, Y.; Yang, B.Y.; Kuang, H.X. α-Tetralone glycosides from the green walnut husks of Juglans mandshurica Maxim. and their cytotoxic activities. Nat. Prod. Res. 2020, 34, 1805–1813. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Li, Y.; Dai, Y.; Peng, J. Natural products for the treatment of type 2 diabetes mellitus: Pharmacology and mechanisms. Pharmacol. Res. 2018, 130, 451–465. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-derived natural products in cancer research: Extraction, mechanism of action, and drug formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, T.; Fang, L.; Liu, C.L.; Liu, X.T.; Li, H.M.; Shi, J.H.; Li, M.H.; Min, W.H. Peptides from walnut (Juglans mandshurica Maxim.) protect hepatic HepG2 cells from high glucose-induced insulin resistance and oxidative stress. Food Funct. 2020, 11, 8112–8121. [Google Scholar] [CrossRef]
- Luan, F.; Wang, Z.Y.; Yang, Y.; Ji, Y.; Lv, H.Z.; Han, K.Q.; Liu, D.H.; Shang, X.F.; He, X.R.; Zeng, N. Juglans mandshurica Maxim.: A review of its traditional usages, phytochemical constituents, and pharmacological properties. Front. Pharmacol. 2021, 11, 569800. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, Y.; Zhu, J.; Li, H.; Mao, J.; Jin, X.; Wang, X.; Du, Y.; Lu, J. The anti-tumor effect and biological activities of the extract JMM6 from the stem-barks of the Chinese Juglans mandshurica Maxim on human hepatoma cell line BEL-7402. Afr. J. Tradit. Complement. Altern. Med. 2012, 10, 258–269. [Google Scholar] [CrossRef] [Green Version]
- Sun, Z.; Zhao, M.; Zuo, L.; Zhou, S.; Fan, F.; Jia, Q.; Xue, L.; Li, H.; Kang, J.; Zhang, X. Rapid qualitative profiling and quantitative analysis of Juglandis mandshuricae Cortex and seven flavonoids by ultra-high performance liquid chromatography-quadrupole/orbitrap high-resolution mass spectrometry. J. Sep. Sci. 2022, 45, 518–528. [Google Scholar] [CrossRef]
- Jucá, M.M.; Cysne Filho, F.; de Almeida, J.C.; Mesquita, D.; Barriga, J.; Dias, K.; Barbosa, T.M.; Vasconcelos, L.C.; Leal, L.; Ribeiro, J.E.; et al. Flavonoids: Biological activities and therapeutic potential. Nat. Prod. Res. 2020, 34, 692–705. [Google Scholar] [CrossRef]
- Lou, L.L.; Cheng, Z.Y.; Guo, R.; Yao, G.D.; Song, S.J. Alkaloids from Juglans mandshurica maxim induce distinctive cell death in hepatocellular carcinoma cells. Nat. Prod. Res. 2019, 33, 911–914. [Google Scholar] [CrossRef] [PubMed]
- Huo, J.; Du, X.; Sun, G.; Meng, Y.; Wang, W. Comparison of the chemical profiles of fresh-raw and dry-processed Juglans mandshurica. J. Sep. Sci. 2017, 40, 646–662. [Google Scholar] [CrossRef]
- Kopustinskiene, D.M.; Jakstas, V.; Savickas, A.; Bernatoniene, J. Flavonoids as anticancer agents. Nutrients 2020, 12, 457. [Google Scholar] [CrossRef] [Green Version]
- Raffa, D.; Maggio, B.; Raimondi, M.V.; Plescia, F.; Daidone, G. Recent discoveries of anticancer flavonoids. Eur. J. Med. Chem. 2017, 142, 213–228. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Zhang, Y.; Bao, Y.; Wu, Y.; Sun, L.; Yu, C.; Huang, Y.; Wang, E.; Li, Y. Secondary metabolites from the stem bark of Juglans mandshurica. Biochem. Syst. Ecol. 2013, 51, 184–188. [Google Scholar] [CrossRef]
- Pham, D.C.; Nguyen, H.C.; Nguyen, T.L.; Ho, H.L.; Trinh, T.K.; Riyaphan, J.; Weng, C.F. Optimization of ultrasound-assisted extraction of flavonoids from Celastrus hindsii leaves using response surface methodology and evaluation of their antioxidant and antitumor activities. Biomed. Res. Int. 2020, 15, 3497107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, J.O.; de Souza, M.C.; da Silva, L.C.; Lachos-Perez, D.; Torres-Mayanga, P.C.; Machado, A.; Forster-Carneiro, T.; Vázquez-Espinosa, M.; González-de-Peredo, A.V.; Barbero, G.F.; et al. Extraction of flavonoids from natural sources using modern techniques. Front. Chem. 2020, 8, 507887. [Google Scholar] [CrossRef] [PubMed]
- Ghitescu, R.E.; Volf, I.; Carausu, C.; Bühlmann, A.M.; Gilca, I.A.; Popa, V.I. Optimization of ultrasound-assisted extraction of polyphenols from spruce wood bark. Ultrason. Sonochem. 2015, 22, 535–541. [Google Scholar] [CrossRef] [PubMed]
- Nie, J.; Chen, D.; Ye, J.; Lu, Y.; Dai, Z. Optimization and kinetic modeling of ultrasonic-assisted extraction of fucoxanthin from edible brown algae Sargassum fusiforme using green solvents. Ultrason. Sonochem. 2021, 77, 105671. [Google Scholar] [CrossRef] [PubMed]
- Oroian, M.; Ursachi, F.; Dranca, F. Influence of ultrasonic amplitude, temperature, time and solvent concentration on bioactive compounds extraction from propolis. Ultrason. Sonochem. 2020, 64, 105021. [Google Scholar] [CrossRef] [PubMed]
- Christou, A.; Stavrou, I.J.; Kapnissi-Christodoulou, C.P. Continuous and pulsed ultrasound-assisted extraction of carob’s antioxidants: Processing parameters optimization and identification of polyphenolic composition. Ultrason. Sonochem. 2021, 76, 105630. [Google Scholar] [CrossRef] [PubMed]
- Anaya-Esparza, L.M.; Ramos-Aguirre, D.; Zamora-Gasga, V.M.; Yahia, E.; Montalvo-González, E. Optimization of ultrasonic-assisted extraction of phenolic compounds from Justicia spicigera leaves. Food Sci. Biotechnol. 2018, 27, 1093–1102. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Zhang, Z.; Sun, D.W.; Sivagnanam, S.P.; Tiwari, B.K. Combination of emerging technologies for the extraction of bioactive compounds. Crit. Rev. Food Sci. Nutr. 2020, 60, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.S.; Fan, M.Y.; Hu, J.W.; Ruan, W.Q.; Wu, X.L.; Wei, X.H. Artificial intelligence based optimization for the Se(IV) removal from aqueous solution by reduced graphene oxide-supported nanoscale zero-valent iron composites. Materials 2018, 11, 428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarley, C.; Silveira, G.; Dos Santos, W.; Matos, G.; Da Silva, E.; Bezerra, M.; Miro, M.; Ferreira, S. Chemometric tools in electroanalytical chemistry: Methods for optimization based on factorial design and response surface methodology. Microchem. J. 2009, 92, 58–67. [Google Scholar] [CrossRef]
- Stalikas, C.; Fiamegos, Y.; Sakkas, V.; Albanis, T. Developments on chemometric approaches to optimize and evaluate microextraction. J. Chromatogr. A 2009, 1216, 175–189. [Google Scholar] [CrossRef] [PubMed]
- Peris-Díaz, M.D.; Rodak, O.; Sweeney, S.R.; Krężel, A.; Sentandreu, E. Chemometrics-assisted optimization of liquid chromatography-quadrupole-time-of-flight mass spectrometry analysis for targeted metabolomics. Talanta 2019, 199, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.Q.; Gan, R.Y.; Ge, Y.Y.; Zhang, D.; Corke, A.H. Ultrasonic treatment increases extraction rate of common bean (Phaseolus vulgaris L.) antioxidants. Antioxidants 2019, 8, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benmeziane, A.; Boulekbache-Makhlouf, L.; Mapelli-Brahm, P.; Khaled Khodja, N.; Remini, H.; Madani, K.; Meléndez-Martínez, A.J. Extraction of carotenoids from cantaloupe waste and determination of its mineral composition. Food Res. Int. 2018, 111, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zheng, Y.; Lin, Z.; Han, X.; Liao, W. Macroporous resin purification and characterization of flavonoids from Platycladus orientalis (L.) Franco and their effects on macrophage inflammatory response. Food Funct. 2017, 8, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Senoner, T.; Dichtl, W. Oxidative stress in cardiovascular diseases: Still a therapeutic target? Nutrients 2019, 11, 2090. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poprac, P.; Jomova, K.; Simunkova, M.; Kollar, V.; Rhodes, C.J.; Valko, M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol. Sci. 2017, 38, 592–607. [Google Scholar] [CrossRef] [PubMed]
- Neha, K.; Haider, M.R.; Pathak, A.; Yar, M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019, 178, 687–704. [Google Scholar] [CrossRef] [PubMed]
- Pohl, F.; Kong Thoo Lin, P. The potential use of plant natural products and plant extracts with antioxidant properties for the prevention/treatment of neurodegenerative diseases: In vitro, in vivo and clinical trials. Molecules 2018, 23, 3283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.P.; Li, Y.; Meng, X.; Zhou, T.; Zhou, Y.; Zheng, J.; Zhang, J.J.; Li, H.B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017, 18, 96. [Google Scholar] [CrossRef]
- Kamiyama, M.; Shibamoto, T. Flavonoids with potent antioxidant activity found in young green barley leaves. J. Agric. Food Chem. 2012, 60, 6260–6267. [Google Scholar] [CrossRef] [PubMed]
- El-Desoky, A.H.; Abdel-Rahman, R.F.; Ahmed, O.K.; El-Beltagi, H.S.; Hattori, M. Anti-inflammatory and antioxidant activities of naringin isolated from Carissa carandas L.: In vitro and in vivo evidence. Phytomedicine 2018, 42, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wen, Y.Q.; Xue, C.H.; Xu, L.L.; Wang, X.H.; Bi, S.J.; Xue, Q.Q.; Zhang, T.; Xue, Y.; Li, Z.J.; Chen, G.D.; et al. Application of Plackett-Burman design in screening of natural antioxidants suitable for anchovy oil. Antioxidants 2019, 8, 627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, F.; Chen, L.; Jiang, S.; He, J.; Zhang, X.; Peng, J.; Xu, Q.; Li, R. Optimization of methazolamide-loaded solid lipid nanoparticles for ophthalmic delivery using Box-Behnken design. J. Liposome Res. 2014, 24, 171–181. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Wang, R.; Zhang, S.J.; Li, D.Y.; Sun, H.Y.; Wang, L.M. Prediction of pilling of polyester-cotton blended woven fabric using artificial neural network models. J. Eng. Fiber. Fabr. 2020, 15, 1558925019900152. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Liu, G.; Dou, Y.; Liang, L.; Zhang, H.; Ren, Y. Quantitative analysis of diclofenac sodium powder via near-infrared spectroscopy combined with artificial neural network. J. Pharm. Biomed. Anal. 2009, 50, 158–163. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.X.; Xing, G.Y.; Li, Y.T.; Song, J.; Wang, Y.Z.; Meng, Q.F.; Lu, J.H.; Zhou, Y.L.; Liu, Y.; Wang, D.; et al. The optimization of Marasmius androsaceus submerged fermentation conditions in five-liter fermentor. Saudi J. Biol. Sci. 2016, 23, S99–S105. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Lu, M.F.; Liao, H.B.; Li, Y.X.; Han, W.; Yuan, K. Content determination of the flavonoids in the different parts and different species of Abelmoschus esculentus L. by reversed phase-high performance liquid chromatograph and colorimetric method. Pharmacogn. Mag. 2014, 10, 278–284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, R.; Bhunia, B.; Mondal, A.; Kanti Bandyopadhyay, T.; Devi, I.; Oinam, G.; Prasanna, R.; Abraham, G.; Nath Tiwari, O. Statistical optimization of process parameters for improvement of phycobiliproteins (PBPs) yield using ultrasound-assisted extraction and its kinetic study. Ultrason. Sonochem. 2020, 60, 104762. [Google Scholar] [CrossRef] [PubMed]
- Perumal, A.B.; Li, X.; Su, Z.; He, Y. Preparation and characterization of a novel green tea essential oil nanoemulsion and its antifungal mechanism of action against Magnaporthae oryzae. Ultrason. Sonochem. 2021, 76, 105649. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Taehwan Kim, A.; Liu, X.; Yan, L.; Moo Kim, S. Antioxidant and antidiabetic activities of vanadium-binding protein and trifuhalol A. J. Food Biochem. 2020, 44, e13540. [Google Scholar] [CrossRef] [PubMed]
- Prommaban, A.; Sriyab, S.; Marsup, P.; Neimkhum, W.; Sirithunyalug, J.; Anuchapreeda, S.; To-Anun, C.; Chaiyana, W. Comparison of chemical profiles, antioxidation, inhibition of skin extracellular matrix degradation, and anti-tyrosinase activity between mycelium and fruiting body of Cordyceps militaris and Isaria tenuipes. Pharm. Biol. 2022, 60, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Su, X.Y.; Wang, Z.Y.; Liu, J.R. In vitro and in vivo antioxidant activity of Pinus koraiensis seed extract containing phenolic compounds. Food Chem. 2009, 117, 681–686. [Google Scholar] [CrossRef]


| Run | X1 | X2 | X3 | X4 | X5 | X6 | X7 | X8 | Y (mg·g−1) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 3.65 ± 0.33 |
| 2 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | −1 | 4.29 ± 0.14 |
| 3 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | 4.26 ± 0.24 |
| 4 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | 5.41 ± 0.14 |
| 5 | 1 | −1 | −1 | −1 | 1 | 1 | 1 | −1 | 5.87 ± 0.17 |
| 6 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 6.16 ± 0.11 |
| 7 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 1 | 5.95 ± 0.14 |
| 8 | 1 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 5.74 ± 0.14 |
| 9 | 1 | −1 | 1 | −1 | −1 | −1 | 1 | 1 | 4.91 ± 0.35 |
| 10 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | −1 | 4.44 ± 0.09 |
| 11 | −1 | −1 | 1 | 1 | 1 | −1 | 1 | 1 | 5.39 ± 0.06 |
| 12 | 1 | −1 | 1 | 1 | −1 | 1 | −1 | −1 | 4.76 ± 0.24 |
| Run | Extraction Temperature (X1, °C) | Ethanol Concentration (X2, %) | Ultrasonic Power (X3, W) | Extraction Rate (Y, mg·g−1) |
|---|---|---|---|---|
| 1 | 50 (−1) | 40 (−1) | 225 (0) | 5.32 ± 0.11 |
| 2 | 50 (−1) | 80 (1) | 225 (0) | 5.63 ± 0.13 |
| 3 | 70 (1) | 40 (−1) | 225 (0) | 5.49 ± 0.17 |
| 4 | 70 (1) | 80 (1) | 225 (0) | 6.04 ± 0.14 |
| 5 | 60 (0) | 40 (−1) | 200 (−1) | 4.96 ± 0.22 |
| 6 | 60 (0) | 40 (−1) | 250 (1) | 4.99 ± 0.14 |
| 7 | 60 (0) | 80 (1) | 200 (−1) | 5.49 ± 0.15 |
| 8 | 60 (0) | 80 (1) | 250 (1) | 5.56 ± 0.06 |
| 9 | 50 (−1) | 60 (0) | 200 (−1) | 5.40 ± 0.09 |
| 10 | 70 (1) | 60 (0) | 200 (−1) | 5.29 ± 0.17 |
| 11 | 50 (−1) | 60 (0) | 250 (1) | 5.29 ± 0.18 |
| 12 | 70 (1) | 60 (0) | 250 (1) | 5.38 ± 0.08 |
| 13 | 60 (0) | 60 (0) | 225 (0) | 6.15 ± 0.05 |
| 14 | 60 (0) | 60 (0) | 225 (0) | 6.24 ± 0.03 |
| 15 | 60 (0) | 60 (0) | 225 (0) | 6.18 ± 0.12 |
| Source | DF | SS | MS | F | Pr > F |
|---|---|---|---|---|---|
| X1 | 1 | 0.0390 | 0.0390 | 3.2752 | 0.1301 |
| X2 | 1 | 0.4831 | 0.4831 | 40.5320 | 0.0014 |
| X3 | 1 | 0.0008 | 0.0008 | 0.0638 | 0.8107 |
| X1 × X1 | 1 | 0.2111 | 0.2111 | 17.7148 | 0.0084 |
| X1 × X2 | 1 | 0.0143 | 0.0143 | 1.1986 | 0.3235 |
| X1 × X3 | 1 | 0.0100 | 0.0100 | 0.8414 | 0.4011 |
| X2 × X2 | 1 | 0.3973 | 0.3973 | 33.3355 | 0.0022 |
| X2 × X3 | 1 | 0.0004 | 0.0004 | 0.0365 | 0.8561 |
| X3 × X3 | 1 | 1.3804 | 1.3804 | 115.8228 | 0.0001 |
| Model | 9 | 2.3317 | 0.2591 | 21.7385 | 0.0017 |
| (Linear) | 3 | 0.5229 | 0.1743 | 14.6236 | 0.0066 |
| (Quadratic) | 3 | 1.7841 | 0.5947 | 49.8997 | 0.0004 |
| (Cross-product) | 3 | 0.0247 | 0.0082 | 0.6922 | 0.5951 |
| Error | 5 | 0.0596 | 0.0119 | ||
| (Lack of fit) | 3 | 0.0552 | 0.0184 | 8.3424 | 0.1089 |
| (Pure error) | 2 | 0.0044 | 0.0022 | ||
| Total | 14 | 2.3913 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chu, G.; Liang, R.; Wan, C.; Yang, J.; Li, J.; Wang, R.; Du, L.; Lin, R. Ultrasonic-Assisted Extraction of Flavonoids from Juglans mandshurica Maxim.: Artificial Intelligence-Based Optimization, Kinetics Estimation, and Antioxidant Potential. Molecules 2022, 27, 4837. https://doi.org/10.3390/molecules27154837
Chu G, Liang R, Wan C, Yang J, Li J, Wang R, Du L, Lin R. Ultrasonic-Assisted Extraction of Flavonoids from Juglans mandshurica Maxim.: Artificial Intelligence-Based Optimization, Kinetics Estimation, and Antioxidant Potential. Molecules. 2022; 27(15):4837. https://doi.org/10.3390/molecules27154837
Chicago/Turabian StyleChu, Guodong, Rui Liang, Chenmeng Wan, Jing Yang, Jing Li, Ruinan Wang, Linna Du, and Ruixin Lin. 2022. "Ultrasonic-Assisted Extraction of Flavonoids from Juglans mandshurica Maxim.: Artificial Intelligence-Based Optimization, Kinetics Estimation, and Antioxidant Potential" Molecules 27, no. 15: 4837. https://doi.org/10.3390/molecules27154837
APA StyleChu, G., Liang, R., Wan, C., Yang, J., Li, J., Wang, R., Du, L., & Lin, R. (2022). Ultrasonic-Assisted Extraction of Flavonoids from Juglans mandshurica Maxim.: Artificial Intelligence-Based Optimization, Kinetics Estimation, and Antioxidant Potential. Molecules, 27(15), 4837. https://doi.org/10.3390/molecules27154837





